Multifunctional Medical Grade Resin with Enhanced Mechanical and Antibacterial Properties: The Effect of Copper Nano-Inclusions in Vat Polymerization (VPP) Additive Manufacturing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Copper Nanoparticles Inspection
2.3. Samples Preparation
2.4. Thermal and Spectroscopic Analysis
2.5. Quality Characteristics and Mechanical Characterization
2.6. Morphological Examination
2.7. Design of Experiment, Statistical Analysis, and Optimization of the Experimental Results
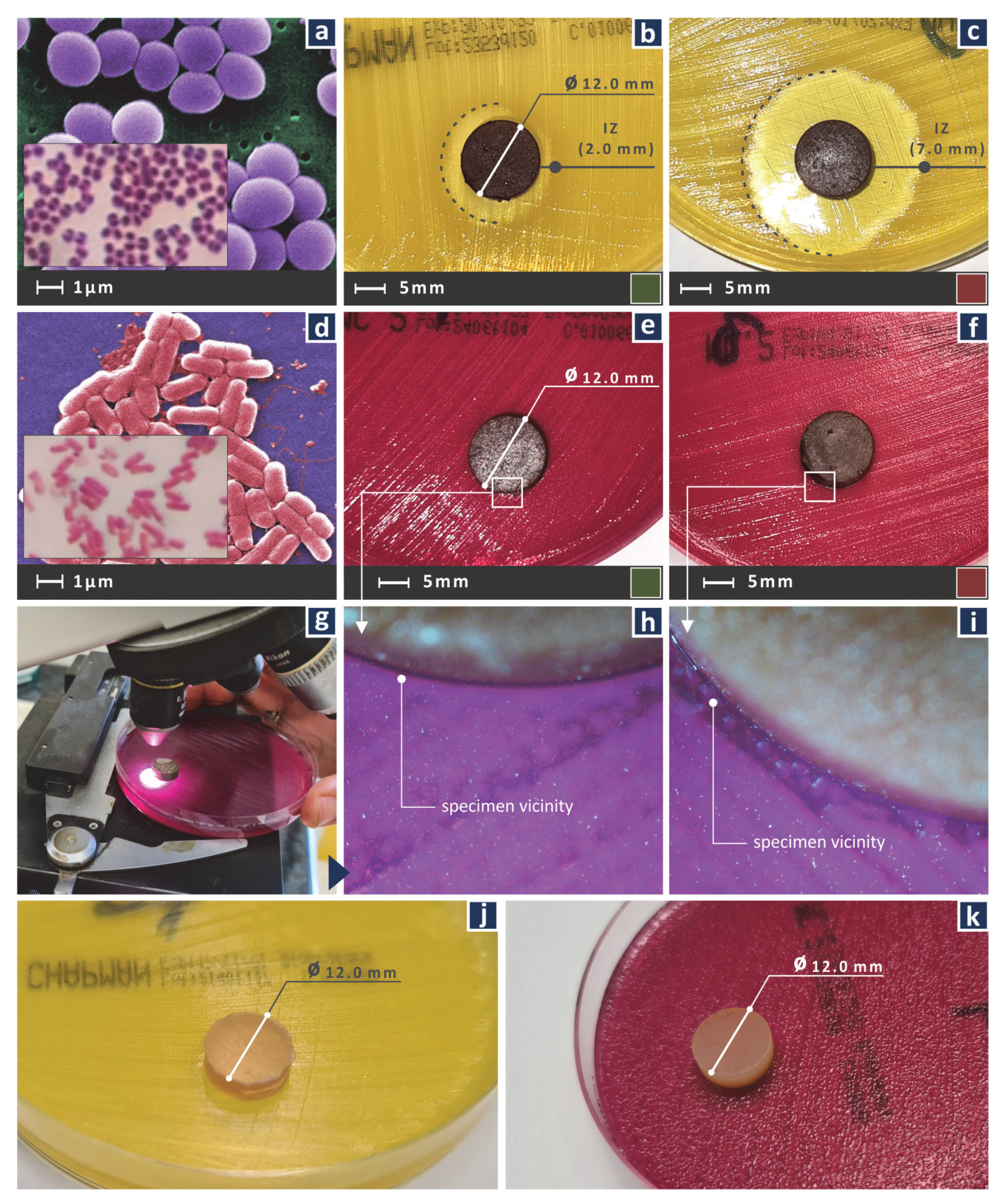
2.8. Antibacterial Performance of the Nanocomposites
3. Results
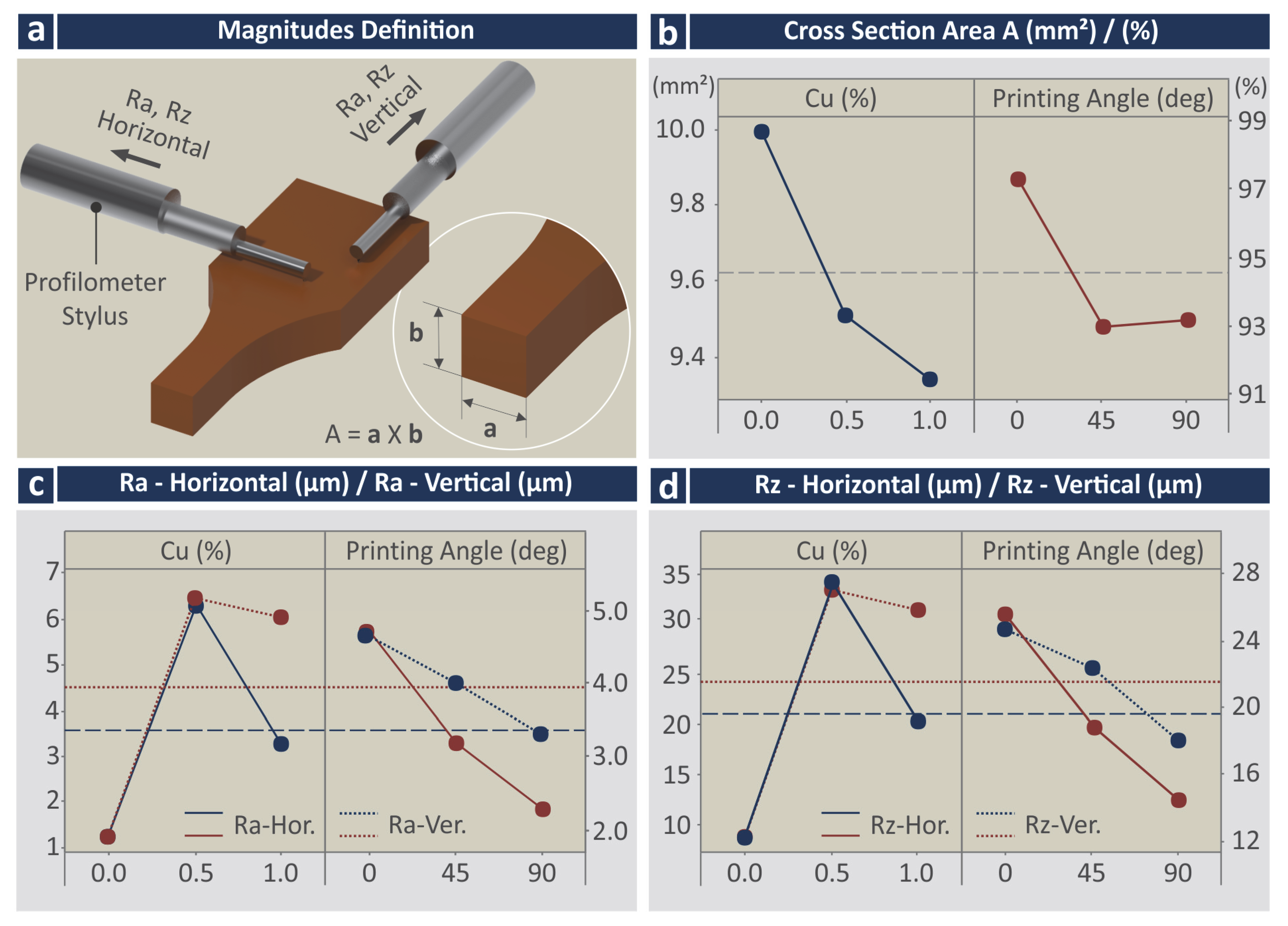
3.1. Copper Nanoparticles Inspection
3.2. Thermal and Spectroscopic Analysis
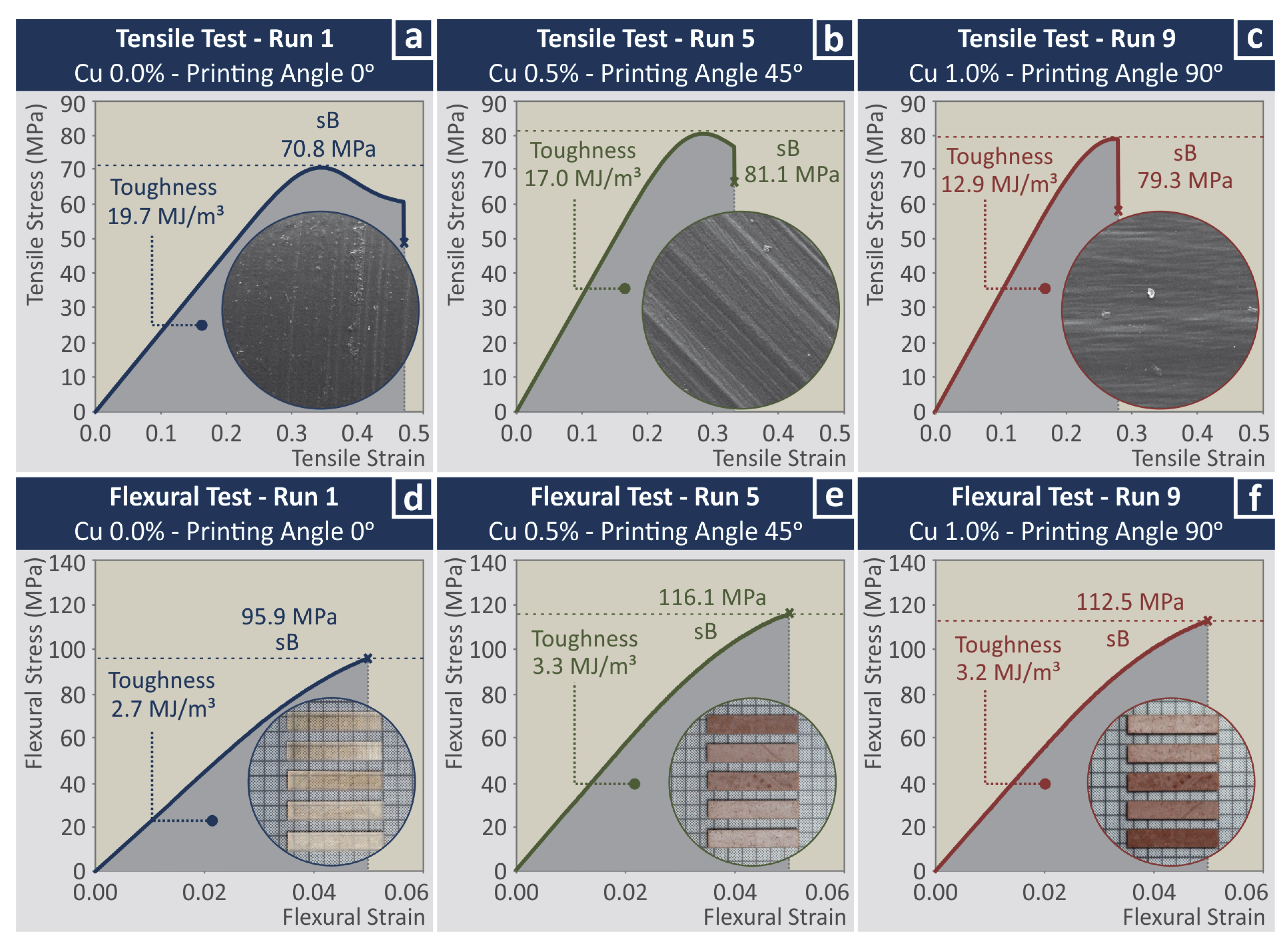
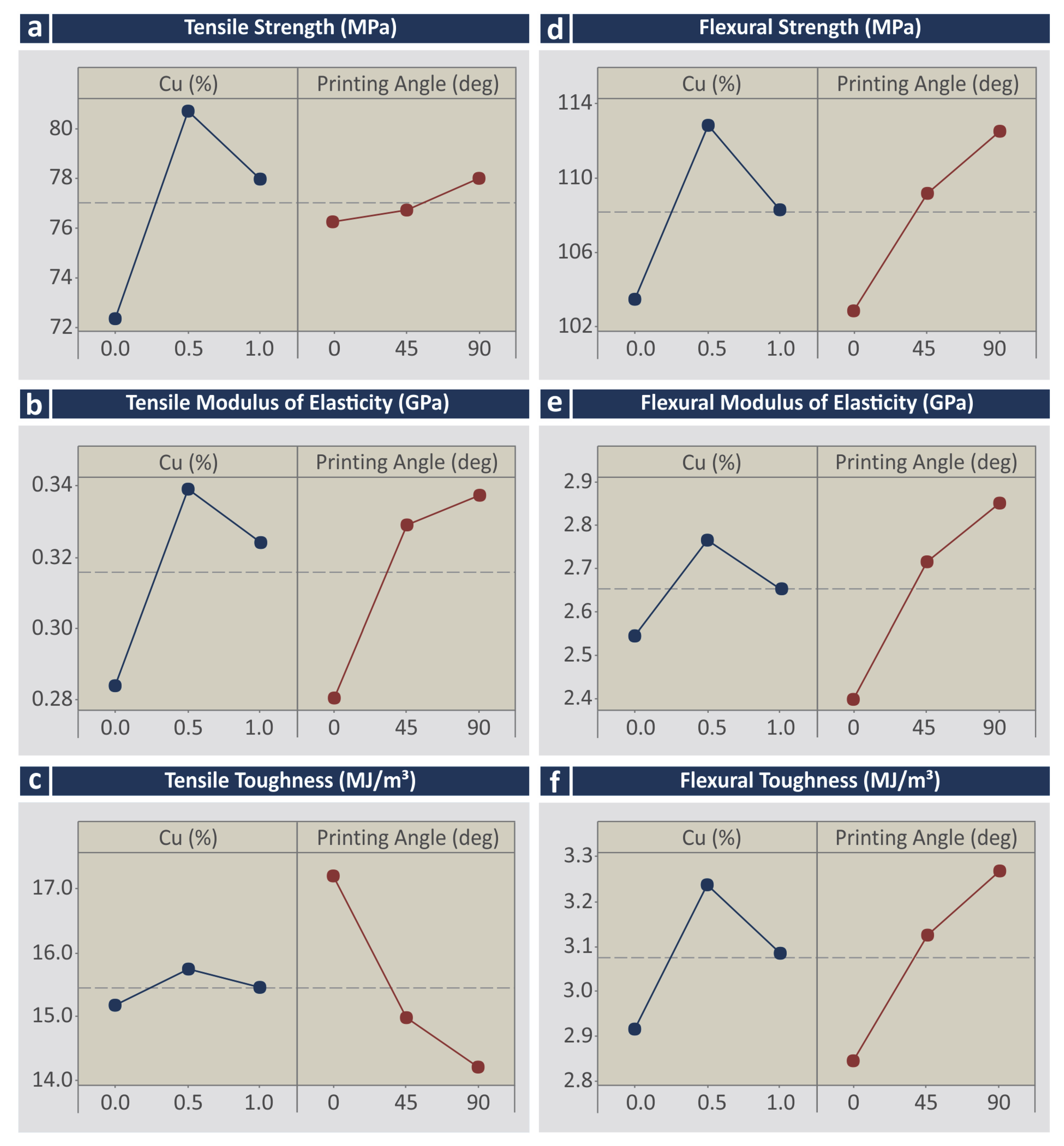
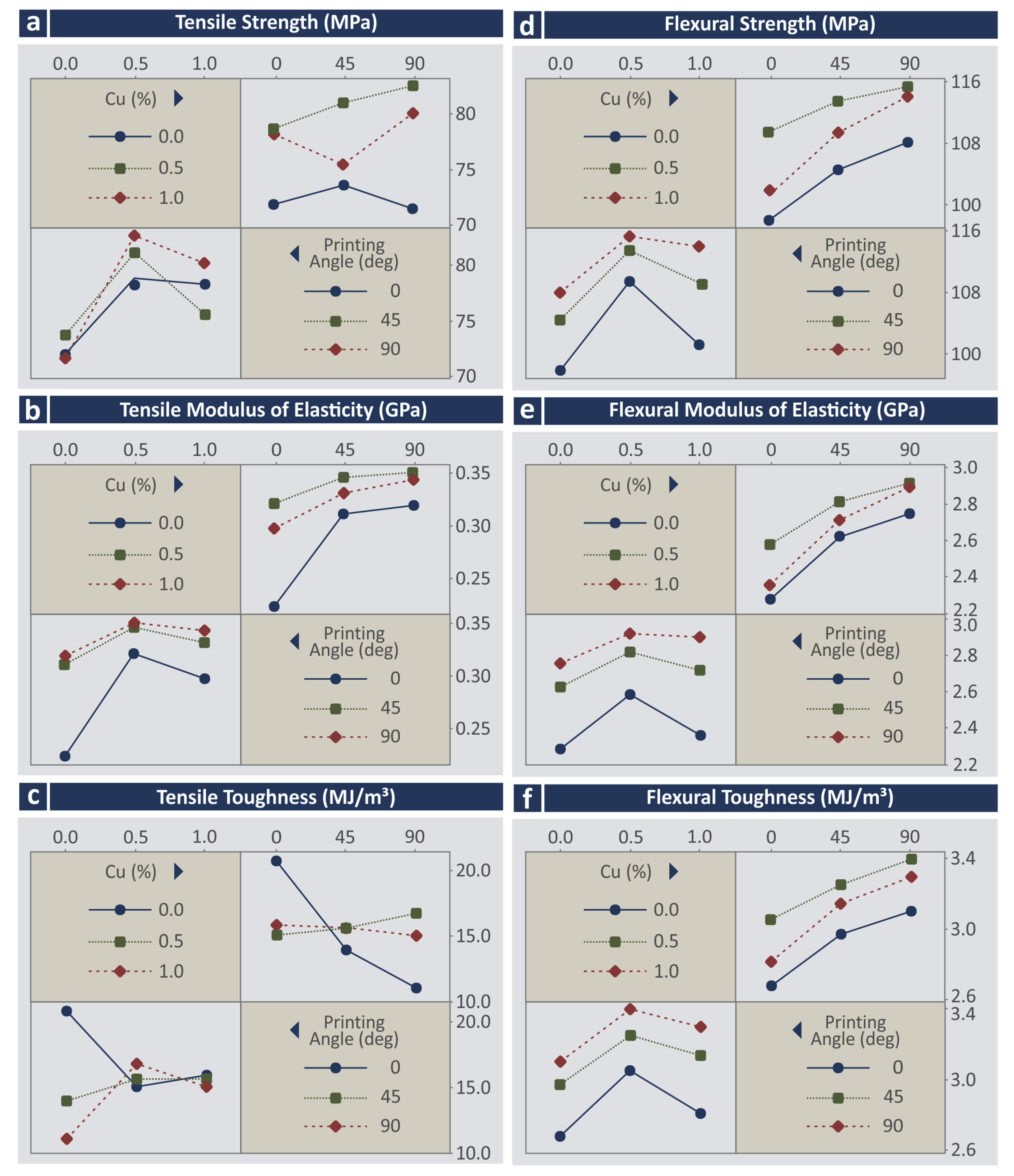
3.3. Mechanical Characterization
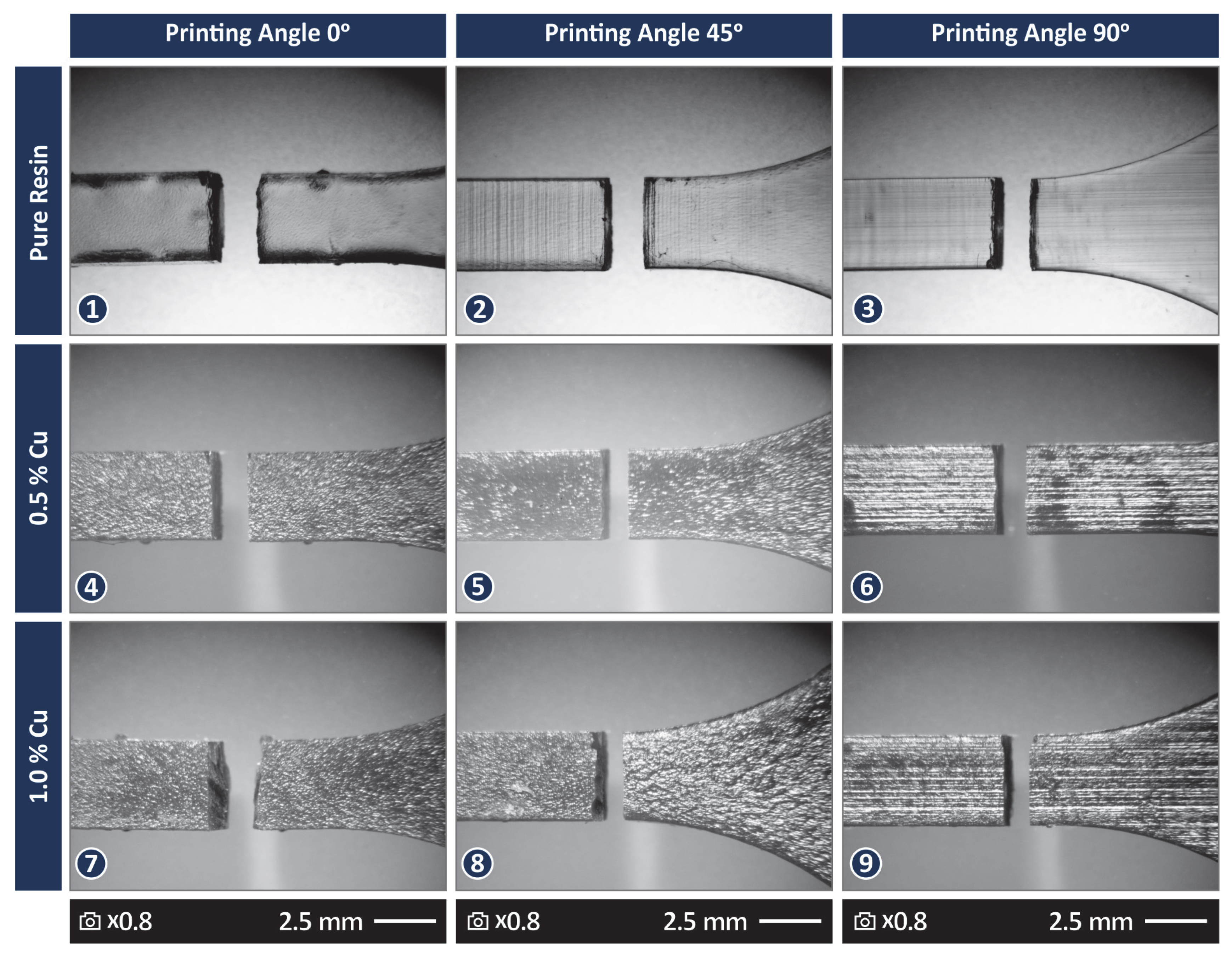
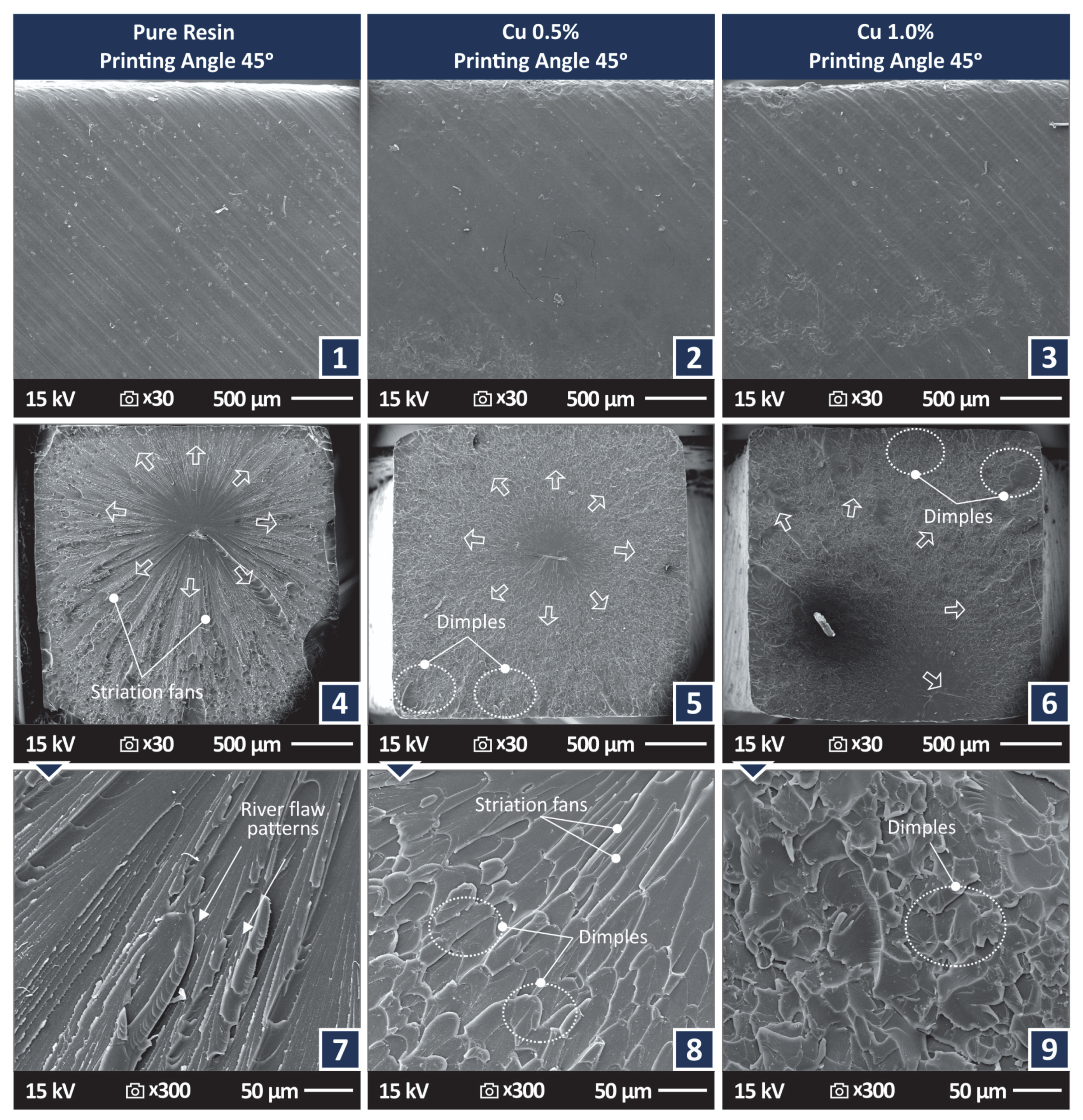
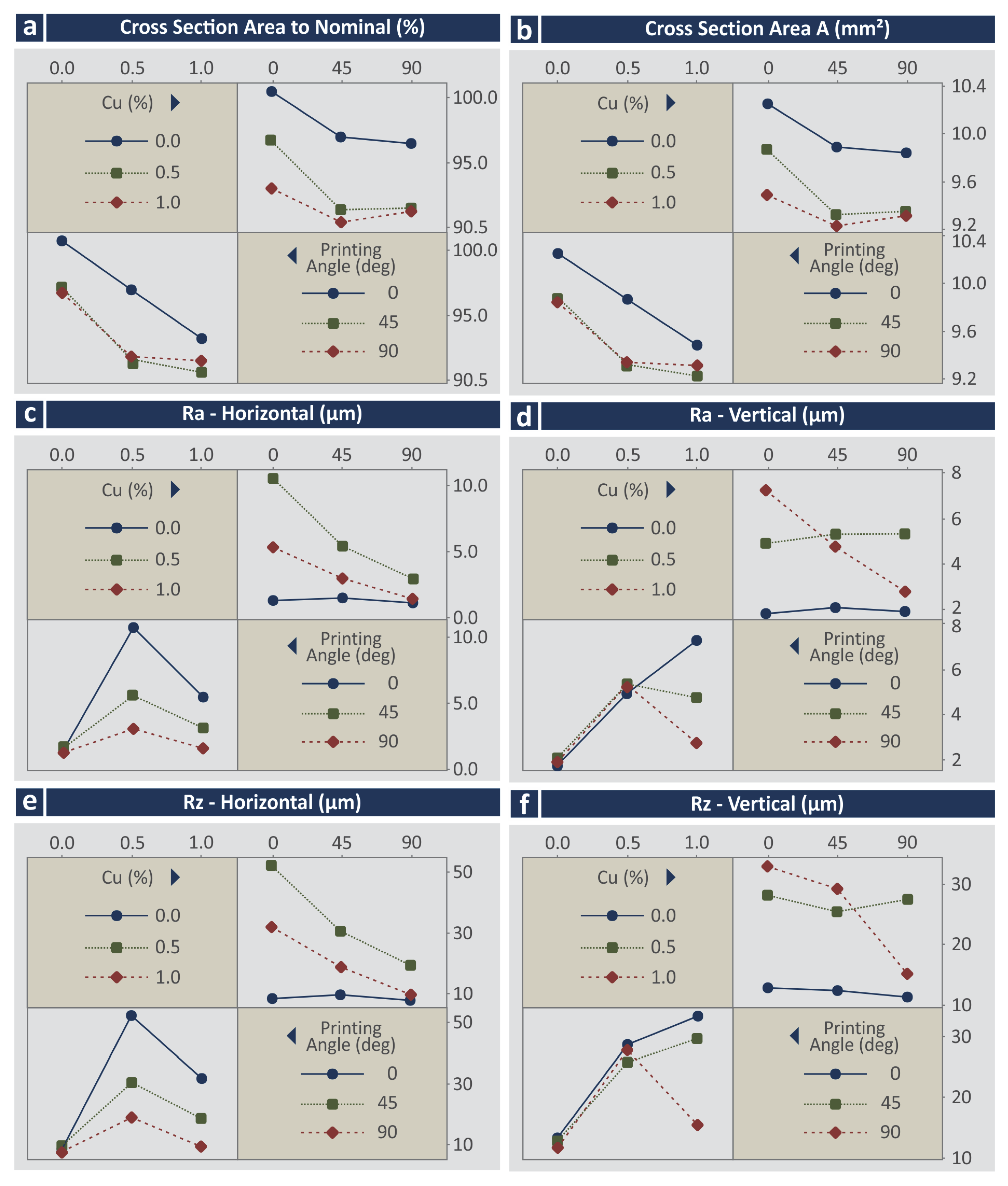
3.4. Morphological Examination
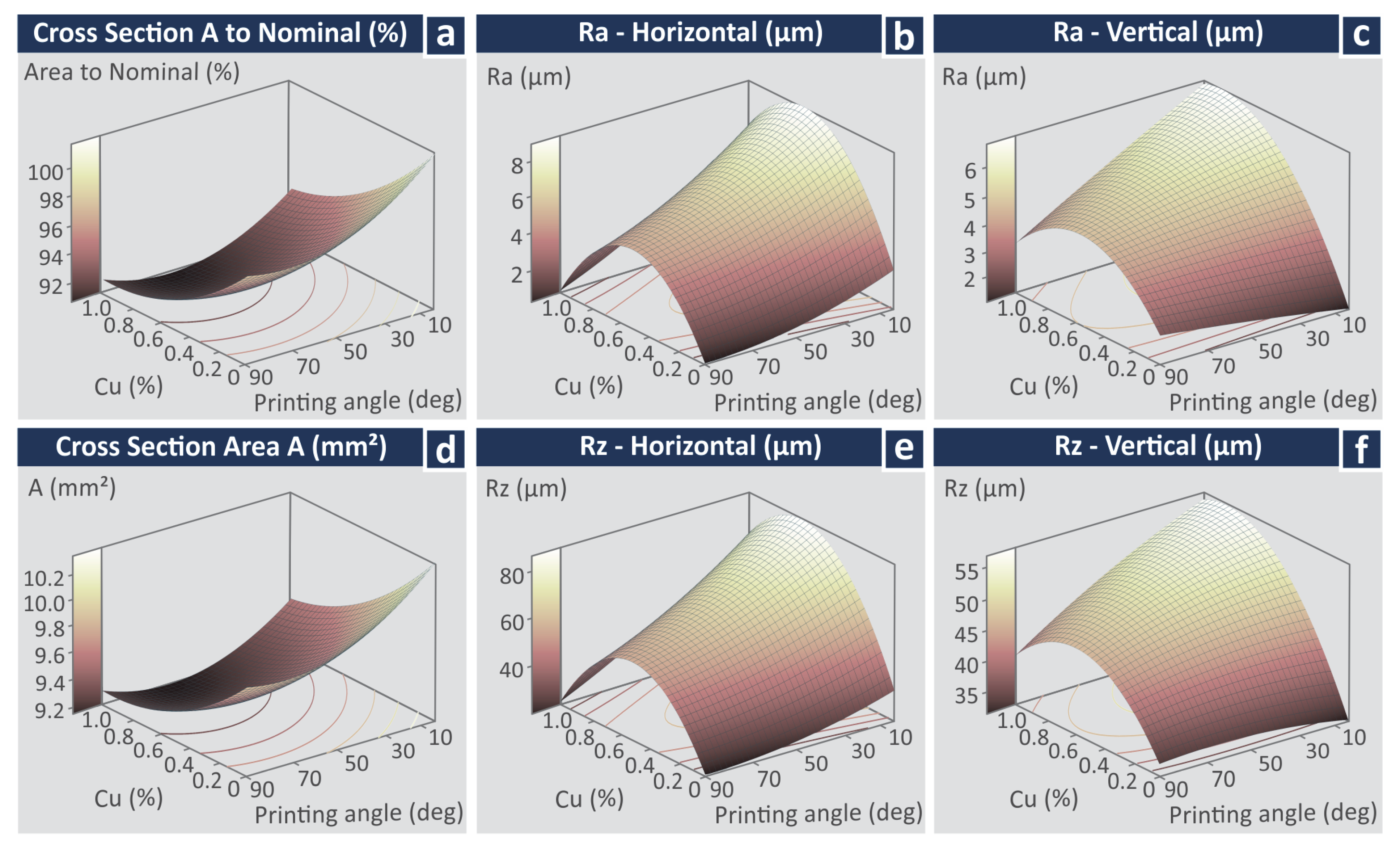
3.5. Statistical Analysis and Optimization of the Experimental Results
3.6. Regression and ANOVA
- All parameters for the cross-section area to nominal (%);
- All parameters for the cross-section area (mm2);
- Cu, Cu2, and Cu × Deg for the Ra in the horizontal direction;
- Cu, Cu2, and Cu × Deg for the Ra in the vertical direction;
- Cu, Cu2, and Cu × Deg for the Rz in the horizontal direction;
- Cu, Cu2, and Cu × Deg for the Rz in the vertical direction;
- Cu and Cu2 for the tensile strength (MPa);
- All parameters for the tensile modulus of elasticity (GPa);
- Cu×Deg and Deg for the tensile toughness (MJ/m3);
- Cu, Cu2, and Deg for the flexural strength (MPa);
- Cu, Cu2, Deg, and Deg2 for the flexural modulus of elasticity (GPa);
- Cu, Cu2, Deg, and Deg2 for the flexural toughness (MJ/m3).
3.7. Antibacterial Performance of the Nanocomposites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, J.S.; Dowling, L.; Kennedy, J.; Trimble, D. Investigations of the mechanical properties on different print orientations in SLA 3D printed resin. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2020, 234, 2279–2293. [Google Scholar] [CrossRef]
- Hu, G.; Cao, Z.; Hopkins, M.; Lyons, J.G.; Brennan-Fournet, M.; Devine, D.M. Nanofillers can be used to enhance the thermal conductivity of commercially available SLA resins. Procedia Manuf. 2019, 38, 1236–1243. [Google Scholar] [CrossRef]
- Miedzińska, D.; Gieleta, R.; Małek, E. Experimental study of strength properties of SLA resins under low and high strain rates. Mech. Mater. 2020, 141, 103245. [Google Scholar] [CrossRef]
- Riccio, C.; Civera, M.; Grimaldo Ruiz, O.; Pedullà, P.; Rodriguez Reinoso, M.; Tommasi, G.; Vollaro, M.; Burgio, V.; Surace, C. Effects of Curing on Photosensitive Resins in SLA Additive Manufacturing. Appl. Mech. 2021, 2, 942–955. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Raju, R.; Ellakwa, A. Effect of Printing Layer Thickness and Postprinting Conditions on the Flexural Strength and Hardness of a 3D-Printed Resin. Biomed. Res. Int. 2022, 2022, 8353137. [Google Scholar] [CrossRef]
- Fry, C.; Mihalko, A.; Michael, R.; Piovesan, D. Mechanical Property Determination of a Stereolithographic Resin Subjected to Compressive Loading. Volume 3: Biomedical and Biotechnology Engineering. In Proceedings of the ASME 2018 International Mechanical Engineering Congress and Exposition, Pittsburgh, Pennsylvania, USA, 9–15 November 2018; pp. 6–11. [Google Scholar] [CrossRef]
- Maines, E.M.; Porwal, M.K.; Ellison, C.J.; Reineke, T.M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021, 23, 6863–6897. [Google Scholar] [CrossRef]
- Xiao, R.; Ding, M.; Wang, Y.; Gao, L.; Fan, R.; Lu, Y. Stereolithography (SLA) 3D printing of carbon fiber-graphene oxide (CF-GO) reinforced polymer lattices. Nanotechnology 2021, 32, 235702. [Google Scholar] [CrossRef]
- Podsiadły, B.; Skalski, A.; Rozpiórski, W.; Słoma, M. Are We Able to Print Components as Strong as Injection Molded?—Comparing the Properties of 3D Printed and Injection Molded Components Made from ABS Thermoplastic. Appl. Sci. 2021, 11, 6946. [Google Scholar] [CrossRef]
- Kaynak, C.; Varsavas, S.D. Performance comparison of the 3D-printed and injection-molded PLA and its elastomer blend and fiber composites. J. Thermoplast. Compos. Mater. 2018, 32, 501–520. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pal, A.K.; Rodriguez, A.U.; Wu, F.; Misra, M.; Mielewski, D.F.; Kiziltas, A.; Mohanty, A.K. Recycled poly(lactic acid)–based 3D printed sustainable biocomposites: A comparative study with injection molding. Mater. Today Sustain. 2020, 7–8, 100027. [Google Scholar] [CrossRef]
- Komal, U.K.; Kasaudhan, B.K.; Singh, I. Comparative Performance Analysis of Polylactic Acid Parts Fabricated by 3D Printing and Injection Molding. J. Mater. Eng. Perform. 2021, 30, 6522–6528. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Vairis, A.; Savvakis, K.; Maniadi, A. A parametric determination of bending and Charpy’s impact strength of ABS and ABS-plus fused deposition modeling specimens. Prog. Addit. Manuf. 2019, 4, 323–330. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Vairis, A.; Savvakis, K.; Maniadi, A. On the compressive behavior of an FDM Steward Platform part. J. Comput. Des. Eng. 2017, 4, 339–346. [Google Scholar] [CrossRef]
- Garcia, E.A.; Ayranci, C.; Qureshi, A.J. Material Property-Manufacturing Process Optimization for Form 2 Vat-Photo Polymerization 3D Printers. J. Manuf. Mater. Process. 2020, 4, 12. [Google Scholar] [CrossRef]
- Park, G.-S.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y.; Seo, D.-G. Effects of Printing Parameters on the Fit of Implant-Supported 3D Printing Resin Prosthetics. Materials 2019, 12, 2533. [Google Scholar] [CrossRef]
- Baino, F.; Schwentenwein, M.; Verné, E. Modelling the Mechanical Properties of Hydroxyapatite Scaffolds Produced by Digital Light Processing-Based Vat Photopolymerization. Ceramics 2022, 5, 593–600. [Google Scholar] [CrossRef]
- Martín-Montal, J.; Pernas-Sánchez, J.; Varas, D. Experimental characterization framework for SLA additive manufacturing materials. Polymers 2021, 13, 1147. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.; Xu, M.; Hu, R.; Zheng, L. Study of photocurable energetic resin based propellants fabricated by 3D printing. Mater. Des. 2021, 207, 109891. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D printing review: Materials, process, and design strategies for medical applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Son, J.; Lee, H. Preliminary study on polishing SLA 3D-printed ABS-like resins for surface roughness and glossiness reduction. Micromachines 2020, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Vaxevanidis, N.; Kechagias, J. Surface roughness investigation of poly-jet 3D printing. Mathematics 2020, 8, 758. [Google Scholar] [CrossRef]
- Kechagias, J.; Chaidas, D.; Vidakis, N.; Salonitis, K.; Vaxevanidis, N.M. Key parameters controlling surface quality and dimensional accuracy: A critical review of FFF process. Mater. Manuf. Process. 2022, 37, 963–984. [Google Scholar] [CrossRef]
- Ling, L.; Taremi, N.; Malyala, R. A Novel Low-Shrinkage Resin for 3D Printing. J. Dent. 2022, 118, 103957. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, C.; Shannon, A.; Sullivan, A.O.; Sullivan, K.J.O.; Sullivan, L.W.O. Impact of Increased UV Curing Time on the Curing Depth of Photosensitive Resins for 3D Printing; Research Square: Durham, NC, USA, 2022; pp. 1–20. [Google Scholar] [CrossRef]
- Puškar, T.; Trifković, B.; Koprivica, D.D.; Kojić, V.; Jevremović, A.; Mirković, S.; Eggbeer, D. In vitro cytotoxicity assessment of the 3D printed polymer based epoxy resin intended for use in dentistry. Vojnosanit. Pregl. 2019, 76, 502–509. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kasetaite, S.; Rekštytė, S.; Lileikis, S.; Ostrauskaite, J.; Malinauskas, M. A Bio-Based Resin for a Multi-Scale Optical 3D Printing. Sci. Rep. 2020, 10, 9758. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Strating, T.; Schnelting, G.H.M.; Dijkstra, P.; Tietema, M.; Xu, J.; Woortman, A.J.J.; Loos, K.; Jager, J.; Folkersma, R. Biobased Acrylate Photocurable Resin Formulation for Stereolithography 3D Printing. ACS Omega 2018, 3, 1403–1408. [Google Scholar] [CrossRef]
- Kaufmann, R.; Zech, C.J.; Takes, M.; Brantner, P.; Thieringer, F.; Deutschmann, M.; Hergan, K.; Scharinger, B.; Hecht, S.; Rezar, R.; et al. Vascular 3D Printing with a Novel Biological Tissue Mimicking Resin for Patient-Specific Procedure Simulations in Interventional Radiology: A Feasibility Study. J. Digit. Imaging 2022, 35, 9–20. [Google Scholar] [CrossRef]
- Guerra, A.J.; Lara-Padilla, H.; Becker, M.L.; Rodriguez, C.A.; Dean, D. Photopolymerizable Resins for 3D-Printing Solid-Cured Tissue Engineered Implants. Curr. Drug Targets 2019, 20, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Portillo, D.J.; Bayliss, L.; Rivas, S.; Pineda, G.; Kaur, S.; Bunegin, L.; Hood, R.L. Characterizing and Tuning Perfusion Parameters Within an Innovative, Versatile Oxygenating Perfusion System. Ann. Biomed. Eng. 2021, 49, 3154–3164. [Google Scholar] [CrossRef] [PubMed]
- Beitler, B.; Roytman, G.R.; Parmer, G.; Tommasini, S.M.; Wiznia, D.H. Evaluating surface coatings to reduce bone cement adhesion to point of care 3D printed molds in the intraoperative setting. 3D Print. Med. 2022, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Hao, L.; Yang, Y.; Tang, T.; Tang, D.; Xiong, W. Graphene-reinforced biodegradable resin composites for stereolithographic 3D printing of bone structure scaffolds. J. Nanomater. 2019, 2019, 9710264. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Adeoye, A.O.M. 3D printing of bone scaffolds with hybrid biomaterials. Compos. Part B Eng. 2019, 158, 428–436. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Bao, Y.; Paunović, N.; Leroux, J.C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864. [Google Scholar] [CrossRef]
- Keßler, A.; Dosch, M.; Reymus, M.; Folwaczny, M. Influence of 3D-printing method, resin material, and sterilization on the accuracy of virtually designed surgical implant guides. J. Prosthet. Dent. 2022, 128, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Tsikritzis, D.; Gkagkanatsiou, A.; Kanellopoulou, S. Investigation of the Biocidal Performance of Multi-Functional Resin/Copper Nanocomposites with Superior Mechanical Response in SLA 3D Printing. Biomimetics 2022, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, C.; Shannon, A.; O’Sullivan, A.; O’Sullivan, K.J.; O’Sullivan, L.W. Biocompatible 3D printing resins for medical applications: A review of marketed intended use, biocompatibility certification, and post-processing guidance. Ann. 3D Print. Med. 2022, 5, 100044. [Google Scholar] [CrossRef]
- García-Sevilla, M.; Mediavilla-Santos, L.; Ruiz-Alba, M.T.; Pérez-Mañanes, R.; Calvo-Haro, J.A.; Pascau, J. Patient-specific desktop 3D-printed guides for pelvic tumour resection surgery: A precision study on cadavers. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Karipott, S.S.; Fear, K.; Nelson, B.; Leguineche, K.; Lin, A.; Shekhar, S.; Guldberg, R.E.; Ong, K.G. A magnetoelastic bone fixation device for controlled mechanical stimulation at femoral fractures in rodents. Eng. Res. Express 2021, 3, 035028. [Google Scholar] [CrossRef]
- Burhamah, W.; Alshawaf, S.M.; Alwazzan, S.; AlYouha, S.; Al-Sabah, S. The Utilization of Three-Dimensional Printing in Creating a Surgical Instrument: An Areola Cookie Cutter. Aesthetic Surg. J. Open Forum. 2022, 4, 1–5. [Google Scholar] [CrossRef]
- Franchi, L.; Vichi, A.; Marti, P.; Lampus, F.; Guercio, S.; Recupero, A.; Giuntini, V.; Goracci, C. 3D Printed Customized Facemask for Maxillary Protraction in the Early Treatment of a Class III Malocclusion: Proof-of-Concept Clinical Case. Materials 2022, 15, 3747. [Google Scholar] [CrossRef]
- Lundquist, J.; Horstmann, B.; Pestov, D.; Ozgur, U.; Avrutin, V.; Topsakal, E. Energy-Efficient, On-Demand Activation of Biosensor Arrays for Long-Term Continuous Health Monitoring. Biosensors 2022, 12, 358. [Google Scholar] [CrossRef]
- Liu, L.; Dharmadhikari, S.; Spector, B.M.; Tan, Z.H.; Van Curen, C.E.; Agarwal, R.; Nyirjesy, S.; Shontz, K.; Sperber, S.A.; Breuer, C.K.; et al. Tissue-engineered composite tracheal grafts create mechanically stable and biocompatible airway replacements. J. Tissue Eng. 2022, 13, 20417314221108791. [Google Scholar] [CrossRef]
- Viswanath, D.I.; Liu, H.C.; Capuani, S.; Vander Pol, R.S.; Saunders, S.Z.; Chua, C.Y.X.; Grattoni, A. Engineered implantable vaccine platform for continuous antigen-specific immunomodulation. Biomaterials 2022, 281, 121374. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Lincoln, D.M.; Allahyari, Z.; Widom, L.P.; Robert, N.; Gaborski, T.R. Effects of Oxygen Plasma Treatment on Parylene C and Parylene N Membrane Biocompatibility for Tissue Barrier Models. bioRxiv 2022. [Google Scholar] [CrossRef]
- Musgrove, H.B.; Catterton, M.A.; Pompano, R.R. Analytica Chimica Acta Applied tutorial for the design and fabrication of biomicrofluidic devices by resin 3D printing. Anal. Chim. Acta 2022, 1209, 339842. [Google Scholar] [CrossRef] [PubMed]
- Pose-d, A.; Moreta-martinez, R.; Garc, D.; Calvo-haro, J.A.; Mediavilla-santos, L. HoloLens 1 vs. HoloLens 2: Improvements in the New Model for Orthopedic Oncological Interventions. Sensors 2022, 22, 4915. [Google Scholar]
- Pham, Y.L.; Beauchamp, J.; Clement, A.; Wiegandt, F.; Holz, O. 3D-printed mouthpiece adapter for sampling exhaled breath in medical applications. 3D Print. Med. 2022, 8, 1–8. [Google Scholar] [CrossRef]
- Velentza-Almpani, A.; Ibeanu, N.; Liu, T.; Redhead, C.; Khaw, P.T.; Brocchini, S.; Awwad, S.; Bouremel, Y. Effects of Flow Hydrodynamics and Eye Movements on Intraocular Drug Clearance. Pharmaceutics 2022, 14, 1267. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Kechagias, J.D.; Mountakis, N.; Argyros, A.; Boura, O.; Grammatikos, S. High-performance medical-grade resin radically reinforced with cellulose nanofibers for 3D printing. J. Mech. Behav. Biomed. Mater. 2022, 134, 105408. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Papadakis, V.; Korlos, A.; Mountakis, N.; Argyros, A. Multi-Functional 3D-Printed Vat Photopolymerization Biomedical-Grade Resin Reinforced with Binary Nano Inclusions: The Effect of Cellulose Nanofibers and Antimicrobial Nanoparticle Agents. Polymers 2022, 14, 1903. [Google Scholar] [CrossRef]
- Xiang, Z.; Cao, D. Porous covalent-organic materials: Synthesis, clean energy application and design. J. Mater. Chem. A 2013, 1, 2691–2718. [Google Scholar] [CrossRef]
- Makowski, P.; Thomas, A.; Kuhn, P.; Goettmann, F. Organic materials for hydrogen storage applications: From physisorption on organic solids to chemisorption in organic molecules. Energy Environ. Sci. 2009, 2, 480–490. [Google Scholar] [CrossRef]
- Feringa, B.L.; Jager, W.F.; de Lange, B. Organic materials for reversible optical data storage. Tetrahedron 1993, 49, 8267–8310. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, M.; Pathak, D.; Wagner, T.; Nunzi, J.M. Organic materials for photovoltaic applications: Review and mechanism. Synth. Met. 2014, 190, 20–26. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, S.; Zhang, X.; Ren, X.; Li, R.; Dong, H.; Hu, W. 2D Organic Materials for Optoelectronic Applications. Adv. Mater. 2018, 30, 1702415. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.; Mendes, L.C.; Ferreira, M.; Benzi, M.R. Degree of conversion versus the depth of polymerization of an organically modified ceramic dental restoration composite by fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 2007, 104, 325–330. [Google Scholar] [CrossRef]
- Gajewski, V.E.S.; Pfeifer, C.S.; Fróes-Salgado, N.R.G.; Boaro, L.C.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- De Souza Venter, S.A.; Fávaro, S.L.; Radovanovic, E.; Girotto, E.M. Hardness and degree of conversion of dental restorative compositesbased on an organic-inorganic hybrid. Mater. Res. 2013, 16, 898–902. [Google Scholar] [CrossRef]
- Mendes, L.C.; Tedesco, A.D.; Miranda, M.S. Determination of degree of conversion as function of depth of a photo-initiated dental restoration composite. Polym. Test. 2005, 24, 418–422. [Google Scholar] [CrossRef]
- Amirouche-Korichi, A.; Mouzali, M.; Watts, D.C. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent. Mater. 2009, 25, 1411–1418. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Khanna, P.K.; Gaikwad, S.; Adhyapak, P.V.; Singh, N.; Marimuthu, R. Synthesis and characterization of copper nanoparticles. Mater. Lett. 2007, 61, 4711–4714. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, P.; Yu, Z.; Shi, H.; Wu, D.; Yan, H.; Ye, X.; Lu, Q.; Tian, Y. A review on additive manufacturing of pure copper. Coatings 2021, 11, 740. [Google Scholar] [CrossRef]
- Roccetti Campagnoli, M.; Galati, M.; Saboori, A. On the processability of copper components via powder-based additive manufacturing processes: Potentials, challenges and feasible solutions. J. Manuf. Process. 2021, 72, 320–337. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Silva, J.C.; Graça, M.P.F. Copper-doped 45S5 bioactive glass for implants: Synthesis and physical-biological characterization. SSRN 2022, 1–19. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Mohamed, S.H. Abdel-wahab Photocatalytic activity in nanostructured zinc oxide thin films doped with metallic copper. Phys. B Phys. Condens. Matter 2022, 646, 414352. [Google Scholar] [CrossRef]
- Bao, X.; Li, Y.; Liu, X.; Feng, Y.; Xu, X.; Sun, G.; Wang, W.; Li, B.; Li, Z.; Yang, J. Fish and Shellfish Immunology Effect of acute Cu exposure on immune response mechanisms of golden cuttlefish ( Sepia esculenta). Fish Shellfish Immunol. 2022, 130, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Yosri, N.; Khalifa, S.A.M.; Guo, Z.; Xu, B.; Zou, X.; El-Seedi, H.R. Marine organisms: Pioneer natural sources of polysaccharides/proteins for green synthesis of nanoparticles and their potential applications. Int. J. Biol. Macromol. 2021, 193, 1767–1798. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; El-Wahed, A.A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Basay, C.P.; Paterno, E.S.; Delmo-Organo, N.; Villegas, L.C.; Fernando, L.M. Effects of Nanoformulated Plant Growth Regulator on Culturable Bacterial Population, Microbial Biomass and Enzyme Activities in Two Soil Types. Mindanao J. Sci. Technol. 2021, 19, 145–163. [Google Scholar]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Aguilar-Perez, D.; Vargas-Coronado, R.; Cervantes-Uc, J.M.; Rodriguez-Fuentes, N.; Aparicio, C.; Covarrubias, C.; Alvarez-Perez, M.; Garcia-Perez, V.; Martinez-Hernandez, M.; Cauich-Rodriguez, J.V. Antibacterial activity of a glass ionomer cement doped with copper nanoparticles. Dent. Mater. J. 2020, 39, 389–396. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef]
- Marovic, D.; Par, M.; Tauböck, T.T.; Haugen, H.J.; Negovetic Mandic, V.; Wüthrich, D.; Burrer, P.; Zheng, K.; Attin, T.; Tarle, Z.; et al. Impact of Copper-Doped Mesoporous Bioactive Glass Nanospheres on the Polymerisation Kinetics and Shrinkage Stress of Dental Resin Composites. Int. J. Mol. Sci. 2022, 23, 8195. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Goossens, L.R.; Kinds, Y.; Van Hooreweder, B.; Vanmeensel, K. Laser-based powder bed fusion additive manufacturing of pure copper. Addit. Manuf. 2021, 42, 101990. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Dhekne, P.P.; Dadbakhsh, S.; Kruth, J.P.; Van Humbeeck, J.; Vanmeensel, K. Surface Modified Copper Alloy Powder for Reliable Laser-based Additive Manufacturing. Addit. Manuf. 2020, 35, 101418. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Murr, L.E.; Li, S.J.; Tian, Y.X.; Martinez, E.; Martinez, J.L.; Machado, B.I.; Gaytan, S.M.; Medina, F.; Wicker, R.B. Open-cellular copper structures fabricated by additive manufacturing using electron beam melting. Mater. Sci. Eng. A 2011, 528, 5379–5386. [Google Scholar] [CrossRef]
- Sriraman, M.R.; Babu, S.S.; Short, M. Bonding characteristics during very high power ultrasonic additive manufacturing of copper. Scr. Mater. 2010, 62, 560–563. [Google Scholar] [CrossRef]
- Petousis, M.; Vidakis, N.; Velidakis, E.; Kechagias, J.D.; David, C.N.; Papadakis, S.; Mountakis, N. Affordable Biocidal Ultraviolet Cured Cuprous Oxide Filled Vat Photopolymerization Resin Nanocomposites with Enhanced Mechanical Properties. Biomimetics 2022, 7, 12. [Google Scholar] [CrossRef]
- Petousis, M.; Vidakis, N.; Mountakis, N.; Papadakis, V.; Kanellopoulou, S.; Gaganatsiou, A.; Stefanoudakis, N.; Kechagias, J. Multifunctional Material Extrusion 3D-Printed Antibacterial Polylactic Acid ( PLA ) with Binary Inclusions: The Effect of Cuprous Oxide and Cellulose Nanofibers. Fibers 2022, 10, 5. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Grammatikos, S.; David, C.N.; Mountakis, N.; Argyros, A.; Boura, O. Development and Optimization of Medical-Grade MultiFunctional Polyamide 12-Cuprous Oxide Nanocomposites with Superior Mechanical and Antibacterial Properties for Cost-Effective 3D Printing. Nanomaterials 2022, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Mountakis, N.; Korlos, A.; Papadakis, V.; Moutsopoulou, A. Trilateral Multi-Functional Polyamide 12 Nanocomposites with Binary Inclusions for Medical Grade Material Extrusion 3D Printing: The Effect of Titanium Nitride in Mechanical Reinforcement and Copper/Cuprous Oxide as Antibacterial Agents. J. Funct. Biomater. 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zeng, J.; Long, H.; Xiao, J.; Luo, Y.; Gu, J.; Zhou, W.; Wei, Y.; Dong, X. Micrometer copper-zinc alloy particles-reinforced wood plastic composites with high gloss and antibacterial properties for 3d printing. Polymers 2020, 12, 621. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Mountakis, N.; Maravelakis, E.; Zaoutsos, S.; Kechagias, J.D. Mechanical response assessment of antibacterial PA12/TiO2 3D printed parts: Parameters optimization through artificial neural networks modeling. Int. J. Adv. Manuf. Technol. 2022, 121, 785–803. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Tzounis, L.; Liebscher, M.; Grammatikos, S.A. Enhanced mechanical, thermal and antimicrobial properties of additively manufactured polylactic acid with optimized nano silica content. Nanomaterials 2021, 11, 1012. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Liebscher, M.; Tzounis, L. Three-Dimensional Printed Antimicrobial Objects of Polylactic Acid (PLA)-Silver Nanoparticle Nanocomposite Filaments Produced by an In-Situ Reduction Reactive Melt Mixing Process. Biomimetics 2020, 5, 42. [Google Scholar] [CrossRef]
- Tzounis, L.; Bangeas, P.I.; Exadaktylos, A.; Petousis, M.; Vidakis, N. Three-dimensional printed polylactic acid (PLA) surgical retractors with sonochemically immobilized silver nanoparticles: The next generation of low-cost antimicrobial surgery equipment. Nanomaterials 2020, 10, 985. [Google Scholar] [CrossRef]
- Bayraktar, I.; Doganay, D.; Coskun, S.; Kaynak, C.; Akca, G.; Unalan, H.E. 3D printed antibacterial silver nanowire/polylactide nanocomposites. Compos. Part B Eng. 2019, 172, 671–678. [Google Scholar] [CrossRef]
- Sa, L.; Kaiwu, L.; Shenggui, C.; Junzhong, Y.; Yongguang, J.; Lin, W.; Li, R. 3D printing dental composite resins with sustaining antibacterial ability. J. Mater. Sci. 2019, 54, 3309–3318. [Google Scholar] [CrossRef]
- Maróti, P.; Kocsis, B.; Ferencz, A.; Nyitrai, M.; Lőrinczy, D. Differential thermal analysis of the antibacterial effect of PLA-based materials planned for 3D printing. J. Therm. Anal. Calorim. 2020, 139, 367–374. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Kourinou, M.; Velidakis, E.; Mountakis, N.; Fischer-Griffiths, P.E.; Grammatikos, S.; Tzounis, L. Additive manufacturing of multifunctional polylactic acid (PLA)—Multiwalled carbon nanotubes (MWCNTs) nanocomposites. Nanocomposites 2021, 7, 184–199. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Fischer-Griffiths, P.E.; Grammatikos, S.; Tzounis, L. Fused Filament Fabrication Three-Dimensional Printing Multi-Functional of Polylactic Acid/Carbon Black Nanocomposites. C 2021, 7, 52. [Google Scholar] [CrossRef]
- Du, B.D.; Van Phu, D.; Quoc, L.A.; Hien, N.Q. Synthesis and Investigation of Antimicrobial Activity of Cu2O Nanoparticles/Zeolite. J. Nanoparticles 2017, 2017, 7056864. [Google Scholar] [CrossRef]
- Malas, A.; Isakov, D.; Couling, K.; Gibbons, G.J. Fabrication of High Permittivity Resin Composite for Vat Photopolymerization 3D Printing: Morphology, Thermal, Dynamic Mechanical and Dielectric Properties. Materials 2019, 12, 3818. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, C.; Brown, J.R.; Feller, K.; Scott, P.J.; Meenakshisundaram, V.; Williams, C.; Long, T. Vat Photopolymerization of Reinforced Styrene–Butadiene Elastomers: A Degradable Scaffold Approach. ACS Appl. Mater. Interfaces 2022, 14, 18965–18973. [Google Scholar] [CrossRef]
- Medellin, A.; Du, W.; Miao, G.; Zou, J.; Pei, Z.; Ma, C. Vat Photopolymerization 3D Printing of Nanocomposites: A Literature Review. J. Micro Nano-Manufacturing 2019, 7, 031006. [Google Scholar] [CrossRef]
- Resta, V.; Quarta, G.; Lomascolo, M.; Maruccio, L.; Calcagnile, L. Raman and Photoluminescence spectroscopy of polycarbonate matrices irradiated with different energy 28Si+ ions. Vacuum 2015, 116, 82–89. [Google Scholar] [CrossRef]
- Giordano, D.; Russell, J.K.; González-García, D.; Bersani, D.; Dingwell, D.B.; Del Negro, C. Raman spectroscopy from laboratory and proximal to remote sensing: A tool for the volcanological sciences. Remote Sens. 2020, 12, 805. [Google Scholar] [CrossRef]
- Stuart, B.H. Temperature studies of polycarbonate using Fourier transform Raman spectroscopy. Polym. Bull. 1996, 36, 341–346. [Google Scholar] [CrossRef]
- Zimmerer, C.; Matulaitiene, I.; Niaura, G.; Reuter, U.; Janke, A.; Boldt, R.; Sablinskas, V.; Steiner, G. Nondestructive characterization of the polycarbonate—Octadecylamine interface by surface enhanced Raman spectroscopy. Polym. Test. 2019, 73, 152–158. [Google Scholar] [CrossRef]
- Lin, Z.N.; Guo, X.M.; He, Z.P.; Liang, X.R.; Wang, M.M.; Jin, G. Thermal degradation kinetics study of molten polylactide based on Raman spectroscopy. Polym. Eng. Sci. 2021, 61, 201–210. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing cellulose structures with vibrational spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Łydżba-Kopczyńska, B.; Sentandreu, E. Raman spectroscopy coupled to chemometrics to discriminate provenance and geological age of amber. J. Raman Spectrosc. 2018, 49, 842–851. [Google Scholar] [CrossRef]
- Singh, S.S.; Chakraborty, P.; Kitey, R. Deformation characteristics of glass-filled epoxy composite under compression: Role of filler shape and volume fraction. Polym. Compos. 2019, 40, 4726–4741. [Google Scholar] [CrossRef]
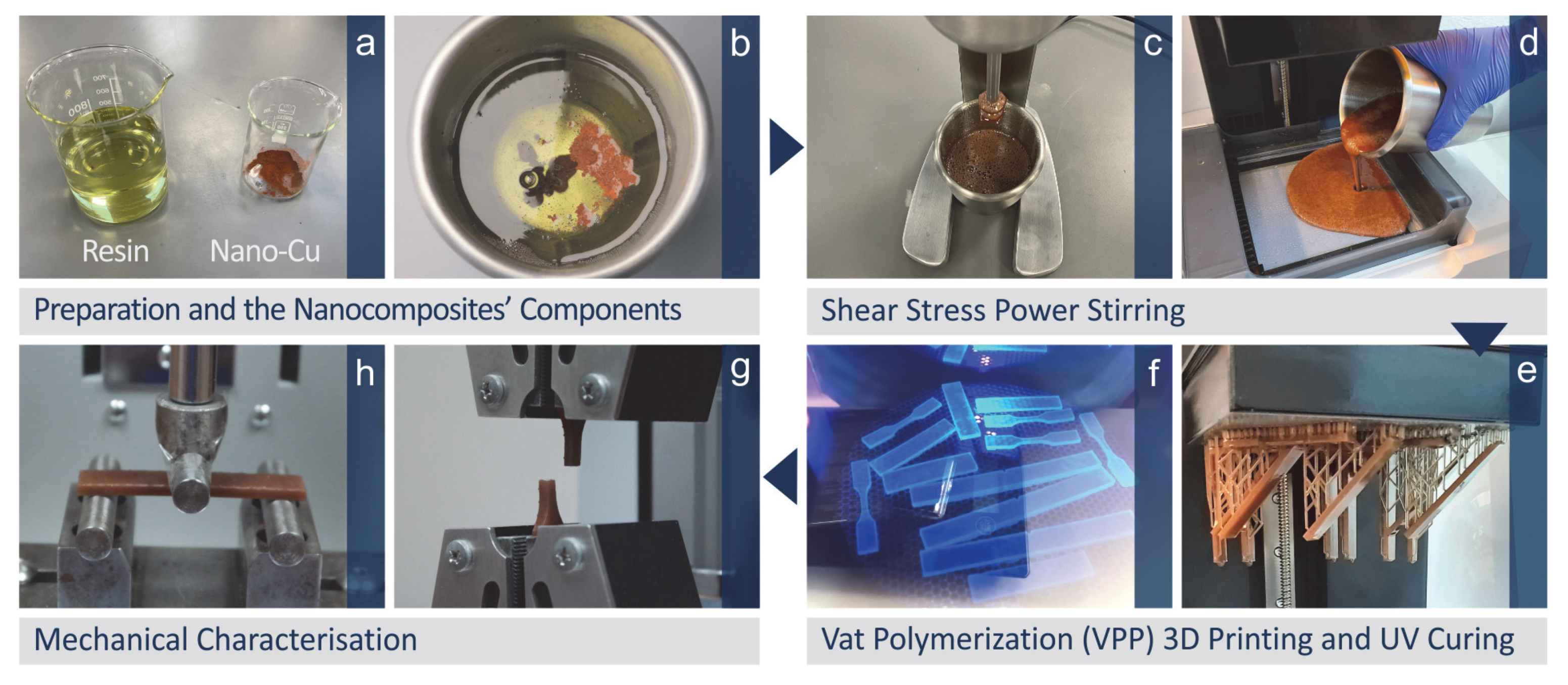
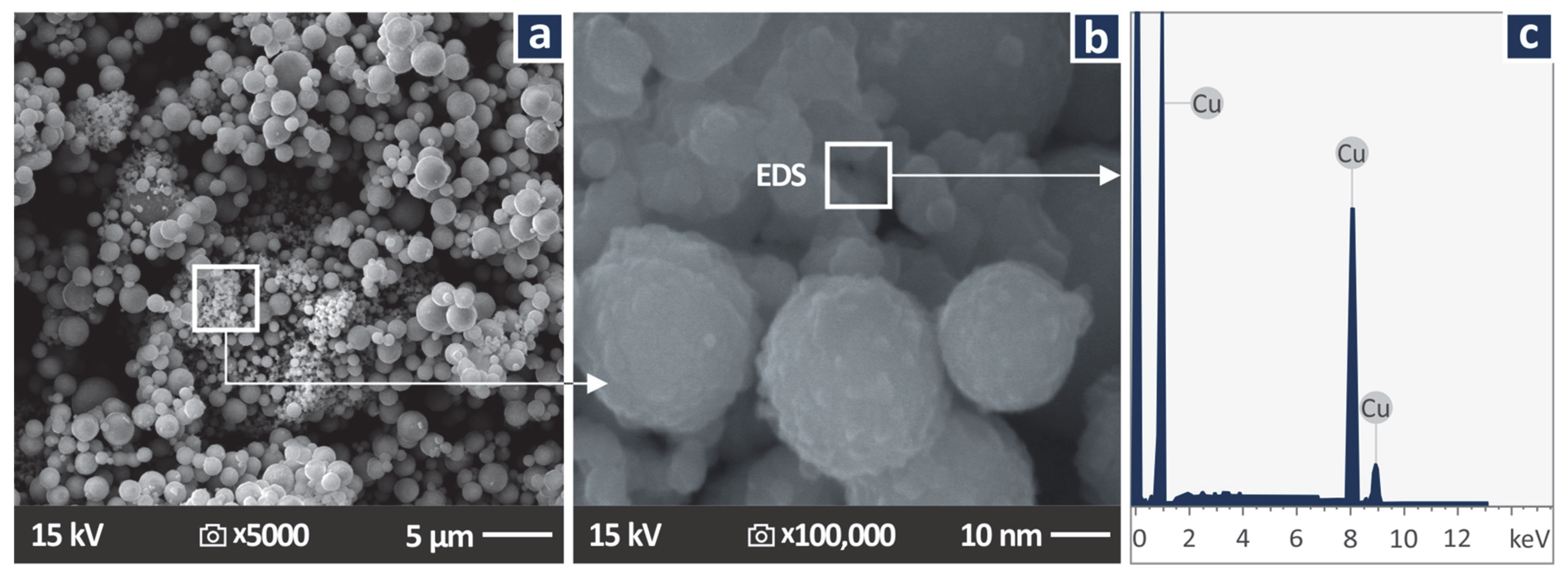
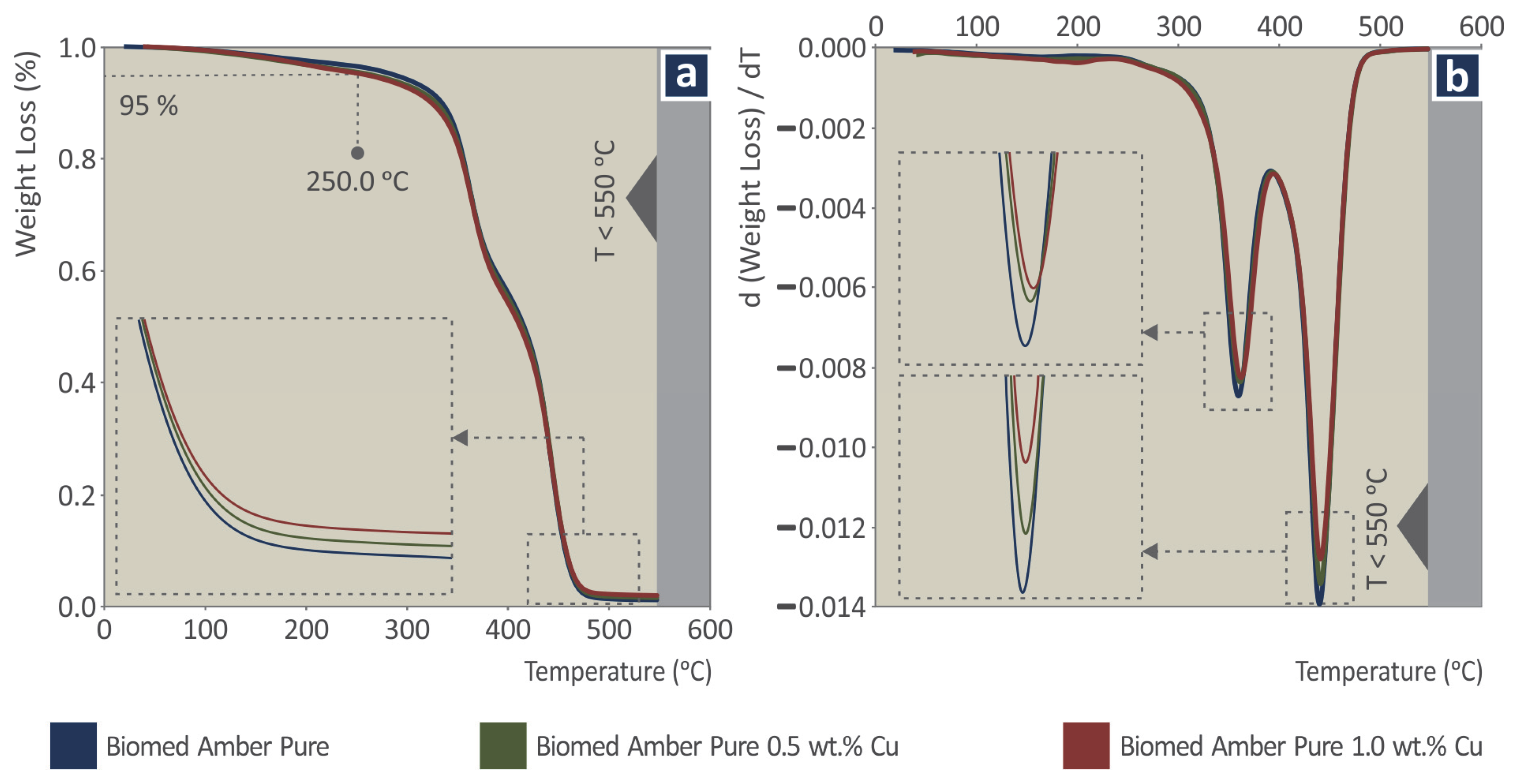
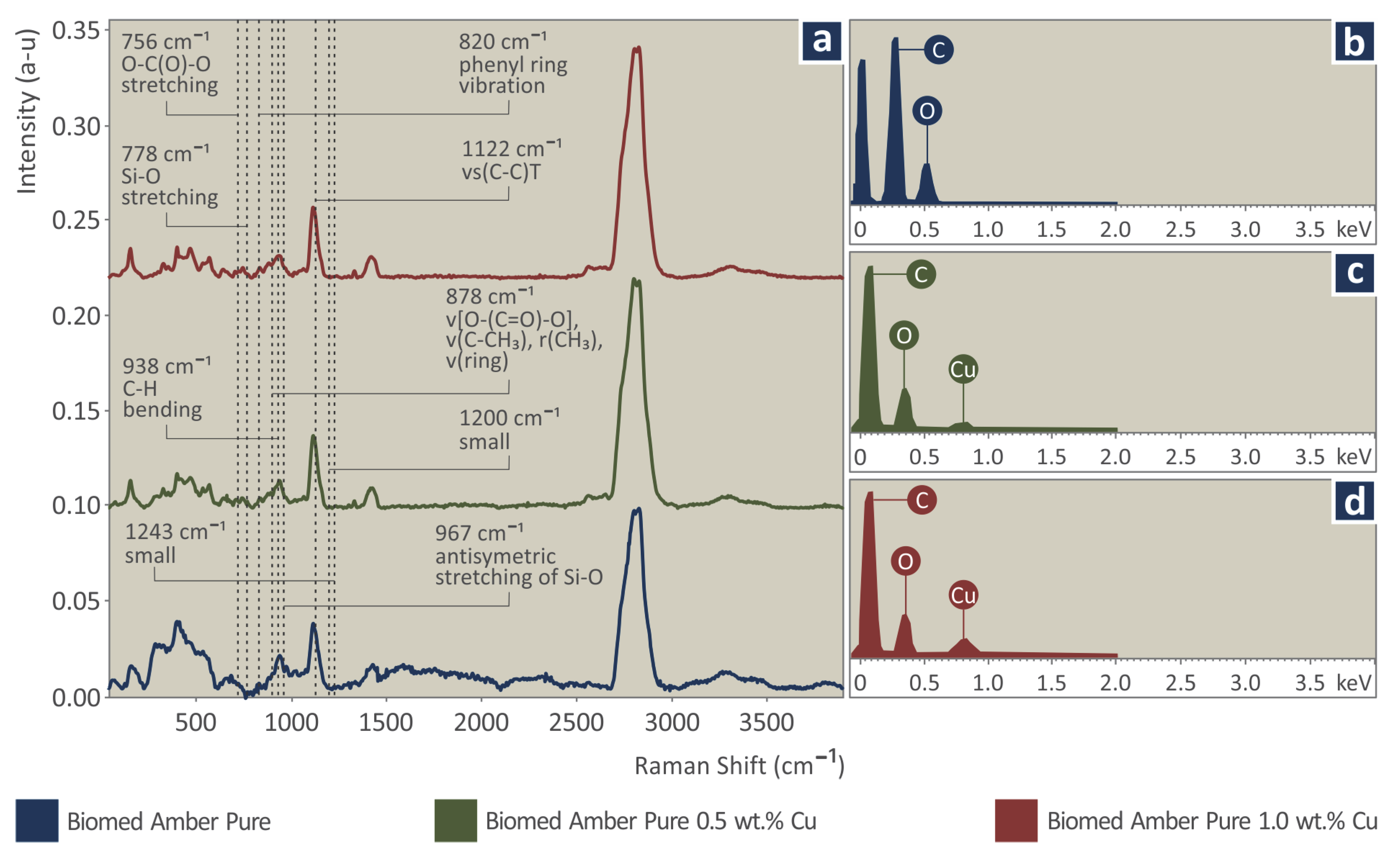


| # | Filler Consideration wt.% Cu | Resin |
|---|---|---|
| 1 | 0.0 | Biomed Amber |
| 2 | 0.5 | Biomed Amber |
| 3 | 1.0 | Biomed Amber |
| Run | Filler Consideration wt.% Cu | Printing Angle/PA (deg.) |
|---|---|---|
| 1 | 0.0 | 0 |
| 2 | 0.0 | 45 |
| 3 | 0.0 | 90 |
| 4 | 0.5 | 0 |
| 5 | 0.5 | 45 |
| 6 | 0.5 | 90 |
| 7 | 1.0 | 0 |
| 8 | 1.0 | 45 |
| 9 | 1.0 | 90 |
| Wavenumber (cm−1) | Raman Peak Assignment |
|---|---|
| 756 | O-C(O)-O stretching [109] |
| 778 | Si-O stretching [110], O-C(O)-O stretching [111] |
| 820 | phenyl ring vibration [111,112] |
| 878 | ν[O−(C=O)−O], ν(C−CH3), r(CH3), ν(ring) [109,111,113] |
| 938 | C-H bending [109,111] |
| 1200 | C-O-C stretching [109] |
| 1243 | C-O-C symmetric stretch [109] |
| 1294 | C-O-C symmetric stretch [109,111] or CH2 [112] |
| 1364 | C–C-H, C-O–H, and O-C-H [114] |
| 1449 | CH3 bending [109,111,113] |
| 1638 | v (C=C) [115] |
| 1723 | ν (C=O) [115] |
| 2935 | CH2 asymmetric stretching [114] |
| 2953 | CH2 asymmetric stretching [114] |
| Wavenumber (cm−1) | Raman Peak Assignment | Change |
|---|---|---|
| 756 | O-C(O)-O stretching | Strong increase |
| 778 | Si-O stretching | Strong increase |
| 820 | phenyl ring vibration | Strong increase |
| 878 | ν[O−(C=O)−O], ν(C−CH3), r(CH3), ν(ring) | Strong increase |
| 938 | C-H bending | Small increase |
| 967 | Antisymmetric stretching of Si-O | A new and strong peak |
| 1122 | νs(C−C)T | A new and small peak |
| 1200 | small | Small increase |
| 1243 | small | Small increase |
| Run | Cross Section Area (mm2) | Area to Nom [%] | Ra-Hor. (μm) | Rz-Hor. (μm) | Ra-Ver. (μm) | Rz-Ver. (μm) |
|---|---|---|---|---|---|---|
| 1 | 10.25 ± 0.05 | 100.73 ± 0.45 | 1.27 ± 0.07 | 8.46 ± 0.62 | 1.77 ± 0.32 | 12.88 ± 2.76 |
| 2 | 9.89 ± 0.10 | 97.19 ± 0.95 | 1.49 ± 0.04 | 9.82 ± 0.39 | 2.04 ± 0.18 | 12.50 ± 1.14 |
| 3 | 9.84 ± 0.03 | 96.70 ± 0.28 | 1.16 ± 0.21 | 8.00 ± 0.88 | 1.86 ± 0.08 | 11.42 ± 0.19 |
| 4 | 9.87 ± 0.11 | 96.95 ± 1.12 | 10.56 ± 0.59 | 52.20 ± 14.65 | 4.88 ± 0.82 | 28.20 ± 2.17 |
| 5 | 9.32 ± 0.08 | 91.59 ± 0.75 | 5.42 ± 0.39 | 30.60 ± 3.58 | 5.28 ± 0.92 | 25.40 ± 6.77 |
| 6 | 9.34 ± 0.08 | 91.78 ± 0.80 | 2.92 ± 0.36 | 19.20 ± 1.30 | 5.30 ± 1.09 | 27.40 ± 3.71 |
| 7 | 9.48 ± 0.09 | 93.19 ± 0.91 | 5.34 ± 0.42 | 32.00 ± 12.51 | 7.24 ± 0.81 | 33.00 ± 3.67 |
| 8 | 9.22 ± 0.03 | 90.64 ± 0.34 | 2.98 ± 0.58 | 18.80 ± 2.68 | 4.72 ± 0.73 | 29.20 ± 5.02 |
| 9 | 9.31 ± 0.04 | 91.48 ± 0.39 | 1.43 ± 0.20 | 9.74 ± 2.02 | 2.72 ± 0.59 | 15.20 ± 4.76 |
| Run | sB Tension (MPa) | E Tension (MPa) | Toughness Tension (MJ/m3) | sB Flexural (MPa) | E Flexural (MPa) | Toughness Flexural (MJ/m3) |
|---|---|---|---|---|---|---|
| 1 | 71.91 ± 1.03 | 222.04 ± 20.77 | 20.73 ± 2.57 | 97.86 ± 0.59 | 2275.98 ± 22.19 | 2.68 ± 0.02 |
| 2 | 73.68 ± 0.58 | 310.31 ± 11.03 | 13.87 ± 0.98 | 104.49 ± 2.33 | 2616.88 ± 80.58 | 2.97 ± 0.08 |
| 3 | 71.49 ± 1.47 | 319.12 ± 14.97 | 10.97 ± 2.13 | 108.06 ± 1.79 | 2746.75 ± 63.12 | 3.10 ± 0.06 |
| 4 | 78.63 ± 1.96 | 320.85 ± 16.06 | 14.97 ± 1.24 | 109.42 ± 1.52 | 2573.82 ± 38.04 | 3.05 ± 0.04 |
| 5 | 80.96 ± 0.74 | 345.99 ± 12.10 | 15.53 ± 1.40 | 113.49 ± 2.48 | 2811.18 ± 68.65 | 3.26 ± 0.07 |
| 6 | 82.53 ± 0.24 | 350.18 ± 10.44 | 16.69 ± 1.11 | 115.37 ± 2.63 | 2911.61 ± 75.71 | 3.40 ± 0.09 |
| 7 | 78.20 ± 1.14 | 297.76 ± 14.04 | 15.84 ± 0.30 | 101.27 ± 0.80 | 2350.87 ± 24.04 | 2.81 ± 0.03 |
| 8 | 75.53 ± 2.73 | 330.86 ± 7.98 | 15.56 ± 6.19 | 109.33 ± 2.51 | 2710.62 ± 60.43 | 3.14 ± 0.06 |
| 9 | 80.04 ± 0.58 | 343.17 ± 2.53 | 14.97 ± 1.78 | 114.03 ± 1.11 | 2892.06 ± 73.77 | 3.30 ± 0.06 |
| Nano Compound wt.% Cu | S. aureus IZ (mm) | E. coli IZ (mm) |
|---|---|---|
| 0.0 | 0.0 | 0.0 |
| 0.5 | 2.0 | ~0.5 |
| 1.0 | 7.0 | ~0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidakis, N.; Petousis, M.; Papadakis, V.M.; Mountakis, N. Multifunctional Medical Grade Resin with Enhanced Mechanical and Antibacterial Properties: The Effect of Copper Nano-Inclusions in Vat Polymerization (VPP) Additive Manufacturing. J. Funct. Biomater. 2022, 13, 258. https://doi.org/10.3390/jfb13040258
Vidakis N, Petousis M, Papadakis VM, Mountakis N. Multifunctional Medical Grade Resin with Enhanced Mechanical and Antibacterial Properties: The Effect of Copper Nano-Inclusions in Vat Polymerization (VPP) Additive Manufacturing. Journal of Functional Biomaterials. 2022; 13(4):258. https://doi.org/10.3390/jfb13040258
Chicago/Turabian StyleVidakis, Nectarios, Markos Petousis, Vassilis M. Papadakis, and Nikolaos Mountakis. 2022. "Multifunctional Medical Grade Resin with Enhanced Mechanical and Antibacterial Properties: The Effect of Copper Nano-Inclusions in Vat Polymerization (VPP) Additive Manufacturing" Journal of Functional Biomaterials 13, no. 4: 258. https://doi.org/10.3390/jfb13040258
APA StyleVidakis, N., Petousis, M., Papadakis, V. M., & Mountakis, N. (2022). Multifunctional Medical Grade Resin with Enhanced Mechanical and Antibacterial Properties: The Effect of Copper Nano-Inclusions in Vat Polymerization (VPP) Additive Manufacturing. Journal of Functional Biomaterials, 13(4), 258. https://doi.org/10.3390/jfb13040258








