Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review
Abstract
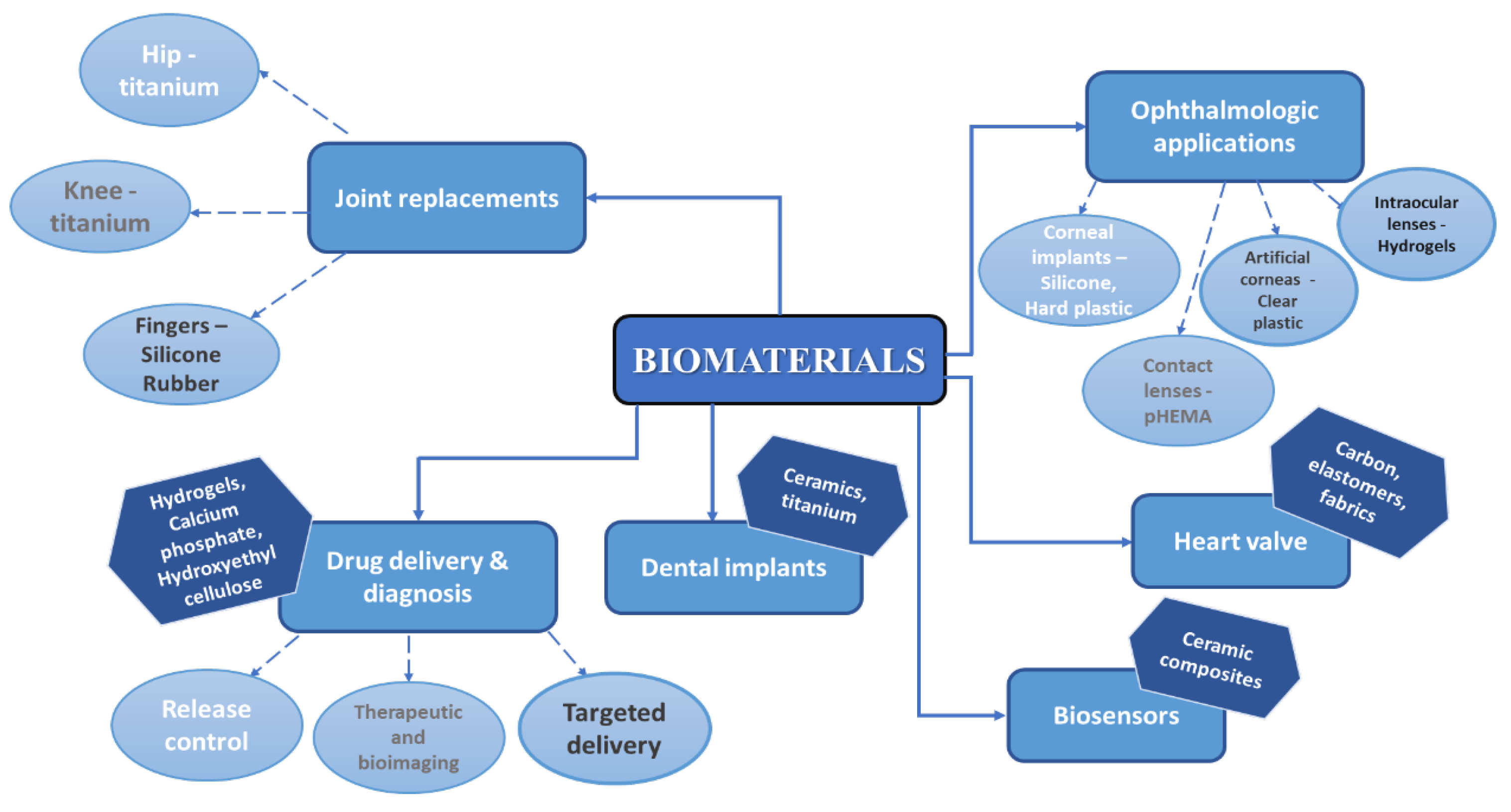
1. Introduction
2. Focus on Hydroxyapatite
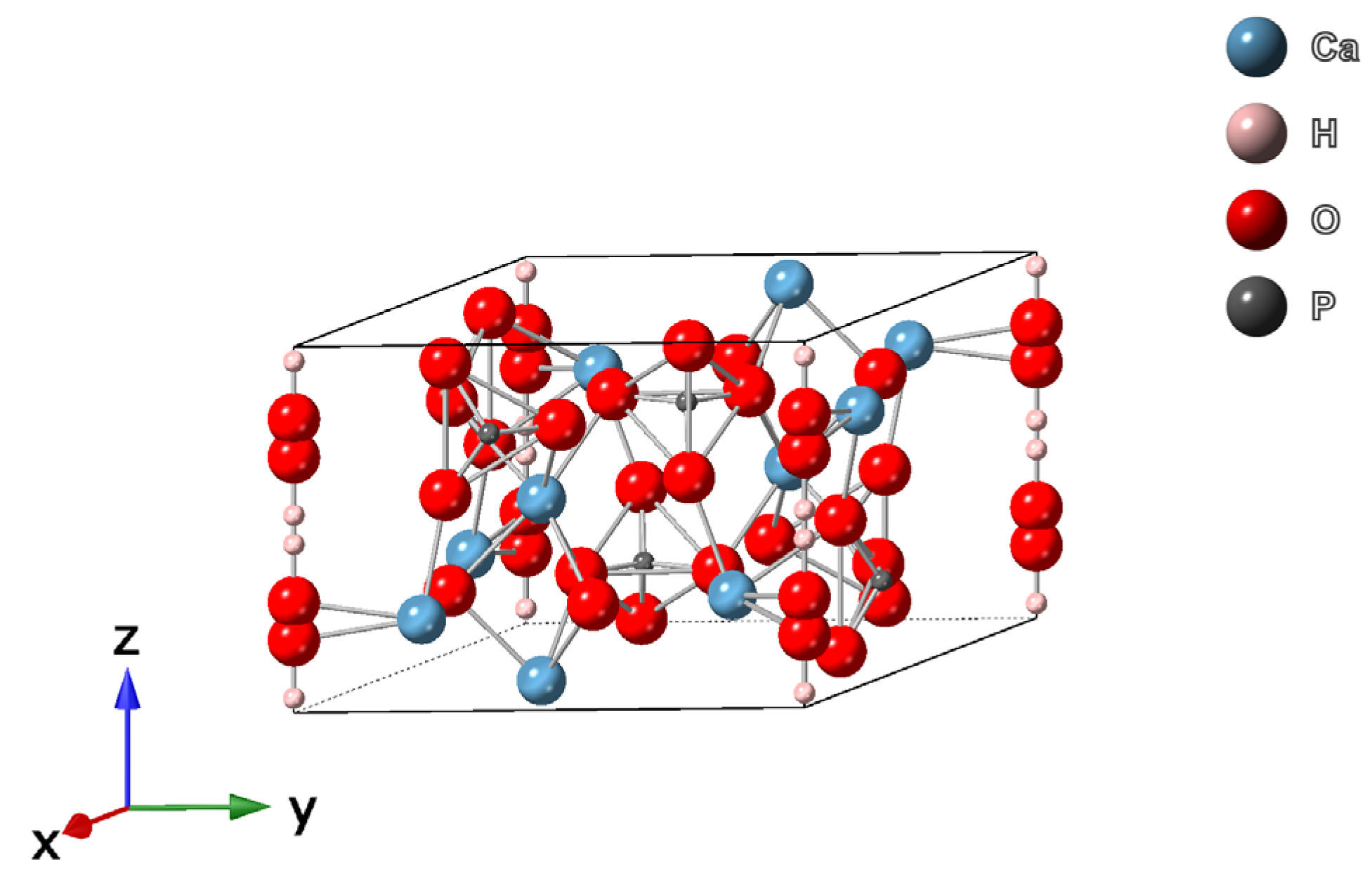
2.1. Structure of Hydroxyapatite
2.2. Synthesis of Hydroxyapatite
- Wet–chemical synthesis: precipitation, hydrothermal, and sol–gel method;
- Dry–chemical synthesis: solid-state reactions and mechanochemical method;
- High-temperature methods: combustion, spray pyrolysis, and thermal decomposition;
- Use of bioresources: animal sources (sheep, pig, and goat teeth and bones); marine sources (bones, red algae, fish scale, corals, or seashells); and plant sources (bamboo, potato orange banana peels, calendula flowers, etc.).
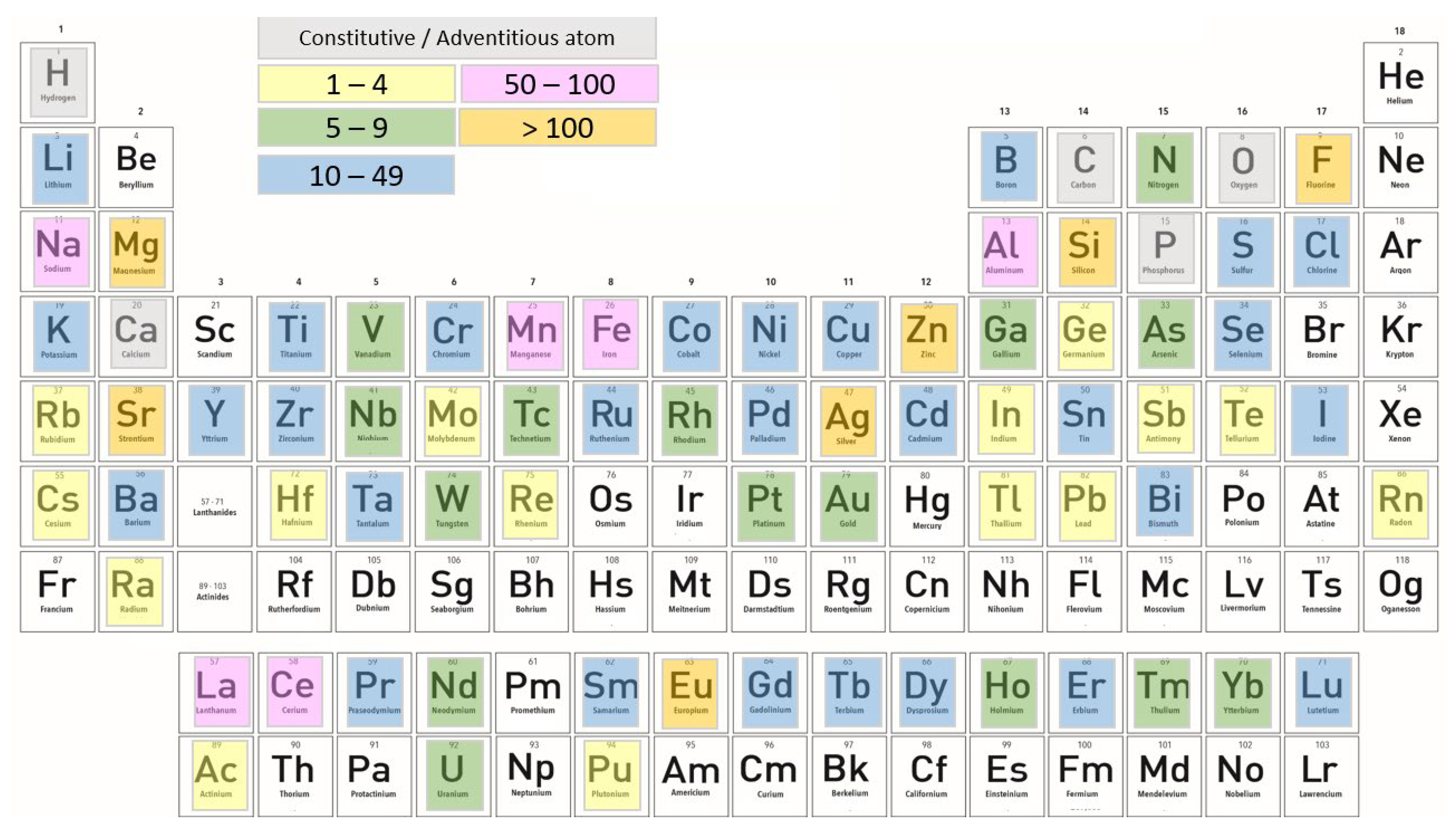
2.3. Substitution of Various Ions in HAp
2.3.1. Cationic Substitutions
2.3.2. Anionic Substitutions
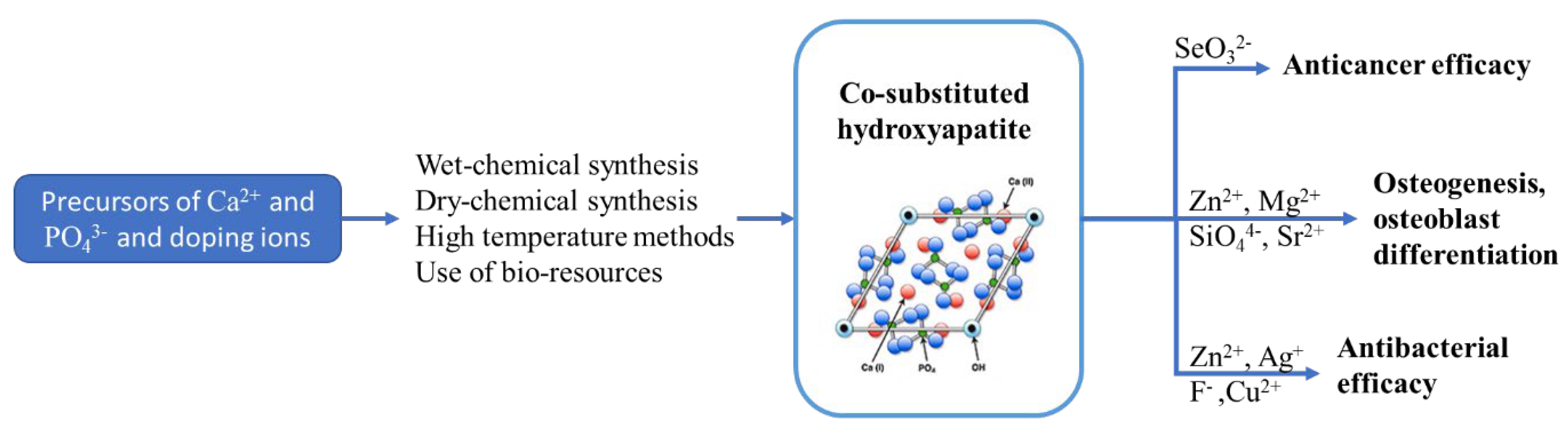
2.3.3. Effects of Co-Doping on Physicochemical, Antibacterial, and Biological Properties of HAp
| Co-Doping Agents | Synthesis Method | Biological Performance |
|---|---|---|
| Zn2+, SeO32− [120] | Precipitation method | Cytotoxic for BALB/c 3T3 mouse fibroblasts |
| Zn2+, Cu2+ [122] | Electrolytic deposition | Antibacterial properties, promoting the MC3T3-E1 osteoblasts cell viability |
| Zn2+, Sr2+ [127] | Hydrothermal method | Antibacterial properties, improving the cytotoxic limit of Zn2+, adhesion |
| Sr2+, Ag+ [124] | Hydrothermal method | Antibacterial properties, high osteoinductive properties, promote ALP activity in vitro |
| Sr2+, Bi3+ [121] | Microwave-assisted precipitation method | Antibacterial properties |
| Sr2+, F− [121] | Hydrothermal method | Antibacterial properties, promote ALP activity |
| SiO44−, Ag+ [128] | Precipitation method | Enhanced osteoconductive properties in vitro |
| Sr2+, Ag+, F− [125] | Electrolytic deposition | Induce bone formation, high early bone integration ability |
3. Focus on Bioactive Glasses, Bioglass® 45S5
3.1. Structure and Synthesis of Bioglass
3.2. Clinical Applications of 45S5 Bioglass® and Bioceramics
| Material | Composition and Structure | References |
|---|---|---|
| PerioGlas® | Contains calcium, sodium, phosphate and silica; it is a granulated form of 45S5 Bioglass® Particle size: 90–710 µm | [148] |
| Biogran® | Same composition as 45S5 Bioglass® (calcium, sodium, phosphate, and silica); particles of narrow size range Particle size: 300–360 µm | [149] |
| BonAlive® | Special bioglass (S53P4), consists of 53% SiO2, 23% Na2O, 20% CaO, and 4% P2O5 as weight percent Particle size: 500–800 μm | [150,151] |
| NovaMin® | Identical with 45S5 Bioglass®, contains only calcium, sodium, phosphate, and silica in an amorphous matrix Particle size: 18 µm | [152] |
3.3. Derived Bioglass Bioactive Materials
4. Hydroxyapatite/Bioglass 45S5 Composites
| Information about Material | Material | ||
|---|---|---|---|
| Hydroxyapatite | 45S5 Bioglass® | ||
| Composition | Ca10(PO4)6(OH)2 | 45 wt.% SiO2, 6 wt.% P2O5, 24.5 wt.% CaO, and 24.5 wt.% Na2O | |
| Structure | Crystalline, hexagonal symmetry | Amorphous | |
| Average particle size | 10 nm and 500 nm | Commercial products have different particle size, from 18 µm to 800 µm | |
| Molar mass (g/mol) | 502.31 | - | |
| Density (g/cm3) | 3.16 | 2.7 | |
| Melting point (°C) | 1614 | Approximately 1100–1200 [172] | |
| Solubility in water | Poorly soluble in water | More soluble than other bioactive materials such as HAp | |
| Mechanical properties | Compression strength (MPa) | >400 | Approximately 500 |
| Tensile strength (MPa) | Approximately 40 | 42 | |
| Elastic modulus (GPa) | Approximately 100 | 35 | |
| Fracture toughness (MPa) | Approximately 1.0 | 0.5 to 1 | |
| Uses and advantages | Desirable bone replacement material due to high hardness and high bioactivity, which accelerate early stage of bone growth | Repair of bone injuries or defects due to high biocompatibility, bioactivity, and osteoconductivity Applications in tissue engineering and tooth enamel reconstruction with high antibacterial resistance | |
| Antibacterial effect | Low antibacterial properties, doping bacteriostatic ions such as Sr, Zn, Ce, and Ag are used for substitution | Effective against a wide selection of aerobic and anaerobic bacteria [173] | |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ishikawa, K.; Matsuya, S.; Miyamoto, Y.; Kawate, K. 9.05 Bioceramics. In Bioengineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; pp. 169–214. [Google Scholar]
- Riman, R.E.; Suchanek, W.L.; Byrappa, K.; Chen, C.-W.; Shuk, P.; Oakes, C.S. Solution Synthesis of Hydroxyapatite Designer Particulates. Solid State Ion. 2002, 151, 393–402. [Google Scholar] [CrossRef]
- Cheng, K.; Weng, W.; Han, G.; Du, P.; Shen, G.; Yang, J.; Ferreira, J.M.F. The Effect of Triethanolamine on the Formation of Sol-Gel Derived Fluoroapatite/Hydroxyapatite Solid Solution. J. Mater. Chem. Phys. 2003, 78, 767–771. [Google Scholar] [CrossRef]
- Weng, W.; Zhang, S.; Cheng, K.; Qu, H.; Du, P.; Shen, G.; Yuan, J.; Han, G. Sol-Gel Preparation of Bioactive Apatite Films. Surf. Coat Technol. 2003, 167, 292–296. [Google Scholar] [CrossRef]
- Choi, D.; Marra, K.G.; Kumta, P.N. Chemical Synthesis of Hydroxyapatite/Poly(ε-Caprolactone) Composites. Mater. Res. Bull. 2004, 39, 417–432. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Gul, H.; Khan, M.; Khan, A.S. Bioceramics: Types and Clinical Applications. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–83. ISBN 9780081028346. [Google Scholar]
- Das, A.; Pamu, D. A Comprehensive Review on Electrical Properties of Hydroxyapatite Based Ceramic Composites. Mater. Sci. Eng. C 2019, 101, 539–563. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Hasegawa, M.; Doi, Y.; Uchida, A. Cell-Mediated Bioresorption of Sintered Carbonate Apatite in Rabbits. J. Bone Jt. Surg.—Ser. B 2003, 85, 142–147. [Google Scholar] [CrossRef]
- Zhao, F.; Lei, B.; Li, X.; Mo, Y.; Wang, R.; Chen, D.; Chen, X. Promoting in Vivo Early Angiogenesis with Sub-Micrometer Strontium-Contained Bioactive Microspheres through Modulating Macrophage Phenotypes. Biomaterials 2018, 178, 36–47. [Google Scholar] [CrossRef]
- Jones, J.R.; Gibson, I.R. Ceramics, Glasses, and Glass-Ceramics: Basic Principles. Mater. Sci. 2013. [Google Scholar] [CrossRef]
- Dasgupta, S.; Banerjee, S.S.; Bandyopadhyay, A.; Bose, S. Zn- and Mg-Doped Hydroxyapatite Nanoparticles for Controlled Release of Protein. Langmuir 2010, 26, 4958–4964. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Huang, S.S.; Yu, W.X.; Hsu, Y.W.; Hsu, F.Y. Fabrication and Characteristics of Porous Hydroxyapatite-CaO Composite Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Alshemary, A.Z.; Evis, Z. Co-Doped Hydroxyapatites as Potential Materials for Biomedical Applications. Microchem. J. 2019, 144, 443–453. [Google Scholar] [CrossRef]
- Tomoda, K.; Ariizumi, H.; Nakaji, T.; Makino, K. Hydroxyapatite Particles as Drug Carriers for Proteins. Colloids Surf. B Biointerfaces 2010, 76, 226–235. [Google Scholar] [CrossRef]
- Kester, M.; Heakal, Y.; Fox, T.; Sharma, A.; Robertson, G.P.; Morgan, T.T.; Altinoǧlu, E.I.; Tabaković, A.; Parette, M.R.; Rouse, S.M.; et al. Calcium Phosphate Nanocomposite Particles for in Vitro Imaging and Encapsulated Chemotherapeutic Drug Delivery to Cancer Cells. Nano Lett. 2008, 8, 4116–4121. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium Phosphate Ceramic Systems in Growth Factor and Drug Delivery for Bone Tissue Engineering: A Review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Evolution of Ceramics with Medical Applications. Z. Anorg. Allg. Chem. 2007, 633, 1762–1773. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding Mechanisms at the Interface of Ceramic Prosthetic Materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Zhao, X. Bioactive Materials in Orthopaedics. In Bioactive Materials in Medicine: Design and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 124–154. ISBN 9781845696245. [Google Scholar]
- Fernando, S.; McEnery, M.; Guelcher, S.A. Polyurethanes for Bone Tissue Engineering. In Advances in Polyurethane Biomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 481–501. ISBN 9780081006221. [Google Scholar]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharm. Transl. Sci. 2021, 4, 8–54. [Google Scholar] [CrossRef]
- Park, J.; Um, S.H.; Seo, Y.; Lee, J.; Kim, Y.C.; Ok, M.R.; Hwang, S.W.; Sun, J.Y.; Han, H.S.; Jeon, H. Improving Hydroxyapatite Coating Ability on Biodegradable Metal through Laser-Induced Hydrothermal Coating in Liquid Precursor: Application in Orthopedic Implants. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Sulyok, A.; Kis, V.K.; Balázsi, K.; Mihály, J.; Balázsi, C. Preparation and Morphological Investigation on Bioactive Ion-Modified Carbonated Hydroxyapatite-Biopolymer Composite Ceramics as Coatings for Orthopaedic Implants. Ceram. Int. 2022, 48, 760–768. [Google Scholar] [CrossRef]
- Hikku, G.S.; Arthi, C.; Jeen Robert, R.B.; Jeyasubramanian, K.; Murugesan, R. Calcium Phosphate Conversion Technique: A Versatile Route to Develop Corrosion Resistant Hydroxyapatite Coating over Mg/Mg Alloys Based Implants. J. Magnes. Alloy. 2022, 10, 1821–1845. [Google Scholar] [CrossRef]
- Nagai, M.; Nishino, T. Surface conduction of porous hydroxyapatite ce1laa’viics at elevated temperatures. Solid State Ion. 1988, 28–30, 1456–1461. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; Tenhuisen, K.S. Mechanochemical-Hydrothermal Synthesis of Calcium Phosphate Powders with Coupled Magnesium and Carbonate Substitution. J. Solid State Chem. 2004, 177, 793–799. [Google Scholar] [CrossRef]
- Chahkandi, M. Mechanism of Congo Red Adsorption on New Sol-Gel-Derived Hydroxyapatite Nano-Particle. Mater. Chem. Phys. 2017, 202, 340–351. [Google Scholar] [CrossRef]
- Bohner, M. Calcium Orthophosphates in Medicine: From Ceramics to Calcium Phosphate Cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium Phosphate Nanoparticles as Novel Non-Viral Vectors for Targeted Gene Delivery. Int. J. Pharm. 2003, 250, 25–33. [Google Scholar] [CrossRef]
- Lai, C.; Tang, S.Q.; Wang, Y.J.; Wei, K. Formation of Calcium Phosphate Nanoparticles in Reverse Microemulsions. Mater. Lett. 2005, 59, 210–214. [Google Scholar] [CrossRef]
- Talal, A.; Hamid, S.K.; Khan, M.; Khan, A.S. Structure of Biological Apatite: Bone and Tooth. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. ISBN 9780081028346. [Google Scholar]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Doi, Y.; Shibutani, T.; Moriwaki, Y.; Kajimoto, T.; Iwayama, Y. Sintered Carbonate Apatites as Bioresorbable Bone Substitutes. J. Biomed. Mater. Res. 1998, 39, 603–610. [Google Scholar] [CrossRef]
- Nachtigall, P.; Arean, C.O. Themed Issue on Characterization of Adsorbed Species. Phys. Chem. Chem. Phys. 2010, 12, 6307–6308. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.Y.; Brown, P.W. Computer Simulation of Stoichiometric Hydroxyapatite: Structure and Substitutions. J. Phys. Chem. Solids 2007, 68, 431–437. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent Progress on Fabrication and Drug Delivery Applications of Nanostructured Hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium Phosphate Bone Cements for Clinical Applications Part II: Precipitate Formation during Setting Reactions. J. Mater. Sci. Mater. Med. 1999, 10, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis Methods for Nanosized Hydroxyapatite with Diverse Structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J. The Transformation of Single-Crystal Calcium Phosphate Ribbon-like Fibres to Hydroxyapatite Spheres Assembled from Nanorods. Nanotechnology 2008, 19, 155608. [Google Scholar] [CrossRef]
- Tas, A.C. Synthesis of Biomimetic Ca-Hydroxyapatite Powders at 373C in Synthetic Body fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar]
- Mobasherpour, I.; Heshajin, M.S.; Kazemzadeh, A.; Zakeri, M. Synthesis of Nanocrystalline Hydroxyapatite by Using Precipitation Method. J. Alloys Compd. 2007, 430, 330–333. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Balakrishnan, A.; Chu, M.C.; Lee, Y.J.; Kim, T.N.; Cho, S.J. Sol-Gel Synthesis and Characterization of Hydroxyapatite Nanorods. Particuology 2009, 7, 466–470. [Google Scholar] [CrossRef]
- Szterner, P.; Biernat, M. The Synthesis of Hydroxyapatite by Hydrothermal Process with Calcium Lactate Pentahydrate: The Effect of Reagent Concentrations, pH, Temperature, and Pressure. Bioinorg. Chem. Appl. 2022, 2022, 3481677. [Google Scholar] [CrossRef]
- Cihlar, J.; Castkova, K. Direct Synthesis of N Anocrystalline Hydroxyapatite by Hydrothermal Hydrolysis of Alkylphosphates. Mon. Für Chem.-Chem. Mon. 2002, 133, 761–771. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Wang, M.; Cheung, W.L.; Guo, B.C.; Jia, D.M. Synthesis of Carbonated Hydroxyapatite Nanospheres through Nanoemulsion. J. Mater. Sci. Mater. Med. 2008, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Vukomanović, M.; Braçko, I.; Poljanşek, I.; Uskoković, D.; Şkapin, S.D.; Suvorov, D. The Growth of Silver Nanoparticles and Their Combination with Hydroxyapatite to Form Composites via a Sonochemical Approach. Cryst. Growth Des. 2011, 11, 3802–3812. [Google Scholar] [CrossRef]
- Ekka, B.; Nayak, S.R.; Achary, L.S.K.; Sarita; Kumar, A.; Mawatwal, S.; Dhiman, R.; Dash, P.; Patel, R.K. Synthesis of Hydroxyapatite-Zirconia Nanocomposite through Sonochemical Route: A Potential Catalyst for Degradation of Phenolic Compounds. J. Environ. Chem. Eng. 2018, 6, 6504–6515. [Google Scholar] [CrossRef]
- Jevtić, M.; Mitrić, M.; Škapin, S.; Jančar, B.; Ignjatović, N.; Uskoković, D. Crystal Structure of Hydroxyapatite Nanorods Synthesized by Sonochemical Homogeneous Precipitation. Cryst. Growth Des. 2008, 8, 2217–2222. [Google Scholar] [CrossRef]
- Sahni, G.; Gopinath, P.; Jeevanandam, P. A Novel Thermal Decomposition Approach to Synthesize Hydroxyapatite-Silver Nanocomposites and Their Antibacterial Action against GFP-Expressing Antibiotic Resistant E. coli. Colloids Surf. B Biointerfaces 2013, 103, 441–447. [Google Scholar] [CrossRef]
- Barralet, J.; Knowles, J.C.; Best, S.; Bonfield, W. Thermal Decomposition of Synthesised Carbonate Hydroxyapatite. J. Mater. Sci. Mater. Med. 2002, 13, 529–533. [Google Scholar] [CrossRef]
- Chun, Y.A.N.G.; Guo, Y.K.; Zhang, M.L. Thermal Decomposition and Mechanical Properties of Hydroxyapatite Ceramic. Trans. Nonferrous Met. Soc. China 2010, 20, 254–258. [Google Scholar] [CrossRef]
- Obada, D.O.; Salami, K.A.; Oyedeji, A.N.; Fasanya, O.O.; Suleiman, M.U.; Ibisola, B.A.; Atta, A.Y.; Dodoo-Arhin, D.; Kuburi, L.S.; Dauda, M.; et al. Solution Combustion Synthesis of Strontium-Doped Hydroxyapatite: Effect of Sintering and Low Compaction Pressure on the Mechanical Properties and Physiological Stability. Mater. Lett. 2021, 304, 130613. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Roy, S.K.; Kundu, B.; Datta, S.; Basu, D. Synthesis of Nano-Sized Hydroxyapatite Powders through Solution Combustion Route under Different Reaction Conditions. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2011, 176, 14–21. [Google Scholar] [CrossRef]
- Taş, A.C. Molten Salt Synthesis of Calcium Hydroxyapatite Whiskers. J. Am. Ceram. Soc. 2001, 84, 295–300. [Google Scholar] [CrossRef]
- Zhang, H.G.; Zhu, Q. Preparation of Fluoride-Substituted Hydroxyapatite by a Molten Salt Synthesis Route. J. Mater. Sci. Mater. Med. 2006, 17, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pal, U.; Dey, A. Natural Origin Hydroxyapatite Scaffold as Potential Bone Tissue Engineering Substitute. Ceram. Int. 2016, 42, 18338–18346. [Google Scholar] [CrossRef]
- Sobczak, A.; Kida, A.; Kowalski, Z.; Wzorek, Z. Evaluation of the Biomedical Properties of Hydroxyapatite Obtained from Bone Waste. Pol. J. Chem. Technol. 2009, 11, 37–43. [Google Scholar] [CrossRef]
- Wu, S.C.; Tsou, H.K.; Hsu, H.C.; Hsu, S.K.; Liou, S.P.; Ho, W.F. A Hydrothermal Synthesis of Eggshell and Fruit Waste Extract to Produce Nanosized Hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Charlena; Suparto, I.H.; Putri, D.K. Synthesis of Hydroxyapatite from Rice Fields Snail Shell (Bellamya Javanica) through Wet Method and Pore Modification Using Chitosan. Procedia Chem. 2015, 17, 27–35. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium Phosphates as Substitution of Bone Tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Chirică, I.M.; Enciu, A.M.; Tite, T.; Dudău, M.; Albulescu, L.; Iconaru, S.L.; Predoi, D.; Pasuk, I.; Enculescu, M.; Radu, C.; et al. The Physico-Chemical Properties and Exploratory Real-Time Cell Analysis of Hydroxyapatite Nanopowders Substituted with Ce, Mg, Sr, and Zn (0.5–5 at.%). Materials 2021, 14, 3808. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Arcos, D. Silicon Substituted Hydroxyapatites. A Method to Upgrade Calcium Phosphate Based Implants. J. Mater. Chem. 2005, 15, 1509–1516. [Google Scholar] [CrossRef]
- Gibson, I.R.; Best, S.M.; Bonfield, W. Chemical Characterization of Silicon-Substituted Hydroxyapatite. J. Biomed. Mater. Res. 1999, 15, 422–428. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-Doped Hydroxyapatite: An Impasse or the Road to Follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A Mineralogical Perspective on the Apatite in Bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic Substitutions in Calcium Phosphates Synthesized at Low Temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K. First-Principles Study of Substitutional Magnesium and Zinc in Hydroxyapatite and Octacalcium Phosphate. J. Chem. Phys. 2008, 128, 06B618. [Google Scholar] [CrossRef] [PubMed]
- Sprio, S.; Dapporto, M.; Preti, L.; Mazzoni, E.; Iaquinta, M.R.; Martini, F.; Tognon, M.; Pugno, N.M.; Restivo, E.; Visai, L.; et al. Enhancement of the Biological and Mechanical Performances of Sintered Hydroxyapatite by Multiple Ions Doping. Front. Mater. 2020, 7, 224. [Google Scholar] [CrossRef]
- Salam, N.; Gibson, I.R. Lithium Ion Doped Carbonated Hydroxyapatite Compositions: Synthesis, Physicochemical Characterisation and Effect on Osteogenic Response in Vitro. Biomater. Adv. 2022, 140, 213068. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Cao, S.; Li, K.; Chen, M.; Xu, Z.; Lu, J.; Zhang, L. Na-Doped Hydroxyapatite Coating on Carbon/Carbon Composites: Preparation, in Vitro Bioactivity and Biocompatibility. Appl. Surf. Sci. 2012, 263, 163–173. [Google Scholar] [CrossRef]
- Riaz, M.; Zia, R.; Ijaz, A.; Hussain, T.; Mohsin, M.; Malik, A. Synthesis of Monophasic Ag Doped Hydroxyapatite and Evaluation of Antibacterial Activity. Mater. Sci. Eng. C 2018, 90, 308–313. [Google Scholar] [CrossRef]
- Jelinek, M.; Kocourek, T.; Remsa, J.; Weiserová, M.; Jurek, K.; Mikšovský, J.; Strnad, J.; Galandáková, A.; Ulrichová, J. Antibacterial, Cytotoxicity and Physical Properties of Laser—Silver Doped Hydroxyapatite Layers. Mater. Sci. Eng. C 2013, 33, 1242–1246. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Jiang, X.; Sun, Y.; Yang, H.; Liu, Q.; Cao, Y.; Zhang, Y.; Cheng, H. Synthesis of Strontium (Sr) Doped Hydroxyapatite (HAp) Nanorods for Enhanced Adsorption of Cr (VI) Ions from Wastewater. Ceram. Int. 2021, 47, 16730–16736. [Google Scholar] [CrossRef]
- de Lima, C.O.; de Oliveira, A.L.M.; Chantelle, L.; Silva Filho, E.C.; Jaber, M.; Fonseca, M.G. Zn-Doped Mesoporous Hydroxyapatites and Their Antimicrobial Properties. Colloids Surf. B Biointerfaces 2021, 198, 111471. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Fan, F.; Xu, W.; Zhang, H.; Liu, N. The Structural and Surface Properties of Al-Doped Hydroxyapatite (Ca5(PO4)3OH) Nanorods and Their Applications for PH-Induced Drug Delivery. J. Alloy. Compd. 2021, 879, 160414. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Nikolaev, A.M.; Lähderanta, E.; Frank-Kamenetskaya, O.V.; Kuz’mina, M.A. Structural and Luminescence Properties of Ce3+-Doped Hydroxyapatite Nanocrystalline Powders. Opt. Mater. 2020, 99, 109550. [Google Scholar] [CrossRef]
- Vladescu, A.; Padmanabhan, S.C.; Ak Azem, F.; Braic, M.; Titorencu, I.; Birlik, I.; Morris, M.A.; Braic, V. Mechanical Properties and Biocompatibility of the Sputtered Ti Doped Hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2016, 63, 314–325. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Z.; Cheng, L.; Gao, X.; Sun, J.; Chen, L. Improvement of the Mechanical Properties and Osteogenic Activity of 3D-Printed Polylactic Acid Porous Scaffolds by Nano-Hydroxyapatite and Nano-Magnesium Oxide. Heliyon 2022, 8, e09748. [Google Scholar] [CrossRef]
- Li, C.; Qin, W.; Lakshmanan, S.; Ma, X.; Sun, X.; Xu, B. Hydroxyapatite Based Biocomposite Scaffold: A Highly Biocompatible Material for Bone Regeneration. Saudi J. Biol. Sci. 2020, 27, 2143–2148. [Google Scholar] [CrossRef]
- Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Magnesium Incorporated Hydroxyapatite: Synthesis and Structural Properties Characterization. Ceram. Int. 2014, 40, 6021–6029. [Google Scholar] [CrossRef]
- Cacciotti, I.; Bianco, A.; Lombardi, M.; Montanaro, L. Mg-Substituted Hydroxyapatite Nanopowders: Synthesis, Thermal Stability and Sintering Behaviour. J. Eur. Ceram. Soc. 2009, 29, 2969–2978. [Google Scholar] [CrossRef]
- Bauer, L.; Antunović, M.; Rogina, A.; Ivanković, M.; Ivanković, H. Bone-Mimetic Porous Hydroxyapatite/Whitlockite Scaffolds: Preparation, Characterization and Interactions with Human Mesenchymal Stem Cells. J. Mater. Sci. 2021, 56, 3947–3969. [Google Scholar] [CrossRef]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.R.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium- and Strontium-Co-Substituted Hydroxyapatite: The Effects of Doped-Ions on the Structure and Chemico-Physical Properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Buton, N.; Megier, C. Obtaining and Characterizing Thin Layers of Magnesium Doped Hydroxyapatite by Dip Coating Procedure. Coatings 2020, 10, 510. [Google Scholar] [CrossRef]
- Ito, A.; Kawamura, H.; Otsuka, M.; Ikeuchi, M.; Ohgushi, H.; Ishikawa, K.; Onuma, K.; Kanzaki, N.; Sogo, Y.; Ichinose, N. Zinc-Releasing Calcium Phosphate for Stimulating Bone Formation. Mater. Sci. Eng. C 2002, 22, 21–25. [Google Scholar] [CrossRef]
- Tang, Y.; Chappell, H.F.; Dove, M.T.; Reeder, R.J.; Lee, Y.J. Zinc Incorporation into Hydroxylapatite. Biomaterials 2009, 30, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic Substituted Hydroxyapatite for Bone Regeneration Applications: A Review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and Characterization of Zn-Doped Hydroxyapatite: Scaffold Application, Antibacterial and Bioactivity Studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.A.; Buton, N.; Megier, C.; Beuran, M. Development of Iron-Doped Hydroxyapatite Coatings. Coatings 2021, 11, 186. [Google Scholar] [CrossRef]
- Jose, S.; Senthilkumar, M.; Elayaraja, K.; Haris, M.; George, A.; Raj, A.D.; Sundaram, S.J.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K. Preparation and Characterization of Fe Doped N-Hydroxyapatite for Biomedical Application. Surf. Interfaces 2021, 25, 101185. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–68. [Google Scholar]
- Paduraru, A.V.; Musuc, A.M.; Oprea, O.C.; Trusca, R.; Iordache, F.; Vasile, B.S.; Andronescu, E. Synthesis and Characterization of Photoluminescent Ce(Iii) and Ce(Iv) Substituted Hydroxyapatite Nanomaterials by Co-Precipitation Method: Cytotoxicity and Biocompatibility Evaluation. Nanomaterials 2021, 11, 1911. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Utara, S.; Khemthong, P.; Kamonwannasit, S. Structural Characterization of Cerium-Doped Hydroxyapatite Nanoparticles Synthesized by an Ultrasonic-Assisted Sol-Gel Technique. Results Phys. 2018, 10, 956–963. [Google Scholar] [CrossRef]
- Kanchana, P.; Navaneethan, M.; Sekar, C. Fabrication of Ce Doped Hydroxyapatite Nanoparticles Based Non-Enzymatic Electrochemical Sensor for the Simultaneous Determination of Norepinephrine, Uric Acid and Tyrosine. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2017, 226, 132–140. [Google Scholar] [CrossRef]
- Kaygili, O.; Dorozhkin, S.V.; Keser, S. Synthesis and Characterization of Ce-Substituted Hydroxyapatite by Sol-Gel Method. Mater. Sci. Eng. C 2014, 42, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Gopi, D.; Ramya, S.; Rajeswari, D.; Karthikeyan, P.; Kavitha, L. Strontium, Cerium Co-Substituted Hydroxyapatite Nanoparticles: Synthesis, Characterization, Antibacterial Activity towards Prokaryotic Strains and in Vitro Studies. Colloids Surf. A Phys. Eng. Asp. 2014, 451, 172–180. [Google Scholar] [CrossRef]
- Sanosh, K.P.; Chu, M.-C.; Balakrishnan, A.; Kim, T.N.; Cho, S.-J. Preparation and Characterization of Nano-Hydroxyapatite Powder Using Sol-Gel Technique. Bull. Mater. Sci. 2009, 32, 465–470. [Google Scholar] [CrossRef]
- Stoica, T.F.; Morosanu, C.; Slav, A.; Stoica, T.; Osiceanu, P.; Anastasescu, C.; Gartner, M.; Zaharescu, M. Hydroxyapatite Films Obtained by Sol-Gel and Sputtering. Thin Solid Film. 2008, 516, 8112–8116. [Google Scholar] [CrossRef]
- Amjad, Z. Calcium Phosphates in Biological and Industrial Systems; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Rogers, K.D.; Daniels, P. An X-Ray Diffraction Study of the Effects of Heat Treatment on Bone Mineral Microstructure. Biomaterials 2002, 23, 2577–2585. [Google Scholar] [CrossRef]
- Merry, J.C.; Gibson, I.R.; Best, S.M.; Bonfield, W. Synthesis and Characterization of Carbonate Hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 779–783. [Google Scholar] [CrossRef]
- El Feki, E.; Savariault, J.M.; Salah, A.B. Structure Refinements by the Rietveld Method of Partially Substituted: Ca9Na0.5(PO4)4(CO3)1.5(OH)2. J. Alloy. Compd. 1999, 287, 114–120. [Google Scholar] [CrossRef]
- Lala, S.; Brahmachari, S.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Biocompatible Nanocrystalline Natural Bonelike Carbonated Hydroxyapatite Synthesized by Mechanical Alloying in a Record Minimum Time. Mater. Sci. Eng. C 2014, 42, 647–656. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A Review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nazarian, H.; Pourbaghi-Masouleh, M.; Borhan, S. Comparative Study of Mesenchymal Stem Cells Osteogenic Differentiation on Low-Temperature Biomineralized Nanocrystalline Carbonated Hydroxyapatite and Sintered Hydroxyapatite. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Germaini, M.M.; Detsch, R.; Grünewald, A.; Magnaudeix, A.; Lalloue, F.; Boccaccini, A.R.; Champion, E. Osteoblast and Osteoclast Responses to A/B Type Carbonate-Substituted Hydroxyapatite Ceramics for Bone Regeneration. Biomed. Mater. 2017, 12, 035008. [Google Scholar] [CrossRef] [PubMed]
- Spence, G.; Patel, N.; Brooks, R.; Rushton, N. Carbonate Substituted Hydroxyapatite: Resorption by Osteoclasts Modifies the Osteoblastic Response. J. Biomed. Mater. Res. A 2009, 90, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Uysal, I.; Severcan, F.; Evis, Z. Structural and Mechanical Characteristics of Nanohydroxyapatite Doped with Zinc and Chloride. Adv. Appl. Ceram. 2013, 112, 149–157. [Google Scholar] [CrossRef]
- Gomes, S.; Nedelec, J.M.; Jallot, E.; Sheptyakov, D.; Renaudin, G. Unexpected Mechanism of Zn2+ Insertion in Calcium Phosphate Bioceramics. Chem. Mater. 2011, 23, 3072–3085. [Google Scholar] [CrossRef]
- Basar, B.; Tezcaner, A.; Keskin, D.; Evis, Z. Improvements in Microstructural, Mechanical, and Biocompatibility Properties of Nano-Sized Hydroxyapatites Doped with Yttrium and Fluoride. Ceram. Int. 2010, 36, 1633–1643. [Google Scholar] [CrossRef]
- Basar, B.; Evis, Z. Structural Investigation of Nanohydroxyapatite Doped with Y3+ and F− Ions. Mater. Sci. Technol. 2009, 25, 794–798. [Google Scholar] [CrossRef]
- Evis, Z.; Basar, B.; Sun, Z.P. Diametral Strength Testing of Hydroxyapatites Doped with Yttrium and Fluoride. Adv. Appl. Ceram. 2010, 109, 383–388. [Google Scholar] [CrossRef]
- Yilmaz, B.; Evis, Z. Raman Spectroscopy Investigation of Nano Hydroxyapatite Doped with Yttrium and Fluoride Ions. Spectrosc. Lett. 2014, 47, 24–29. [Google Scholar] [CrossRef]
- Tahmasebifar, A.; Evis, Z. Structural and Mechanical Characteristics of Hydroxyapatite and Tri-Calcium Phosphates Doped with Al3+ and F- Ions. J. Ceram. Process. Res. 2013, 14, 549–556. [Google Scholar] [CrossRef]
- İnce, T.; Kaygili, O.; Tatar, C.; Bulut, N.; Koytepe, S.; Ates, T. The Effects of Ni-Addition on the Crystal Structure, Thermal Properties and Morphology of Mg-Based Hydroxyapatites Synthesized by a Wet Chemical Method. Ceram. Int. 2018, 44, 14036–14043. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Engin Pazarceviren, A.; Tezcaner, A.; Evis, Z. Fe3+/SeO42− Dual Doped Nano Hydroxyapatite: A Novel Material for Biomedical Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Laskus, A.; Zgadzaj, A.; Kolmas, J. Zn2+ and SeO32− Co-Substituted Hydroxyapatite: Physicochemical Properties and Biological Usefulness. Ceram. Int. 2019, 45, 22707–22715. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Mansour, S.F.; Mostafa, M.S.; Darwesh, R.; El-dek, S.I. Structural, Mechanical and Thermal Features of Bi and Sr Co-Substituted Hydroxyapatite. J. Mater. Sci. 2019, 54, 1977–1991. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Mao, H.; Li, T.; Zhao, R.; Yan, Y.; Pang, X. Osteoblastic Cell Responses and Antibacterial Efficacy of Cu/Zn Co-Substituted Hydroxyapatite Coatings on Pure Titanium Using Electrodeposition Method. RSC Adv. 2015, 5, 17076–17086. [Google Scholar] [CrossRef]
- Stanić, V.; Janaćković, D.; Dimitrijević, S.; Tanasković, S.B.; Mitrić, M.; Pavlović, M.S.; Krstić, A.; Jovanović, D.; Raičević, S. Synthesis of Antimicrobial Monophase Silver-Doped Hydroxyapatite Nanopowders for Bone Tissue Engineering. Appl. Surf. Sci. 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Li, P.; Jia, Z.; Wang, Q.; Tang, P.; Wang, M.; Wang, K.; Fang, J.; Zhao, C.; Ren, F.; Ge, X.; et al. A Resilient and Flexible Chitosan/Silk Cryogel Incorporated Ag/Sr Co-Doped Nanoscale Hydroxyapatite for Osteoinductivity and Antibacterial Properties. J. Mater. Chem. B 2018, 6, 7427–7438. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Ma, L.; Xie, L.; Yang, H.; Li, Y.; Wang, S.; Qiao, H.; Lin, H.; Lan, J.; et al. Chemical Stability, Antibacterial and Osteogenic Activities Study of Strontium-Silver Co-Substituted Fluorohydroxyapatite Nanopillars: A Potential Multifunctional Biological Coating. Ceram. Int. 2020, 46, 27758–27773. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Efficient Destruction of Bacteria with Ti(IV) and Antibacterial Ions in Co-Substituted Hydroxyapatite Films. Appl. Catal. B 2007, 73, 345–353. [Google Scholar] [CrossRef]
- Ullah, I.; Siddiqui, M.A.; Kolawole, S.K.; Liu, H.; Zhang, J.; Ren, L.; Yang, K. Synthesis, Characterization and in Vitro Evaluation of Zinc and Strontium Binary Doped Hydroxyapatite for Biomedical Application. Ceram. Int. 2020, 46, 14448–14459. [Google Scholar] [CrossRef]
- Lim, P.N.; Konishi, T.; Wang, Z.; Feng, J.; Wang, L.; Han, J.; Yang, Z.; Thian, E.S. Enhancing Osteoconductivity and Biocompatibility of Silver-Substituted Apatite in Vivo through Silicon Co-Substitution. Mater. Lett. 2018, 212, 90–93. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Kannan, S.; Michel, J.; Rebelo, A.H.S.; Ferreira, J.M.F. Development and in Vitro Characterization of Sol-Gel Derived CaO-P2O5-SiO2-ZnO Bioglass. Acta Biomater. 2007, 3, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Beckham, C.A.; Greenlee, T.K., Jr.; Crebo, A.R. Bone Formation at a Ceramic Implant Interface. Calcif. Tissue Int. 1971, 8, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Griss, P.; Greeenspan, D.C.; Heimke, G.; Krempien, B.; Buchinger, R.; Hench, L.L.; Jentschura, G. Evaluation of a Bioglass-Coated Al2O3 Total Hip Prosthesis in Sheep. J. Biomed. Mater. Res. 1976, 10, 511–518. [Google Scholar] [CrossRef]
- Hench, L.L. Chronology of Bioactive Glass Development and Clinical Applications. New J. Glass Ceram. 2013, 03, 67–73. [Google Scholar] [CrossRef]
- Wilson, J.; Pigott, G.H.; Schoent, F.J.; Hench, L.L. Toxicology and Biocompatibility of Bioglasses. J. Biomed. Mater. Res. 1981, 15, 805–817. [Google Scholar] [CrossRef]
- Stanley, H.R.; Hench, L.; Going, R.; Bennett, C.; Chellemi, S.J.; King, C.; Ingersoll, N.; Ethridge, E.; Kreutziger, K. The Implantation of Natural Tooth Form Bioglasses in Baboons A Preliminary Report. Oral Surg. Oral Med. Oral Pathol. 1976, 42, 339–356. [Google Scholar] [CrossRef]
- Stanley, H.R.; Hall, M.B.; Colaizzi, F.; Clark, A.E. Residual Alveolar Ridge Maintenance with a New Endosseous Implant Material. J. Prosthet. Dent. 1987, 58, 607–613. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An Investigation of Bioactive Glass Powders by Sol-Gel Processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Polka, J.M.; Xynos, I.D.; Buttery, L.D.K. Bioactive Materials to Control Cell Cycle. Mat. Res. Innov. 2000, 3, 313–323. [Google Scholar] [CrossRef]
- Jones, J.R. Bioactive Glasses. In Bioceramics and Their Clinical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 266–283. ISBN 9781845692049. [Google Scholar]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of Melt-Derived 45S5 and Sol-Gel-Derived 58S Bioactive Glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Arai, Y.; Masaki, T.; Ishikawa, T.; Yoda, S.; Kohara, S.; Taniguchi, H.; Itoh, M.; Kuroiwa, Y. Fabrication of BaTi2O5 Glass-Ceramics with Unusual Dielectric Properties during Crystallization. Chem. Mater. 2006, 18, 2169–2173. [Google Scholar] [CrossRef]
- Krishnan, V.; Lakshmi, T. Bioglass: A Novel Biocompatible Innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Seabra, C.M.; Palma, P.J.; Cardoso, A.L.; Peça, J.; Santos, J.M. Effects of a New Bioceramic Material on Human Apical Papilla Cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and Future Options for Dental Pulp Therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef]
- Lisa, C.N.; Abraham, S.; Shama Rao, H.N.; Sridhar, N.; Moon, N.; Barde, D.H. A Clinical and Radiographic Evaluation of Periodontal Regenerative Potential of PerioGlas®: A Synthetic, Resorbable Material in Treating Periodontal Infrabony Defects. J. Int. Oral Health 2014, 6, 20–26. [Google Scholar]
- Tadjeodin, E.S.; de Lange, G.L.; Lyaruu, D.M.; Kuiper, L.; Burger, E.H. High Concentrations of Bioactive Glass Material (BioGranA) vs. Autogenous Bone for Sinus Floor Elevation. Clin. Oral Impl. Res. 2002, 13, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, T.A.G.; Geurts, J.A.P.; Blokhuis, T.J. Treatment of Infected Tibial Non-Unions Using a BMAC and S53P4 BAG Combination for Reconstruction of Segmental Bone Defects: A Clinical Case Series. Injury 2021, 52, S67–S71. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.G.J.; van Gestel, N.A.P.; Bevers, R.; Hofmann, S.; Geurts, J.; van Loo, I.H.M.; Arts, J.J. Assessment of Growth Reduction of Five Clinical Pathogens by Injectable S53P4 Bioactive Glass Material Formulations. Front. Bioeng. Biotechnol. 2020, 8, 634. [Google Scholar] [CrossRef]
- Greenspan, D.C. NovaMin® and Tooth Sensitivity—An Overview. J. Clin. Dent. 2010, 21, 61–65. [Google Scholar] [PubMed]
- Esteban-Tejeda, L.; Smirnov, A.; Prado, C.; Moya, J.S.; Torrecillas, R.; Bartolomé, J.F. Multifunctional Ceramic-Metal Biocomposites with Zinc Containing Antimicrobial Glass Coatings. Ceram. Int. 2016, 42, 7023–7029. [Google Scholar] [CrossRef]
- Pazarçeviren, A.E.; Tahmasebifar, A.; Tezcaner, A.; Keskin, D.; Evis, Z. Investigation of Bismuth Doped Bioglass/Graphene Oxide Nanocomposites for Bone Tissue Engineering. Ceram. Int. 2018, 44, 3791–3799. [Google Scholar] [CrossRef]
- Tripathi, H.; Rath, C.; Kumar, A.S.; Manna, P.P.; Singh, S.P. Structural, Physico-Mechanical and in-Vitro Bioactivity Studies on SiO2–CaO–P2O5–SrO–Al2O3 Bioactive Glasses. Mater. Sci. Eng. C 2019, 94, 279–290. [Google Scholar] [CrossRef]
- Li, Y.; Stone, W.; Schemitsch, E.H.; Zalzal, P.; Papini, M.; Waldman, S.D.; Towler, M.R. Antibacterial and Osteo-Stimulatory Effects of a Borate-Based Glass Series Doped with Strontium Ions. J. Biomater. Appl. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Araujo, M.S.; Silva, A.C.; Cabal, B.; Bartolomé, J.F.; Mello-Castanho, S. In Vitro Bioactivity and Antibacterial Capacity of 45S5 Bioglass®-Based Compositions Containing Alumina and Strontium. J. Mater. Res. Technol. 2021, 13, 154–161. [Google Scholar] [CrossRef]
- Leonelli, C.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Tonelli, M. Synthesis and Characterization of Cerium-Doped Glasses and in Vitro Evaluation of Bioactivity. J. Non-Cryst. Solids 2003, 316, 198–216. [Google Scholar] [CrossRef]
- Saranti, A.; Koutselas, I.; Karakassides, M.A. Bioactive Glasses in the System CaO-B2O3-P2O5: Preparation, Structural Study and in Vitro Evaluation. J. Non-Cryst. Solids 2006, 352, 390–398. [Google Scholar] [CrossRef]
- Singh, R.K.; Kothiyal, G.P.; Srinivasan, A. Magnetic and Structural Properties of CaO-SiO2-P2O5-Na2O-Fe2O3 Glass Ceramics. J. Magn. Magn. Mater. 2008, 320, 1352–1356. [Google Scholar] [CrossRef]
- Kang, X.; Cheng, Z.; Li, C.; Yang, D.; Shang, M.; Ma, P.; Li, G.; Liu, N.; Lin, J. Core-Shell Structured up-Conversion Luminescent and Mesoporous NaYF 4:Yb3+/Er3+@nSiO2@mSiO2 Nanospheres as Carriers for Drug Delivery. J. Phys. Chem. C 2011, 115, 15801–15811. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Gnanasammandhan, M.K.; Zhang, Y. Small Upconverting Fluorescent Nanoparticles for Biomedical Applications. Small 2010, 6, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xing, M.; Chen, Z.; Wang, X.; Zhao, C.; Qiu, J.; Yu, J.; Chang, J. Er3+/Yb3+ Co-Doped Bioactive Glasses with up-Conversion Luminescence Prepared by Containerless Processing. Ceram. Int. 2016, 42, 13168–13175. [Google Scholar] [CrossRef]
- Kalaivani, S.; Srividiya, S.; Vijayalakshmi, U.; Kannan, S. Bioactivity and Up-Conversion Luminescence Characteristics of Yb3+/Tb3+ Co-Doped Bioglass System. Ceram. Int. 2019, 45, 18640–18647. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and Challenges in Biomaterials Used for Bone Tissue Engineering: Bioactive Glasses and Elastomeric Composites. Prog. Biomater. 2012, 1, 1–22. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Sharif Zein, S.H.; Othman, M.R.; Yang, F.; Jansen, J.A. Nanophase Hydroxyapatite as a Biomaterial in Advanced Hard Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2013, 19, 431–441. [Google Scholar] [CrossRef]
- Oonishi, H.; Hench, L.L.; Wilson, J.; Sugihara, F.; Tsuji, E.; Kushitani, S.; Iwaki, H. Comparative Bone Growth Behavior in Granules of Bioceramic Materials of Various Sizes. J. Biomed. Mater. Res. 1999, 44, 31–43. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Ojaghi Ilkhchi, M. Effect of Suspension Medium on the Characteristics of Electrophoretically Deposited Bioactive Glass Coatings on Titanium Substrate. J. Non-Cryst. Solids 2019, 503–504, 232–242. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Ojaghi-Ilkhchi, M.; Farrokhi-Rad, M. Evaluation of Bioglass and Hydroxyapatite Based Nanocomposite Coatings Obtained by Electrophoretic Deposition. Ceram. Int. 2020, 46, 26069–26077. [Google Scholar] [CrossRef]
- Ravarian, R.; Moztarzadeh, F.; Hashjin, M.S.; Rabiee, S.M.; Khoshakhlagh, P.; Tahriri, M. Synthesis, Characterization and Bioactivity Investigation of Bioglass/Hydroxyapatite Composite. Ceram. Int. 2010, 36, 291–297. [Google Scholar] [CrossRef]
- Rizwan, M.; Hamdi, M.; Basirun, W.J.; Kondoh, K.; Umeda, J. Low Pressure Spark Plasma Sintered Hydroxyapatite and Bioglass® Composite Scaffolds for Bone Tissue Repair. Ceram. Int. 2018, 44, 23052–23062. [Google Scholar] [CrossRef]
- de Siqueira, L.; Grenho, L.; Fernandes, M.H.; Monteiro, F.J.; Trichês, E.S. 45S5 Bioglass-Derived Glass-Ceramic Scaffolds Containing Niobium Obtained by Gelcasting Method. Mater. Res. 2021, 24, 1–6. [Google Scholar] [CrossRef]
- Hu, S.; Chang, J.; Liu, M.; Ning, C. Study on Antibacterial Effect of 45S5 Bioglass®. J. Mater. Sci. Mater. Med. 2009, 20, 281–286. [Google Scholar] [CrossRef]
| Synthesis Technology | Advantages | Morphology and Size |
|---|---|---|
| Co–precipitation [40,41,42,43] | The most efficient method at room temperatureThe particle size depends on the pH and ionic strength of the reaction media | Rod, sphere, fiber, tube, filament, whisker, flower Size: 3 nm–1000 µm |
| Sol–gel method [40,44] | The mixing of reactant at molecular level may improve the chemical homogeneity | Sphere, needle, tube, rod, filament, platelet Size: 3 nm–1000 µm |
| Hydrothermal method [40,45,46,47] | Enhanced solubility of precursors | Irregular, rod, needle, filer, whisker, feathery structures Size: 5 nm–8 µm |
| Sonochemical [40,48,49,50] | The possibility of producing monodispersed nanoparticles of different shapes | Sphere, filament, tube, rod Size: 5 nm–1000 µm |
| Thermal decomposition [40,51,52,53] | Method that allows a good particle size control and good crystallinity | Flake, plate, sheet, formless particles Size: 5 nm–200 µm |
| Combustion [40,54,55] | A quick method with high purity and a single step procedure | Sphere, oval, irregular spherical shapes Size: 5 nm–200 µm |
| Solid-state dry synthesis [40,56,57] | Particles with a well-crystallized structure | Irregular, rod, needle, whisker Size: 5 nm–1000 µm |
| Biosources [58,59,60,61] | Ecofriendly technique to transform waste from various natural sources to wealth | Different shapes: flakes, plate, rod, tubular shape, etc. Size: 10 nm–2000 µm |
| Year | Development Steps |
|---|---|
| 1969 | 45S5 Bioglass® was discovered at the University of Florida |
| 1971 | Based on the research activity, bonding of bone to bioactive glasses and glass–ceramics study was published [20,130] |
| 1975 | Bioglass-coated alumina was used for bone bonding on sheep hip implants [131] |
| 1977 | Bioglass coating on metals and alumina ceramics was patented [132] |
| 1981 | Discovery of soft connective tissue bonding to 45S5 Bioglass® [133] |
| 1988 | Bioglass is approved by FDA to be used on ERMI implant [132,134,135] |
| 1991 | Sol–gel synthesis was investigated for bioactive gel-glasses [136,137] |
| 1993 | PerioGlas® was approved by FDA to be used for bone and dental repair [138] |
| 1996 | PerioGlas® (bioglass used for bone grafting) is used in tooth extraction and alveolar augmentation [132] |
| 1999 | TheraSphere® was approved by FDA to be used for cancer treatment [138] |
| 2000 | Studies were made to examine if 45S5 Bioglass® can be used to control osteoblast cell cycles [139] |
| 2004–2005 | NovaMin® (particular type of 45S5 Bioglass®) is developed for use as repair agent in toothpaste [132] |
| 2010 | Cardiac tissue engineering [138] |
| 2012 | Spinal cord repair [138] |
| 2018 | TheraSphere® is used for liver metastatic colorectal carcinoma [138] |
| Medical Field | Applications |
|---|---|
| Orthopedics products | Trauma: Fractures of long bones (alone or with internal fixation) Repair of femoral Tibial plateau fracture Arthroplasty: Filler around implants Impaction bone grafting Spine fusion: Posterolateral lumbar spinal fusion Recovery of bones after tumor removal |
| Cranial–facial products | Cranioplasty: Facial reconstruction Augmentation of alveolar ridge General dental/oral improvement: Sinus lift surgery Cutting or reshaping of bones Repair of periodontal defects |
| Dental–maxillofacial products | Toothpaste and treatments to prevent hypersensitivity Obliteration of frontal sinus Direct and indirect pulp capping Repair of orbital floor fracture |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. https://doi.org/10.3390/jfb13040248
Filip DG, Surdu V-A, Paduraru AV, Andronescu E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. Journal of Functional Biomaterials. 2022; 13(4):248. https://doi.org/10.3390/jfb13040248
Chicago/Turabian StyleFilip, Diana Georgiana, Vasile-Adrian Surdu, Andrei Viorel Paduraru, and Ecaterina Andronescu. 2022. "Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review" Journal of Functional Biomaterials 13, no. 4: 248. https://doi.org/10.3390/jfb13040248
APA StyleFilip, D. G., Surdu, V.-A., Paduraru, A. V., & Andronescu, E. (2022). Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. Journal of Functional Biomaterials, 13(4), 248. https://doi.org/10.3390/jfb13040248







