Biodegradable Mg-Sc-Sr Alloy Improves Osteogenesis and Angiogenesis to Accelerate Bone Defect Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Mg Alloy Fabrication
2.2. Microstructure Characterization
2.3. Angiogenesis
2.4. Osteogenesis
2.5. Statistical Analysis
3. Results
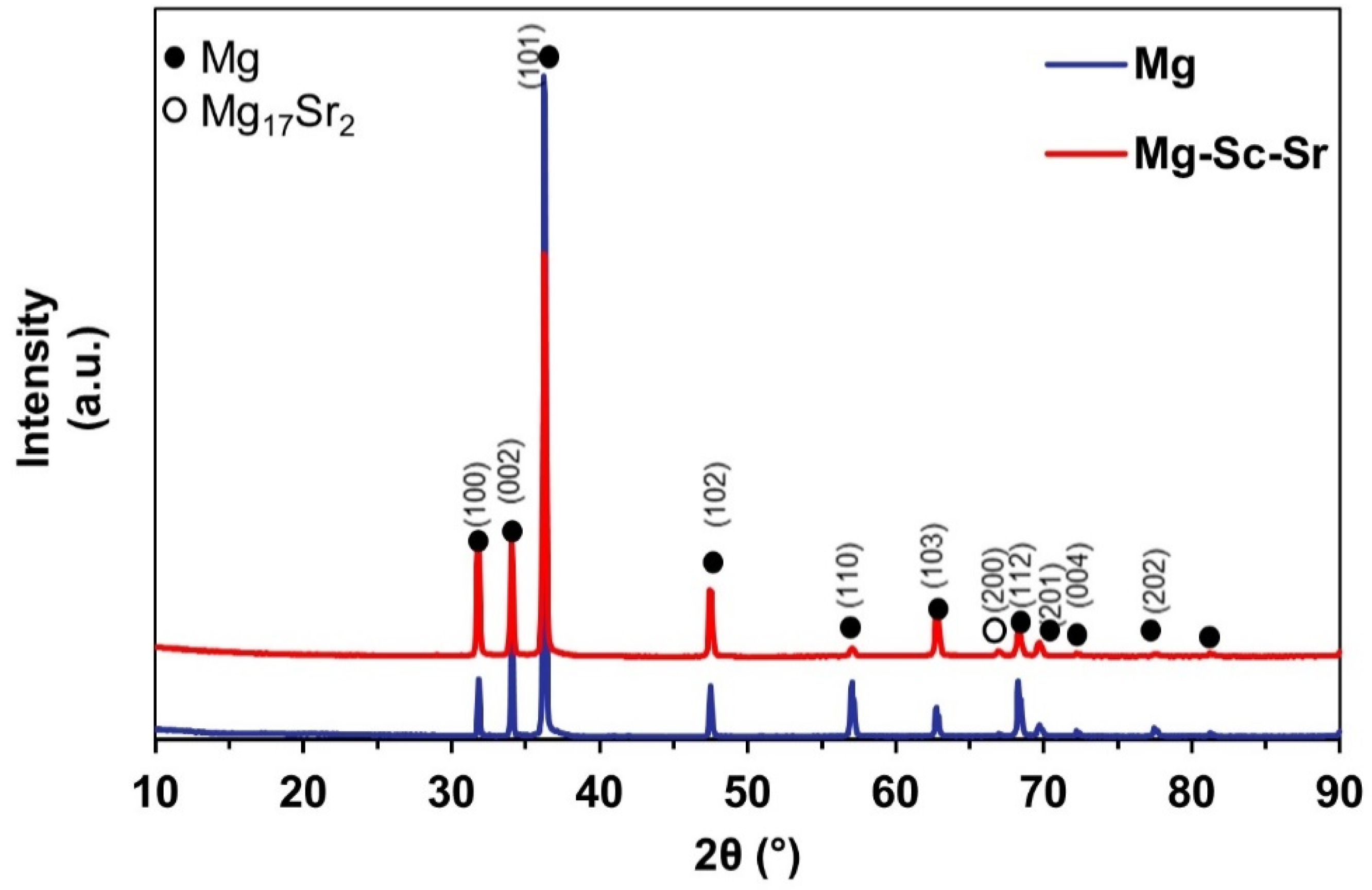
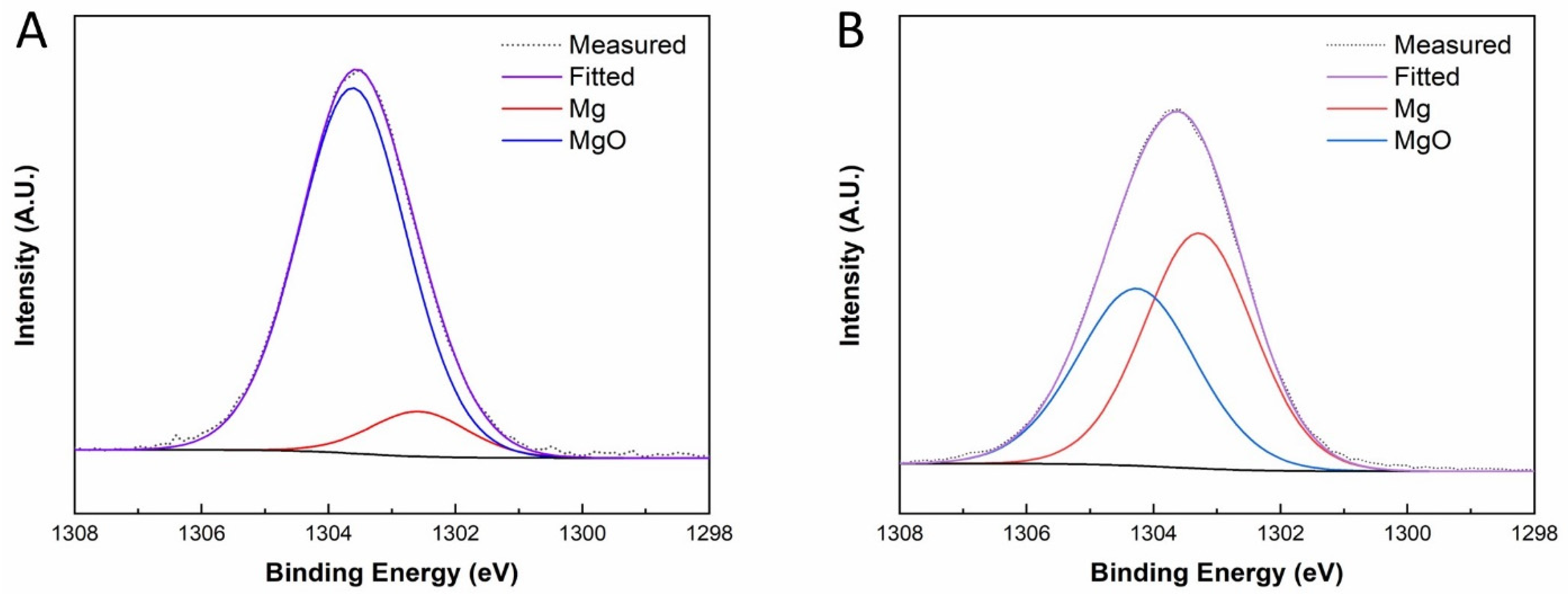
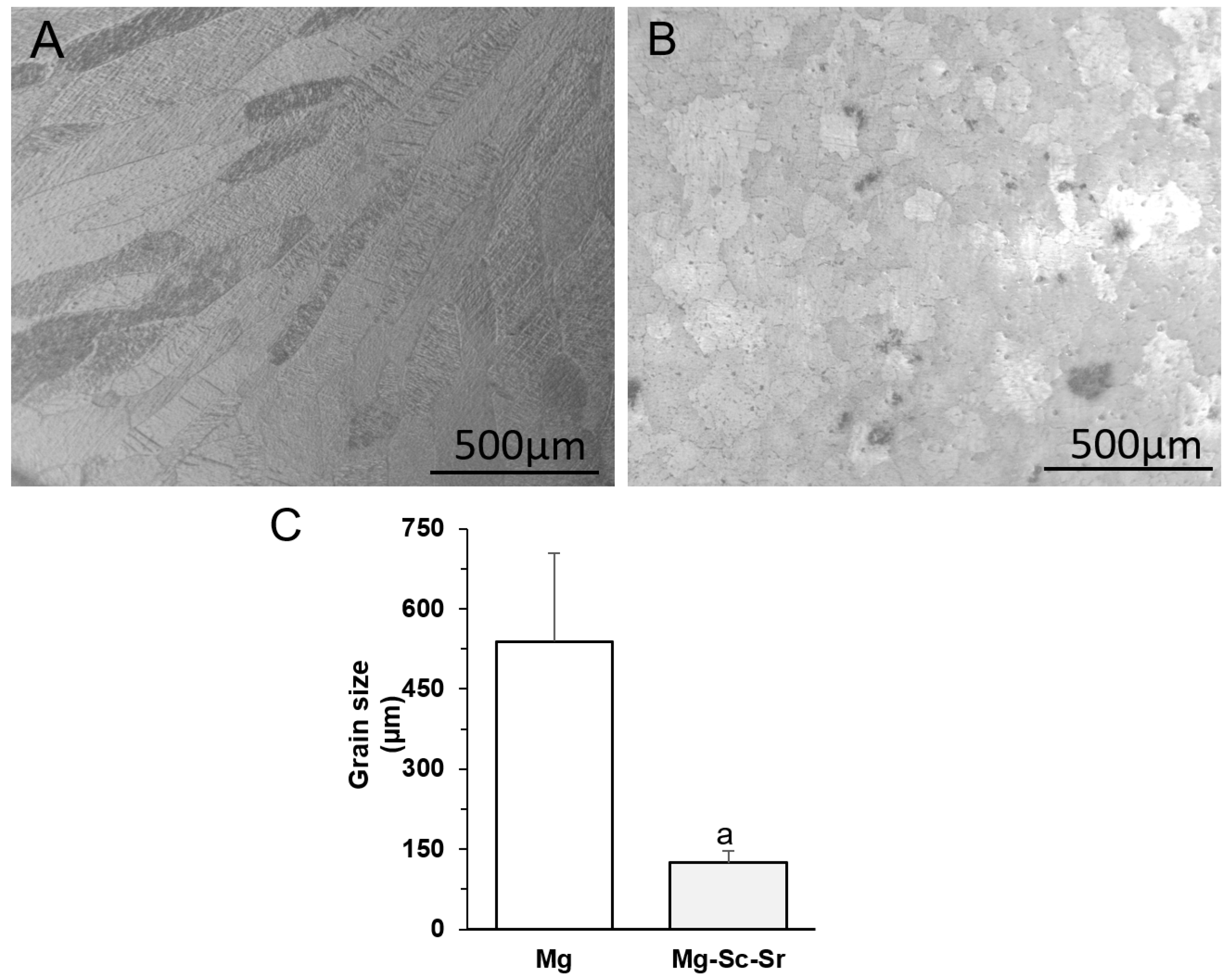
3.1. Microstructure Characterization
3.2. Angiogenesis
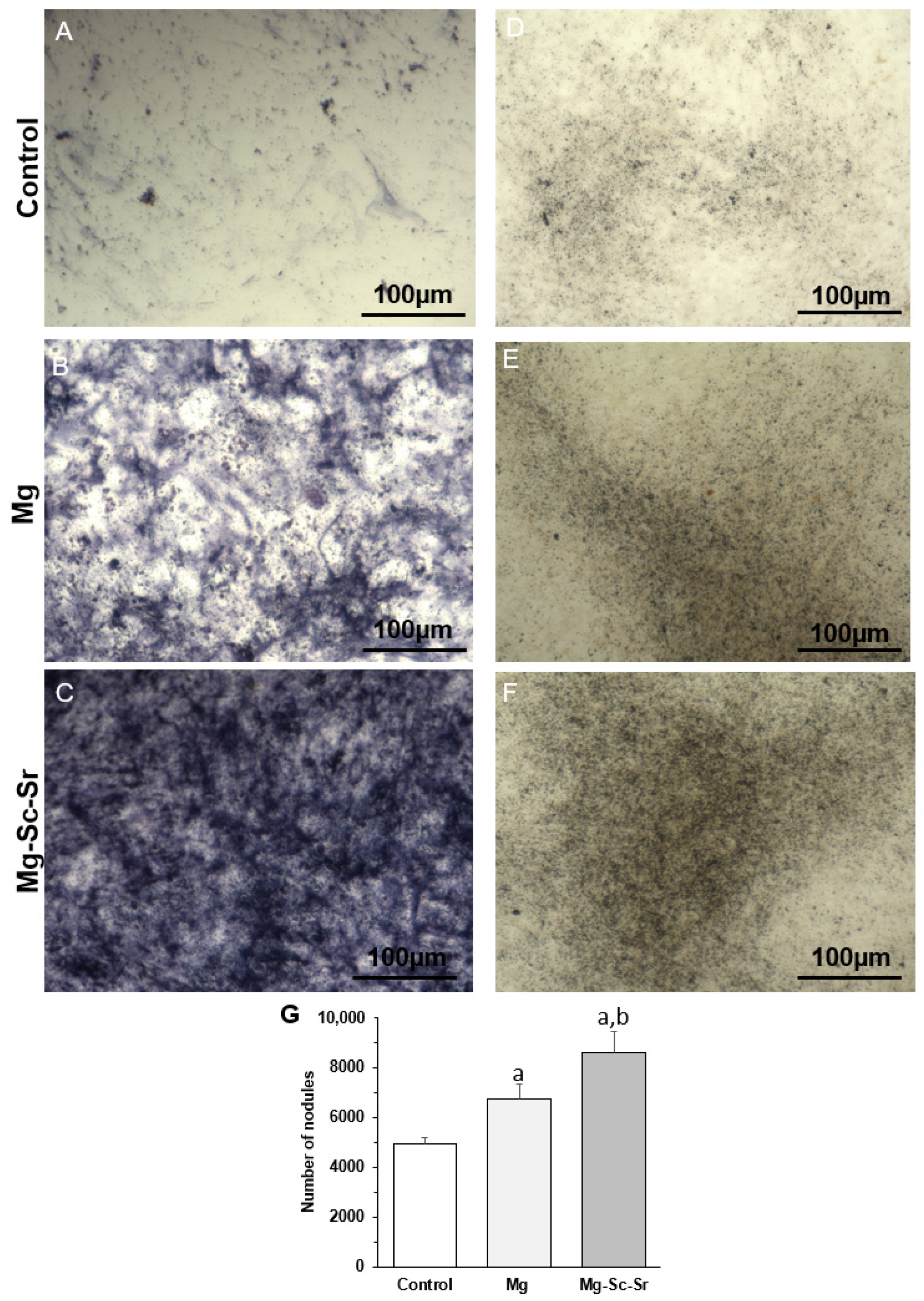
3.3. Osteogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C.J.M. Design, Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Wen, C.; Wright, P.F.; Li, Y.J.A.B. Mechanical, corrosion, and biocompatibility properties of Mg-Zr-Sr-Sc alloys for biodegradable implant applications. Acta Biomater. 2020, 102, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Kean, T.; Seal, S.; Coathup, M.J. Multi-functional cerium oxide nanoparticles regulate inflammation and enhance osteogenesis. Mater. Sci. Eng. C 2021, 124, 112041. [Google Scholar] [CrossRef]
- Kiernan, C.; Knuth, C.; Farrell, E. Endochondral Ossification: Recapitulating Bone Development for Bone Defect Repair, Developmental Biology and Musculoskeletal Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 125–148. [Google Scholar]
- Zhang, N.; Wang, W.; Zhang, X.; Nune, K.C.; Zhao, Y.; Liu, N.; Misra, R.; Yang, K.; Tan, L.; Yan, J. The effect of different coatings on bone response and degradation behavior of porous magnesium-strontium devices in segmental defect regeneration. Bioact. Mater. 2020, 6, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhao, Y.; An, Z.; Cheng, M.; Wang, Q.; Cheng, T.; Wang, Q.; Wang, J.; Jiang, Y.; Zhang, X. Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn-Zr alloy. Biomaterials 2015, 53, 211–220. [Google Scholar] [CrossRef]
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of advancement and development trend on magnesium alloy. J. Magnes. Alloy. 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Li, W.; Qiao, W.; Liu, X.; Bian, D.; Shen, D.; Zheng, Y.; Wu, J.; Kwan, K.Y.; Wong, T.M.; Cheung, K.M. Biomimicking bone–implant interface facilitates the bioadaption of a new degradable magnesium alloy to the bone tissue microenvironment. Adv. Sci. 2021, 8, 2102035. [Google Scholar] [CrossRef]
- Gao, P.; Fan, B.; Yu, X.; Liu, W.; Wu, J.; Shi, L.; Yang, D.; Tan, L.; Wan, P.; Hao, Y. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioact. Mater. 2020, 5, 680–693. [Google Scholar] [CrossRef]
- Chen, X.B.; Nisbet, D.R.; Li, R.W.; Smith, P.; Abbott, T.B.; Easton, M.A.; Zhang, D.-H.; Birbilis, N. Controlling initial biodegradation of magnesium by a biocompatible strontium phosphate conversion coating. Acta Biomater. 2014, 10, 1463–1474. [Google Scholar] [CrossRef]
- Sun, K.; Fu, R.; Liu, X.; Xu, L.; Wang, G.; Chen, S.; Zhai, Q.; Pauly, S. Osteogenesis and angiogenesis of a bulk metallic glass for biomedical implants. Bioact. Mater. 2021, 8, 253–266. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Li, Y.; Zhao, J.; Qin, L.; Lai, Y. Corrosion and biocompatibility improvement of magnesium-based alloys as bone implant materials: A review. Regen. Biomater. 2017, 4, 129–137. [Google Scholar] [CrossRef]
- al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and human health: Perspectives and research directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef]
- Guan, R.-G.; Cipriano, A.F.; Zhao, Z.-y.; Lock, J.; Tie, D.; Zhao, T.; Cui, T.; Liu, H. Development and evaluation of a magnesium–zinc–strontium alloy for biomedical applications—Alloy processing, microstructure, mechanical properties, and biodegradation. Mater. Sci. Eng. C 2013, 33, 3661–3669. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, S.; Liu, X.; Qian, S.; Chu, P.K.; Zheng, Y.; Cheung, K.M.; Zhao, Y.; Yeung, K.W. A surface-engineered multifunctional TiO2 based nano-layer simultaneously elevates the corrosion resistance, osteoconductivity and antimicrobial property of a magnesium alloy. Acta Biomater. 2019, 99, 495–513. [Google Scholar] [CrossRef]
- Zocchi, M.; Locatelli, L.; Zuccotti, G.V.; Mazur, A.; Béchet, D.; Maier, J.A.; Castiglioni, S. Magnesium homeostasis in myogenic differentiation—A focus on the regulation of TRPM7, MagT1 and SLC41A1 transporters. Int. J. Mol. Sci. 2022, 23, 1658. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Liu, W.; Guo, S.; Tang, Z.; Wei, X.; Gao, P.; Wang, N.; Li, X.; Guo, Z. Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB. Biochem. Biophys. Res. Commun. 2020, 528, 664–670. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, W.; Tang, Z.; Wu, H.; Liu, Y.; Dong, H.; Wang, N.; Huang, H.; Bao, S.; Shi, L. Magnesium surface-activated 3D printed porous PEEK scaffolds for in vivo osseointegration by promoting angiogenesis and osteogenesis. Bioact. Mater. 2023, 20, 16–28. [Google Scholar] [CrossRef]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Xu, J.; TerBush, J.; Li, W.; Setty, M.; Guan, S.; Nguyen, T.D.; Qin, L.; Zheng, Y. Biodegradable metal-derived magnesium and sodium enhances bone regeneration by angiogenesis aided osteogenesis and regulated biological apatite formation. Chem. Eng. J. 2020, 410, 127616. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-based alloys used in orthopedic surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Liu, X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, Q.; Yu, K.; Xiao, T.; Liu, L.; Dai, Y.; Xiong, L.; Zhang, B.; Li, A. Mg–Zn–Mn alloy extract induces the angiogenesis of human umbilical vein endothelial cells via FGF/FGFR signaling pathway. Biochem. Biophys. Res. Commun. 2019, 514, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; He, Y.; Zhou, J.; Tang, S.; Yang, Y.; Wang, X.J.M.L. Microstructure and mechanical property of biodegradable Mg–1.5 Zn–0.6 Zr alloy with varying contents of scandium. Mater. Lett. 2018, 229, 60–63. [Google Scholar] [CrossRef]
- Cheng, S.; Ke, J.; Yao, M.; Shao, H.; Zhou, J.; Wang, M.; Ji, X.; Zhong, G.; Peng, F.; Ma, L. Improved osteointegration and angiogenesis of strontium-incorporated 3D-printed tantalum scaffold via bioinspired polydopamine coating. J. Mater. Sci. Technol. 2021, 69, 106–118. [Google Scholar] [CrossRef]
- Zhao, F.; Lei, B.; Li, X.; Mo, Y.; Wang, R.; Chen, D.; Chen, X. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials 2018, 178, 36–47. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lee, A.K.-X.; Ho, C.-C.; Fang, M.-J.; Kuo, T.-Y.; Shie, M.-Y. The effects of a 3D-printed magnesium-/strontium-doped calcium silicate scaffold on regulation of bone regeneration via dual-stimulation of the AKT and WNT signaling pathways. Mater. Sci. Eng. C 2022, 133, 112660. [Google Scholar] [CrossRef]
- Li, T.; He, H.; Yang, Z.; Wang, J.; Zhang, Y.; He, G.; Huang, J.; Song, D.; Ni, J.; Zhou, X. Strontium-doped gelatin scaffolds promote M2 macrophage switch and angiogenesis through modulating the polarization of neutrophils. Biomater. Sci. 2021, 9, 2931–2946. [Google Scholar] [CrossRef]
- Wang, Y.; Tie, D.; Guan, R.; Wang, N.; Shang, Y.; Cui, T.; Li, J. Microstructures, mechanical properties, and degradation behaviors of heat-treated Mg-Sr alloys as potential biodegradable implant materials. J. Mech. Behav. Biomed. Mater. 2018, 77, 47–57. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Chu, P.K.; Wang, L.; Pan, H.; Zheng, Y.; Wu, S.; Liu, X.; Cheung, K.M.; Wong, T. A functionalized TiO2/Mg2TiO4 nano-layer on biodegradable magnesium implant enables superior bone-implant integration and bacterial disinfection. Biomaterials 2019, 219, 119372. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Vitale-Brovarone, C.; Ciapetti, G. Protocol of co-culture of human osteoblasts and osteoclasts to test biomaterials for bone tissue engineering. Methods Protoc. 2022, 5, 8. [Google Scholar] [CrossRef]
- Gu, X.; Xie, X.; Li, N.; Zheng, Y.; Qin, L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H.; He, Y.; Wen, N.; Wang, X. Microstructure, mechanical properties and in vitro degradation behavior of a novel biodegradable Mg–1.5 Zn–0.6 Zr–0.2 Sc alloy. J. Mater. Sci. Technol. 2015, 31, 744–750. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Yan, B.; Zou, C.; Wei, Z. The effect of grain refinement and precipitation strengthening induced by Sc or Er alloying on the mechanical properties of cast Al-Li-Cu-Mg alloys at elevated temperatures. Mater. Sci. Eng. A 2021, 822, 141641. [Google Scholar] [CrossRef]
- Suresh, M.; Sharma, A.; More, A.; Nayan, N.; Suwas, S. Effect of Scandium addition on evolution of microstructure, texture and mechanical properties of thermo-mechanically processed Al-Li alloy AA2195. J. Alloys Compd. 2018, 740, 364–374. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Guo, S.; Jiang, W.; Liu, Q. Effect of Sr on the microstructure and biodegradable behavior of Mg–Zn–Ca-Mn alloys for implant application. Mater. Des. 2018, 153, 308–316. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hasanpur, E.; Bahmani, A.; Shin, K.S. Corrosion behaviour of AZ31 magnesium alloy containing various levels of strontium. Corros. Sci. 2018, 141, 117–126. [Google Scholar] [CrossRef]
- Cheng, R.; Pan, F.; Jiang, S.; Li, C.; Jiang, B.; Jiang, X. Effect of Sr addition on the grain refinement of AZ31 magnesium alloys. Prog. Nat. Sci. Mater. Int. 2013, 23, 7–12. [Google Scholar] [CrossRef]
- StJohn, D.; Easton, M.; Qian, M.; Taylor, J. Grain refinement of magnesium alloys: A review of recent research, theoretical developments, and their application. Met. Mater. Trans. A 2013, 44, 2935–2949. [Google Scholar] [CrossRef]
- Li, W.; Shen, Y.; Shen, J.; Shen, D.; Liu, X.; Zheng, Y.; Yeung, K.W.; Guan, S.; Kulyasova, O.B.; Valiev, R. In vitro and in vivo studies on pure Mg, Mg–1Ca and Mg–2Sr alloys processed by equal channel angular pressing. Nano Mater. Sci. 2020, 2, 96–108. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.H.; Zhai, C.Q. Effect of strontium on mechanical properties and corrosion resistance of AZ91D. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2007; pp. 567–570. [Google Scholar]
- Liu, J.; Lin, Y.; Bian, D.; Wang, M.; Lin, Z.; Chu, X.; Li, W.; Liu, Y.; Shen, Z.; Liu, Y. In vitro and in vivo studies of Mg-30Sc alloys with different phase structure for potential usage within bone. Acta Biomater. 2019, 98, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Neumann, A.; Ng, H.; Lapovok, R.; Kasper, C.; Lowe, T.; Anumalasetty, V.; Estrin, Y. Combined effect of grain refinement and surface modification of pure titanium on the attachment of mesenchymal stem cells and osteoblast-like SaOS-2 cells. Mater. Sci. Eng. C 2017, 71, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, O.; Joseph, O.; Mubiayi, M.; Durodola, B.; Gabriel, O. Microstructural evolution and characterization of super-induced MgO composite on zinc-rich coatings. Egypt. J. Basic Appl. Sci. 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir, M.R.A.; Izman, S.; Marvibaigi, M. The effect of MgO on the biodegradation, physical properties and biocompatibility of a Mg/HA/MgO nanocomposite manufactured by powder metallurgy method. J. Alloys Compd. 2016, 655, 266–280. [Google Scholar] [CrossRef]
- Yao, H.-L.; Xia, J.; Yi, D.-L.; Yang, C.; Zhang, M.-X.; Bai, X.-B.; Chen, Q.-Y.; Wang, H.-T.; Li, S.-B. Microstructure and corrosion properties of biodegradable Mg/MgO composite coating on Mg alloy prepared by high velocity suspension flame spraying. J. Therm. Spray Technol. 2021, 30, 1544–1556. [Google Scholar] [CrossRef]
- Stippich, F.; Vera, E.; Wolf, G.; Berg, G.; Friedrich, C. Enhanced corrosion protection of magnesium oxide coatings on magnesium deposited by ion beam-assisted evaporation. Surf. Coat. Technol. 1998, 103, 29–35. [Google Scholar] [CrossRef]
- Banerjee, P.; Hasda, R.; Murmu, M.; Hirani, H. MgO as corrosion inhibitor. In Inorganic Anticorrosive Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 183–210. [Google Scholar]
- Ke, D.; Tarafder, S.; Vahabzadeh, S.; Bose, S. Effects of MgO, ZnO, SrO, and SiO2 in tricalcium phosphate scaffolds on in vitro gene expression and in vivo osteogenesis. Mater. Sci. Eng. C 2019, 96, 10–19. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Mao, X. Magnesium enhances osteogenesis of BMSCs by tuning osteoimmunomodulation. BioMed Res. Int. 2019, 2019, 7908205. [Google Scholar] [CrossRef]
- Lu, W.-C.; Pringa, E.; Chou, L. Effect of magnesium on the osteogenesis of normal human osteoblasts. Magnes. Res. 2017, 30, 42–52. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Yuan, Q.; Liu, L.; Li, H.; Xiong, L.; Guo, X.; Yan, Y.; Yu, K.; Dai, Y. In vitro and in vivo assessment of the effect of biodegradable magnesium alloys on osteogenesis. Acta Biomater. 2022, 141, 454–465. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef]
- Aimaiti, A.; Maimaitiyiming, A.; Boyong, X.; Aji, K.; Li, C.; Cui, L. Low-dose strontium stimulates osteogenesis but high-dose doses cause apoptosis in human adipose-derived stem cells via regulation of the ERK1/2 signaling pathway. Stem Cell Res. Ther. 2017, 8, 282. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Jang, J.H.; Song, H. Positive modulation of osteogenesis-and osteoclastogenesis-related gene expression with strontium-containing microstructured Ti implants in rabbit cancellous bone. J. Biomed. Mater. Res. Part A 2013, 101, 298–306. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Guo, X.; Cui, W.; Yang, S.; Wang, J.; Qu, Y.; Shao, Z.; Xu, S. Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo. Biomed. Mater. 2017, 13, 015018. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, H.; Zhang, X.; Li, G.; Chang, Q.; Zhao, J.; Qiao, Y.; Ding, X.; Yang, G.; Liu, X. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016, 8, 5291–5301. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloy. 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, Z.; Huang, Y.; Jia, Z.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Yang, X. Unraveling the osteogenesis of magnesium by the activity of osteoblasts in vitro. J. Mater. Chem. B 2018, 6, 6615–6621. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Wang, W.; Zhang, Y.; Wan, P.; Yang, K.; Han, Y. Evaluation of the osteo-inductive potential of hollow three-dimensional magnesium-strontium substitutes for the bone grafting application. Mater. Sci. Eng. C 2017, 73, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, P.; Wu, Y.; Zhang, Y.; Xia, H.; Zheng, Y.; Han, Y. Stimulatory effects of the degradation products from Mg-Ca-Sr alloy on the osteogenesis through regulating ERK signaling pathway. Sci. Rep. 2016, 6, 32323. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, J.; Lu, S.; Zhang, L. Enhanced osteoblast functions of narrow interligand spaced Sr-HA nano-fibers/rods grown on microporous titania coatings. RSC Adv. 2013, 3, 11169–11184. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, P.; Ge, X.; Li, P.; Lv, C.; Wang, M.; Wang, K.; Fang, L.; Lu, X. Experimental and simulation studies of strontium/zinc-codoped hydroxyapatite porous scaffolds with excellent osteoinductivity and antibacterial activity. Appl. Surf. Sci. 2018, 462, 118–126. [Google Scholar] [CrossRef]
- Boda, S.K.; Thrivikraman, G.; Panigrahy, B.; Sarma, D.D.; Basu, B. Competing roles of substrate composition, microstructure, and sustained strontium release in directing osteogenic differentiation of hMSCs. ACS Appl. Mater. Interfaces 2017, 9, 19389–19408. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef]
- Yan, R.; Li, J.; Wu, Q.; Zhang, X.; Hu, L.; Deng, Y.; Jiang, R.; Wen, J.; Jiang, X. Trace element-augmented titanium implant with targeted angiogenesis and enhanced osseointegration in osteoporotic rats. Front. Chem. 2022, 10, 839062. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Qin, W.; Kolooshani, A.; Kolahdooz, A.; Saber-Samandari, S.; Khazaei, S.; Khandan, A.; Ren, F.; Toghraie, D. Coating the magnesium implants with reinforced nanocomposite nanoparticles for use in orthopedic applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126581. [Google Scholar] [CrossRef]
- Sahmani, S.; Saber-Samandari, S.; Khandan, A.; Aghdam, M.M. Influence of MgO nanoparticles on the mechanical properties of coated hydroxyapatite nanocomposite scaffolds produced via space holder technique: Fabrication, characterization and simulation. J. Mech. Behav. Biomed. Mater. 2019, 95, 76–88. [Google Scholar] [CrossRef]



| Element Concentration (%) | Mg | Sc | Sr | P | Cl | Ca | Si | Zn | Cr | Ti | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | 98.4340 | - | - | 0.7580 | 0.4790 | 0.2270 | 0.0294 | 0.0048 | 0.0158 | - | 0.0322 | 0.0188 |
| Mg-Sc-Sr | 94.120 | 1.369 | 2.003 | 1.223 | 0.657 | 0.488 | - | - | 0.0074 | 0.0058 | 0.0824 | 0.0355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboutalebianaraki, N.; Neal, C.J.; Seal, S.; Razavi, M. Biodegradable Mg-Sc-Sr Alloy Improves Osteogenesis and Angiogenesis to Accelerate Bone Defect Restoration. J. Funct. Biomater. 2022, 13, 261. https://doi.org/10.3390/jfb13040261
Aboutalebianaraki N, Neal CJ, Seal S, Razavi M. Biodegradable Mg-Sc-Sr Alloy Improves Osteogenesis and Angiogenesis to Accelerate Bone Defect Restoration. Journal of Functional Biomaterials. 2022; 13(4):261. https://doi.org/10.3390/jfb13040261
Chicago/Turabian StyleAboutalebianaraki, Nadia, Craig J. Neal, Sudipta Seal, and Mehdi Razavi. 2022. "Biodegradable Mg-Sc-Sr Alloy Improves Osteogenesis and Angiogenesis to Accelerate Bone Defect Restoration" Journal of Functional Biomaterials 13, no. 4: 261. https://doi.org/10.3390/jfb13040261
APA StyleAboutalebianaraki, N., Neal, C. J., Seal, S., & Razavi, M. (2022). Biodegradable Mg-Sc-Sr Alloy Improves Osteogenesis and Angiogenesis to Accelerate Bone Defect Restoration. Journal of Functional Biomaterials, 13(4), 261. https://doi.org/10.3390/jfb13040261







