Effect of a Self-Assembly Peptide on Surface Roughness and Hardness of Bleached Enamel
Abstract
:1. Introduction
2. Materials and Methods
3. Results
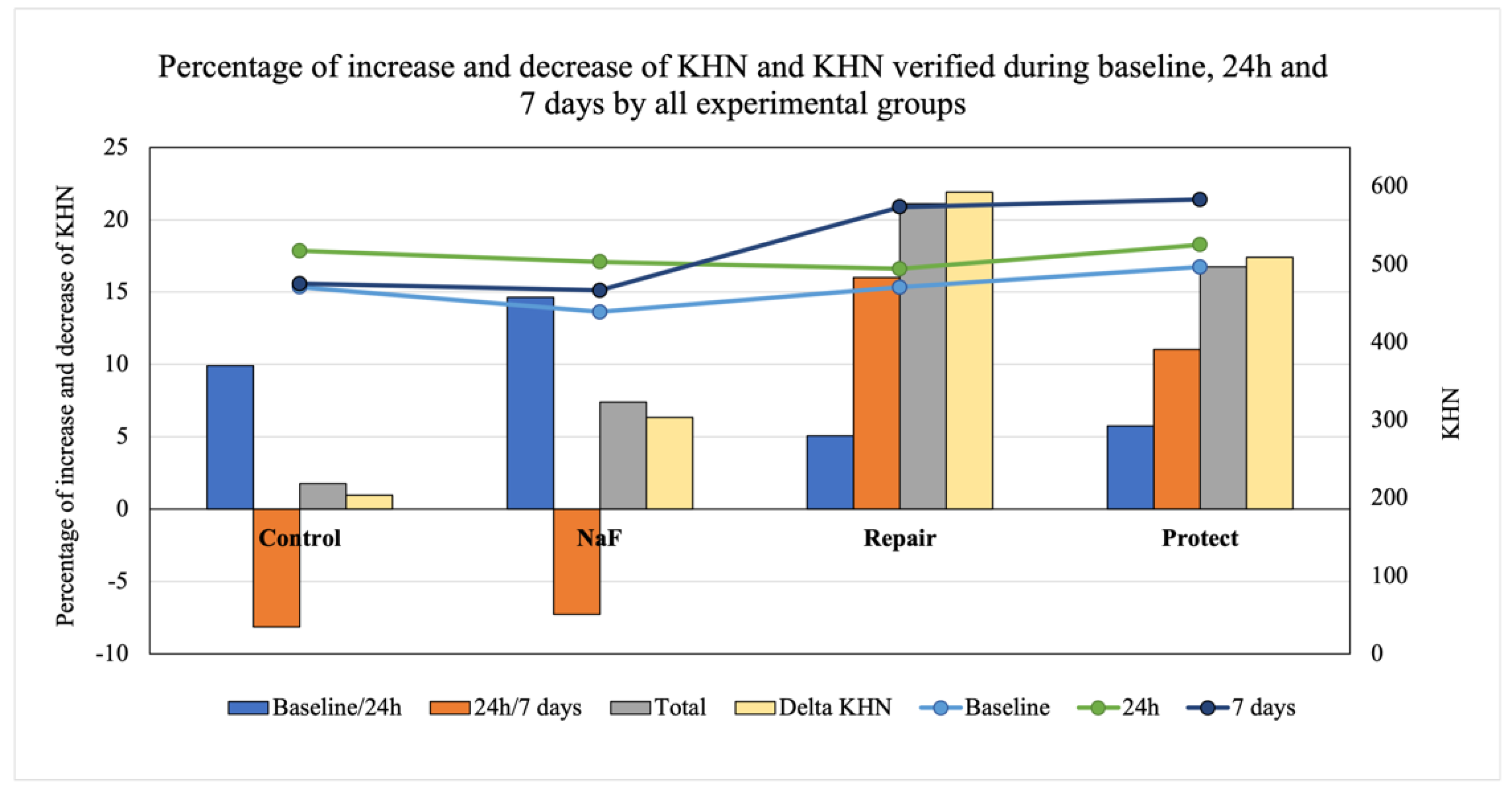
3.1. Knoop Microhardness (KHN)
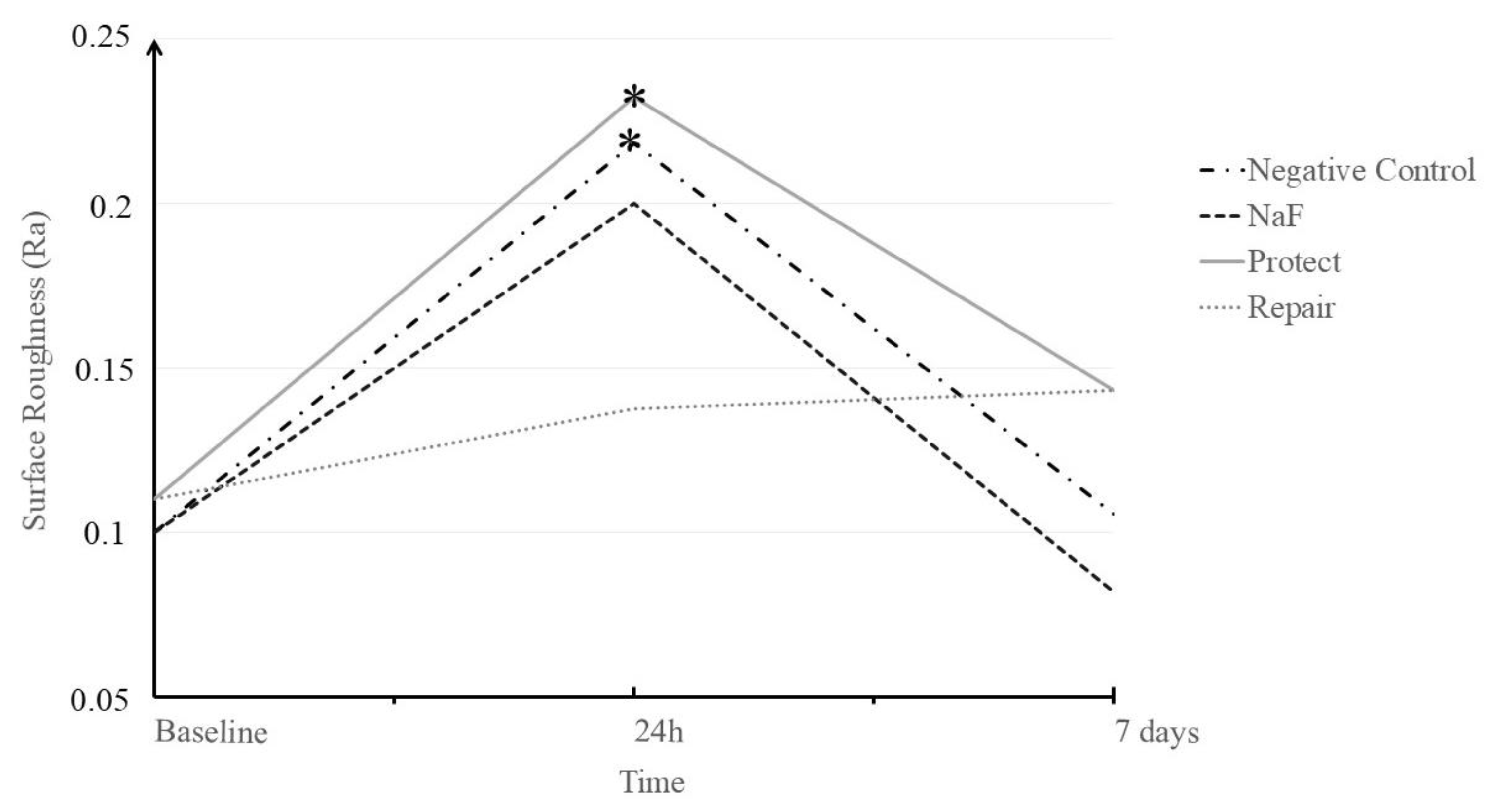
3.2. Surface Roughness (Ra)
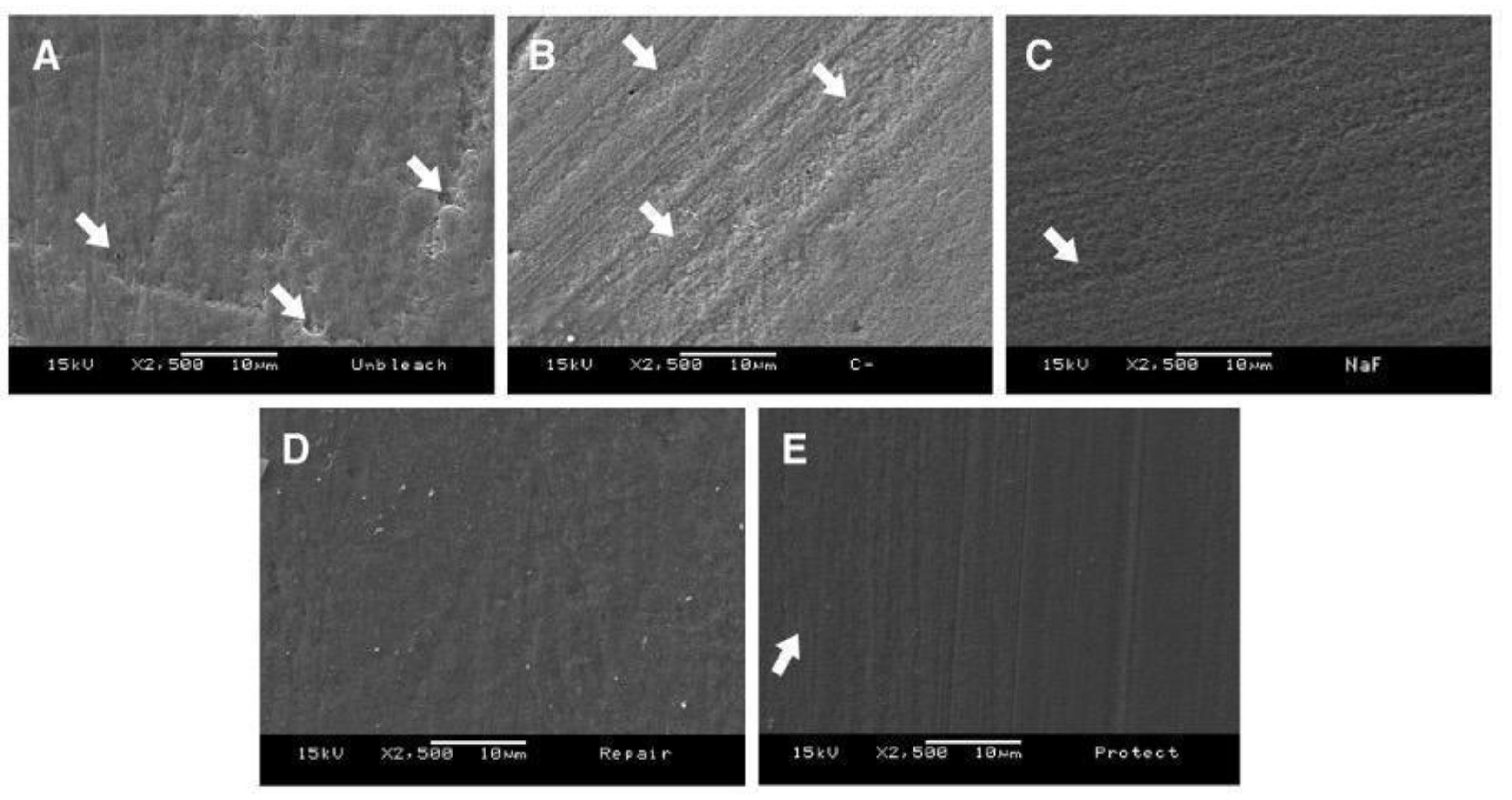
3.3. Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haywood, V.B.; Al Farawati, F. Bleaching update and the future impact on prosthodontics. Br. Dent. J. 2019, 226, 753–760. [Google Scholar] [CrossRef]
- Joiner, A.; Luo, W. Tooth colour and whiteness: A review. J. Dent. 2017, 67, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.R.; Wertz, P.W. Review of the Mechanism of Tooth Whitening. J. Esthet. Restor. Dent. 2015, 27, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Tsujimoto, Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J. Endod. 2004, 30, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Meireles, S.S.; Sarmento, H.R.; Dantas, R.V.; Botero, T.; Tarquinio, S.B. Erosion and abrasion on dental structures undergoing at-home bleaching. Clin. Cosmet. Investig. Dent. 2011, 3, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Li, R.; Sa, Y.; Liang, S.; Sun, L.; Jiang, T.; Wang, Y. Separate contribution of enamel and dentine to overall tooth colour change in tooth bleaching. J. Dent. 2011, 39, 739–745. [Google Scholar] [CrossRef]
- Coceska, E.; Gjorgievska, E.; Coleman, N.J.; Gabric, D.; Slipper, I.J.; Stevanovic, M.; Nicholson, J.W. Enamel alteration following tooth bleaching and remineralization. J. Microsc. 2016, 262, 232–244. [Google Scholar] [CrossRef]
- Magalhães, J.G.; Marimoto, A.R.; Torres, C.R.; Pagani, C.; Teixeira, S.C.; Barcellos, D.C. Microhardness change of enamel due to bleaching with in-office bleaching gels of different acidity. Acta Odontol. Scand. 2012, 70, 122–126. [Google Scholar] [CrossRef]
- Eimar, H.; Siciliano, R.; Abdallah, M.N.; Nader, S.A.; Amin, W.M.; Martinez, P.P.; Celemin, A.; Cerruti, M.; Tamimi, F. Hydrogen peroxide whitens teeth by oxidizing the organic structure. J. Dent. 2012, 40 (Suppl. 2), e25–e33. [Google Scholar] [CrossRef]
- Bitter, N.C. A scanning electron microscopy study of the effect of bleaching agents on enamel: A preliminary report. J. Prosthet. Dent. 1992, 67, 852–855. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A.; Moritz, A.; Flury, S. Enamel Surface Changes After Exposure to Bleaching Gels Containing Carbamide Peroxide or Hydrogen Peroxide. Oper. Dent. 2016, 41, E39–E47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayrak, S.; Tunc, E.S.; Sonmez, I.S.; Egilmez, T.; Ozmen, B. Effects of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) application on enamel microhardness after bleaching. Am. J. Dent. 2009, 22, 393–396. [Google Scholar] [PubMed]
- Kutuk, Z.B.; Ergin, E.; Cakir, F.Y.; Gurgan, S. Effects of in-office bleaching agent combined with different desensitizing agents on enamel. J. Appl. Oral. Sci. 2018, 27, e20180233. [Google Scholar] [CrossRef] [PubMed]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology-A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [Green Version]
- Crastechini, E.; Borges, A.B.; Torres, C. Effect of Remineralizing Gels on Microhardness, Color and Wear Susceptibility of Bleached Enamel. Oper. Dent. 2019, 44, 76–87. [Google Scholar] [CrossRef]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef]
- Schmidlin, P.; Zobrist, K.; Attin, T.; Wegehaupt, F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J. Appl. Oral. Sci. 2016, 24, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Bröseler, F.; Tietmann, C.; Bommer, C.; Drechsel, T.; Heinzel-Gutenbrunner, M.; Jepsen, S. Randomised clinical trial investigating self-assembling peptide P(11)-4 in the treatment of early caries. Clin. Oral. Investig. 2020, 24, 123–132. [Google Scholar] [CrossRef]
- Doberdoli, D.; Bommer, C.; Begzati, A.; Haliti, F.; Heinzel-Gutenbrunner, M.; Juric, H. Randomized Clinical Trial investigating Self-Assembling Peptide P(11)-4 for Treatment of Early Occlusal Caries. Sci. Rep. 2020, 10, 4195. [Google Scholar] [CrossRef]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef]
- Kobeissi, R.; Badr, S.B.; Osman, E. Effectiveness of Self-assembling Peptide P(11)-4 Compared to Tricalcium Phosphate Fluoride Varnish in Remineralization of White Spot Lesions: A Clinical Randomized Trial. Int. J. Clin. Pediatr. Dent. 2020, 13, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Sedlakova Kondelova, P.; Mannaa, A.; Bommer, C.; Abdelaziz, M.; Daeniker, L.; di Bella, E.; Krejci, I. Efficacy of P(11)-4 for the treatment of initial buccal caries: A randomized clinical trial. Sci. Rep. 2020, 10, 20211. [Google Scholar] [CrossRef]
- Welk, A.; Ratzmann, A.; Reich, M.; Krey, K.F.; Schwahn, C. Effect of self-assembling peptide P(11)-4 on orthodontic treatment-induced carious lesions. Sci. Rep. 2020, 10, 6819. [Google Scholar] [CrossRef] [Green Version]
- Diaferia, C.; Netti, F.; Ghosh, M.; Sibillano, T.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Bi-functional peptide-based 3D hydrogel-scaffolds. Soft Matter 2020, 16, 7006–7017. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Lee, J.; Nam, K.T.; Adler-Abramovich, L. Arginine-Presenting Peptide Hydrogels Decorated with Hydroxyapatite as Biomimetic Scaffolds for Bone Regeneration. Biomacromolecules 2017, 18, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Chng, H.K.; Ramli, H.N.; Yap, A.U.; Lim, C.T. Effect of hydrogen peroxide on intertubular dentine. J. Dent. 2005, 33, 363–369. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.P.; Carvalho, R.G.; Barbosa-Martins, L.F.; Torquato, R.J.S.; Mugnol, K.C.U.; Nascimento, F.D.; Tersariol, I.L.S.; Puppin-Rontani, R.M. The Self-Assembling Peptide P(11)-4 Prevents Collagen Proteolysis in Dentin. J. Dent. Res. 2019, 98, 347–354. [Google Scholar] [CrossRef]
- Ceci, M.; Mirando, M.; Beltrami, R.; Chiesa, M.; Colombo, M.; Poggio, C. Effect of self-assembling peptide P11 -4 on enamel erosion: AFM and SEM studies. Scanning 2016, 38, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Hojabri, N.; Kaisarly, D.; Kunzelmann, K.H. Adhesion and whitening effects of P11-4 self-assembling peptide and HAP suspension on bovine enamel. Clin. Oral. Investig. 2021, 25, 3237–3247. [Google Scholar] [CrossRef]
- Mushashe, A.M.; Coelho, B.S.; Garcia, P.P.; Rechia, B.N.; da Cunha, L.F.; Correr, G.M.; Gonzaga, C.C. Effect of different bleaching protocols on whitening efficiency and enamel superficial microhardness. J. Clin. Exp. Dent. 2018, 10, e772–e775. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Timmer, K.; Shariati, M.; Featherstone, J.D. Effect of timing of fluoride treatment on enamel de- and remineralization in vitro: A pH-cycling study. Caries Res. 1988, 22, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, A.; Bell, M.; Boden, N.; Keen, J.N.; Knowles, P.F.; McLeish, T.C.; Pitkeathly, M.; Radford, S.E. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric beta-sheet tapes. Nature 1997, 386, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Perticaroli, S.; Nickels, J.D.; Ehlers, G.; Sokolov, A.P. Rigidity, secondary structure, and the universality of the boson peak in proteins. Biophys. J. 2014, 106, 2667–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilzy, M.; Santamaria, R.M.; Schmoeckel, J.; Splieth, C.H. Treatment of Carious Lesions Using Self-Assembling Peptides. Adv. Dent. Res. 2018, 29, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, A.; Bell, M.; Carrick, L.M.; Fishwick, C.W.; Harding, R.; Mawer, P.J.; Radford, S.E.; Strong, A.E.; Boden, N. pH as a trigger of peptide beta-sheet self-assembly and reversible switching between nematic and isotropic phases. J. Am. Chem. Soc. 2003, 125, 9619–9628. [Google Scholar] [CrossRef]
- Shetty, S.; Kohad, R.; Yeltiwar, R. Hydroxyapatite as an in-office agent for tooth hypersensitivity: A clinical and scanning electron microscopic study. J. Periodontol. 2010, 81, 1781–1789. [Google Scholar] [CrossRef]
- Dogan, S.; Fong, H.; Yucesoy, D.T.; Cousin, T.; Gresswell, C.; Dag, S.; Huang, G.; Sarikaya, M. Biomimetic Tooth Repair: Amelogenin-Derived Peptide Enables in Vitro Remineralization of Human Enamel. ACS Biomater. Sci. Eng. 2018, 4, 1788–1796. [Google Scholar] [CrossRef]
- Sindhura, V.; Uloopi, K.S.; Vinay, C.; Chandrasekhar, R. Evaluation of enamel remineralizing potential of self-assembling peptide P(11)-4 on artificially induced enamel lesions in vitro. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 352–356. [Google Scholar] [CrossRef]
- Haywood, V.B. Frequently asked questions about bleaching. Compend. Contin. Educ. Dent. 2003, 24, 324–338. [Google Scholar]
- de Arruda, A.M.; dos Santos, P.H.; Sundfeld, R.H.; Berger, S.B.; Briso, A.L. Effect of hydrogen peroxide at 35% on the morphology of enamel and interference in the de-remineralization process: An in situ study. Oper. Dent. 2012, 37, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Bitter, N.C. A scanning electron microscope study of the long-term effect of bleaching agents on the enamel surface in vivo. Gen Dent 1998, 46, 84–88. [Google Scholar] [PubMed]
- Nascimento, W.C.; Gomes Ydo, S.; Alexandrino, L.D.; Costi, H.T.; Silva, J.O., Jr.; Silva, C.M. Influence of fluoride concentration and pH Value of 35% hydrogen peroxide on the hardness, roughness and morphology of bovine enamel. J. Contemp Dent. Pract. 2014, 15, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Dionysopoulos, D.; Koliniotou-Koumpia, E.; Tolidis, K.; Gerasimou, P. Effect of Fluoride Treatments on Bleached Enamel Microhardness and Surface Morphology. Oral. Health Prev. Dent. 2017, 15, 169–175. [Google Scholar] [CrossRef]
- Moosavi, H.; Darvishzadeh, F. The Influence of Post Bleaching Treatments in Stain Absorption and Microhardness. Open Dent. J. 2016, 10, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.; De Ataide, I.N.; Fernandes, M.; Lambor, R. Assessment of Enamel Remineralisation After Treatment with Four Different Remineralising Agents: A Scanning Electron Microscopy (SEM) Study. J. Clin. Diagn. Res. 2017, 11, Zc136–Zc141. [Google Scholar] [CrossRef]
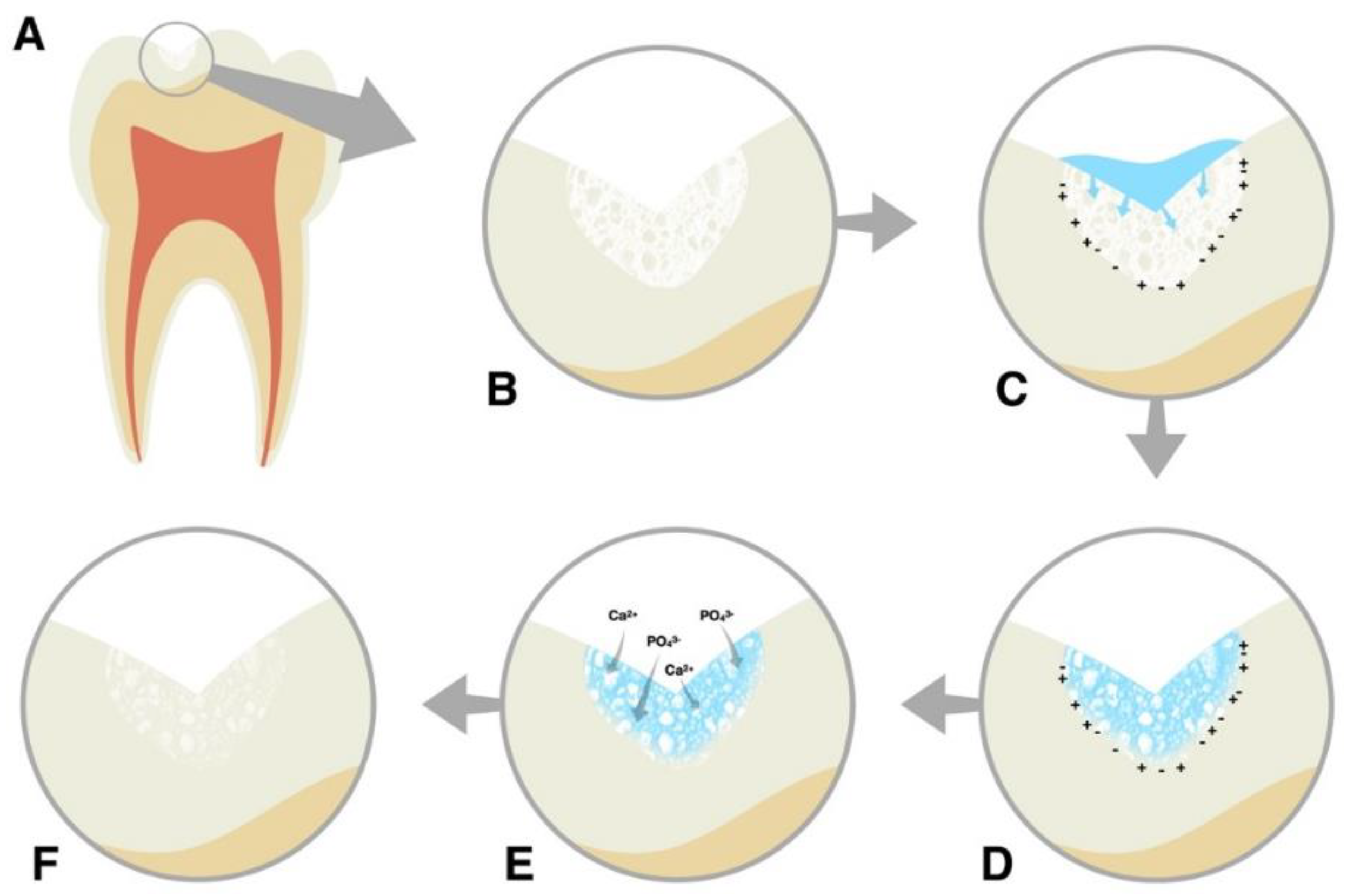

| Groups | Treatment Protocols |
|---|---|
| C- | 35% Carbamide Peroxide, with storage in artificial saliva |
| NaF | 35% Carbamide Peroxide, 2% NaF 9.000 ppm, supersaturated Ca2+ and PO4 solution, with storage in artificial saliva |
| Repair | 35% Carbamide Peroxide, Curodont™ Repair, supersaturated Ca2+ and PO4 solution, with storage in artificial saliva for 1 or 7 days |
| Protect | 35% Carbamide Peroxide, Curodont™ Protect, supersaturated Ca2+ and PO4 solution, with storage in artificial saliva |
| Material (Manufacturers) | Composition | Application Time |
|---|---|---|
| Whiteness Hp Maxx 35% (FGM, Joinville, SC, Brazil) | Hydrogen Peroxide 35%, thickener, red dye, glycol, and water | 3 × 15 min |
| Flugel (Nova DFL, Rio de Janeiro, RJ, Brazil | 2% NaF, 9000 ppm | 1 min |
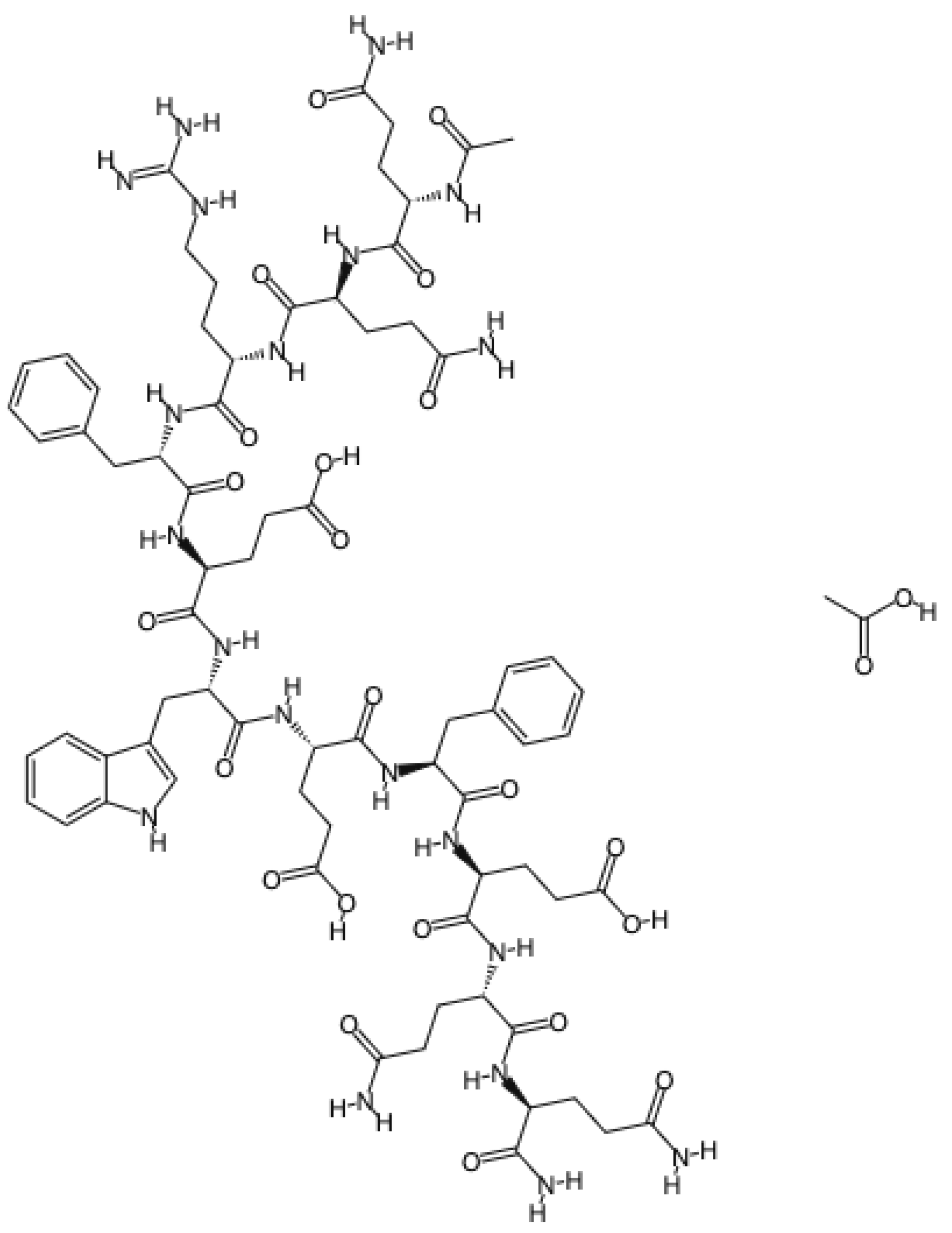
| Curodont™ Repair (Credentis AG, Dorfstrasse, Windisch, Switzerland) | Peptide P11-4 (amino acid sequence: Ace-Gln-Gln-Arg-Phe-Glu-Trp-Glu-Phe-Glu-Gln-Gln-NH2) | 5 min |
| Curodont™ Protect (Credentis AG, Dorfstrasse, Windisch, Switzerland) | Hydrogenated Starch Hydrolysate, Aqua, Hydrated Silica, PEG-8, Cellulose Gum, Sodium Monofluorophosphate, Aroma, Sodium Saccharin, Citric Acid, Sodium Hydroxide, Dicalcium Phosphate, Oligopeptide-104, Calcium Glycerophosphate, Sodium Chloride, Sodium Sulfate, Limonene, Cinnamal, CI 42090 | 5 min |
| Ca2+ and PO43- solution | Saturated solution of Ca2+ and PO43− (1.5 mmol/L calcium, 0.9 mmol/L phosphate, and 150 mol/L KCl in 20 mmol/L cacodylic buffer, pH 7.0) | 1 min |
| Artificial saliva | 1.5 mM CaCl2, 0.9 mM KH2PO4, 130 mM KCl, and 20 mM Hepes, pH 6.5 | Stored for 24 h and 7 days |
| Groups | 24 h | 7 Days |
|---|---|---|
| Negative control | 517.44 (46.41) Aa | 475.22 (58.95) Ba |
| NaF | 503.00 (37.30) Aa | 465.50 (41.50) Ba |
| Repair | 494.33 (28.94) Ab | 572.50 (79.04) Aa * |
| Protect | 525.17 (51.58) Ab * | 583.00 (74.76) Aa * |
| Groups | Baseline | 24 h | 7 days | Average |
|---|---|---|---|---|
| Negative control | 0.122 (0.054) | 0.221 (0.059) | 0.117 (0.033) | 0.222 (0.074) |
| NaF | 0.117 (0.059) | 0.205 (0.068) | 0.168 (0.169) | 0.163 (0.044) |
| Repair | 0.118 (0.020) | 0.133 (0.035) | 0.140 (0.039) | 0.126 (0.010) |
| Protect | 0.123 (0.029) | 0.236 (0.068) | 0.141 (0.085) | 0.189 (0.067) |
| Average | 0.120 (0.041) B | 0.199 (0.069) A | 0.142 (0.094) B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, G.d.A.P.; Fraga, M.A.A.; de Souza Araújo, I.J.; Pacheco, R.R.; Correr, A.B.; Puppin-Rontani, R.M. Effect of a Self-Assembly Peptide on Surface Roughness and Hardness of Bleached Enamel. J. Funct. Biomater. 2022, 13, 79. https://doi.org/10.3390/jfb13020079
Magalhães GdAP, Fraga MAA, de Souza Araújo IJ, Pacheco RR, Correr AB, Puppin-Rontani RM. Effect of a Self-Assembly Peptide on Surface Roughness and Hardness of Bleached Enamel. Journal of Functional Biomaterials. 2022; 13(2):79. https://doi.org/10.3390/jfb13020079
Chicago/Turabian StyleMagalhães, Gabriela de A. P., May Anny A Fraga, Isaac J. de Souza Araújo, Rafael R. Pacheco, Américo B. Correr, and Regina M. Puppin-Rontani. 2022. "Effect of a Self-Assembly Peptide on Surface Roughness and Hardness of Bleached Enamel" Journal of Functional Biomaterials 13, no. 2: 79. https://doi.org/10.3390/jfb13020079
APA StyleMagalhães, G. d. A. P., Fraga, M. A. A., de Souza Araújo, I. J., Pacheco, R. R., Correr, A. B., & Puppin-Rontani, R. M. (2022). Effect of a Self-Assembly Peptide on Surface Roughness and Hardness of Bleached Enamel. Journal of Functional Biomaterials, 13(2), 79. https://doi.org/10.3390/jfb13020079








