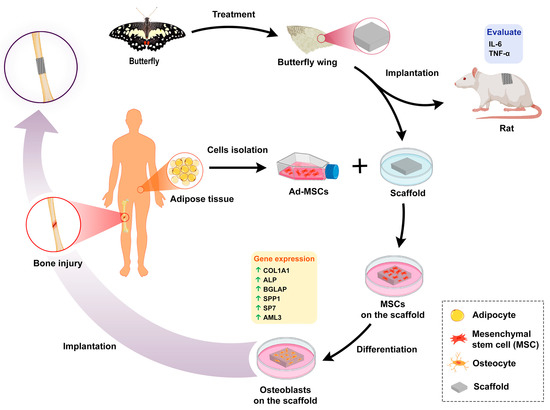
Crossing Phylums: Butterfly Wing as a Natural Perfusable Three-Dimensional (3D) Bioconstruct for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Materials
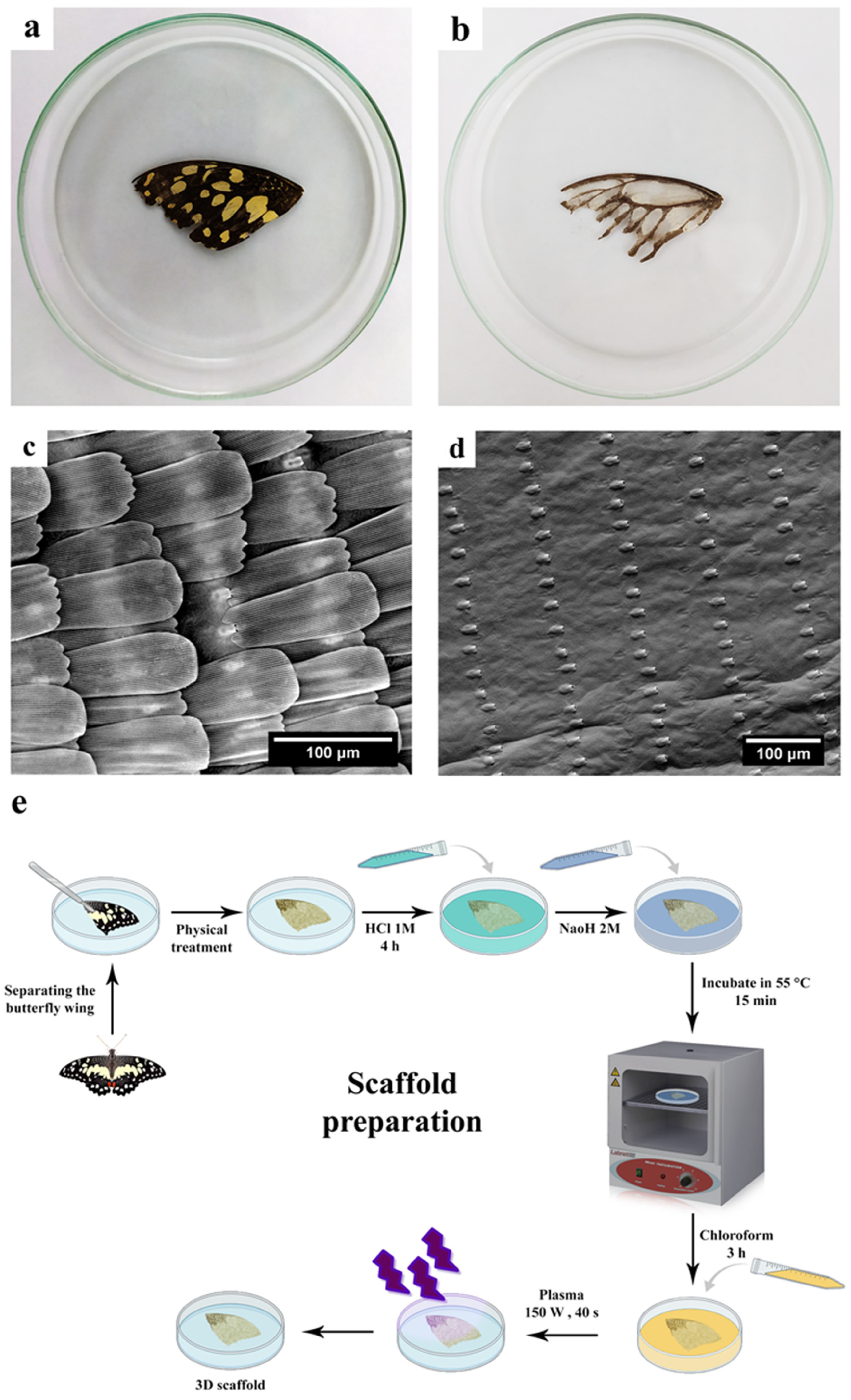
2.3. Scaffold Preparation
2.3.1. Physico-Chemical Treatment of the Butterfly Wings
2.3.2. Hydrophilizing the Butterfly Wings
2.4. Scanning Electron Microscopy
2.5. Atomic Force Microscopy (AFM)
2.6. Fourier Transform Infrared (FTIR) Analysis
2.7. Contact Angle Measurement
2.8. Surface Area and Pore Volume Determination
2.9. In Vivo Animal Tests
2.10. Cell Culture
2.11. Cytotoxicity
2.12. Biocompatibility Assays
2.12.1. DAPI Staining
2.12.2. Field Emission Scanning Electron Microscope (FE-SEM)
2.13. Alizarin Red-S Staining
2.14. Alkaline Phosphatase (ALP) Activity Measurement
2.15. Real-Time RT-PCR
2.16. Immunocytochemistry (ICC) Test
2.17. Statistical Analysis
3. Results
3.1. Scaffold Preparation
3.2. Scaffold Characterization
3.2.1. Morphology and Topography of the Scaffold
3.2.2. Atomic Force Microscopy (AFM) Analysis
3.2.3. Fourier Transform Infrared (FTIR) Spectra Analysis
3.2.4. Water Contact Angle
3.2.5. Brunauer–Emmett–Teller (BET) Analysis
3.3. In Vivo Experiments
3.4. Cell Proliferation and Viability
3.5. DAPI Staining
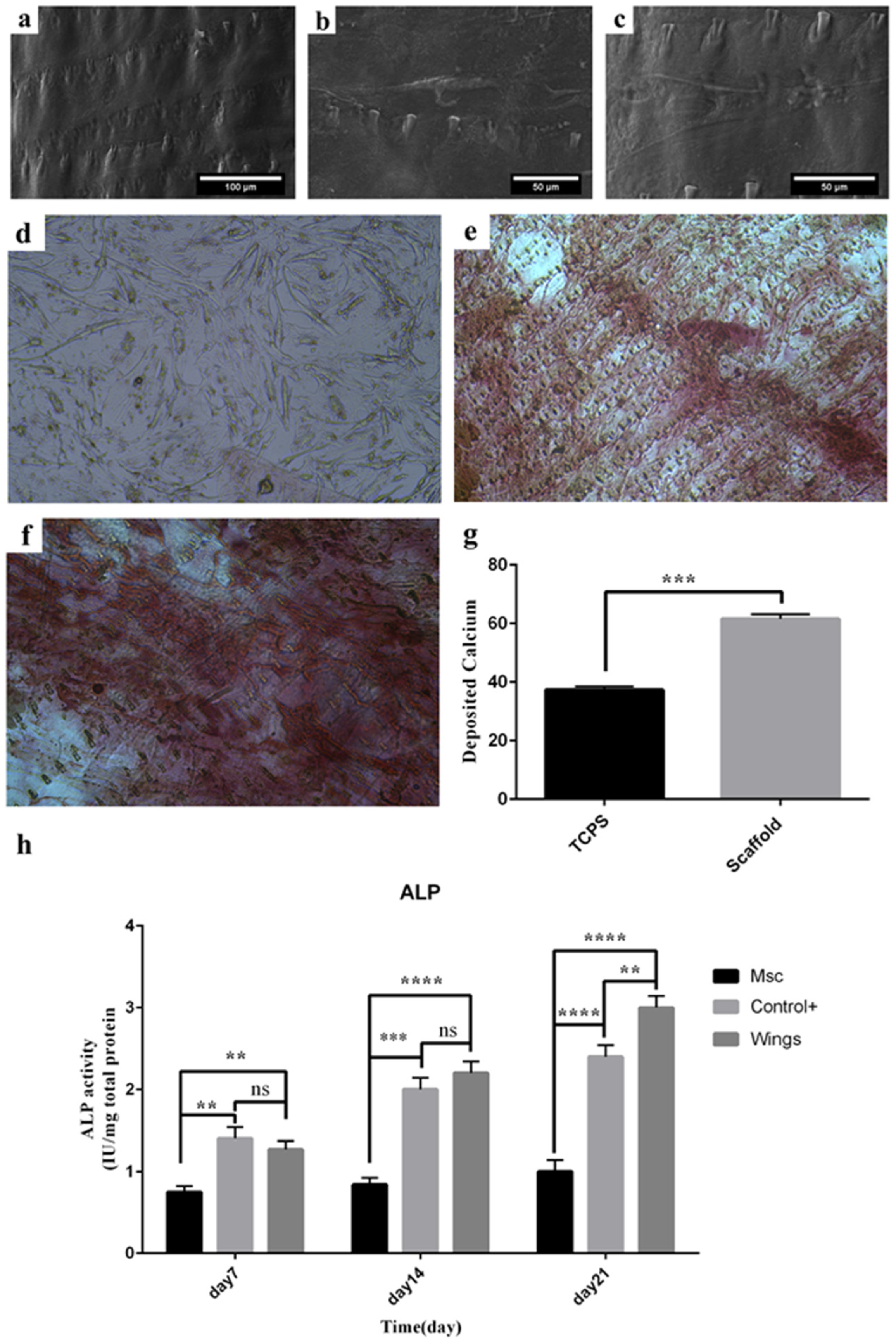
3.6. Cell Morphology
3.7. Calcium Deposition
3.8. Alkaline Phosphatase Activity
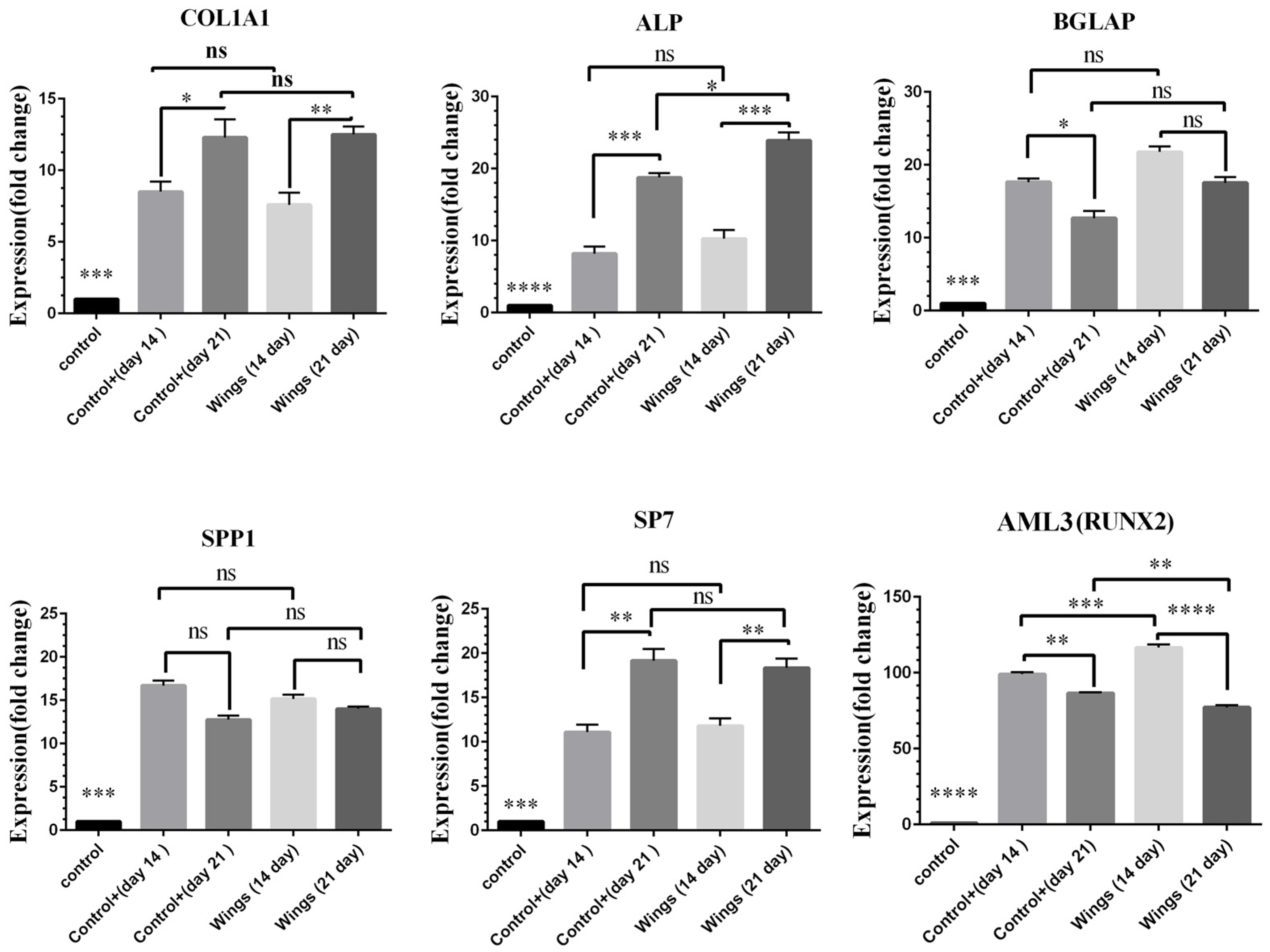
3.9. Gene Expression Analysis
3.10. Immunocytochemistry (ICC) Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Niinikoski, J.; Hunt, T. Oxygen and healing wounds: Tissue-bone repair enhancement. In Handbook on Hyperbaric Medicine; Springer: Berlin/Heidelberg, Germany, 1996; pp. 485–507. [Google Scholar] [CrossRef]
- Wiita, B. Osteoporosis: Causes and consequences. Curr. Sci. 1995, 68, 446–450. [Google Scholar]
- Woolf, A.D. The bone and joint decade 2000–2010. Ann. Rheum. Dis. 2000, 59, 81–82. [Google Scholar] [CrossRef] [Green Version]
- Moradi, S.L.; Golchin, A.; Hajishafieeha, Z.; Khani, M.M.; Ardeshirylajimi, A. Bone tissue engineering: Adult stem cells in combination with electrospun nanofibrous scaffolds. J. Cell. Physiol. 2018, 233, 6509–6522. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Lasanianos, N.G.; Kanakaris, N.K.; Giannoudis, P.V. Current management of long bone large segmental defects. Orthop. Trauma 2010, 24, 149–163. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Zadeh, J.K.; Shahri, N.M.; Abady, K.N.S.; Cheravi, M.; Baharara, J.; Rad, S.A.B.; Bahrami, A.R. Three-dimensional scaffold from decellularized human gingiva for cell cultures: Glycoconjugates and cell behavior. Cell J. 2013, 15, 166. [Google Scholar]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 2016, 71, 353–366. [Google Scholar] [CrossRef]
- He, Y.; Lu, F. Development of synthetic and natural materials for tissue engineering applications using adipose stem cells. Stem Cells Int. 2016, 2016, 5786257. [Google Scholar] [CrossRef] [Green Version]
- Surmenev, R.A.; Ivanov, A.N.; Cecilia, A.; Baumbach, T.; Chernozem, R.V.; Mathur, S.; Surmeneva, M.A. Electrospun composites of poly-3-hydroxybutyrate reinforced with conductive fillers for in vivo bone regeneration. Open Ceram. 2022, 9, 100237. [Google Scholar] [CrossRef]
- He, L.; Liu, B.; Xipeng, G.; Xie, G.; Liao, S.; Quan, D.; Cai, D.; Lu, J.; Ramakrishna, S. Microstructure and properties of nano-fibrous PCL-b-PLLA scaffolds for cartilage tissue engineering. Eur. Cell Mater. 2009, 18, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Fu, F.; Yu, Y.; Wang, H.; Shang, Y.; Zhao, Y. Cardiomyocytes-Actuated Morpho Butterfly Wings. Adv. Mater. 2019, 31, 1805431. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Gao, B.; He, Z.; Gu, Z. Hepatocyte aggregate formation on chitin-based anisotropic microstructures of butterfly wings. Biomimetics 2018, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Pan, Y.; Zhong, Q.; Liu, B. Guided cellular orientation concurrently with cell density gradient on butterfly wings. RSC Adv. 2019, 9, 25875–25879. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, A.; Lu, J.; Gao, B.; Zheng, F.; Mu, Z.; Zhao, Y.; Gu, Z. Chitin-based anisotropic nanostructures of butterfly wings for regulating cells orientation. Polymers 2017, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Nwe, N.; Furuike, T.; Tamura, H. The mechanical and biological properties of chitosan scaffolds for tissue regeneration templates are significantly enhanced by chitosan from Gongronella butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef] [Green Version]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Jahangirian, H.; Azizi, S.; Rafiee-Moghaddam, R.; Baratvand, B.; Webster, T.J. Status of plant protein-based green scaffolds for regenerative medicine applications. Biomolecules 2019, 9, 619. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Kuboki, Y.; Miyata, T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol. Bioeng. 2006, 95, 404–411. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Cabbage-derived three-dimensional cellulose scaffold-induced osteogenic differentiation of stem cells. J. Cell. Physiol. 2020, 236, 5306–5316. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Efficient mineralization and osteogenic gene overexpression of mesenchymal stem cells on decellularized spinach leaf scaffold. Gene 2020, 757, 144852. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Evangelopoulou, M.; Casellas, C.P.; Abrishamcar, S.; Jansen, J.; Vermonden, T.; Masereeuw, R. Spinach and Chive for Kidney Tubule Engineering: The Limitations of Decellularized Plant Scaffolds and Vasculature. AAPS J. 2021, 23, 11. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jung, H.; Park, N.; Park, S.-H.; Ju, J.H. Induced osteogenesis in plants decellularized scaffolds. Sci. Rep. 2019, 9, 20194. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-K.; Shen, C.-R.; Yeh, C.-H.; Fang, B.-S.; Huang, T.-L.; Liu, C.-L. N-acetyl glucosamine obtained from chitin by chitin degrading factors in Chitinbacter tainanesis. Int. J. Mol. Sci. 2011, 12, 1187–1195. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Badawy, R.M.; Mohamed, H.I. Chitin extration, composition of different six insect species and their comparable characteristics with that of the shrimp. J. Am. Sci. 2015, 11, 127–134. [Google Scholar]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, M.; Luan, C.-C.; Wang, Y.; Gu, X.; He, J.-H. Biocompatibility and biosafety of butterfly wings for the clinical use of tissue-engineered nerve grafts. Neural Regen. Res. 2021, 16, 1606. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Kaya, M.; Akata, I. Chitin extraction and chitosan production from cell wall of two mushroom species (Lactarius vellereus and Phyllophora ribis). In Proceedings of the AIP Conference Proceedings, İstanbul, Turkey, 1–3 June 2016; p. 020012. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Sargin, I.; Sabeckis, I.; Noreikaite, D.; Erdonmez, D.; Salaberria, A.M.; Labidi, J.; Baublys, V.; Tubelytė, V. Biological, mechanical, optical and physicochemical properties of natural chitin films obtained from the dorsal pronotum and the wing of cockroach. Carbohydr. Polym. 2017, 163, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Machałowski, T.; Rusak, A.; Wiatrak, B.; Haczkiewicz-Leśniak, K.; Popiel, A.; Jaroszewicz, J.; Żak, A.; Podhorska-Okołów, M.; Jesionowski, T. Naturally Formed Chitinous Skeleton Isolated from the Marine Demosponge Aplysina fistularis as a 3D Scaffold for Tissue Engineering. Materials 2021, 14, 2992. [Google Scholar] [CrossRef]
- Shaala, L.A.; Asfour, H.Z.; Youssef, D.T.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K. New source of 3D chitin scaffolds: The Red Sea demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of chitosan and its oligomers from shrimp shell waste, their characterization and antimicrobial effect. Vet. World 2017, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Ziętek, J.; Pantović, S.; Voronkina, A.; Kovalchuk, V. 3D chitin scaffolds of marine demosponge origin for biomimetic mollusk hemolymph-associated biomineralization ex-vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [Green Version]
- Zamani, F.; Merati, A.; Latifi, M.; Ghanbari Alanagh, H.; Nadipour, F. Effect of Plasma Treatment on Cell Culture in Poly Lactic Glycolic Acid Nanofibrous Scaffold. Modares J. Biotechnol. 2018, 9, 635–641. [Google Scholar]
- Gelb, L.D.; Gubbins, K. Characterization of porous glasses: Simulation models, adsorption isotherms, and the Brunauer− Emmett− Teller analysis method. Langmuir 1998, 14, 2097–2111. [Google Scholar] [CrossRef]
- Naderi, M. Surface Area: Brunauer–Emmett–Teller (BET). In Progress in Filtration and Separation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 585–608. [Google Scholar] [CrossRef]
- Azadian, Z.; Shafiei, M.; Hosseini, S.; Kazemi, J.; Alipour, A.; Shahsavarani, H. Efficient In Vitro Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells Into the Cardiomyocyte Using Plant-Derived Natural Compounds. Proc. Singap. Natl. Acad. Sci. 2019, 13, 47–63. [Google Scholar] [CrossRef]
- Palpandi, C.; Shanmugam, V.; Shanmugam, A. Extraction of chitin and chitosan from shell and operculum of mangrove gastropod Nerita (Dostia) crepidularia Lamarck. Int. J. Med. Med. Sci. 2009, 1, 198–205. [Google Scholar]
- Jalal, A.; Risheed, C.; Ibrahim, B. Optimization of chitin extraction from chicken feet. J. Anal. Bioanal. Tech. 2012, 3, 145. [Google Scholar] [CrossRef]
- Sun, G.; Fang, Y.; Cong, Q.; Ren, L.-q. Anisotropism of the non-smooth surface of butterfly wing. J. Bionic Eng. 2009, 6, 71–76. [Google Scholar] [CrossRef]
- Kholghi-Eshkalak, L.; Jalali Sendi, J.; Karimi-Malati, A.; Zibaee, A. Life table parameters and biological characteristics of citrus butterfly Papilio demoleus (Lepidoptera: Papilionidae) on various citrus hosts. J. Crop Prot. 2017, 6, 315–325. [Google Scholar]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Natural cellulose-based scaffold for improvement of stem cell osteogenic differentiation. J. Drug Deliv. Sci. Technol. 2021, 63, 102453. [Google Scholar] [CrossRef]
- Uemura, T.; Dong, J.; Wang, Y.; Kojima, H.; Saito, T.; Iejima, D.; Kikuchi, M.; Tanaka, J.; Tateishi, T. Transplantation of cultured bone cells using combinations of scaffolds and culture techniques. Biomaterials 2003, 24, 2277–2286. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, L.; Tang, S.; Wen, W.; Lu, L.; Liu, M.; Zhou, C.; Luo, B. Fabrication and evaluation of a chitin whisker/poly (L-lactide) composite scaffold by the direct trisolvent-ink writing method for bone tissue engineering. Nanoscale 2020, 12, 18225–18239. [Google Scholar] [CrossRef]
- Thomas, M.; Arora, A.; Katti, D.S. Surface hydrophilicity of PLGA fibers governs in vitro mineralization and osteogenic differentiation. Mater. Sci. Eng. C 2014, 45, 320–332. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Khan, S.P.; Auner, G.G.; Newaz, G.M. Influence of nanoscale surface roughness on neural cell attachment on silicon. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Di Medio, L.; Brandi, M.L. Advances in bone turnover markers. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 105, pp. 101–140. [Google Scholar]
- Kumar, S.; Maurya, R. Plant Drugs in the Treatment of Osteoporosis. In Natural Products and Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 179–212. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, L.; Tang, L.; Zhang, X.; Yang, L.; Zheng, K.; He, A.; Boccaccini, A.R.; Wei, J.; Zhao, J. Highly dispersed lithium doped mesoporous silica nanospheres regulating adhesion, proliferation, morphology, ALP activity and osteogenesis related gene expressions of BMSCs. Colloids Surf. B Biointerfaces 2018, 170, 563–571. [Google Scholar] [CrossRef]
- Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, G.; Cartmell, S. Genes and proteins involved in the regulation of osteogenesis. Top. Tissue Eng. 2007, 3, 1–22. [Google Scholar]
- Santos, E.; Hernández, R.M.; Pedraz, J.L.; Orive, G. Novel advances in the design of three-dimensional bio-scaffolds to control cell fate: Translation from 2D to 3D. Trends Biotechnol. 2012, 30, 331–341. [Google Scholar] [CrossRef]




| Genes | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) | Product Length (bp) |
|---|---|---|---|
| COL1A1 | CATCTCCCCTTCGTTTTTGAC | CCAAATCCGATGTTTCTGCTG | 121 |
| ALP | ACCATTCCCACGTCTTCACAT | GACATTCTCTCGTTCACCGC | 161 |
| BGLAP | TCACACTCCTCGCCCTATTG | CTCTTCACTACCTCGCTGCC | 133 |
| SPP1 | GAGGTGATGTCCTCGTCTGTAG | CACATATGATGGCCGAGGTG | 111 |
| SP7 | TACCCCATCTCCCTTGACTG | GCTGCAAGCTCTCCATAACC | 110 |
| AML3 | AGATGATGACACTGCCACCTC | GGGATGAAATGCTTGGGAACT | 125 |
| GAPDH | ACCCACTCCTCCACCTTTG | CTCTTGTGCTCTTGCTGGG | 178 |
| r-IL-6 | TCCTACCCCAACTTCCAATGC | GTTTGCCGAGTAGACCTCAT | 138 |
| r-GAPDH | GGCAAGTTCAACGGCACAG | CGCCAGTAGACTCCACGAC | 142 |
| Scaffold | as,BET (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Butterfly wing | 20.073 | 0.1461 | 29.119 |
| Sample | TNF-α (pg/mL) | IL-6 (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Day1 | Day7 | Day14 | Day21 | Day1 | Day7 | Day14 | Day21 | |
| Control a | 25.1 | 27.1 | 26.3 | 25.7 | 15.4 | 17.2 | 16.1 | 15.9 |
| Scaffold b | 37.1 | 33.8 | 29.9 | 26.5 | 25.1 | 24.7 | 19.9 | 17.1 |
| Significant (p ≤ 0.05) | **** | *** | * | ns | *** | *** | * | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostofi, F.; Mostofi, M.; Niroomand, B.; Hosseini, S.; Alipour, A.; Homaeigohar, S.; Mohammadi, J.; Shokrgozar, M.A.; Shahsavarani, H. Crossing Phylums: Butterfly Wing as a Natural Perfusable Three-Dimensional (3D) Bioconstruct for Bone Tissue Engineering. J. Funct. Biomater. 2022, 13, 68. https://doi.org/10.3390/jfb13020068
Mostofi F, Mostofi M, Niroomand B, Hosseini S, Alipour A, Homaeigohar S, Mohammadi J, Shokrgozar MA, Shahsavarani H. Crossing Phylums: Butterfly Wing as a Natural Perfusable Three-Dimensional (3D) Bioconstruct for Bone Tissue Engineering. Journal of Functional Biomaterials. 2022; 13(2):68. https://doi.org/10.3390/jfb13020068
Chicago/Turabian StyleMostofi, Fatemeh, Marzieh Mostofi, Behnaz Niroomand, Saadi Hosseini, Atefeh Alipour, Shahin Homaeigohar, Javad Mohammadi, Mohammad Ali Shokrgozar, and Hosein Shahsavarani. 2022. "Crossing Phylums: Butterfly Wing as a Natural Perfusable Three-Dimensional (3D) Bioconstruct for Bone Tissue Engineering" Journal of Functional Biomaterials 13, no. 2: 68. https://doi.org/10.3390/jfb13020068
APA StyleMostofi, F., Mostofi, M., Niroomand, B., Hosseini, S., Alipour, A., Homaeigohar, S., Mohammadi, J., Shokrgozar, M. A., & Shahsavarani, H. (2022). Crossing Phylums: Butterfly Wing as a Natural Perfusable Three-Dimensional (3D) Bioconstruct for Bone Tissue Engineering. Journal of Functional Biomaterials, 13(2), 68. https://doi.org/10.3390/jfb13020068