An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Biosynthesized AgNPs
2.2. Analytical Methods
2.3. Biological Methods
2.4. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
3.1.1. XRD and EDS
3.1.2. Field-Emission Scanning Electron Microscope (FESEM) Results
3.1.3. TEM and SAED
3.1.4. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
3.2. Biological Studies
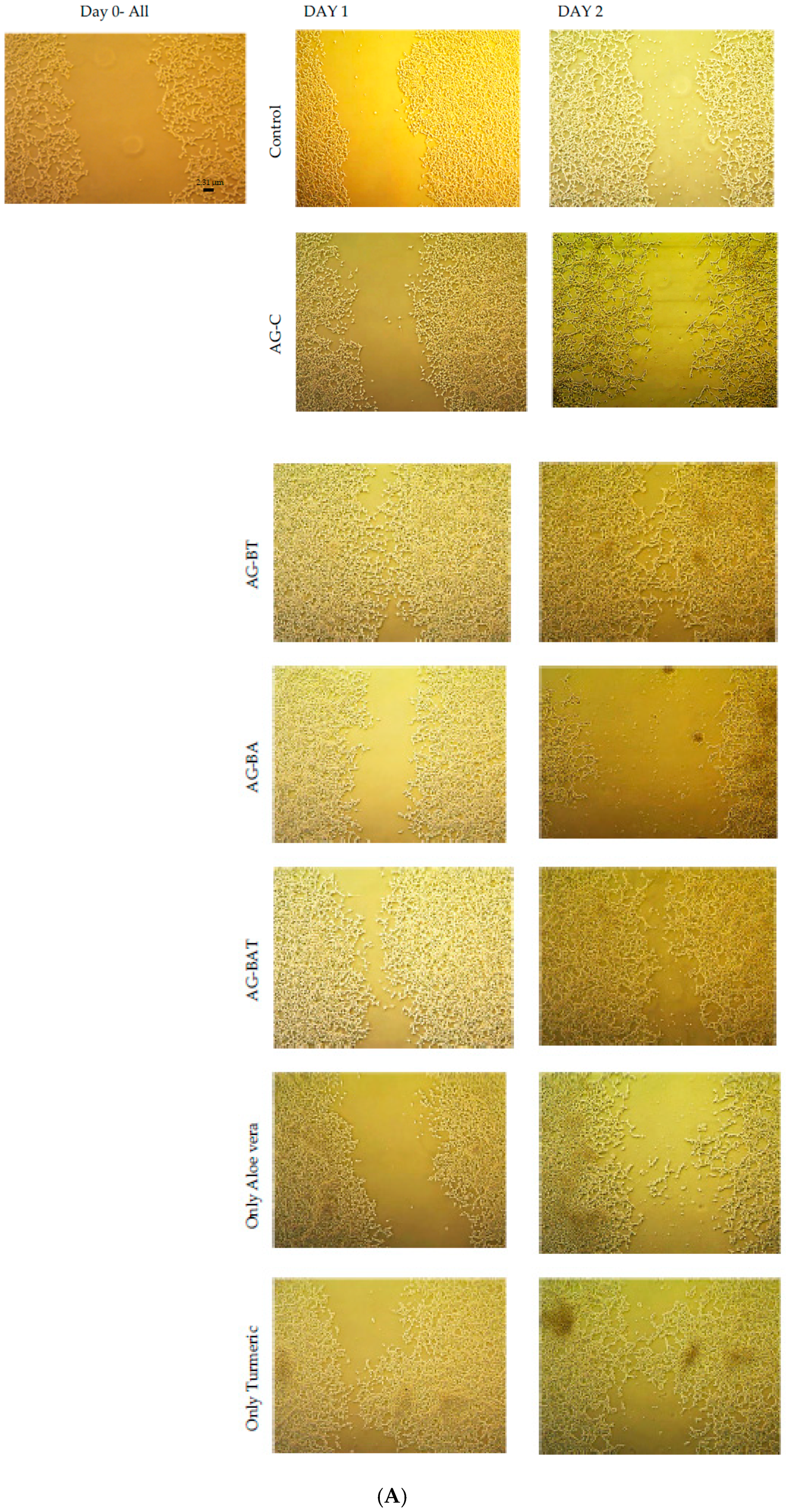
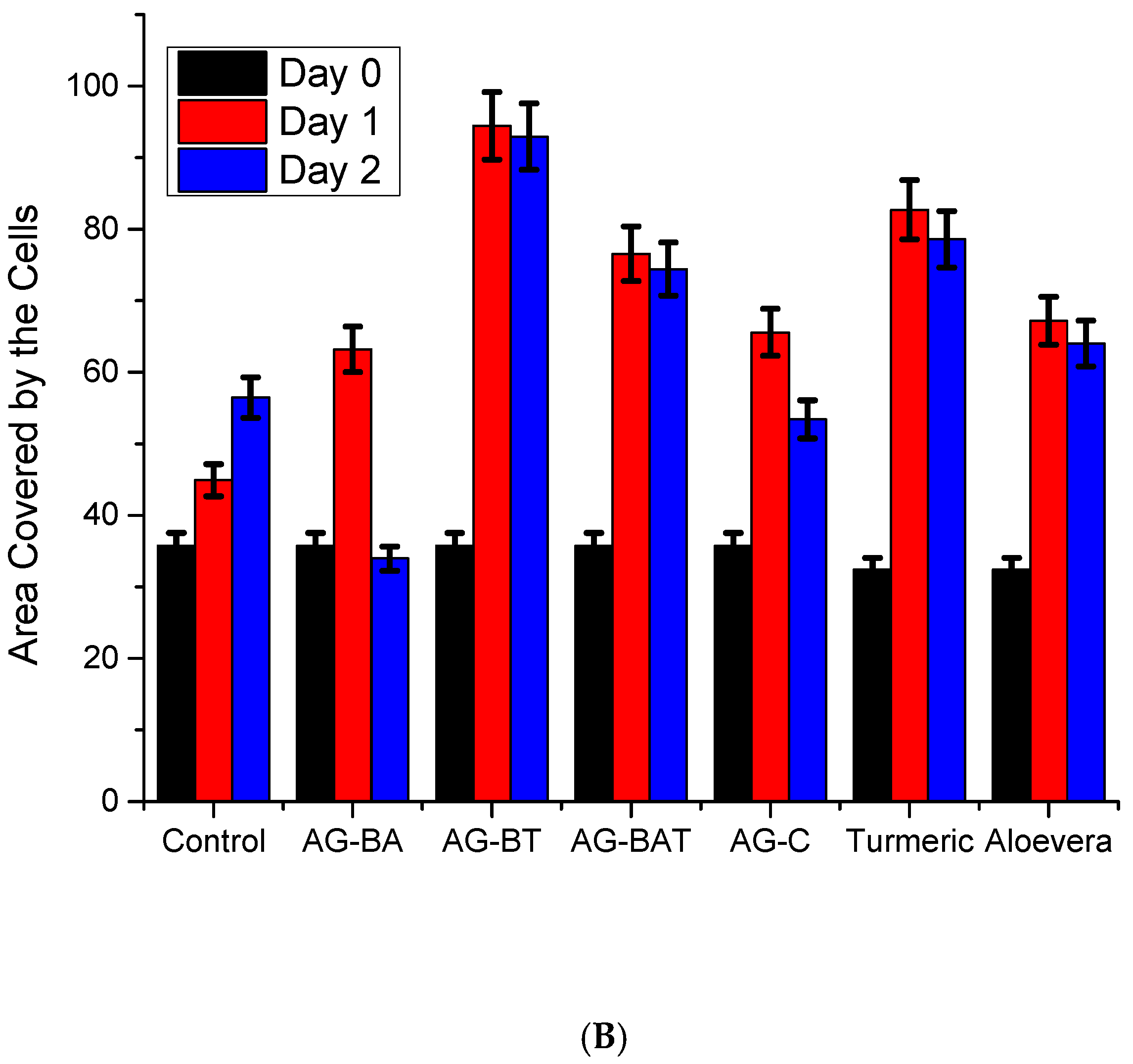
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
Analysis of Larval Development and Adult Eclosion
Climbing Assay
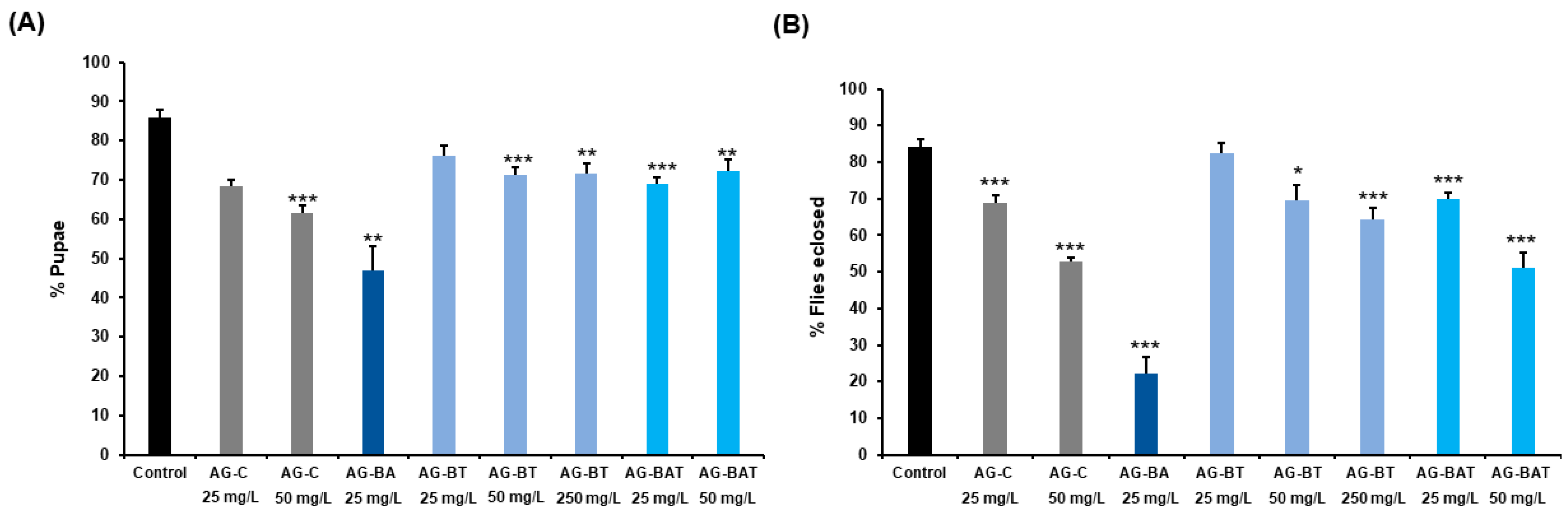
Pupation and Eclosion Rates of Flies Exposed to AgNPs
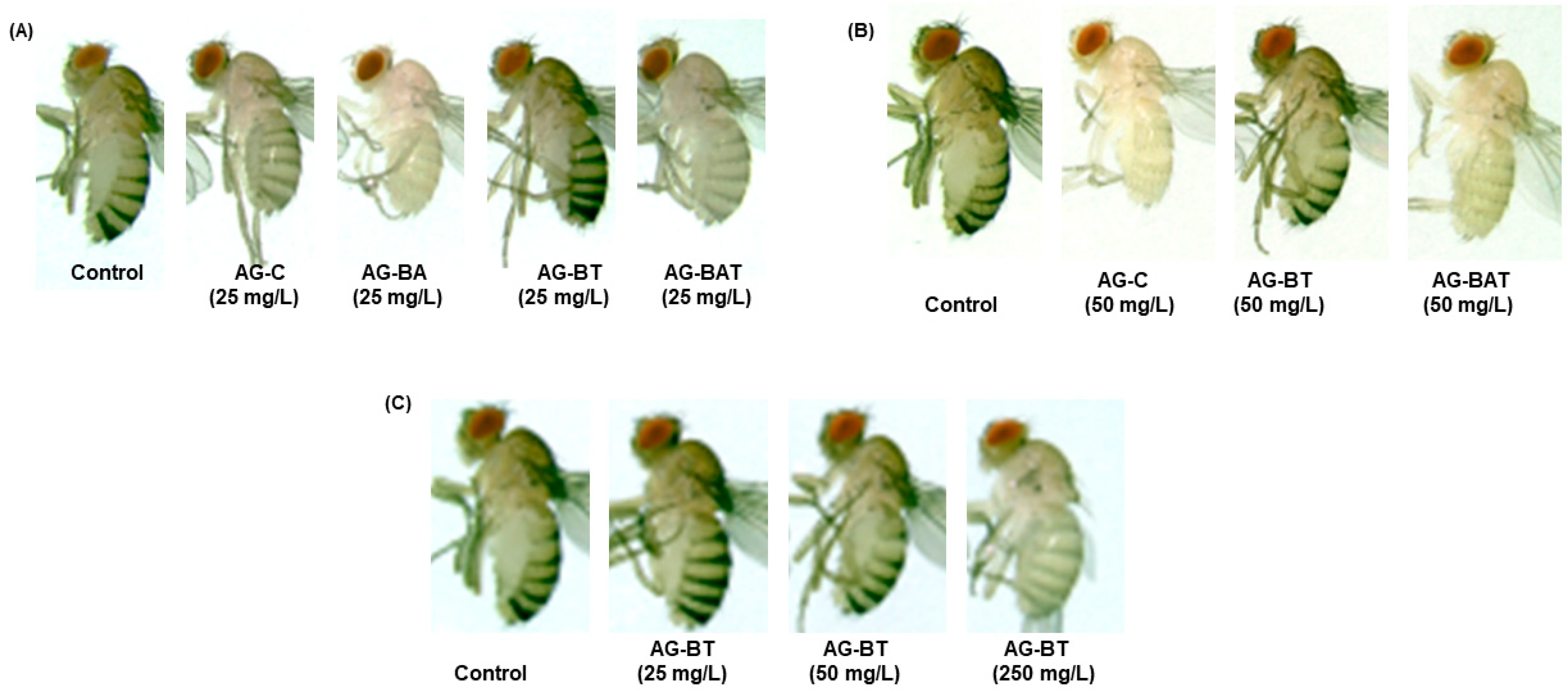
Cuticular Melanization of Flies Exposed to AgNPs
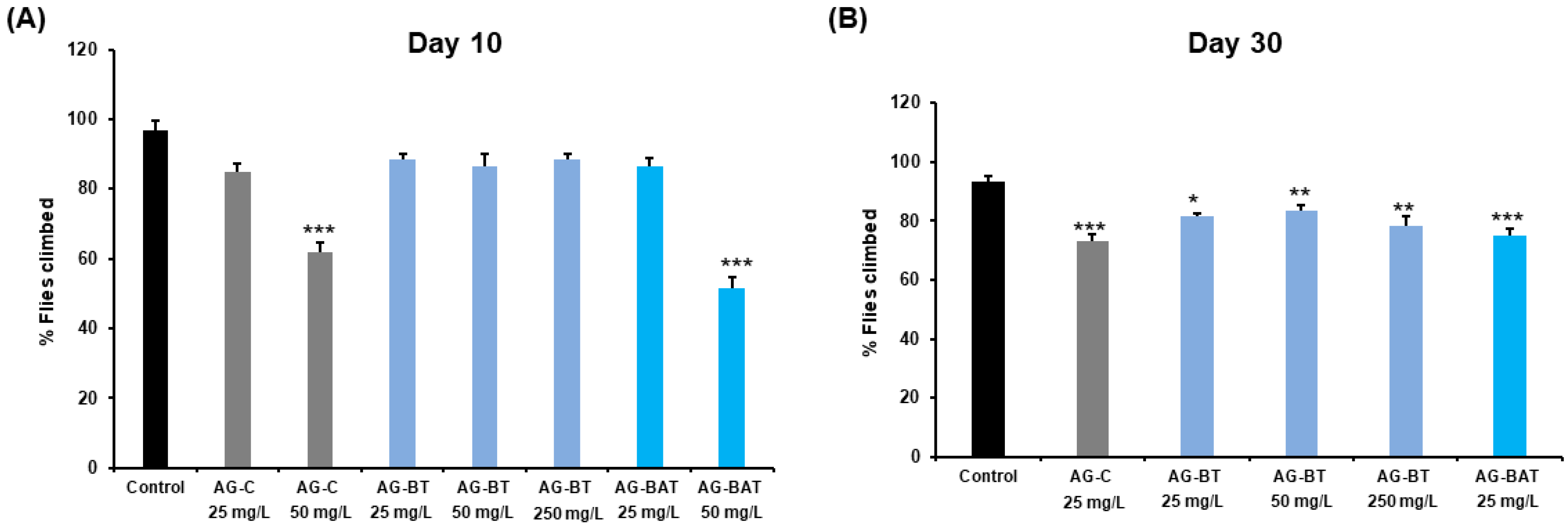
Impact of AgNPs on Degree of Climbing of Flies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rujido-Santos, I.; Herbello-Hermelo, P.; Barciela-Alonso, M.C.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Metal Content in Textile and (Nano)Textile Products. Int. J. Environ. Res. Public Health 2022, 19, 944. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Tavassoli, M.; Alizadeh Sani, M.; Mohammadi, K.; Ehsani, A.; McClements, D.J. Application of Nanotechnology to Improve the Performance of Biodegradable Biopolymer-Based Packaging Materials. Polymers 2021, 13, 4399. [Google Scholar] [CrossRef]
- Yilmaz, H.; Culha, M. A Drug Stability Study Using Surface-Enhanced Raman Scattering on Silver Nanoparticles. Appl. Sci. 2022, 12, 1807. [Google Scholar] [CrossRef]
- Balos, S.; Dramicanin, M.; Janjatovic, P.; Kulundzic, N.; Zabunov, I.; Pilic, B.; Klobcar, D. Influence of Metallic Oxide Nanoparticles on the Mechanical Properties of an A-TIG Welded 304L Austenitic Stainless Steel. Materials 2020, 13, 4513. [Google Scholar] [CrossRef]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef]
- Han, S.K. Basics of wound healing. In Innovations and Advances in Wound Healing; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–37. [Google Scholar]
- Naskar, A.; Kim, K.S. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Razzaq, A.; Khan, Z.U.; Saeed, A.; Shah, K.A.; Khan, N.U.; Menaa, B.; Iqbal, H.; Menaa, F. Development of Cephradine-Loaded Gelatin/Polyvinyl Alcohol Electrospun Nanofibers for Effective Diabetic Wound Healing: In-Vitro and In-Vivo Assessments. Pharmaceutics 2021, 13, 349. [Google Scholar] [CrossRef]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Uzair, B.; Menaa, F.; Braga, V.A. Fabrication, Physical Characterizations, and In Vitro, In Vivo Evaluation of Ginger Extract-Loaded Gelatin/Poly(Vinyl Alcohol) Hydrogel Films Against Burn Wound Healing in Animal Model. AAPS PharmSciTech 2020, 21, 323. [Google Scholar] [CrossRef]
- Shao, J.; Wang, B.; Li, J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antibacterial effect and wound healing ability of silver nanoparticles incorporation into chitosan-based nanofibrous membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Bhatnagar, S.; Ali, S.S.; Dutta, R. Phytofabrication of bioinduced silver nanoparticles for biomedical applications. Int. J. Nanomed. 2015, 10, 7019–7030. [Google Scholar] [CrossRef]
- Dutta, T.; Chowdhury, S.K.; Ghosh, N.N.; Chattopadhyay, A.P.; Das, M.; Mandal, V. Green synthesis of antimicrobial silver nanoparticles using fruit extract of Glycosmis pentaphylla and its theoretical explanations. J. Mol. Struct. 2022, 1247, 131361. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, D.; Song, C.G.; Kang, P.M. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 2015, 10, 2709–2723. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. ActaNaturae 2014, 6, 20. [Google Scholar] [CrossRef]
- Sood, K.; Kaur, J.; Singh, H.; Kumar Arya, S.; Khatri, M. Comparative toxicity evaluation of graphene oxide (GO) and zinc oxide (ZnO) nanoparticles on Drosophila melanogaster. Toxicol. Rep. 2019, 6, 768–781. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural sources of antioxidants–a review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mughal, M.H. Turmeric polyphenols: A comprehensive review. Integr. Food Nutr. Metab. 2019, 6, 1–6. [Google Scholar]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Raj, A.; Padmanabhan, A.; Shah, P.; Agrawal, N. Combating silver nanoparticle-mediated toxicity in Drosophila melanogaster with curcumin. J. Appl. Toxicol. JAT 2021, 41, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Neeru Bhagat, B.P. Synthesis of Antibacterial Oxide of Copper for Potential Application as Antifouling Agent. Curr. Nanosci. 2021, 17, 1–7. [Google Scholar]
- Jalal, M.R.; Hojjati, H.; Jalal, J.R.; Ebrahimi, S. Synthesis of Ag2O2 microparticles combined with Ag2CO3 nanoparticles by a pin-to-solution electrical discharge in atmospheric air. Mater. Lett. 2019, 251, 218–221. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Wang, S.-Y.; Li, J.; Zheng, Y.-X.; Zhang, R.-J.; Zhou, P.; Yang, Y.-M.; Chen, L.-Y. Study of structure and optical properties of silver oxide films by ellipsometry, XRD and XPS methods. Thin Solid Film. 2004, 455, 438–442. [Google Scholar] [CrossRef]
- Hammad, A.; Abdel-Wahab, M.; Alshahrie, A. Structural and morphological properties of sputtered silver oxide thin films: The effect of thin film thickness. Dig. J. Nanomater. Bios. 2016, 11, 1245–1252. [Google Scholar]
- Bielmann, M.; Schwaller, P.; Ruffieux, P.; Gröning, O.; Schlapbach, L.; Gröning, P. AgO investigated by photoelectron spectroscopy: Evidence for mixed valence. Phys. Rev. B 2002, 65, 235431. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Tsendzughul, N.T.; Ogwu, A.A. Physicochemical aspects of the mechanisms of rapid antimicrobial contact-killing by sputtered silver oxide thin films under visible light. ACS Omega 2019, 4, 16847–16859. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, T.C.; Droubay, T.; Chambers, S.A.; Bagus, P.S. Spectroscopic evidence for Ag (III) in highly oxidized silver films by X-ray photoelectron spectroscopy. J. Phys. Chem. C 2010, 114, 21562–21571. [Google Scholar] [CrossRef]
- Lützenkirchen-Hecht, D. Electrochemically grown silver oxide (Ag2O) by XPS. Surf. Sci. Spectra 2011, 18, 96–101. [Google Scholar] [CrossRef]
- Murray, B.; Newberg, J.; Walter, E.; Li, Q.; Hemminger, J.; Penner, R. Reversible resistance modulation in mesoscopic silver wires induced by exposure to amine vapor. Anal. Chem. 2005, 77, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraj, A.; Saoud, K.M.; Elmel, A.; Mansour, S.; Haik, Y. Optoelectronic properties of highly porous silver oxide thin film. SN Appl. Sci. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- de Jesús Ruíz-Baltazar, Á.; Reyes-López, S.Y.; de Lourdes Mondragón-Sánchez, M.; Estevez, M.; Hernández-Martinez, A.R.; Pérez, R. Biosynthesis of Ag nanoparticles using Cynara cardunculus leaf extract: Evaluation of their antibacterial and electrochemical activity. Results Phys. 2018, 11, 1142–1149. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Enhancement of antibacterial activity of capped silver nanoparticles in combination with antibiotics, on model gram-negative and gram-positive bacteria. Bioinorg. Chem. Appl. 2013, 2013, 871097. [Google Scholar] [CrossRef]
- Chircov, C.; Matei, M.-F.; Neacșu, I.A.; Vasile, B.S.; Oprea, O.-C.; Croitoru, A.-M.; Trușcă, R.-D.; Andronescu, E.; Sorescu, I.; Bărbuceanu, F. Iron oxide–silica core–shell nanoparticles functionalized with essential oils for antimicrobial therapies. Antibiotics 2021, 10, 1138. [Google Scholar] [CrossRef]
- Begum, S.; Jones, I.P.; Jiao, C.; Lynch, D.E.; Preece, J.A. Characterisation of hollow Russian doll microspheres. J. Mater. Sci. 2010, 45, 3697–3706. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316. [Google Scholar] [CrossRef]
- Vinken, M.; Blaauboer, B.J. In vitro testing of basal cytotoxicity: Establishment of an adverse outcome pathway from chemical insult to cell death. Toxicol. In Vitro 2017, 39, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Shah, P.; Agrawal, N. Dose-dependent effect of silver nanoparticles (AgNPs) on fertility and survival of Drosophila: An in-vivo study. PLoS ONE 2017, 12, e0178051. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Ramamoorthy, M.; Lyon, D.; Jones, K.; Duttaroy, A. Mechanism of silver nanoparticles action on insect pigmentation reveals intervention of copper homeostasis. PLoS ONE 2013, 8, e53186. [Google Scholar]
- Silver Key, S.C.; Reaves, D.; Turner, F.; Bang, J.J. Impacts of Silver Nanoparticle Ingestion on Pigmentation and Developmental Progression in Drosophila. Atlas J. Biol. 2011, 1, 52–61. [Google Scholar] [CrossRef][Green Version]
- Raj, A.; Shah, P.; Agrawal, N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Sci. Rep. 2017, 7, 15617. [Google Scholar] [CrossRef]
- Dlugosz, O.; Szostak, K.; Staron, A.; Pulit-Prociak, J.; Banach, M. Methods for Reducing the Toxicity of Metal and Metal Oxide NPs as Biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ma, J.; Jo, S.; Lee, S.; Kim, C.S. Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment. Nanomaterials 2020, 10, 938. [Google Scholar] [CrossRef]
- AshaRani, P.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- He, D.; Garg, S.; Waite, T.D. H2O2-mediated oxidation of zero-valent silver and resultant interactions among silver nanoparticles, silver ions, and reactive oxygen species. Langmuir 2012, 28, 10266–10275. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical Applications of Reactive Oxygen Species Generation by Metal Nanoparticles. Materials 2020, 14, 53. [Google Scholar] [CrossRef]
- Yun’an Qing, L.C.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311. [Google Scholar] [CrossRef]
- Almeer, R.S.; Ali, D.; Alarifi, S.; Alkahtani, S.; Almansour, M. Green Platinum Nanoparticles Interaction with HEK293 Cells: Cellular Toxicity, Apoptosis, and Genetic Damage. Dose-Response 2018, 16, 1559325818807382. [Google Scholar] [CrossRef]
- Ast, T.; Mootha, V.K. Oxygen and mammalian cell culture: Are we repeating the experiment of Dr. Ox? Nat. Metab. 2019, 1, 858–860. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Leso, V.; Bergamaschi, A. The effects of nanomaterials as endocrine disruptors. Int. J. Mol. Sci. 2013, 14, 16732–16801. [Google Scholar] [CrossRef]
- Mao, B.H.; Chen, Z.Y.; Wang, Y.J.; Yan, S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef]
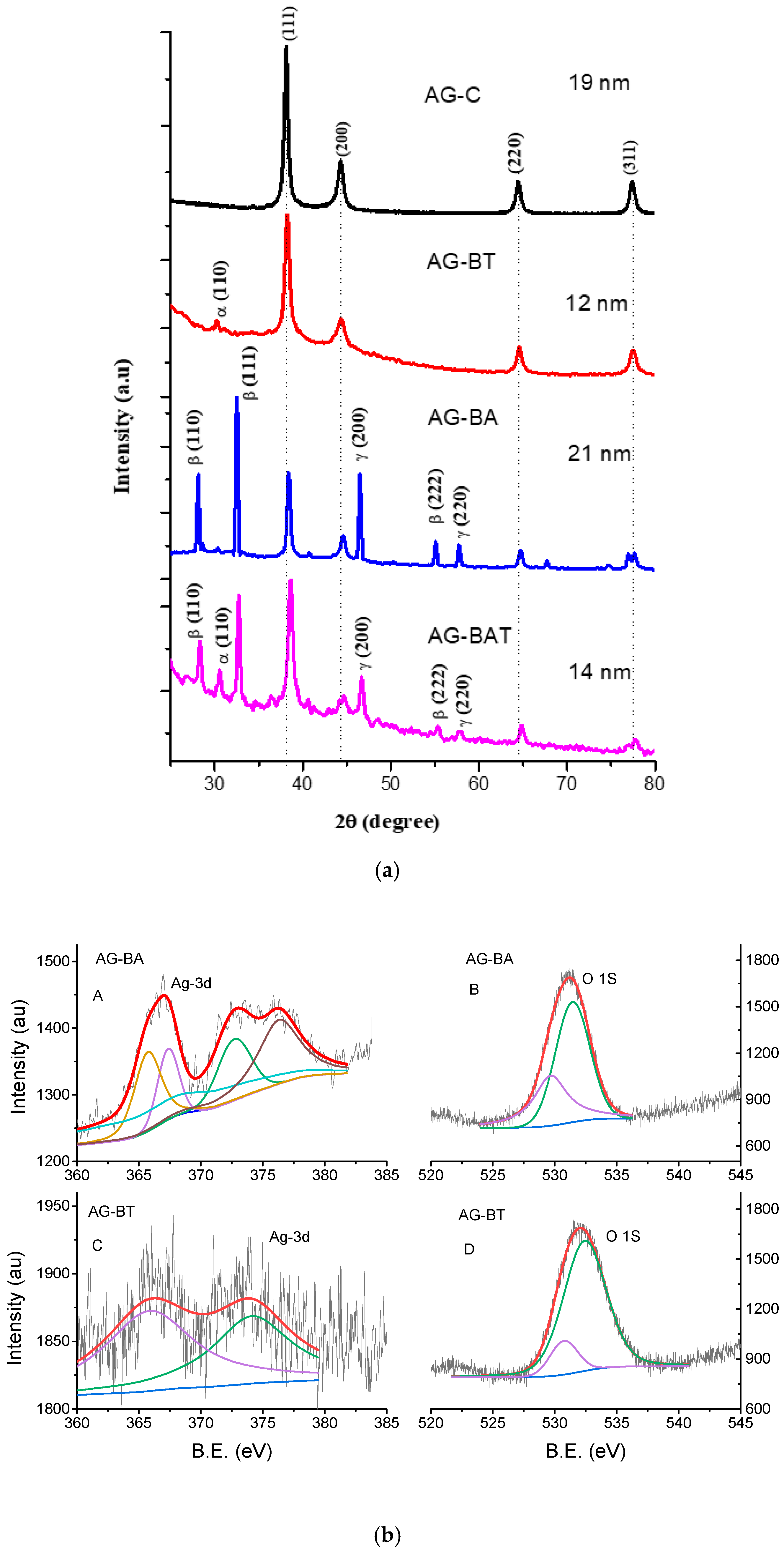

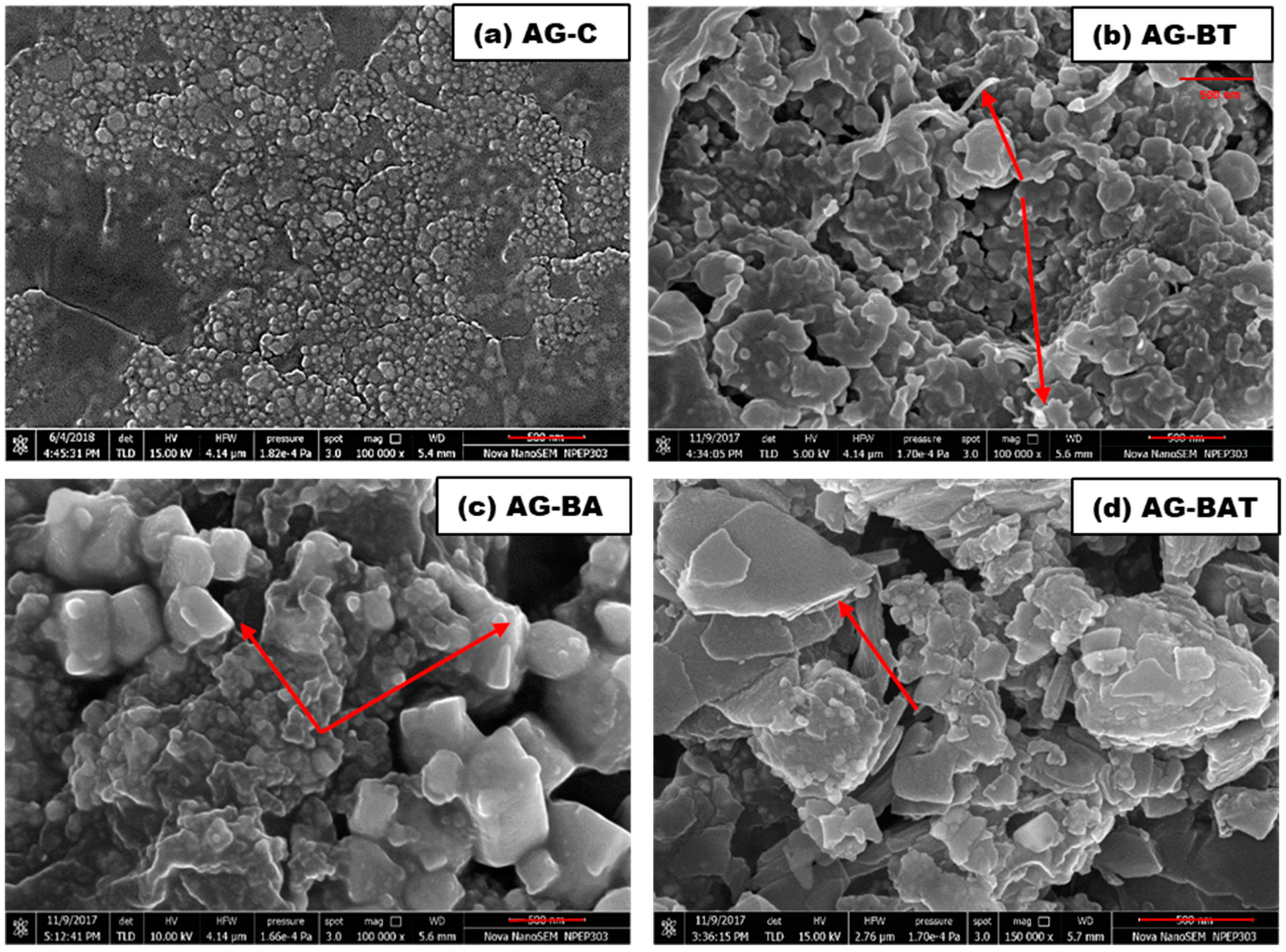
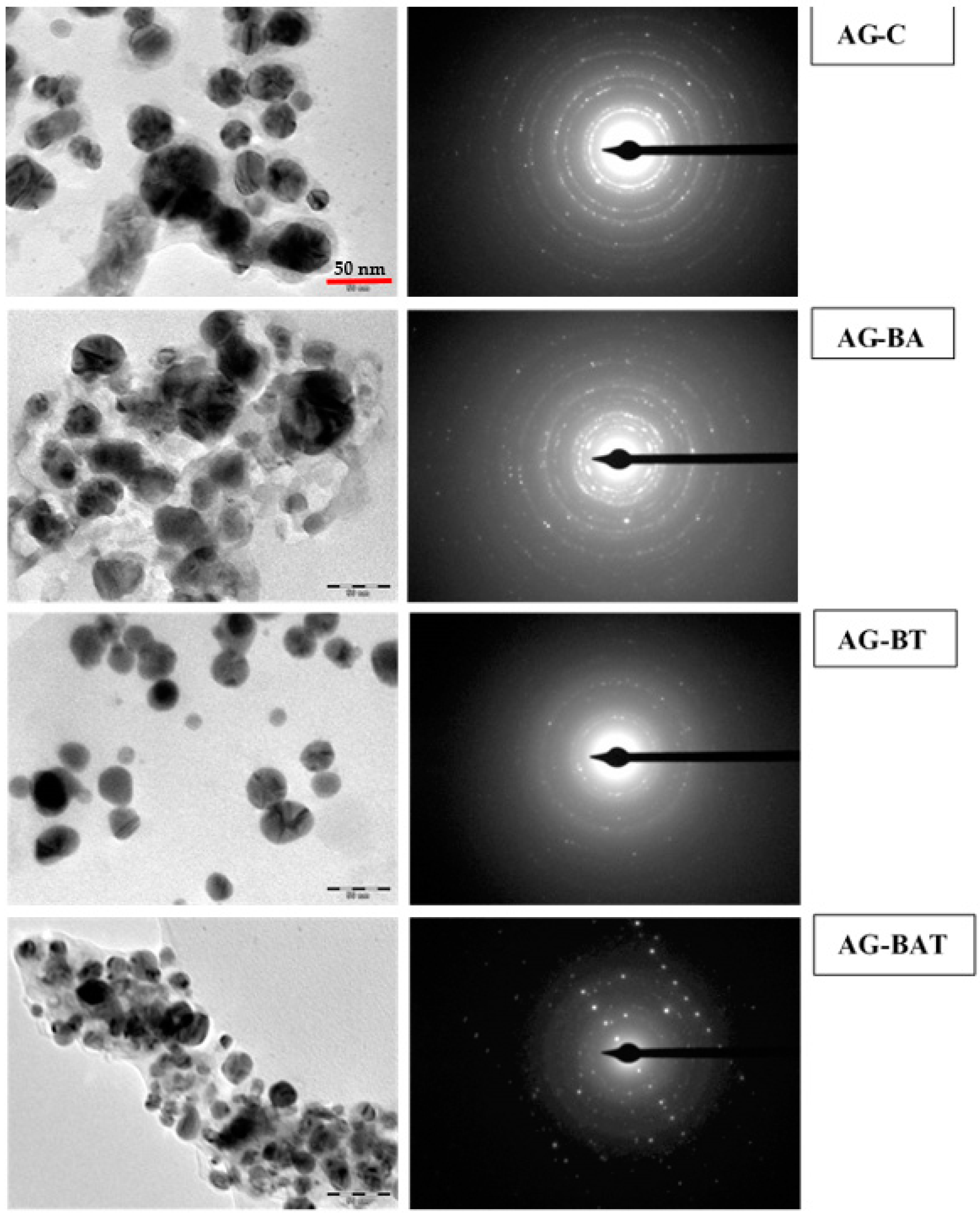

| Sample Name | Silver | Oxygen | Trace Elements |
|---|---|---|---|
| AG-C | 74.9 | 17.6 | 7.5 |
| AG-BT | 17.7 | 59.4 | 22.9 |
| AG-BA | 90.7 | 8.5 | 0.8 |
| AG-BAT | 15.5 | 77 | 7.5 |
| Sample | Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| AG-C (PVP AgNPs) | 124.4 | 0.425 | −4.84 |
| AG-BT (turmeric AgNPs) | 494.5 | 0.589 | −28.9 |
| AG-BAT (aloe vera–turmeric AgNPs) | 778.9 | 0.724 | −23.7 |
| AG-BA (aloe vera AgNPs) | 463.2 | 0.449 | −15.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, A.S.; Singh, A.; Edis, Z.; Haj Bloukh, S.; Shah, P.; Pandey, B.; Agrawal, N.; Bhagat, N. An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles. J. Funct. Biomater. 2022, 13, 54. https://doi.org/10.3390/jfb13020054
Desai AS, Singh A, Edis Z, Haj Bloukh S, Shah P, Pandey B, Agrawal N, Bhagat N. An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles. Journal of Functional Biomaterials. 2022; 13(2):54. https://doi.org/10.3390/jfb13020054
Chicago/Turabian StyleDesai, Anjana S., Akanksha Singh, Zehra Edis, Samir Haj Bloukh, Prasanna Shah, Brajesh Pandey, Namita Agrawal, and Neeru Bhagat. 2022. "An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles" Journal of Functional Biomaterials 13, no. 2: 54. https://doi.org/10.3390/jfb13020054
APA StyleDesai, A. S., Singh, A., Edis, Z., Haj Bloukh, S., Shah, P., Pandey, B., Agrawal, N., & Bhagat, N. (2022). An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles. Journal of Functional Biomaterials, 13(2), 54. https://doi.org/10.3390/jfb13020054









