In Vitro Degradation and Photoactivated Antibacterial Activity of a Hemin-CaP Microsphere-Loaded Coating on Pure Magnesium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
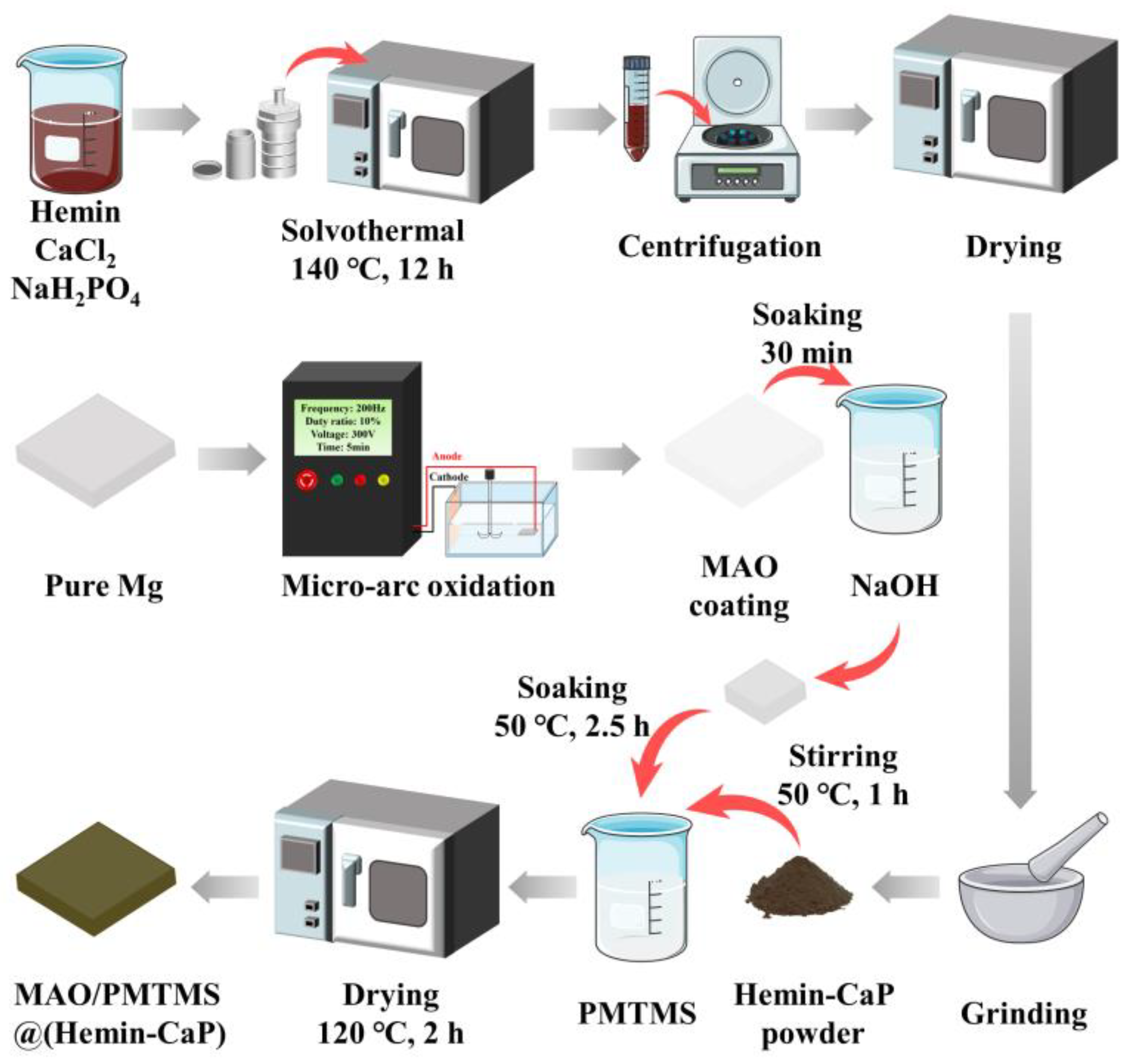
2.2. Preparation of Hemin-CaP Microspheres
2.3. Preparation of MAO Coatings
2.4. Preparation of MAO/PMTMS@(Hemin-CaP) Coatings
2.5. Surface Analysis
2.6. Corrosion Characterization
2.7. Photothermal and Photodynamic Tests
2.8. Antibacterial Test
2.9. Cytotoxicity Test
2.10. Statistical Analysis
3. Results
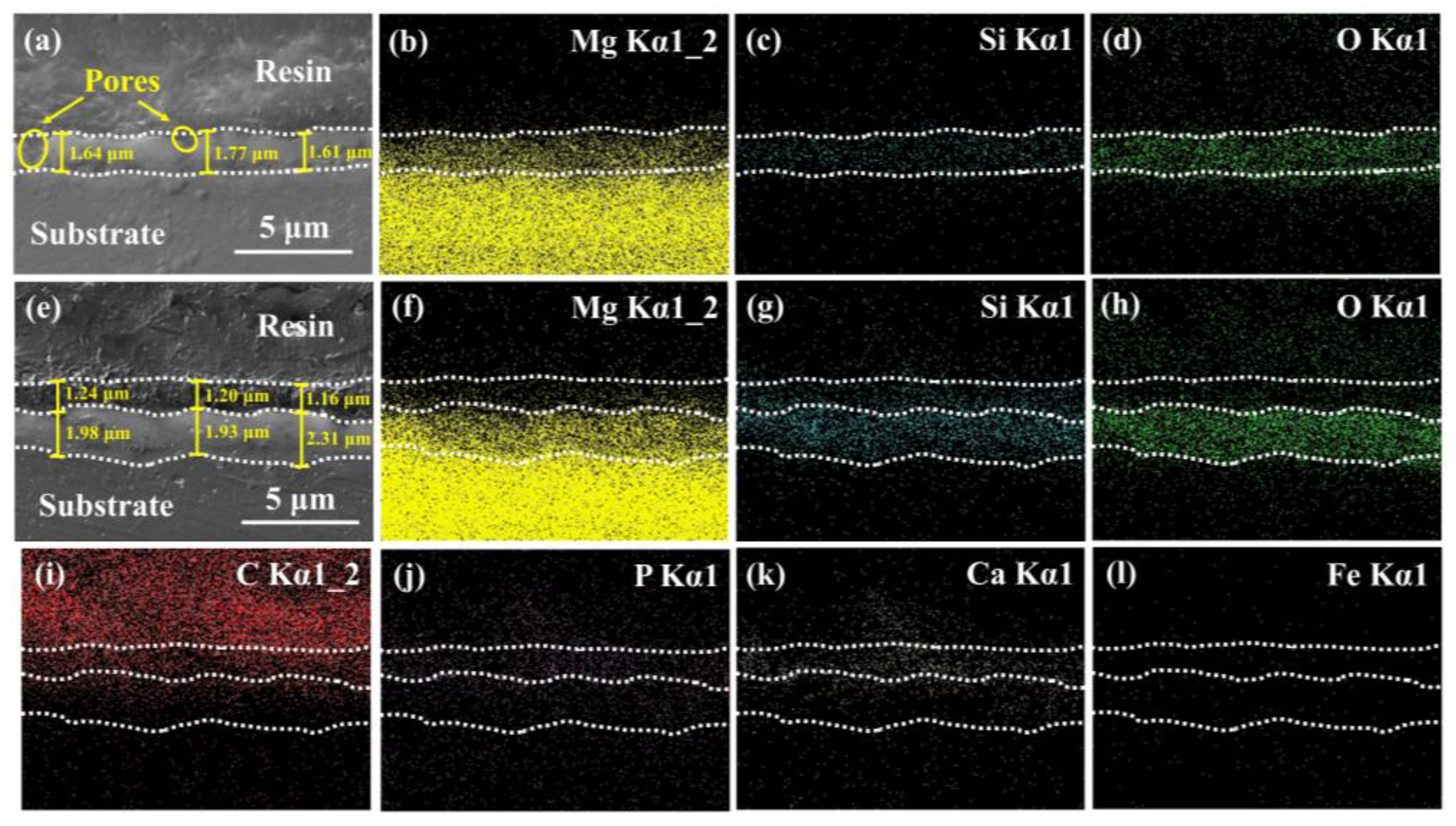
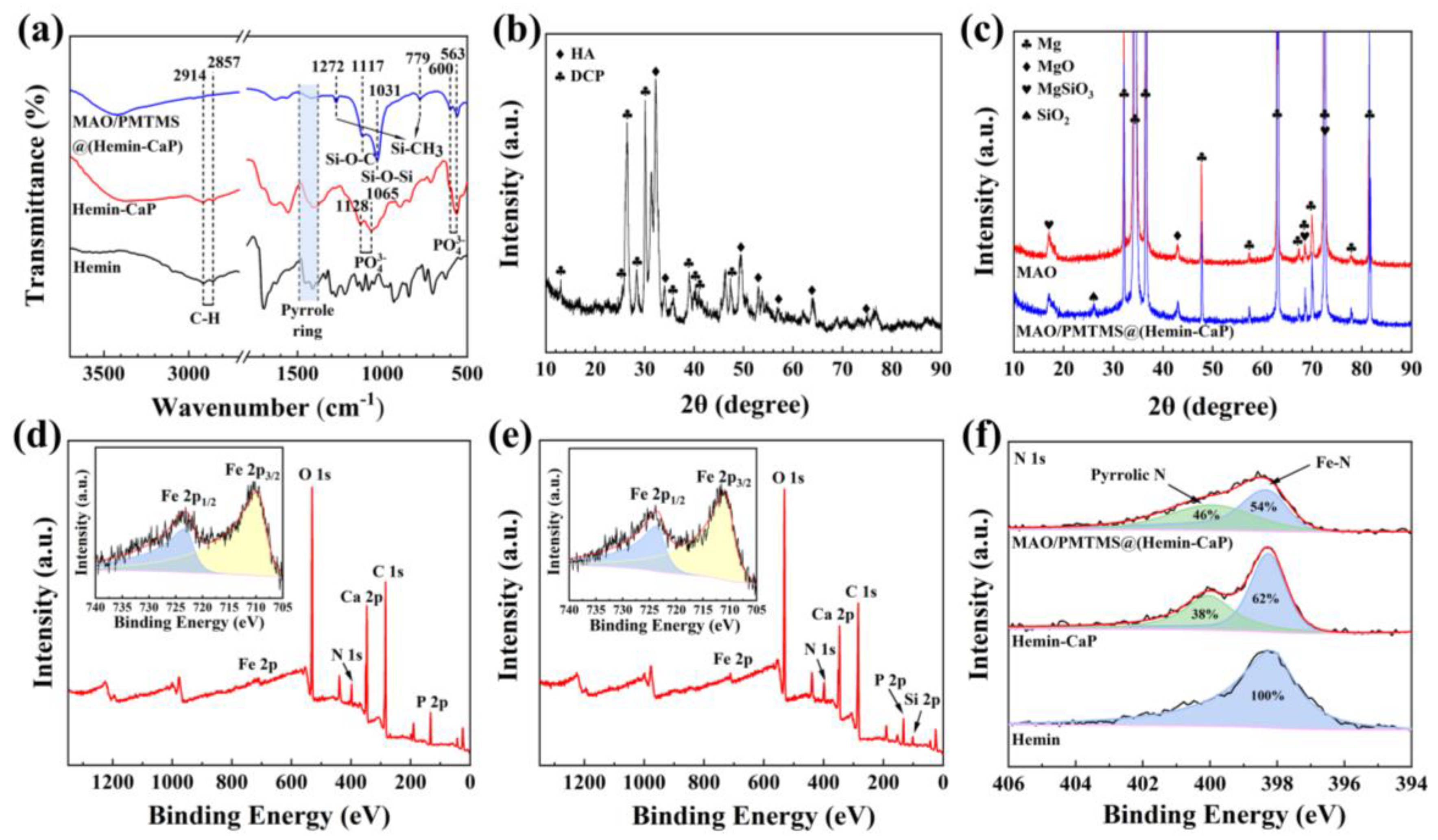
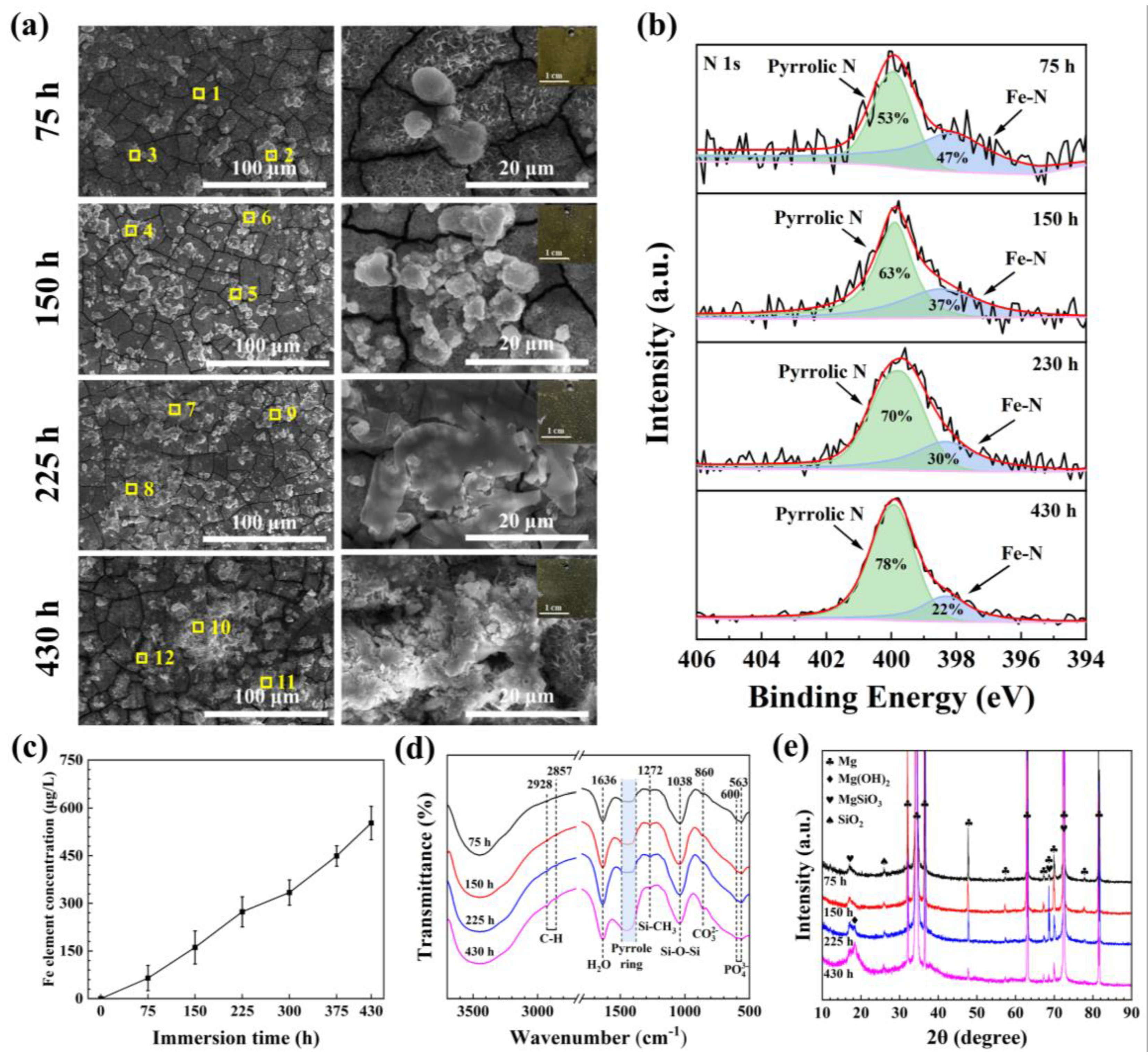
3.1. Surface Analysis
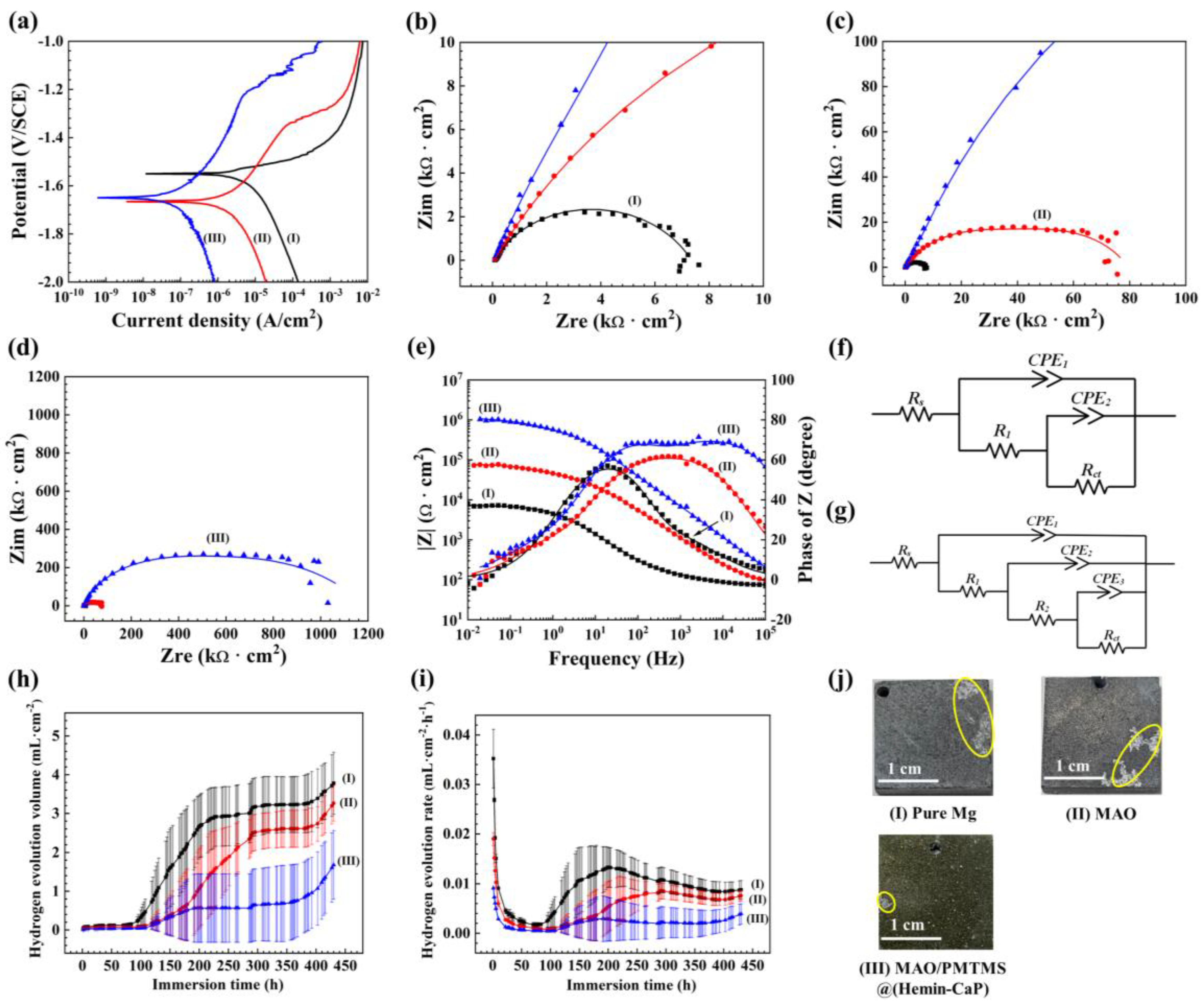
3.2. Corrosion Characterization
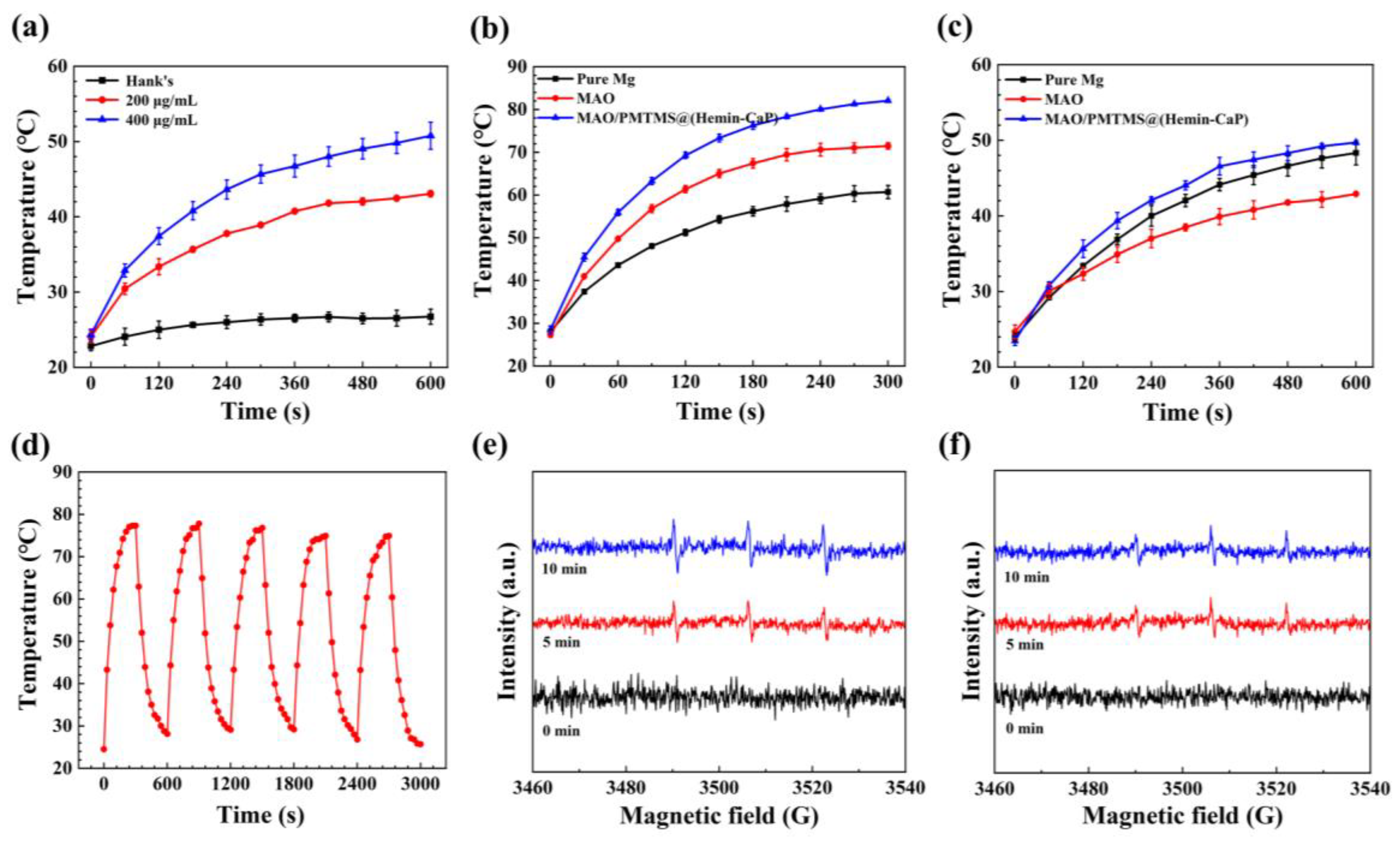
3.3. Photothermal and Photodynamic Effects
3.4. Antibacterial Ability
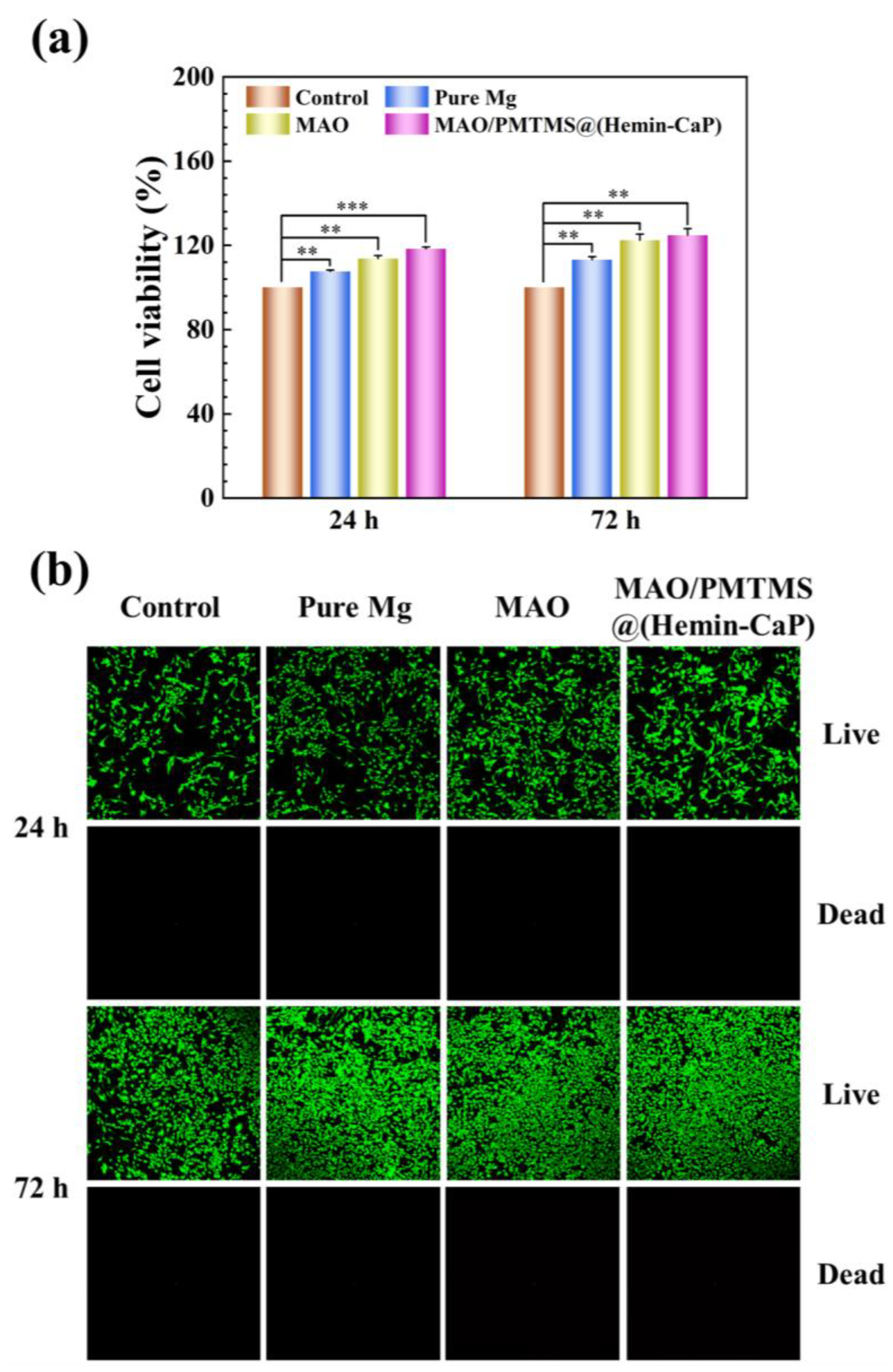
3.5. Cytotoxicity Test
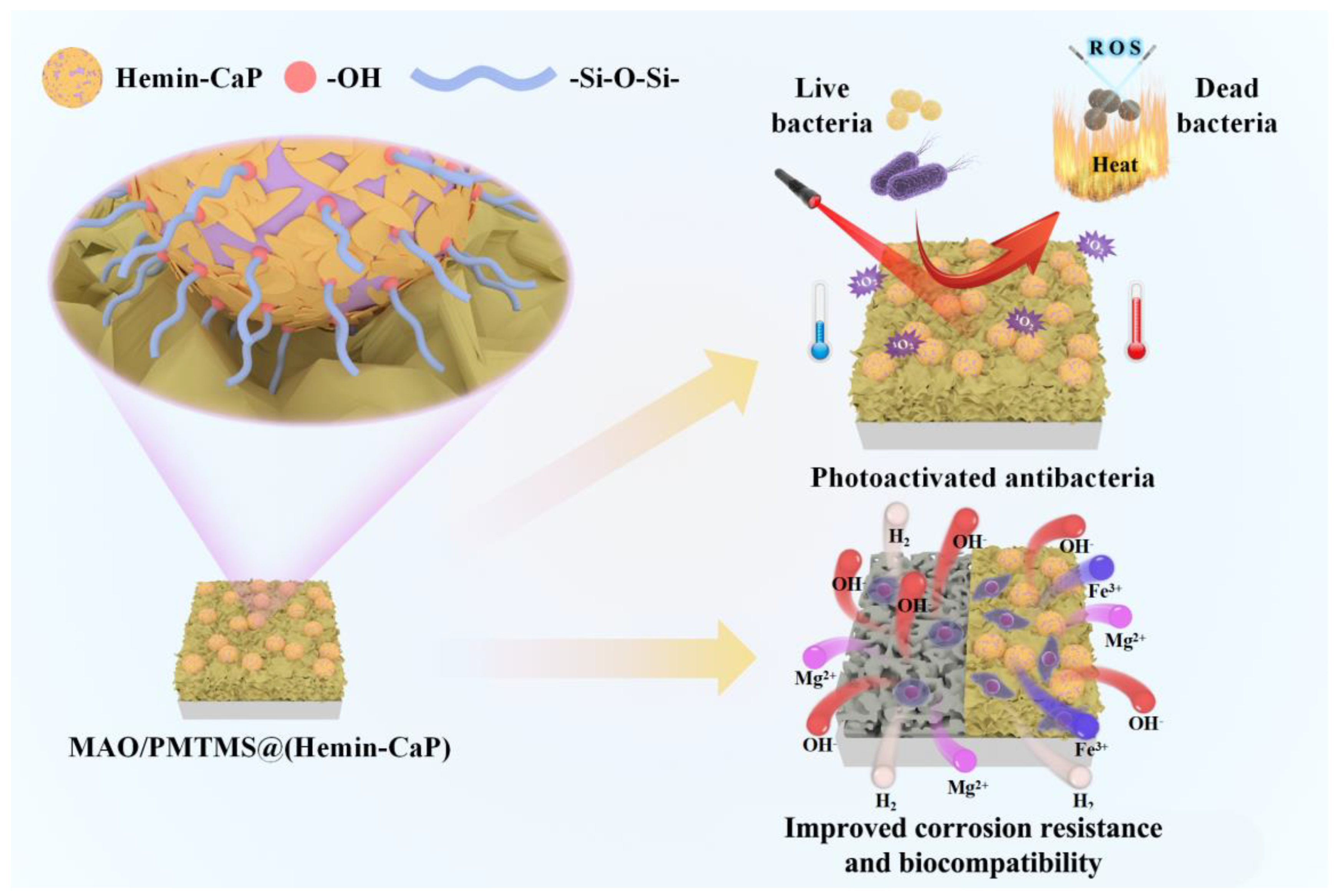
4. Discussion
4.1. Comparison of Coatings on Mg-Based Bone Implants
4.2. Coating Formation and Degradation Mechanisms
- (1)
- After the sample is immersed in Hank’s solution, the microspheres adsorbed on the surface of the MAO/PMTMS@(Hemin-CaP) coating will be continuously degraded. As the flake structures covering the microspheres disappear, the exposed hemin nuclei and the demetallation of hemin will facilitate the occurrence of galvanic corrosion. Since PMTMS acts as a physical barrier, the hydrogen evolution of the sample is not obvious in the early period of immersion.
- (2)
- The corrosion cracks and the permeability of PMTMS become channels for the corrosive medium to penetrate the inner MAO, resulting in a slight release of hydrogen. As the corrosion products continually accumulate in the cracks of the coating, the contact between the solution and substrate is suppressed for a short period of time. The HEV is relatively flat during this period.
- (3)
- With the immersion time extension, the corrosion products become loose and the corrosive medium is once again in contact with the substrate. The galvanic corrosion between Fe3+ ions and the Mg matrix leads to a rapid increase in HEV.
4.3. Antibacterial Activity and Biocompatibility of the Coating
5. Conclusions
- (1)
- The inner nuclei of Hemin-CaP microspheres are hemin and the outer flake structures are DCP and HA. After 808 nm NIR irradiation, the antibacterial efficiency of the Hemin-CaP powder against S. aureus and E. coli reaches as high as 99.9% and 98.3%, respectively, which is attributed to its good photothermal and photodynamic properties.
- (2)
- The MAO/PMTMS@(Hemin-CaP) coating appears as flower-like structures and a cluster of microspheres distributed on the coating surface via physical adsorption. The icorr of the MAO/PMTMS@(Hemin-CaP) coating (4.41 × 10−8 A·cm−2) is two orders of magnitude lower than that of pure Mg (3.12 × 10−6 A·cm−2), indicating the composite coating can provide excellent corrosion protection. Flaky CaP and PMTMS act as physical barriers, restrict the release of Fe3+ ions and prevent the penetration of the corrosion medium, alleviating galvanic corrosion of the Mg substrate.
- (3)
- By virtue of the photothermal and photodynamic performance of the Hemin loaded by microspheres, the antibacterial efficiency of the MAO/PMTMS@(Hemin-CaP) coating against S. aureus and E. coli under 808 nm NIR irradiation is 92.3% and 92.0%, respectively.
- (4)
- The MC3T3-E1 pre-osteoblasts cultured in the 72 h extracts prepared with the MAO/PMTMS@(Hemin-CaP) coating exhibit a broadly healthy fusiform-like shape and display a high degree of spreading regarding morphology. The cell viability is 125%, indicating a positive effect of the coating in promoting bone growth, which is promising for application as bone implants.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, P.; Jia, Z.J.; Zhou, W.H.; Yan, J.L.; Wang, P.; Yuan, W.; Li, Y.Y.; Cheng, Y.; Guan, Z.P.; Zheng, Y.F. Osteogenic and pH stimuli-responsive self-healing coating on biomedical Mg-1Ca alloy. Acta Biomater. 2019, 92, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, J.M.; Yu, X.T.; Chen, X.B.; Li, S.Q.; Chen, D.C.; Guan, S.K.; Zeng, R.C.; Cui, L.Y. Corrosion resistance of bioinspired DNA-induced Ca–P coating on biodegradable magnesium alloy. J. Magnes. Alloy 2019, 7, 144–154. [Google Scholar] [CrossRef]
- Cai, L.; Mei, D.; Zhang, Z.Q.; Huang, Y.D.; Cui, L.Y.; Guan, S.K.; Chen, D.C.; Kannan, M.B.; Zheng, Y.F.; Zeng, R.C. Advances in bioorganic molecules inspired degradation and surface modifications on Mg and its alloys. J. Magnes. Alloy 2022, 10, 670–688. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.A.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.K.; Ahn, R.; Tobias, M.E.; Hansen, L.A.; Luke-Marshall, N.R.; Wild, L.; Campagnari, A.A.; Ehrensberger, M.T. Magnesium alloy AZ91 exhibits antimicrobial properties in vitro but not in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 221–227. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, Z.Y.; Zhu, Y.Y.; Zhao, R.F.; Zhang, S.F.; Shi, X.T.; Li, G.Q.; Chen, Z.Y.; Zhao, Y. Degradation resistance and in vitro cytocompatibility of iron-containing coatings developed on WE43 magnesium alloy by micro-arc oxidation. Coatings 2020, 10, 1138. [Google Scholar] [CrossRef]
- Hafili, F.; Chaharmahali, R.; Babaei, K.; Fattah-alhosseini, A. Duty cycle influence on the corrosion behavior of coatings created by plasma electrolytic oxidation on AZ31B magnesium alloy in simulated body fluid. Corros. Commun. 2021, 3, 62–70. [Google Scholar] [CrossRef]
- Heise, S.; Hohlinger, M.; Hernandez, Y.T.; Palacio, J.J.P.; Ortiz, J.A.R.; Wagener, V.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition and characterization of chitosan/bioactive glass composite coatings on Mg alloy substrates. Electrochim. Acta 2017, 232, 456–464. [Google Scholar] [CrossRef]
- Li, X.J.; Shi, H.; Cui, Y.; Pan, K.; Wei, W.; Liu, X.Y. Dextran-caffeic acid/tetraaniline composite coatings for simultaneous improvement of cytocompatibility and corrosion resistance of magnesium alloy. Prog. Org. Coat. 2020, 149, 105928. [Google Scholar] [CrossRef]
- Cui, L.Y.; Cheng, S.C.; Liang, L.X.; Zhang, J.C.; Li, S.Q.; Wang, Z.L.; Zeng, R.C. In vitro corrosion resistance of layer-by-layer assembled polyacrylic acid multilayers induced Ca-P coating on magnesium alloy AZ31. Bioact. Mater. 2020, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- He, L.J.; Shao, Y.; Li, S.Q.; Cui, L.Y.; Ji, X.J.; Zhao, Y.B.; Zeng, R.C. Advances in layer-by-layer self-assembled coatings upon biodegradable magnesium alloys. Sci. China Mater. 2021, 64, 2093–2106. [Google Scholar] [CrossRef]
- Wu, D.L.; Yao, Z.Q.; Sun, X.Y.; Liu, X.D.; Liu, L.; Zhang, R.L.; Wang, C.G. Mussel-tailored carbon fiber/carbon nanotubes interface for elevated interfacial properties of carbon fiber/epoxy composites. Chem. Eng. J. 2022, 429, 132449. [Google Scholar] [CrossRef]
- Zhang, L.S.; Tong, X.; Lin, J.X.; Li, Y.C.; Wen, C. Enhanced corrosion resistance via phosphate conversion coating on pure Zn for medical applications. Corros. Sci. 2020, 169, 108602. [Google Scholar] [CrossRef]
- Zahedi Asl, V.; Zhao, J.M.; Palizdar, Y.; Junaid Anjum, M. Influence of pH value and Zn/Ce cations ratio on the microstructures and corrosion resistance of LDH coating on AZ31. Corros. Commun. 2022, 5, 73–86. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Zhang, Z.Y.; Wang, D.; Wen, Y.Q.; Peng, N.; Shang, W. ZIF-8-based micro-arc oxidation composite coatings enhanced the corrosion resistance and superhydrophobicity of a Mg alloy. J. Magnes. Alloy 2021, in press. [Google Scholar] [CrossRef]
- Li, L.Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zheng, Y.F.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Jia, Z.J.; Xiong, P.; Shi, Y.Y.; Zhou, W.H.; Cheng, Y.; Zheng, Y.F.; Xi, T.F.; Wei, S.C. Inhibitor encapsulated, self-healable and cytocompatible chitosan multilayer coating on biodegradable Mg alloy: A pH-responsive design. J. Mater. Chem. B 2016, 4, 2498–2511. [Google Scholar] [CrossRef]
- Lu, P.; Fan, H.N.; Liu, Y.; Cao, L.; Wu, X.F.; Xu, X.H. Controllable biodegradability, drug release behavior and hemocompatibility of PTX-eluting magnesium stents. Colloids Surf. B 2011, 83, 23–28. [Google Scholar] [CrossRef]
- Ghanbari, A.; Bordbar-Khiabani, A.; Warchomicka, F.; Sommitsch, C.; Yarmand, B.; Zamanian, A. PEO/Polymer hybrid coatings on magnesium alloy to improve biodegradation and biocompatibility properties. Surf. Interfaces 2023, 36, 102495. [Google Scholar] [CrossRef]
- Cui, L.Y.; Gao, S.D.; Li, P.P.; Zeng, R.C.; Zhang, F.; Li, S.Q.; Han, E.H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Xue, K.; Liang, L.X.; Cheng, S.C.; Liu, H.P.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Wang, Z.L. Corrosion resistance, antibacterial activity and drug release of ciprofloxacin-loaded micro-arc oxidation/silane coating on magnesium alloy AZ31. Prog. Org. Coat. 2021, 158, 106357. [Google Scholar] [CrossRef]
- Li, C.Y.; Fan, X.L.; Cui, L.Y.; Zeng, R.C. Corrosion resistance and electrical conductivity of a nano ATO-doped MAO/methyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2020, 168, 108570. [Google Scholar] [CrossRef]
- Yuan, Z.; He, Y.; Lin, C.C.; Liu, P.; Cai, K.Y. Antibacterial surface design of biomedical titanium materials for orthopedic applications. J. Mater. Sci. Technol. 2021, 78, 51–67. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Chen, H.; Nan, K.H.; Jin, Y.Y.; Sun, L.; Wang, B.L. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J. Mater. Chem. B 2018, 6, 4274–4292. [Google Scholar] [CrossRef] [PubMed]
- Sikder, A.; Chaudhuri, A.; Mondal, S.; Singh, N.D.P. Recent advances on stimuli-responsive combination therapy against multidrug-resistant bacteria and biofilm. ACS Appl. Bio Mater. 2021, 4, 4667–4683. [Google Scholar] [CrossRef]
- Ren, Y.W.; Liu, H.P.; Liu, X.M.; Zheng, Y.F.; Li, Z.Y.; Li, C.Y.; Yeung, K.W.K.; Zhu, S.L.; Liang, Y.Q.; Cui, Z.D.; et al. Photoresponsive materials for antibacterial applications. Cell. Rep. Phys. Sci. 2020, 1, 100245. [Google Scholar] [CrossRef]
- Xie, X.Z.; Mao, C.Y.; Liu, X.M.; Tan, L.; Cui, Z.D.; Yang, X.J.; Zhu, S.L.; Li, Z.Y.; Yuan, X.B.; Zheng, Y.F.; et al. Tuning the bandgap of photo-sensitive polydopamine/Ag3PO4/graphene oxide coating for rapid, noninvasive disinfection of implants. ACS Cent. Sci. 2018, 4, 724–738. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, Y.K.; Teng, W.S.Y.; Zhou, X.Z.; Ye, Y.X.; Zhou, H.; Sun, H.X.; Wang, F.Q.; Liu, A.; Lin, P.; et al. An orthobiologics-free strategy for synergistic photocatalytic antibacterial and osseointegration. Biomaterials 2021, 274, 120853. [Google Scholar] [CrossRef]
- Zhang, D.D.; Cheng, S.; Tan, J.; Xie, J.N.; Zhang, Y.; Chen, S.H.; Du, H.H.; Qian, S.; Qiao, Y.Q.; Peng, F.; et al. Black Mn-containing layered double hydroxide coated magnesium alloy for osteosarcoma therapy, bacteria killing, and bone regeneration. Bioact. Mater. 2022, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Zhou, J.L.; Peng, F.; Tan, J.; Zhang, X.M.; Qian, S.; Qiao, Y.Q.; Zhang, Y.; Liu, X.Y. Mg-Fe LDH sealed PEO coating on magnesium for biodegradation control, antibacteria and osteogenesis. J. Mater. Sci. Technol. 2022, 105, 57–67. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.M.; Cui, Z.D.; Yang, X.J.; Zhu, S.L.; Li, Z.Y.; Yuan, X.B.; Zheng, Y.F.; Yeung, K.W.K.; et al. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv. Mater. 2018, 30, e1801808. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tao, B.L.; He, Y.; Mu, C.Y.; Liu, G.H.; Zhang, J.X.; Liao, Q.; Liu, P.; Cai, K.Y. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 2019, 223, 119479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; An, Y.L.; Wang, X.S.; Cui, L.Y.; Li, S.Q.; Liu, C.B.; Zou, Y.H.; Zhang, F.; Zeng, R.C. In vitro degradation, photo-dynamic and thermal antibacterial activities of Cu-bearing chlorophyllin-induced Ca-P coating on magnesium alloy AZ31. Bioact. Mater. 2022, 18, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Tan, P.H.; Zhao, Z.H.; Cui, L.Y.; Kannan, M.B.; Li, S.Q.; Liu, C.B.; Zou, Y.H.; Zhang, F.; Chen, Z.Y.; et al. In vitro degradation and multi-antibacterial mechanisms of β-cyclodextrin@curcumin embodied Mg(OH)2/MAO coating on AZ31 magnesium alloy. J. Mater. Sci. Technol. 2023, 132, 179–192. [Google Scholar] [CrossRef]
- Wang, L.; Qu, X.Z.; Zhao, Y.X.; Weng, Y.Z.W.; Waterhouse, G.I.N.; Yan, H.; Guan, S.Y.; Zhou, S.Y. Exploiting single atom iron centers in a porphyrin-like MOF for efficient cancer phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 35228–35237. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.F.; Chen, X.H.; Yang, J.A.; Pan, H.B.; Chen, D.F.; Wang, L.N.; Zhang, J.L.; Zhu, D.H.; Wu, S.L.; et al. Fundamental theory of biodegradable metals—Definition, criteria, and design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Gorejová, R.; Haverová, L.; Oriňaková, R.; Oriňak, A.; Oriňak, M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2018, 54, 1913–1947. [Google Scholar] [CrossRef]
- Sahu, A.; Min, K.; Jeon, J.; Yang, H.S.; Tae, G. Catalytic nanographene oxide with hemin for enhanced photodynamic therapy. J Control Release 2020, 326, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.T.; Jin, Y.Y.; Yi, K.; Ju, E.G.; Zhuo, C.Y.; Wei, H.Y.; Wen, X.Q.; Wang, Y.; Li, M.Q.; Tao, Y. Hemin particles-functionalized 3D printed scaffolds for combined photothermal and chemotherapy of osteosarcoma. Chem. Eng. J. 2021, 422, 129919. [Google Scholar] [CrossRef]
- Alsharabasy, A.M.; Pandit, A.; Farras, P. Recent advances in the design and sensing applications of hemin/coordination polymer-based nanocomposites. Adv. Mater. 2021, 33, e2003883. [Google Scholar] [CrossRef]
- Hwang, E.; Jung, H.S. Metal-organic complex-based chemodynamic therapy agents for cancer therapy. ChemComm 2020, 56, 8332–8341. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Shi, X.T.; Wang, Y.; Li, H.Y.; Zhang, S.F.; Zhao, R.F.; Li, G.Q.; Zhang, R.F.; Sheng, Y.; Cao, S.Y.; Zhao, Y.J.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Cui, L.Y.; Wei, G.B.; Han, Z.Z.; Zeng, R.C.; Wang, L.; Zou, Y.H.; Li, S.Q.; Xu, D.K.; Guan, S.K. In vitro corrosion resistance and antibacterial performance of novel tin dioxide-doped calcium phosphate coating on degradable Mg-1Li-1Ca alloy. J. Mater. Sci. Technol. 2019, 35, 254–265. [Google Scholar] [CrossRef]
- Fan, X.L.; Li, C.Y.; Wang, Y.B.; Huo, Y.F.; Li, S.Q.; Zeng, R.C. Corrosion resistance of an amino acid-bioinspired calcium phosphate coating on magnesium alloy AZ31. J. Mater. Sci. Technol. 2020, 49, 224–235. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, H.H.; Pan, H.B.; Zhao, Y. Insight into microbiologically induced corrosion performance of magnesium in tryptic soy broth with S. aureus and E. coli. J. Mater. Sci. Technol. 2022, 115, 221–231. [Google Scholar] [CrossRef]
- He, Y.Z.; Jin, Y.H.; Ying, X.X.; Wu, Q.; Yao, S.L.; Li, Y.Y.; Liu, H.Y.; Ma, G.W.; Wang, X.M. Development of an antimicrobial peptide-loaded mineralized collagen bone scaffold for infective bone defect repair. Regen. Biomater. 2020, 7, 515–525. [Google Scholar] [CrossRef]
- Li, S.L.; Deng, X.Q.; Cheng, H.; Li, X.Z.; Wan, Y.P.; Cao, C.; Yu, J.; Liu, Y.; Yuan, Y.; Wang, K.; et al. Bright near-infrared π-conjugated oligomer nanoparticles for deep-brain three-photon microscopy excited at the 1700 nm window in vivo. ACS Nano 2022, 16, 12480–12487. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Sun, Y.P.; Wei, J.J.; Qiu, X.Y.; Meng, F.H.; Storm, G.; Zhong, Z.Y. Selective transferrin coating as a facile strategy to fabricate BBB-permeable and targeted vesicles for potent RNAi therapy of brain metastatic breast cancer in vivo. J. Control. Release 2021, 337, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhai, Z.H.; Qin, J.Q.; Zhang, X.; Song, Y.J. Pyrolysis of self-assembled hemin on carbon for efficient oxygen reduction reaction. J. Porphyr. Phthalocyanines 2019, 23, 1013–1019. [Google Scholar] [CrossRef]
- Farag, A.A.M. Optical absorption of sodium copper chlorophyllin thin films in UV-vis-NIR region. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.S.; Joshi, M.J. FTIR spectroscopic, thermal and growth morphological studies of calcium hydrogen phosphate dihydrate crystals. Cryst. Res. Technol. 2003, 38, 817–821. [Google Scholar] [CrossRef]
- Rajendran, K.; Keefe, C.D. Growth and characterization of calcium hydrogen phosphate dihydrate crystals from single diffusion gel technique. Cryst. Res. Technol. 2010, 45, 939–945. [Google Scholar] [CrossRef]
- Mulongo-Masamba, R.; El Kassri, T.; Khachani, M.; Arsalane, S.; Halim, M.; El Hamidi, A. Synthesis and thermal dehydroxylation kinetic of anhydrous calcium phosphate monetite CaHPO4. J. Therm. Anal. Calorim. 2015, 124, 171–180. [Google Scholar] [CrossRef]
- Diller, K.; Papageorgiou, A.C.; Klappenberger, F.; Allegretti, F.; Barth, J.V.; Auwarter, W. In vacuo interfacial tetrapyrrole metallation. Chem. Soc. Rev. 2016, 45, 1629–1656. [Google Scholar] [CrossRef]
- Chen, X.; Lu, W.Y.; Xu, T.F.; Li, N.; Qin, D.D.; Zhu, Z.X.; Wang, G.Q.; Chen, W.X. A bio-inspired strategy to enhance the photocatalytic performance of g-C3N4 under solar irradiation by axial coordination with hemin. Appl. Catal. B 2017, 201, 518–526. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Xue, Y.H.; Cao, D.P.; Huang, L.; Chen, J.F.; Dai, L.M. Highly efficient electrocatalysts for oxygen reduction based on 2D covalent organic polymers complexed with non-precious metals. Angew. Chem. Int. Ed. 2014, 53, 2433–2437. [Google Scholar] [CrossRef]
- Dai, Z.F.; Sun, Q.; Liu, X.L.; Bian, C.Q.; Wu, Q.M.; Pan, S.X.; Wang, L.; Meng, X.J.; Deng, F.; Xiao, F.S. Metalated porous porphyrin polymers as efficient heterogeneous catalysts for cycloaddition of epoxides with CO2 under ambient conditions. J. Catal. 2016, 338, 202–209. [Google Scholar] [CrossRef]
- Khan, M.S.; Yang, C.G.; Pan, H.B.; Yang, K.; Zhao, Y. The effect of high temperature aging on the corrosion resistance, mechanical property and antibacterial activity of Cu-2205 DSS. Colloids Surf. B 2022, 211, 112309. [Google Scholar] [CrossRef] [PubMed]
- Prince, L.; Rousseau, M.A.; Noirfalise, X.; Dangreau, L.; Coelho, L.B.; Olivier, M.G. Inhibitive effect of sodium carbonate on corrosion of AZ31 magnesium alloy in NaCl solution. Corros. Sci. 2021, 179, 109131. [Google Scholar] [CrossRef]
- Zhao, Z.; Zong, L.S.; Liu, C.D.; Li, X.Y.; Wang, C.H.; Liu, W.T.; Cheng, X.T.; Wang, J.Y.; Jian, X.G. A novel Mg(OH)2/MgFx(OH)1−x composite coating on biodegradable magnesium alloy for coronary stent application. Corros. Sci. 2022, 208, 110627. [Google Scholar] [CrossRef]
- Chen, D.; Wang, R.Q.; Huang, Z.Q.; Wu, Y.K.; Zhang, Y.; Wu, G.R.; Li, D.L.; Guo, C.H.; Jiang, G.R.; Yu, S.X.; et al. Evolution processes of the corrosion behavior and structural characteristics of plasma electrolytic oxidation coatings on AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 326–335. [Google Scholar] [CrossRef]
- Zhou, W.X.; Zhang, Y.L.; Meng, S.; Xing, C.Y.; Ma, M.Z.; Liu, Z.; Yang, C.B.; Kong, T.T. Micro-/nano-structures on biodegradable magnesium@PLGA and their cytotoxicity, photothermal, and anti-tumor effects. Small Methods 2021, 5, e2000920. [Google Scholar] [CrossRef]
- Honda, M.; Kawanobe, Y.; Nagata, K.; Ishii, K.; Matsumoto, M.; Aizawa, M. Bactericidal and bioresorbable calcium phosphate cements fabricated by silver-containing tricalcium phosphate microspheres. Int. J. Mol. Sci. 2020, 21, 3745. [Google Scholar] [CrossRef]
- Han, D.L.; Han, Y.J.; Li, J.; Liu, X.M.; Yeung, K.W.K.; Zheng, Y.F.; Cui, Z.D.; Yang, X.J.; Liang, Y.Q.; Li, Z.Y.; et al. Enhanced photocatalytic activity and photothermal effects of cu-doped metal-organic frameworks for rapid treatment of bacteria-infected wounds. Appl. Catal. B 2020, 261, 118248. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and cellular behaviors of novel zinc-doped hydroxyapatite/graphene nanocomposite for bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef]
- Zhang, E.L.; Chen, H.Y.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef]
- Zeng, R.C.; Kainer, K.U.; Blawert, C.; Dietzel, W. Corrosion of an extruded magnesium alloy ZK60 component—The role of microstructural features. J. Alloys Compd. 2011, 509, 4462–4469. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Cheng, L.; Lin, J.J.; Sun, D.W.; Zhang, J.; Liu, H.N. In vitro evaluation of degradation, cytocompatibility and antibacterial property of polycaprolactone/hydroxyapatite composite coating on bioresorbable magnesium alloy. J. Magnes. Alloy 2022, 10, 2252–2265. [Google Scholar] [CrossRef]
- Mehrjou, B.; Dehghan-Baniani, D.; Shi, M.; Shanaghi, A.; Wang, G.; Liu, L.; Qasim, A.M.; Chu, P.K. Nanopatterned silk-coated AZ31 magnesium alloy with enhanced antibacterial and corrosion properties. Mater. Sci. Eng. C 2020, 116, 111173. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Yan, R.; Chen, S.G.; Hao, X.P.; Dou, W.W.; Liu, H.; Guo, Z.H.; Kilula, D.; Seok, I. Narrow pH response multilayer films with controlled release of ibuprofen on magnesium alloy. Mater. Sci. Eng. C 2021, 118, 111414. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Daroonparvar, M.; Saebnoori, E.; Chami, A. In vitro degradation behavior, antibacterial activity and cytotoxicity of TiO2-MAO/ZnHA composite coating on Mg alloy for orthopedic implants. Surf. Coat. Technol. 2018, 334, 450–460. [Google Scholar] [CrossRef]
- Saranya, K.; Bhuvaneswari, S.; Suvro, C.; Rajendran, N. Titanate incorporated anodized coating on magnesium alloy for corrosion protection, antibacterial responses and osteogenic enhancement. J. Magnes. Alloy 2022, 10, 1109–1123. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Zhu, W.W.; Fang, L.M.; Ren, F.Z. Mussel-inspired nano-multilayered coating on magnesium alloys for enhanced corrosion resistance and antibacterial property. Colloids Surf. B 2017, 157, 432–439. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, L.; Song, Y.; Tian, X.; Cui, L.; Liu, C.; Li, S.; Wang, Y.; Zeng, R. In Vitro Degradation and Photoactivated Antibacterial Activity of a Hemin-CaP Microsphere-Loaded Coating on Pure Magnesium. J. Funct. Biomater. 2023, 14, 15. https://doi.org/10.3390/jfb14010015
Long L, Song Y, Tian X, Cui L, Liu C, Li S, Wang Y, Zeng R. In Vitro Degradation and Photoactivated Antibacterial Activity of a Hemin-CaP Microsphere-Loaded Coating on Pure Magnesium. Journal of Functional Biomaterials. 2023; 14(1):15. https://doi.org/10.3390/jfb14010015
Chicago/Turabian StyleLong, Lixin, Yang Song, Xiaoyi Tian, Lanyue Cui, Chengbao Liu, Shuoqi Li, Yu Wang, and Rongchang Zeng. 2023. "In Vitro Degradation and Photoactivated Antibacterial Activity of a Hemin-CaP Microsphere-Loaded Coating on Pure Magnesium" Journal of Functional Biomaterials 14, no. 1: 15. https://doi.org/10.3390/jfb14010015
APA StyleLong, L., Song, Y., Tian, X., Cui, L., Liu, C., Li, S., Wang, Y., & Zeng, R. (2023). In Vitro Degradation and Photoactivated Antibacterial Activity of a Hemin-CaP Microsphere-Loaded Coating on Pure Magnesium. Journal of Functional Biomaterials, 14(1), 15. https://doi.org/10.3390/jfb14010015






