Dynamic Responsive Inguinal Scaffold Activates Myogenic Growth Factors Finalizing the Regeneration of the Herniated Groin
Abstract
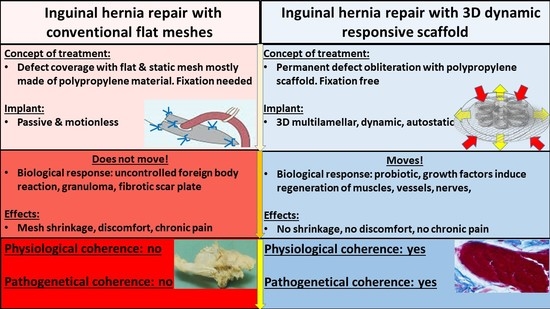
1. Introduction
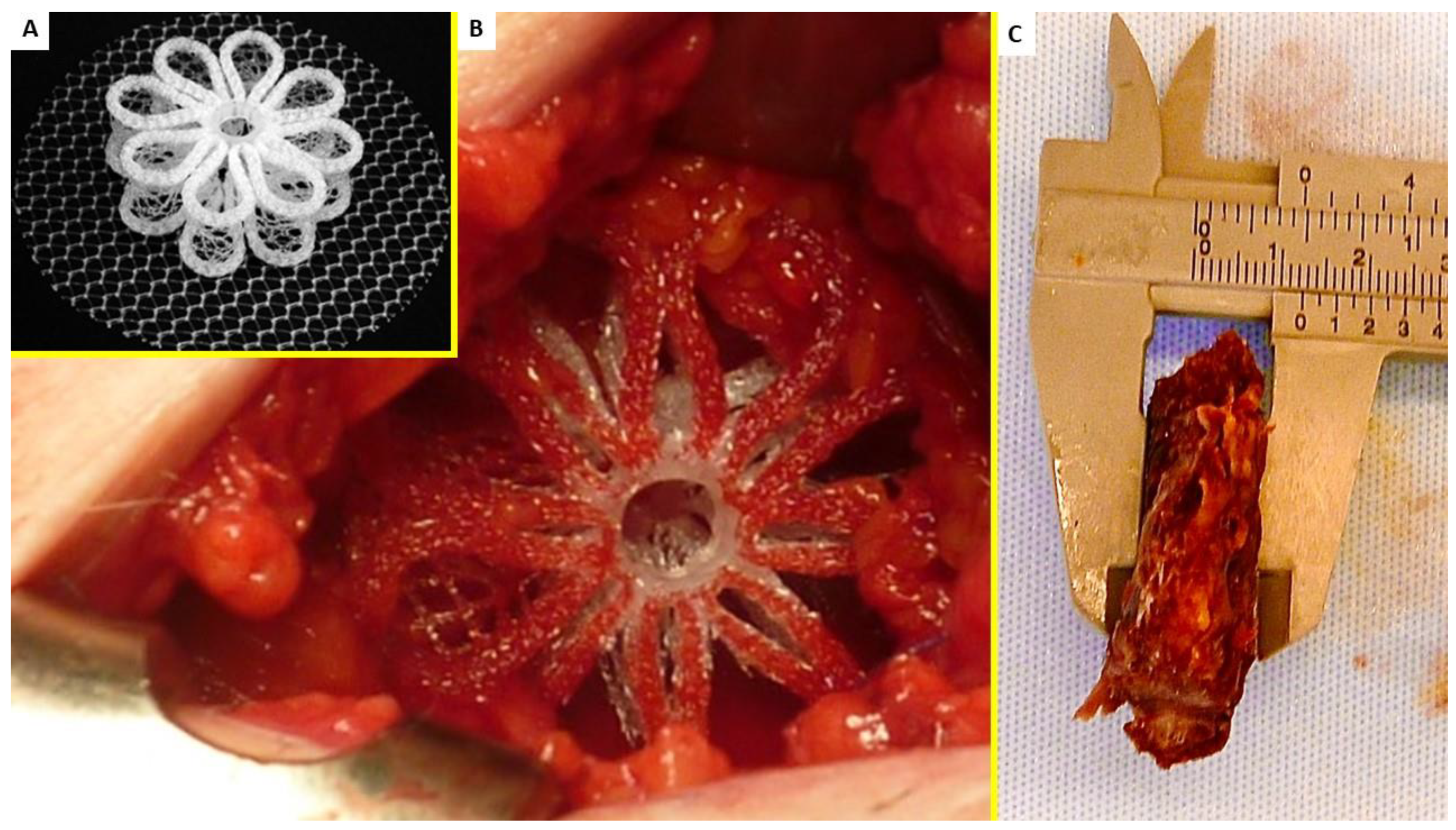
2. Material and Methods
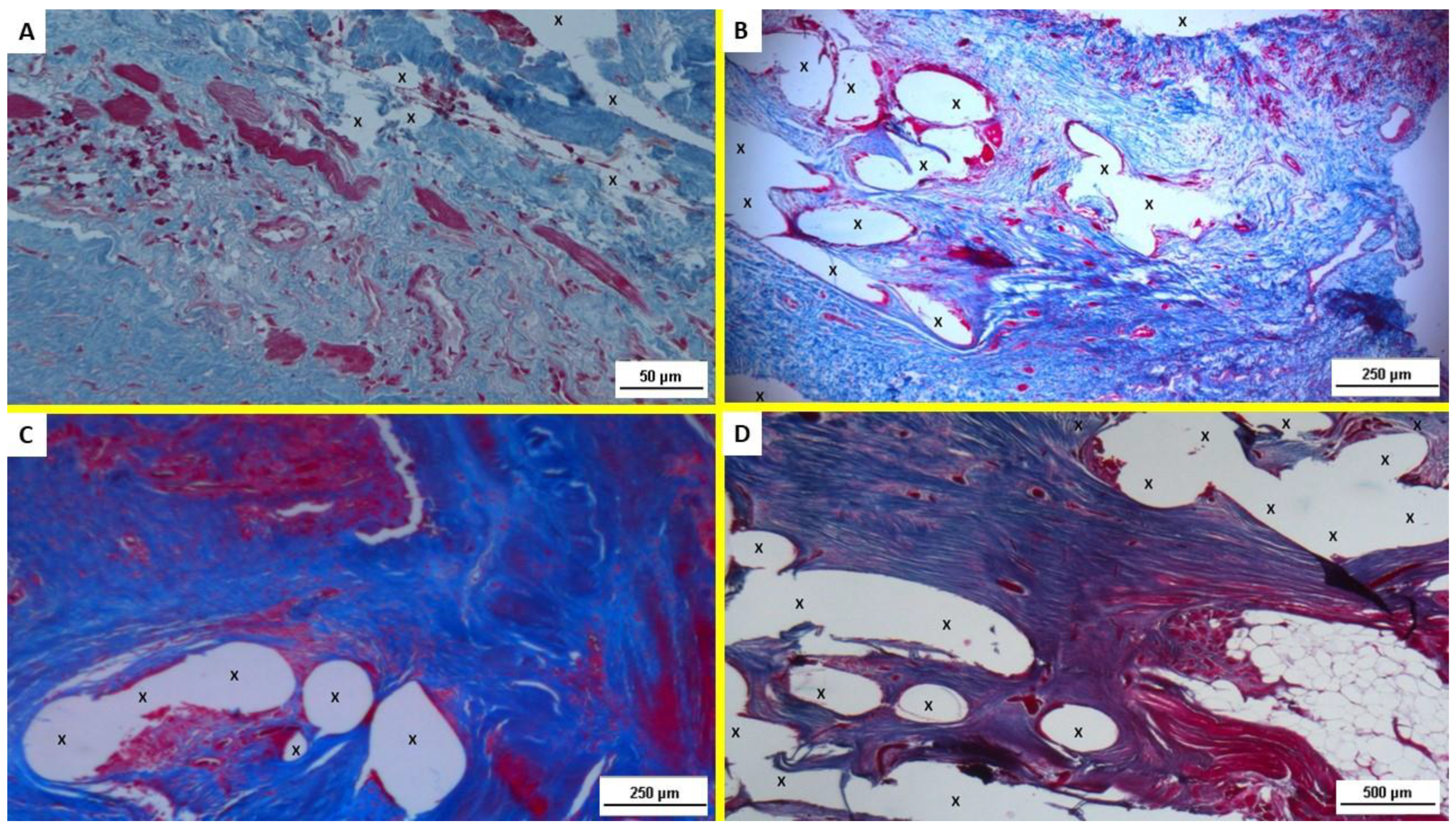
2.1. Histological Evaluation
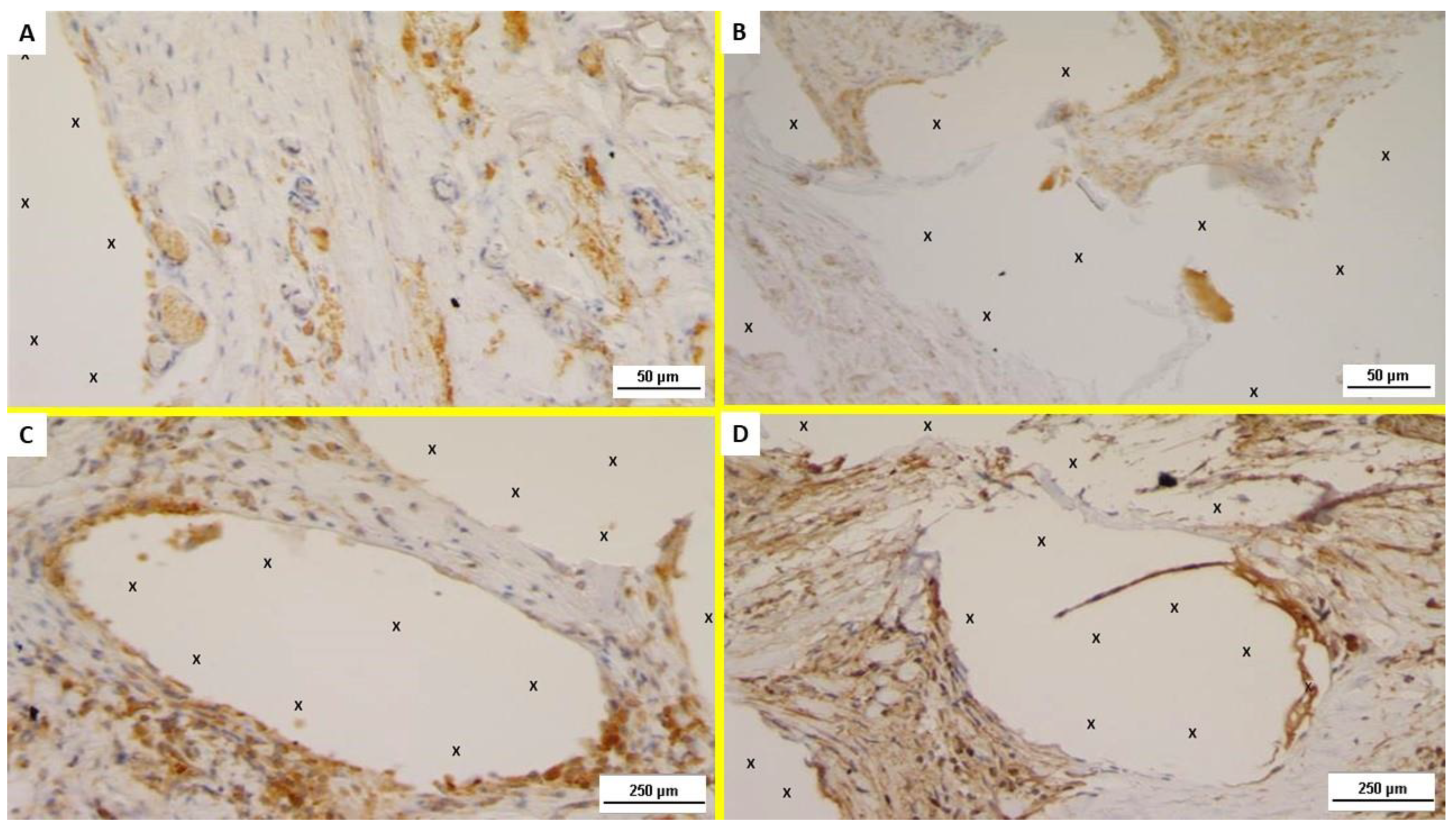
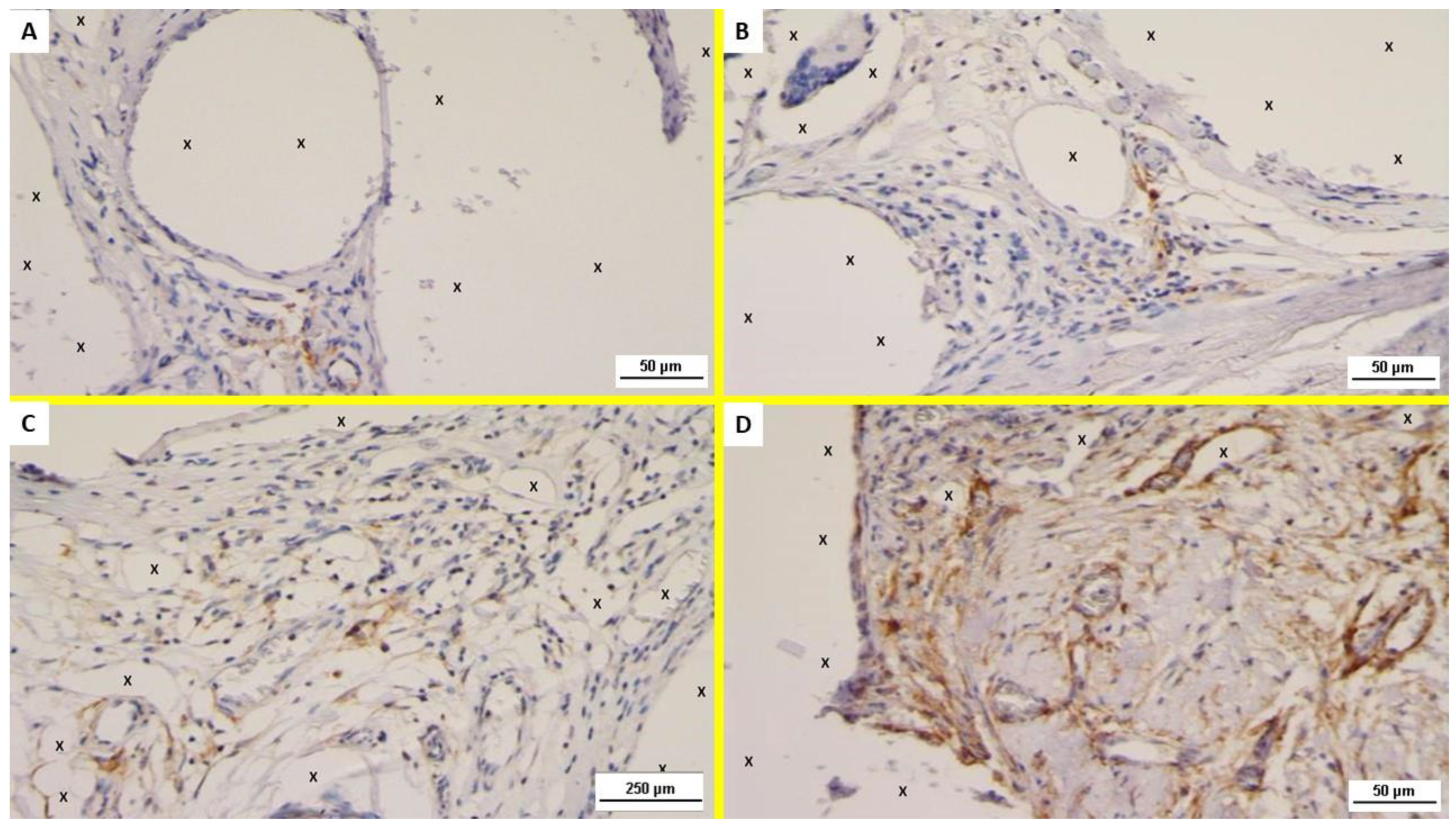
2.2. Immunohistochemistry
- (a)
- The presence of neomyogenetic activity by evidencing the density of specific clusters using an anti-NGF rabbit monoclonal antibody (Abcam, 52 Grove Street Waltham, MA 02453. USA—code: ab52918), dilution: 1:100;
- (b)
- Cross evidence of the NGF active clusters by detecting the NGF receptors in the excised tissue with NGFR p75 mouse monoclonal antibody (Santa Cruz Biotechnology, Inc. 10410 Finnell Street Dallas, Texas 75220 U.S.A., code: sc.13577), dilution: 1:100.
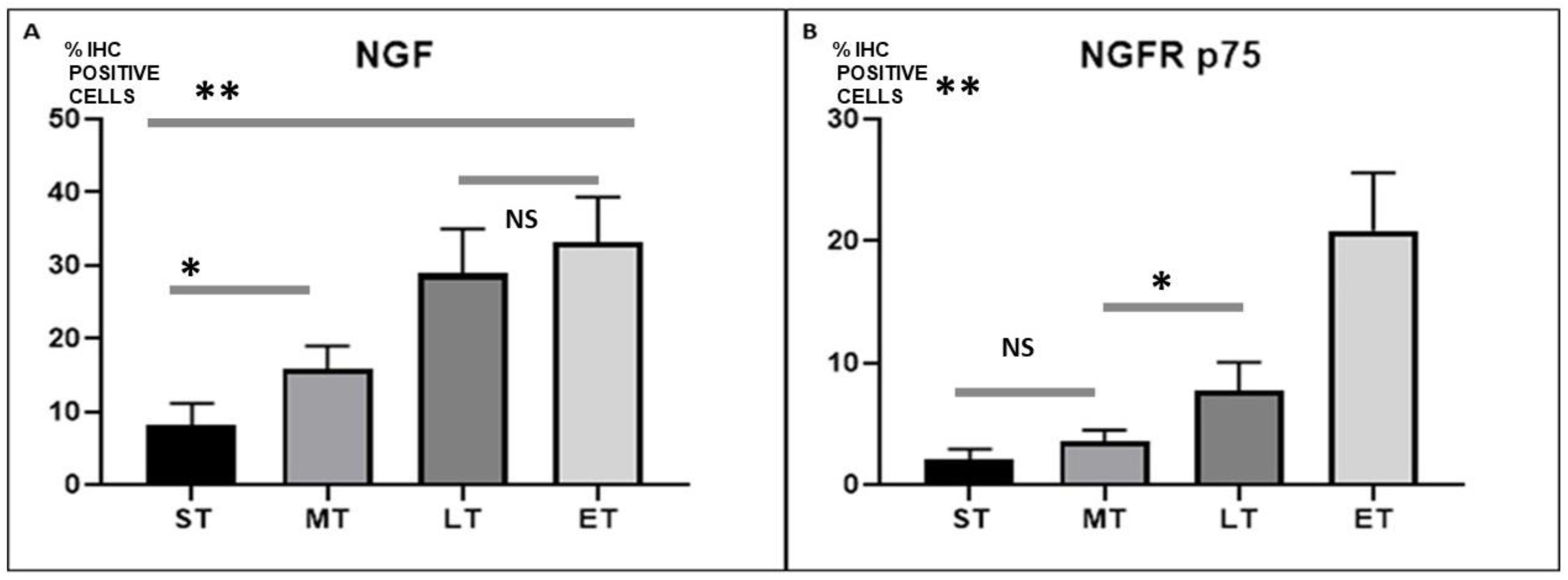
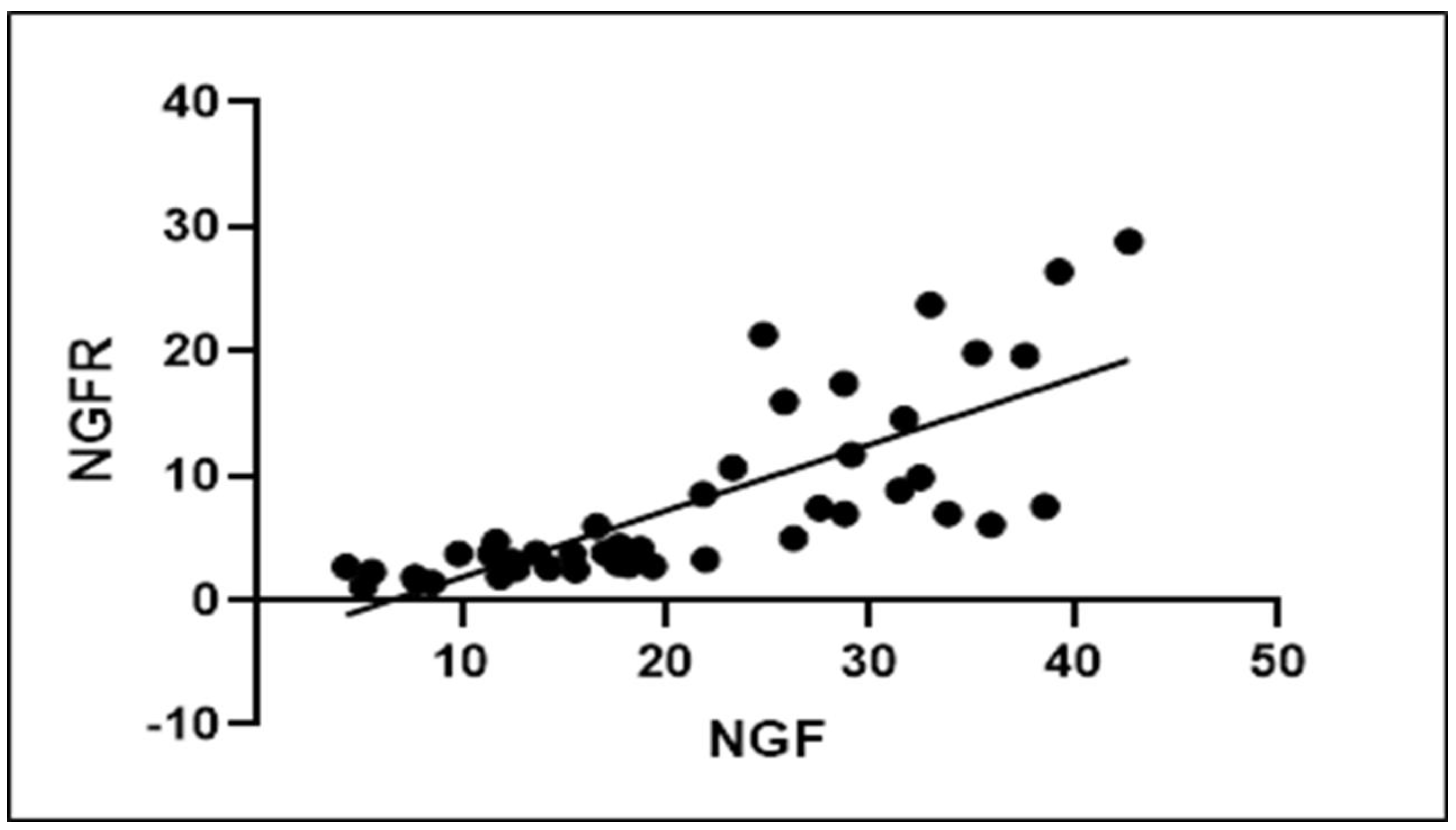
2.3. NGF and NGFR Quantification
2.4. Statistical Analysis
3. Results
3.1. Histological Assessment
3.2. Immunohistochemistry
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usher, F.C.; Gannon, J.P. Marlex mesh, a new plastic mesh for replacing tissue defects. I. Experimental studies. AMA Arch. Surg. 1959, 78, 131–137. [Google Scholar] [CrossRef]
- Lichtenstein, I.L.; Shulman, A.G.; Amid, P.K.; Montllor, M.M. The tension-free hernioplasty. Am. J. Surg. 1989, 157, 188–193. [Google Scholar] [CrossRef]
- Amid, P. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1997, 1, 15–21. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B. Modified classification of surgical meshes for hernia repair based on the analyses of 1000 explanted meshes. Hernia 2012, 16, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Klosterhalfen, B.; Junge, K.; Klinge, U. The lightweight and large porous mesh concept for hernia repair. Expert Rev. Med. Devices 2005, 2, 103–117. [Google Scholar] [CrossRef]
- Kehlet, H.; Bay-Nielsen, M. Nationwide quality improvement of groin hernia repair from the Danish Hernia Database of 87,840 patients from 1998 to 2005. Hernia 2008, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Amid, P.K. Causes, prevention, and surgical treatment of postherniorrhaphy neuropathic inguinodynia: Triple neurectomy with proximal end implantation. Hernia 2004, 8, 343–349. [Google Scholar] [CrossRef]
- Nienhuijs, S.; Staal, E.; Strobbe, L.; Rosman, C.; Groenewoud, H.; Bleichrodt, R. Chronic pain after mesh repair of inguinal hernia: A systematic review. Am. J. Surg. 2007, 194, 394–400. [Google Scholar] [CrossRef]
- Andresen, K.; Rosenberg, J. Management of chronic pain after hernia repair. J. Pain Res. 2018, 11, 675–681. [Google Scholar] [CrossRef]
- Amato, G.; Marasa, L.; Sciacchitano, T.; Bell, S.G.; Romano, G.; Gioviale, M.C.; Lo Monte, A.I.; Romano, M. Histological findings of the internal inguinal ring in patients having indirect inguinal hernia. Hernia 2009, 13, 259–262. [Google Scholar] [CrossRef]
- Amato, G.; Ober, E.; Romano, G.; Salamone, G.; Agrusa, A.; Gulotta, G.; Bussani, R. Nerve degeneration in inguinal hernia specimens. Hernia 2011, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Amato, G.; Romano, G.; Salamone, G.; Agrusa, A.; Saladino, V.A.; Silvestri, F.; Bussani, R. Damage to the vascular structures in inguinal hernia specimens. Hernia 2012, 16, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Amato, G.; Agrusa, A.; Romano, G.; Salamone, G.; Gulotta, G.; Silvestri, F.; Bussani, R. Muscle degeneration in inguinal hernia specimens. Hernia 2012, 16, 327–331. [Google Scholar] [CrossRef]
- Amato, G.; Agrusa, A.; Romano, G.; Salamone, G.; Cocorullo, G.; Mularo, S.A.; Marasa, S.; Gulotta, G. Histological findings in direct inguinal hernia. Hernia 2013, 17, 757–763. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amato, G.; Calò, P.G.; Rodolico, V.; Puleio, R.; Agrusa, A.; Gulotta, L.; Gordini, L.; Romano, G. The Septum Inguinalis: A Clue to Hernia Genesis? J. Investig. Surg. 2018, 31, 1–9. [Google Scholar] [CrossRef]
- Amato, G.; Romano, G.; Goetze, T.; Cicero, L.; Gulotta, E.; Calò, P.G.; Agrusa, A. Fixation free inguinal hernia repair with the 3D dynamic responsive prosthesis ProFlor: Features, procedural steps and long-term results. Int. J. Surg. Open 2019, 21, 34–43. [Google Scholar] [CrossRef]
- Amato, G.; Lo Monte, A.I.; Cassata Damiano, G.; Romano, G.; Bussani, R. A new prosthetic implant for inguinal hernia repair: Its features in a porcine experimental model. Artif. Organs 2011, 35, E181–E190. [Google Scholar] [CrossRef]
- Amato, G.; Romano, G.; Agrusa, A.; Marasa, S.; Cocorullo, G.; Gulotta, G.; Goetze, T.; Puleio, R. Biologic response of inguinal hernia prosthetics: A comparative study of conventional static meshes versus 3D dynamic implants. Artif. Organs 2015, 39, E10–E23. [Google Scholar] [CrossRef]
- Amato, G.; Agrusa Puleio, R.; Micci, G.; Cassata, G.; Cicero, L.; Di Buono, G.; Calò, P.G.; Galia, M.; Romano, G. A regenerative scaffold for inguinal hernia repair. MR imaging and histological cross evidence. Qualitative study. Int. J. Surg. 2021, 96, 106170. [Google Scholar] [CrossRef]
- Amato, G.; Agrusa, A.; Puleio, R.; Calò, P.G.; Goetze, T.; Romano, G. Neo-nervegenesis in 3D dynamic responsive implant for inguinal hernia repair. Qualitative study. Int. J. Surg. 2020, 76, 114–119. [Google Scholar] [CrossRef]
- Amato, G.; Puleio, R.; Rodolico, V.; Agrusa, A.; Calò, P.G.; Di Buono, G.; Romano, G.; Goetze, T. Enhanced angiogenesis in the 3D dynamic responsive implant for inguinal hernia repair ProFlor®. Artif. Organs 2021, 45, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Amato, G.; Romano, G.; Puleio, R.; Agrusa, A.; Goetze, T.; Gulotta, E.; Gordini, L.; Erdas, E.; Calò, P. Neomyogenesis in 3D Dynamic Responsive Prosthesis for Inguinal Hernia Repair. Artif. Organs 2018, 42, 1216–1223. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Puleio, R.; Condorelli, L.; Collura, G.; Giammona, G. A hyaluronic acid/cyclodextrin based injectable hydrogel for local doxorubicin delivery to solid tumors. Int. J. Pharm. 2020, 589, 119879. [Google Scholar] [CrossRef] [PubMed]
- Coda, A.; Lamberti, R.; Martorana, S. Classification of prosthetics used in hernia repair based on weight and biomaterial. Hernia 2012, 16, 9–20. [Google Scholar] [CrossRef]
- Amato, G.; Agrusa, A.; Di Buono, G.; Calò, P.G.; Cassata, G.; Cicero, L.; Romano, G. Inguinal Hernia: Defect Obliteration with the 3D Dynamic Regenerative Scaffold Proflor™. Surg. Technol. Int. 2021, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Amato, G.; Romano, G.; Agrusa, A.; Cocorullo, G.; Gulotta, G.; Goetze, T. Dynamic inguinal hernia repair with a 3d fixation-free and motion-compliant implant: A clinical study. Surg. Technol. Int. 2014, 24, 155–165. [Google Scholar] [PubMed]
- Amato, G.; Agrusa, A.; Romano, G. Fixation-free inguinal hernia repair using a dynamic self-retaining implant. Surg. Technol. Int. 2012, 22, 107–112. [Google Scholar]
- Amato, G.; Romano, G.; Calò, P.G.; Di Buono, G.; Agrusa, A. First-in-man permanent laparoscopic fixation free obliteration of inguinal hernia defect with the 3D dynamic responsive implant ProFlor-E®. Case report. Int. J. Surg. Case Rep. 2020, 77S, S2–S7. [Google Scholar] [CrossRef]
- Amato, G.; Agrusa, A.; Calò, P.G.; Di Buono, G.; Buscemi, S.; Cordova, A.; Zanghì, G.; Romano, G. Fixation free laparoscopic obliteration of inguinal hernia defects with the 3D dynamic responsive scaffold ProFlor. Sci. Rep. 2022, 8, 18971. [Google Scholar] [CrossRef]
- Almada, A.E.; Wagers, A.J. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing, and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 267–279. [Google Scholar] [CrossRef]
- Chevrel, G.; Hohlfeld, R.; Sendtner, M. The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerve 2006, 33, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Deponti, D.; Buono, R.; Catanzaro, G.; De Palma, C.; Longhi, R.; Meneveri, R.; Bresolin, N.; Bassi, M.T.; Cossu, G.; Clementi, E.; et al. The low-affinity receptor for neurotrophins p75NTR plays a key role for satellite cell function in muscle repair acting via RhoA. Mol. Biol. Cell 2009, 20, 3620–3627. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, K.; Lecht, S.; Arien-Zakay, H.; Cohen, G.; Aga-Mizrachi, S.; Yanay, N.; Saragovi, H.U.; Nedev, H.; Marcinkiewicz, C.; Nevo, Y.; et al. Nerve growth factor stimulation of ERK1/2 phosphorylation requires both p75NTR and α9β1 integrin and confers myoprotection towards ischemia in C2C12 skeletal muscle cell model. Cell Signal. 2012, 24, 2378–2388. [Google Scholar] [CrossRef]
- de Perini, A.; Dimauro, I.; Duranti, G.; Fantini, C.; Mercatelli, N.; Ceci, R.; Di Luigi, L.; Sabatini, S.; Caporossi, D. The p75NTR-mediated effect of nerve growth factor in L6C5 myogenic cells. BMC Res. Notes 2017, 10, 686. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef]
- Bibel, M.; Barde, Y.A. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000, 14, 2919–2937. [Google Scholar] [CrossRef] [PubMed]
- Segatto, M.; Fico, E.; Gharbiya, M.; Rosso, P.; Carito, V.; Tirassa, P.; Plateroti, R.; Lambiase, A. VEGF inhibition alters neurotrophin signalling pathways and induces caspase-3 activation and autophagy in rabbit retina. J. Cell. Physiol. 2019, 234, 18297–18307. [Google Scholar] [CrossRef]
- Ruberti, F.; Capsoni, S.; Comparini, A.; Di Daniel, E.; Franzot, J.; Gonfloni, S.; Rossi, G.; Berardi, N.; Cattaneo, A. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J. Neurosci. 2000, 20, 2589–2601. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sobue, G.; Yamamoto, K.; Terao, S.; Mitsuma, T. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem. Res. 1996, 21, 929–938. [Google Scholar] [CrossRef]
- Pallottini, V.; Colardo, M.; Tonini, C.; Martella, N.; Strimpakos, G.; Colella, B.; Tirassa, P.; Bartolomeo, S.D.; Segatto, M. ProNGF/p75NTR Axis Drives Fiber Type Specification by Inducing the Fast-Glycolytic Phenotype in Mouse Skeletal Muscle Cells. Cells 2020, 9, 2232. [Google Scholar] [CrossRef]
- Colombo, E.; Romaggi, S.; Medico, E.; Menon, R.; Mora, M.; Falcone, C.; Lochmüller, H.; Confalonieri, P.; Mantegazza, R.; Morandi, L.; et al. Neurotrophin Receptor p75NTR Defines Differentiation-Oriented Skeletal Muscle Precursor Cells: Implications for Muscle Regeneration. Neuropathol. Exp. Neurol. 2011, 70, 133–142. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, G.; Romano, G.; Rodolico, V.; Puleio, R.; Calò, P.G.; Di Buono, G.; Cicero, L.; Romano, G.; Goetze, T.O.; Agrusa, A. Dynamic Responsive Inguinal Scaffold Activates Myogenic Growth Factors Finalizing the Regeneration of the Herniated Groin. J. Funct. Biomater. 2022, 13, 253. https://doi.org/10.3390/jfb13040253
Amato G, Romano G, Rodolico V, Puleio R, Calò PG, Di Buono G, Cicero L, Romano G, Goetze TO, Agrusa A. Dynamic Responsive Inguinal Scaffold Activates Myogenic Growth Factors Finalizing the Regeneration of the Herniated Groin. Journal of Functional Biomaterials. 2022; 13(4):253. https://doi.org/10.3390/jfb13040253
Chicago/Turabian StyleAmato, Giuseppe, Giorgio Romano, Vito Rodolico, Roberto Puleio, Pietro Giorgio Calò, Giuseppe Di Buono, Luca Cicero, Giorgio Romano, Thorsten Oliver Goetze, and Antonino Agrusa. 2022. "Dynamic Responsive Inguinal Scaffold Activates Myogenic Growth Factors Finalizing the Regeneration of the Herniated Groin" Journal of Functional Biomaterials 13, no. 4: 253. https://doi.org/10.3390/jfb13040253
APA StyleAmato, G., Romano, G., Rodolico, V., Puleio, R., Calò, P. G., Di Buono, G., Cicero, L., Romano, G., Goetze, T. O., & Agrusa, A. (2022). Dynamic Responsive Inguinal Scaffold Activates Myogenic Growth Factors Finalizing the Regeneration of the Herniated Groin. Journal of Functional Biomaterials, 13(4), 253. https://doi.org/10.3390/jfb13040253