Development and Characterization of the Biodegradable Film Derived from Eggshell and Cornstarch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Eggshell Powder (ESP)
2.3. Preparation of Biodegradable ESP/CS Films
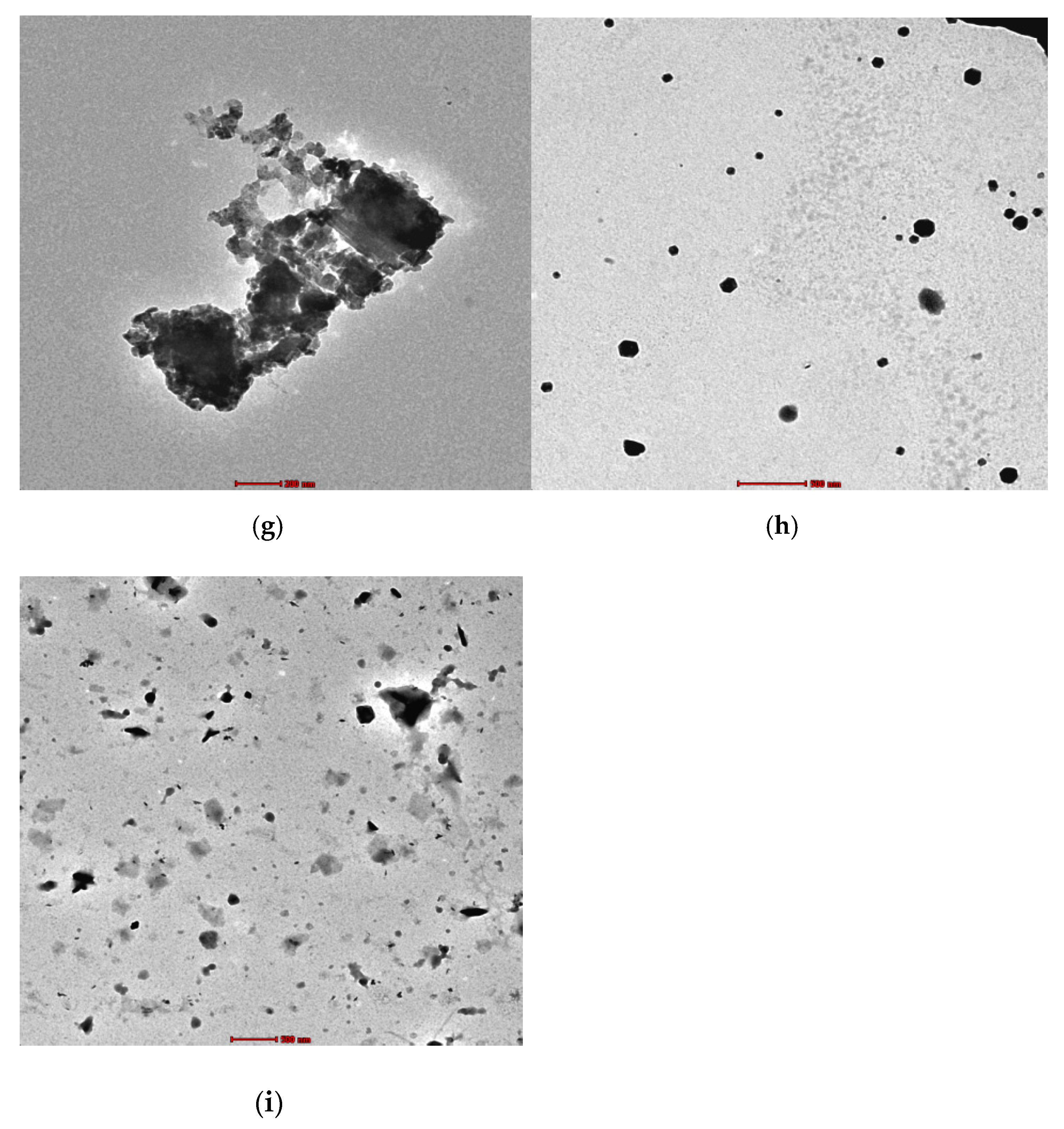
2.4. Morphological Characterization
2.5. Physical Characterization
2.5.1. Thickness (e) and Density (ρ)
2.5.2. Moisture Content (MC), Water Solubility (WS), Water Absorption (WA) and Swelling Power (SP) of Films
2.6. Storage Stability
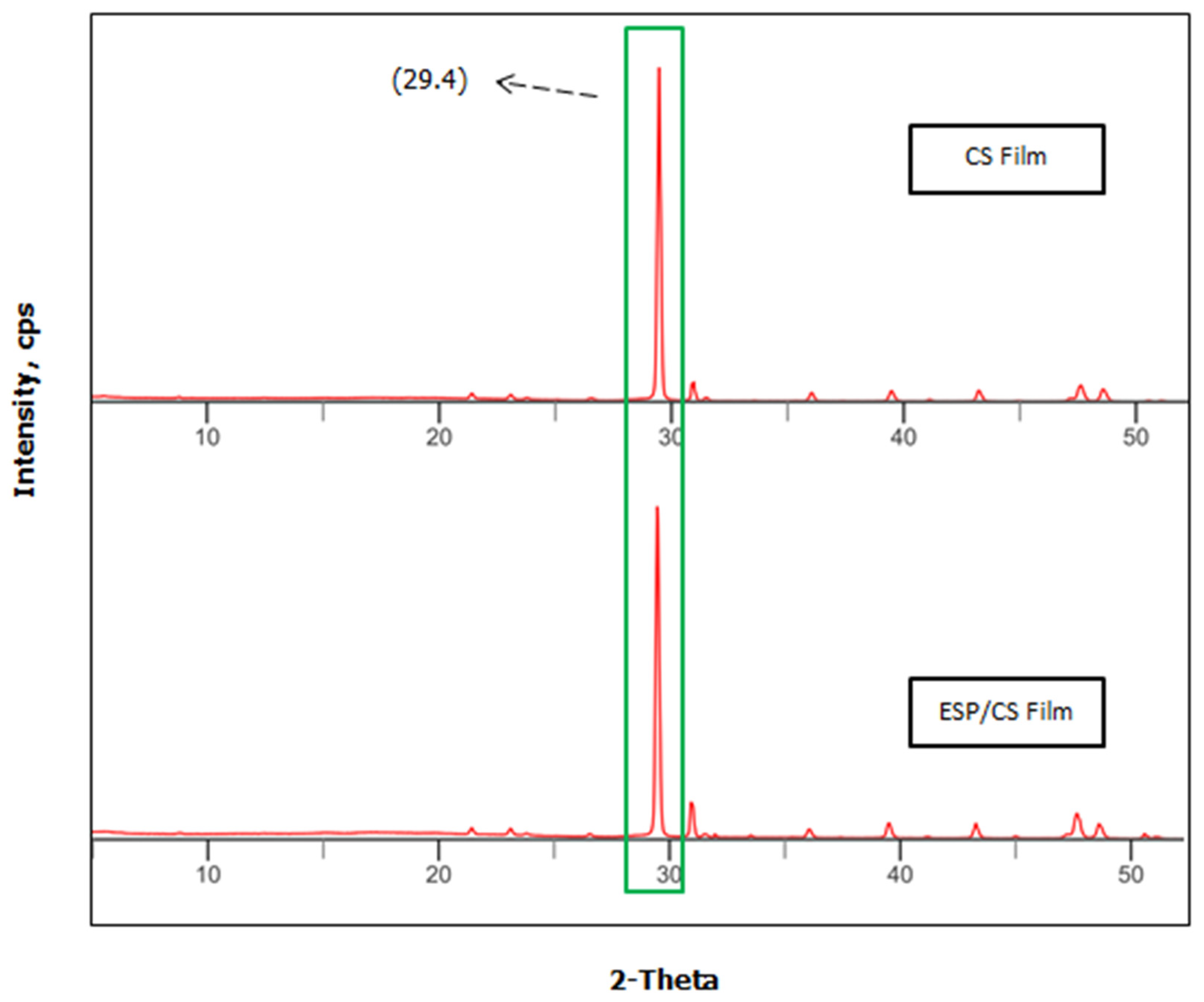
2.7. Fourier Transform Infrared (FTIR) Spectroscopy and X-ray Diffraction (XRD) Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphology Characterization
3.2. Film Thickness and Density Measurement
3.3. Moisture Content (MC)
3.4. Water Solubility (WS)
3.5. Water Adsorption (WA)
3.6. Swelling Power (SP)
3.7. Storage Stability
3.8. Fourier Transform Infrared (FTIR) and X-ray Diffraction (XRD) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| % | Percent |
| °C | Degrees Celsius |
| ° | Degree |
| ø | Theta |
| μm | Micrometer |
| e | Thickness |
| ρ | Density |
| cm | Centimeter |
| g | Gram |
| mA | Milliampere |
| m | Meter |
| mm | Millimeter |
| nm | Nanometer |
| Kv | Kilovolt |
Abbreviations
| ANOVA | Analysis of variance |
| BEP | Bentonite−eggshell composites |
| EDX | Energy dispersive X-ray |
| ES | Eggshell |
| ESP | Eggshell powder |
| FTIR | Fourier Transform Infrared spectroscopy |
| MC | Moisture content |
| SEM | Scanning electron microscopy |
| SP | Swelling power |
| TEM | Transmission electron microscopy |
| WA | Water adsorption |
| WS | Water solubility |
References
- Onwubu, S.C.; Vahed, A.; Singh, S.; Kanny, K. Physicochemical Characterization of a Dental Eggshell Powder Abrasive Material. J. Appl. Biomater. Funct. Mater. 2017, 15, e341–e346. [Google Scholar] [CrossRef] [PubMed]
- Sethupathi, S.; Kai, Y.C.; Kong, L.L.; Munusamy, Y.; Bashir, M.J.K.; Iberahim, N. Preliminary study of sulfur dioxide removal using calcined egg shell. Malays. J. Anal. Sci. 2017, 21, 719–725. [Google Scholar] [CrossRef]
- Liu, Z.; Song, L.; Zhang, F.; He, W.; Linhardt, R. Characteristics of global organic matrix in normal and pimpled chicken eggshells. Poult. Sci. 2017, 96, 3775–3784. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.J.; Varalakshmi, R.; Gargi, S.; Jayasri, M.A.; Suthindhiran, K. Removal of Cr (III) and Ni (II) from tannery effluent using calcium carbonate coated bacterial magnetosomes. NPJ Clean Water 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Cree, D.; Rutter, A. Sustainable Bio-Inspired Limestone Eggshell Powder for Potential Industrialized Applications. ACS Sustain. Chem. Eng. 2015, 3, 941–949. [Google Scholar] [CrossRef]
- Betancourt, N.G.; Cree, D.E. Mechanical Properties of Poly (lactic acid) Composites Reinforced with CaCO3 Eggshell Based Fillers. MRS Adv. 2017, 2, 2545–2550. [Google Scholar] [CrossRef] [Green Version]
- Pirsa, S.; Sharifi, K.A. A review of the applications of bioproteins in the preparation of biodegradable films and polymers. J. Chem. Lett. 2020, 1, 47–58. [Google Scholar] [CrossRef]
- Jabraili, A.; Pirsa, S.; Pirouzifard, M.K.; Amiri, S. Biodegradable Nanocomposite Film Based on Gluten/Silica/Calcium Chloride: Physicochemical Properties and Bioactive Compounds Extraction Capacity. J. Polym. Environ. 2021, 29, 2557–2571. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Pirsa, S.; Farzi, J. Biodegradable nano composite film based on modified starch-albumin/MgO; antibacterial, antioxidant and structural properties. Polym. Test. 2021, 97, 107182. [Google Scholar] [CrossRef]
- García, A.V.; Álvarez-Pérez, O.B.; Rojas, R.; Aguilar, C.N.; Garrigós, M.C. Impact of Olive Extract Addition on Corn Starch-Based Active Edible Films Properties for Food Packaging Applications. Foods 2020, 9, 1339. [Google Scholar] [CrossRef]
- Abotbina, W.; Sapuan, S.M.; Sultan, M.T.H.; Alkbir, M.F.M.; Ilyas, R.A. Development and characterization of cornstarch-based bioplastics packaging film using a combination of different plasticizers. Polymers 2021, 13, 3487. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, L.; Du, X.; Chen, P.; Ji, Y.; Hao, H.; Xu, X. Investigation of glycerol concentration on corn starch morphologies and gelatinization behaviours during heat treatment. Carbohydr. Polym. 2017, 176, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, W.H.; Ye, R.; Liu, A.J.; Xiao, J.D.; Liu, Y.W.; Zhao, Y.N. Mechanical properties and solubility in water of corn starch-collagen composite films: Effect of starch type and concentrations. Food Chem. 2017, 216, 209–216. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Sasidharan, A.; Sabu, S.; Basheer, A.; Navaf, M.; Raghavender, C.; Sinha, S.; George, J. Energetic neutral N2 atoms treatment on the kithul (Caryota urens) starch biodegradable film: Physico-chemical characterization. Food Hydrocoll. 2020, 103, 105650. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Data of thermal degradation and dynamic mechanical properties of starch–glycerol based films with citric acid as crosslinking agent. Data Briefs 2016, 7, 1331–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, H.; Wang, M.; Lv, X.; Zhao, Y.; Xia, L. Development and characterization of irradiated-corn-starch films. Carbohydr. Polym. 2018, 194, 395–400. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R.; Farrag, Y. Effects of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) microparticles on morphological, mechanical, thermal, and barrier properties in thermoplastic potato starch films. Carbohydr. Polym. 2018, 194, 357–364. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch-Stärke 2017, 69, 1500366. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Characteristics of thermoplastic sugar palm Starch/Agar blend: Thermal, tensile, and physical properties. Int. J. Biol. Macromol. 2016, 89, 575–581. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.; Chockchaisawasdee, S.; Golding, J.; Scarlett, C.J.; Stathopoulos, C. Physical, Barrier, and Antioxidant Properties of Pea Starch-Guar Gum Biocomposite Edible Films by Incorporation of Natural Plant Extracts. Food Bioproc. Technol. 2017, 10, 2240–2250. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, N.; Zhang, S.; Zhu, J.; Xiao, H.; Ding, C. Preparation and application of granular bentonite-eggshell composites for heavy metal removal. J. Porous Mater. 2022, 29, 817–826. [Google Scholar] [CrossRef]
- Harripersadth, C.; Musonge, P.; Isa, Y.M.; Morales, M.G.; Sayago, A. The application of eggshells and sugarcane bagasse as potential biomaterials in the removal of heavy metals from aqueous solutions. S. Afr. J. Chem. Eng. 2020, 34, 142–150. [Google Scholar] [CrossRef]
- Mostafavi, S.; Rezaverdinejad, V.; Pirsa, S. Design and fabrication of nanocomposite-based polyurethane filter for improving municipal waste water quality and removing organic pollutants. Adsorpt. Sci. Technol. 2019, 37, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Xiao, D.; Lu, J.; Li, T.; Jiao, C.; Li, S.; Lu, P.; Zhang, Z. Preparation and Properties of Biocomposite Films Based on Poly(vinyl alcohol) Incorporated with Eggshell Powder as a Biological Filler. J. Polym. Environ. 2020, 28, 2020–2028. [Google Scholar] [CrossRef]
- Shukla, S.K.; Al Mushaiqri, N.R.S.; Al Subhi, H.M.; Yoo, K.; Al Sadeq, H. Low-cost activated carbon production from organic waste and its utilization for wastewater treatment. Appl. Water Sci. 2020, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh, H.; Peighambardoust, S.J.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef]
- Ploypetchara, T.; Gohtani, S. Change in characteristics of film based on rice starch blended with sucrose, maltose, and trehalose after storage. J. Food Sci. 2020, 85, 1470–1478. [Google Scholar] [CrossRef]
- Herniou--Julien, C.; Mendieta, J.R.; Gutiérrez, T.J. Characterization of biodegradable/non-compostable films made from cellulose acetate/corn starch blends processed under reactive extrusion conditions. Food Hydrocoll. 2018, 89, 67–79. [Google Scholar] [CrossRef]
- Chong, K.-Y.; Chia, C.-H.; Zakaria, S. Polymorphs calcium carbonate on temperature reaction. AIP Conf. Proc. 2014, 1614, 52–56. [Google Scholar] [CrossRef]
- Weiss, C.A.; Torres-Cancel, K.; Moser, R.D.; Allison, P.G.; Gore, E.R.; Chandler, M.Q.; Malone, P.G. Influence of temperature on calcium carbonate polymorph formed from ammonium carbonate and calcium acetate. J. Nanotech. Smart Mater. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Ooi, C.-W.; Ling, T.C.; Shu-Jen, C.; Chen, S.-Y.; Chang, Y.-K. Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol. Environ. Policy 2020, 22, 773–786. [Google Scholar] [CrossRef]
- Sun, Q.; Xi, T.; Li, Y.; Xiong, L. Characterization of Corn Starch Films Reinforced with CaCO3 Nanoparticles. PLoS ONE 2014, 9, e106727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Li, S.; Wu, Y.; Song, J.; Chen, S.; Li, X.; Sun, H. Preparation and characterization of natural corn starch-based composite films reinforced by eggshell powder. CyTA J. Food 2018, 16, 1045–1054. [Google Scholar] [CrossRef]
- King’ori, A. A Review of the Uses of Poultry Eggshells and Shell Membranes. Int. J. Poult. Sci. 2011, 10, 908–912. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Cui, D.; Liu, M.; Gong, H.; Huang, Y.; Han, F. Corn porous starch: Preparation, characterization and adsorption property. Int. J. Biol. Macromol. 2012, 50, 250–256. [Google Scholar] [CrossRef]
- Gao, J.; Sun, S.-P.; Zhu, W.-P.; Chung, T.-S. Chelating polymer modified P84 nanofiltration (NF) hollow fiber membranes for high efficient heavy metal removal. Water Res. 2014, 63, 252–261. [Google Scholar] [CrossRef]
- Hassan, S.; Aigbodion, V. Effects of eggshell on the microstructures and properties of Al–Cu–Mg/eggshell particulate composites. J. King Saud Univ. Eng. Sci. 2015, 27, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Onwubu, S.C.; Mdluli, P.S.; Singh, S.; Tlapana, T. A novel application of nano eggshell/titanium dioxide composite on occluding dentine tubules: An in vitro study. Braz. Oral Res. 2019, 33, e016. [Google Scholar] [CrossRef]
- Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N.; Pechyen, C. A flower shape-green synthesis and characterization of silver nanoparticles (AgNPs) with different starch as a reducing agent. J. Mater. Res. Technol. 2020, 9, 11003–11012. [Google Scholar] [CrossRef]
- Yin, P.; Liu, J.; Zhou, W.; Li, P. Preparation and Properties of Corn Starch/Chitin Composite Films Cross-Linked by Maleic Anhydride. Polymers 2020, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- Jalu, R.G.; Chamada, T.A.; Kasirajan, R. Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb(II)) from aqueous solution. Environ. Chall. 2021, 4, 100193. [Google Scholar] [CrossRef]
- da Rosa Zavareze, E.; Pinto, V.Z.; Klein, B.; El Halal, S.L.M.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R.G. Development of oxidised and heat–moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Halimatul, M.; Sapuan, S.; Jawaid, M. Water absorption and water solubility properties of sago starch biopolymer composite films filled with sugar palm particles. Polimery 2019, 64, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Hani, N.M.; Nirmal, N.; Fazial, F.F.; Mohtar, N.F.; Romli, S.R. Optical and thermo-mechanical properties of composite films based on fish gelatin/rice flour fabricated by casting technique. Prog. Org. Coat. 2015, 84, 115–127. [Google Scholar] [CrossRef]
- Farahana, R.N.; Supri, A.G.; Teh, P.I. Tensile and water absorption properties of eggshell powder filled recycled high-density polyethylene/ethylene vinyl acetate composites: Effect of 3-aminopropyltriethoxysilane. Adv. Mat. Res. 2015, 5, 1–9. [Google Scholar]
- Valcárcel-Yamani, B.; Rondan-Sanabria, G.G.; Finardi-Filho, F. The physical, chemical and functional characterization of starches from Andean tubers: Oca (Oxalis tuberosa Molina), olluco (Ullucus tuberosus Caldas) and mashua (Tropaeolum tuberosum Ruiz & Pavón). Braz. J. Pharm. Sci. 2013, 49, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Ket-On, A.; Pongmongkol, N.; Somwangthanaroj, A.; Janjarasskul, T.; Tananuwong, K. Properties and storage stability of whey protein edible film with spice powders. J. Food Sci. Technol. 2016, 53, 2933–2942. [Google Scholar] [CrossRef] [Green Version]
- Ji, M.; Li, F.; Li, J.; Li, J.; Zhang, C.; Sun, K.; Guo, Z. Enhanced mechanical properties, water resistance, thermal stability, and biodegradation of the starch-sisal fibre composites with various fillers. Mater. Des. 2021, 198, 109373. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.-S.; Han, J. Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rabadán, A.; Álvarez-Ortí, M.; Pardo, J.E.; Alvarruiz, A. Storage stability and composition changes of three cold-pressed nut oils under refrigeration and room temperature conditions. Food Chem. 2018, 259, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Tizo, M.S.; Blanco, L.A.V.; Cagas, A.C.Q.; Cruz, B.R.B.D.; Encoy, J.C.; Gunting, J.V.; Arazo, R.O.; Mabayo, V.I.F. Efficiency of calcium carbonate from eggshells as an adsorbent for cadmium removal in aqueous solution. Sustain. Environ. Res. 2018, 28, 326–332. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption study of heavy metal ions from aqueous solution by nanoparticle of wild herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Nacke, H.; Gonçalves, D.S.M.; Campagnolo, M.A.; Coelho, G.F.; Schwantes, D.; Dos Santos, M.G.; Briesch, D.L.; Zimmermann, J. Adsorption of Cu (II) and Zn (II) from Water by Jatropha curcas L. as Biosorbent. Open Chem. J. 2016, 14, 103–117. [Google Scholar] [CrossRef]
- Salaheldin, H.I. Optimizing the synthesis conditions of silver nanoparticles using corn starch and their catalytic reduction of 4-nitrophenol. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025013. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef]
- Li, C.; Zhu, W.; Xue, H.; Chen, Z.; Chen, Y.; Wang, X. Physical and structural properties of peanut protein isolate-gum Arabic films prepared by various glycation time. Food Hydrocoll. 2015, 43, 322–328. [Google Scholar] [CrossRef]
- Ji, G.; Zhu, H.; Qi, C.; Zeng, M. Mechanism of interactions of eggshell microparticles with epoxy resins. Polym. Eng. Sci. 2009, 49, 1383–1388. [Google Scholar] [CrossRef]

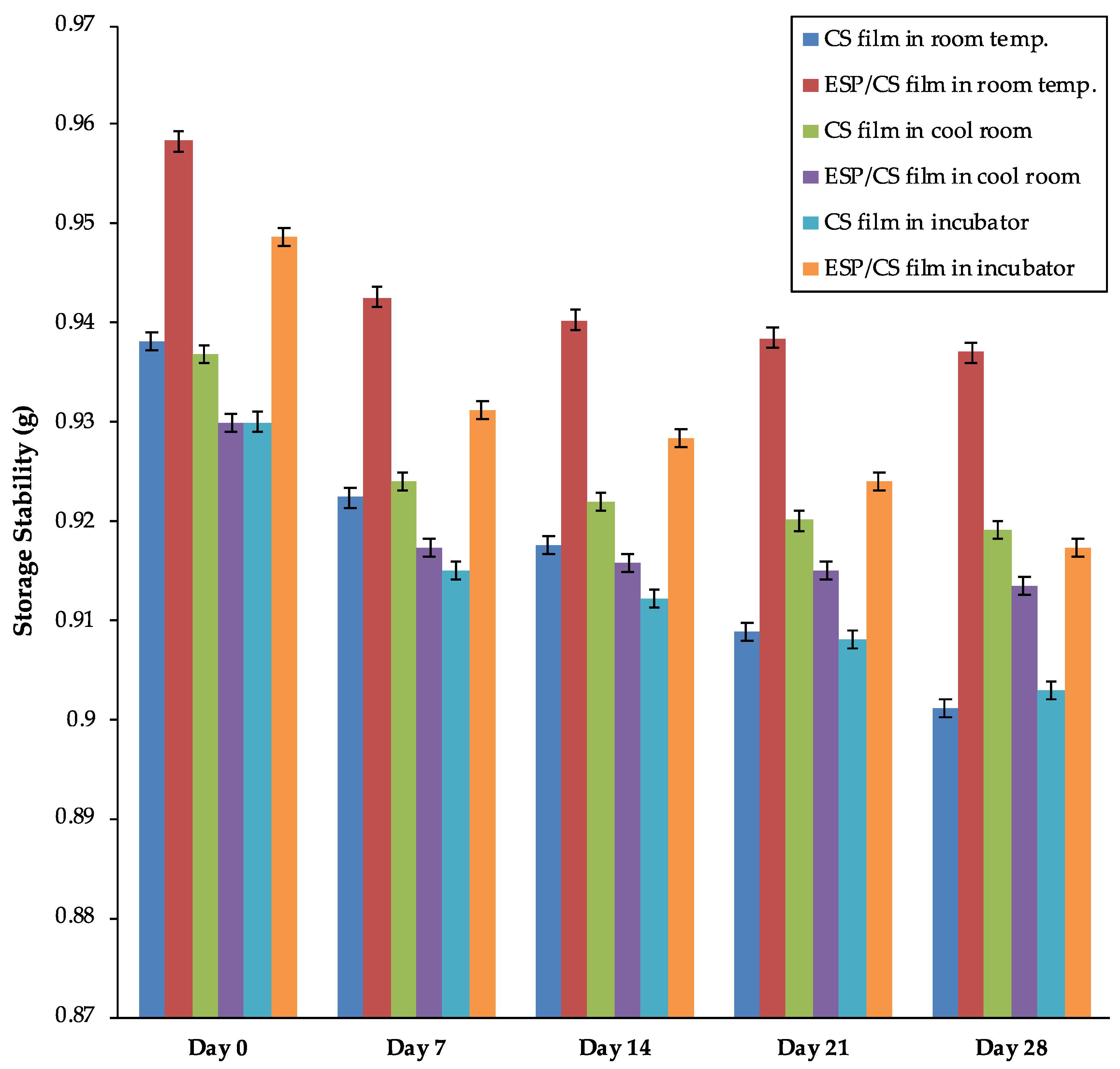

| Parameters | CS Film | ESP/CS Film |
|---|---|---|
| Thickness | 0.018 ± 0.021 a | 0.026 ± 0.018 b |
| Density | 0.535 ± 0.386 c | 0.617 ± 0.210 d |
| MC (%) | 48.533 ± 18.213 | 38.781 ± 16.139 e |
| WS (%) | 46.632 ± 9.109 | 39.022 ± 12.251 |
| WA (%) | 87.072 ± 2.758 | 87.700 ± 3.374 |
| SP (%) | 8.000 ± 2.739 | 7.000 ± 2.739 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vonnie, J.M.; Rovina, K.; Azhar, R.A.; Huda, N.; Erna, K.H.; Felicia, W.X.L.; Nur’Aqilah, M.N.; Halid, N.F.A. Development and Characterization of the Biodegradable Film Derived from Eggshell and Cornstarch. J. Funct. Biomater. 2022, 13, 67. https://doi.org/10.3390/jfb13020067
Vonnie JM, Rovina K, Azhar RA, Huda N, Erna KH, Felicia WXL, Nur’Aqilah MN, Halid NFA. Development and Characterization of the Biodegradable Film Derived from Eggshell and Cornstarch. Journal of Functional Biomaterials. 2022; 13(2):67. https://doi.org/10.3390/jfb13020067
Chicago/Turabian StyleVonnie, Joseph Merillyn, Kobun Rovina, Rasnarisa Awatif Azhar, Nurul Huda, Kana Husna Erna, Wen Xia Ling Felicia, Md Nasir Nur’Aqilah, and Nur Fatihah Abdul Halid. 2022. "Development and Characterization of the Biodegradable Film Derived from Eggshell and Cornstarch" Journal of Functional Biomaterials 13, no. 2: 67. https://doi.org/10.3390/jfb13020067
APA StyleVonnie, J. M., Rovina, K., Azhar, R. A., Huda, N., Erna, K. H., Felicia, W. X. L., Nur’Aqilah, M. N., & Halid, N. F. A. (2022). Development and Characterization of the Biodegradable Film Derived from Eggshell and Cornstarch. Journal of Functional Biomaterials, 13(2), 67. https://doi.org/10.3390/jfb13020067











