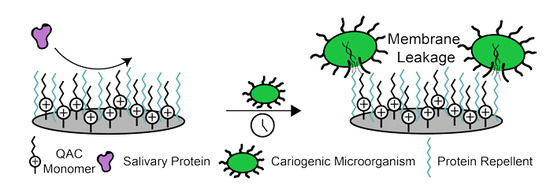
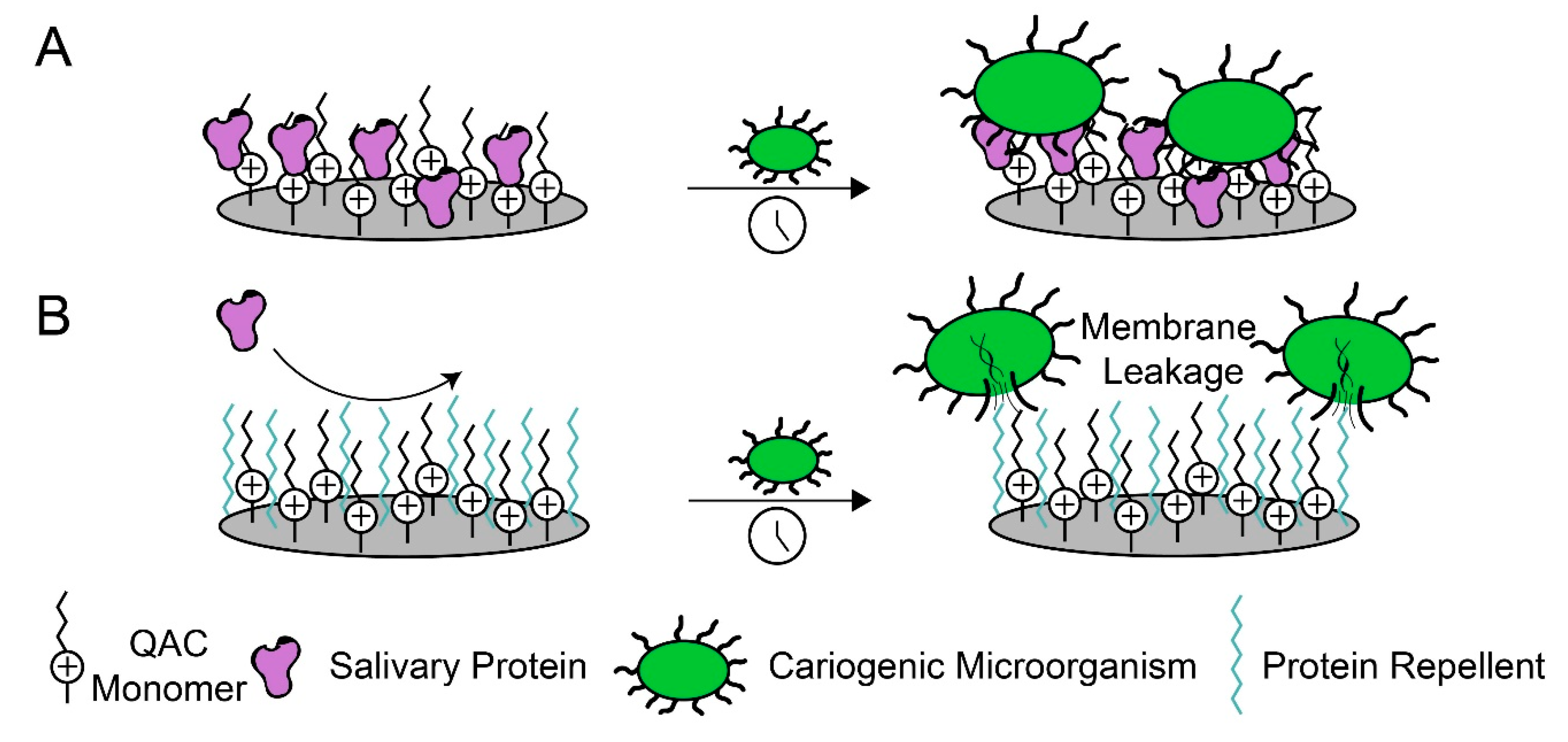
Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials
Abstract
1. Introduction
2. Theoretical and Practical Considerations for Protein Adsorption
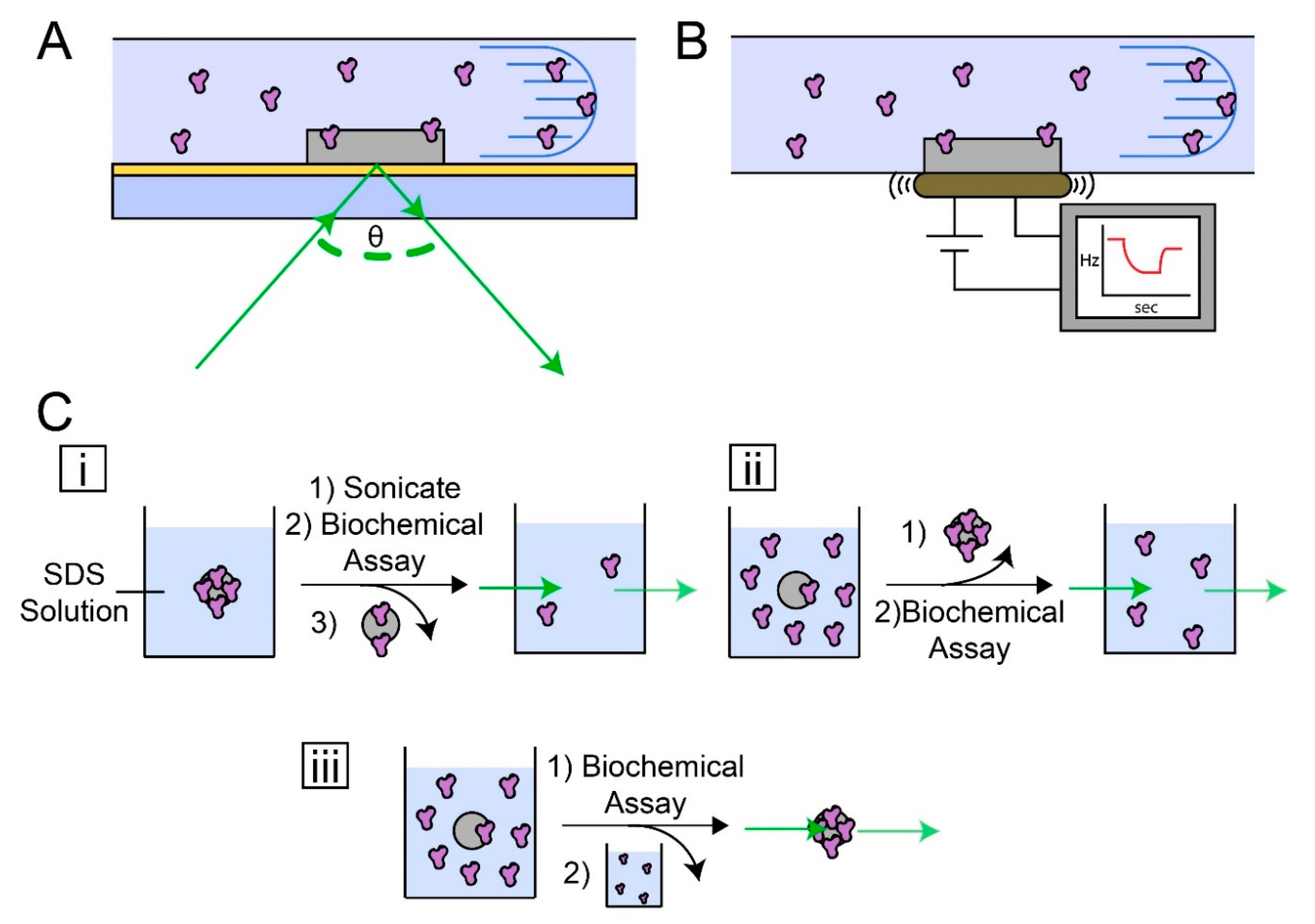
3. Characterization Methods for Quantifying Protein Adsorption
4. Dental Materials with Protein-Repellent Functionality
5. Mouthwash Coating Technology
| Protein Repellent Compound | Bulk Material | Filler | Adsorption Value (ng/cm2) | Quantification Method | Reference |
|---|---|---|---|---|---|
| 3% MPC (w/w) | 25.5% 1:1 BisGMA/TEGDMA | 70% Barium boroaluminosilicate | 1240 | SDS removal + BCA Assay | [58] |
| 3% MPC | 27% 1:1 BisGMA/TEGDMA | 70% Barium boroaluminosilicate | 960 | SDS removal + BCA Assay | [66] |
| 7.5% MPC | 75% 1:1 Scotchbond Multi-Purpose Primer and Adhesive | 15% Amorphous calcium phosphate | 321 | SDS removal + BCA Assay | [68] |
| 3% MPC | 25.5% 50:50 BisGMA/TEGDMA | 70% Barium boroaluminosilicate | 972 (with 180 days water aging) | SDS removal + BCA Assay | [69] |
| 3% MPC | 24% 1:1 EBPM | 20% Amorphous calcium phosphate; 50% barium boroaluminosilicate | 1200 | SDS removal + BCA Assay | [70] |
| 3% MPC | 44.5% PMGDM, 39.5% EBPADMA, 10% 2-hydroxyethyl methacrylate, 5% BisGMA | 30% Amorphous calcium phosphate | 416 | SDS removal + BCA Assay | [72] |
| 3% MPC | 47.75% Nature CrylTM liquid | 47.75% Nature CrylTM powder | 2150 | SDS removal + BCA Assay | [74] |
| 3% MPC | 24% 1:1 EBPM | 20% NACP, 50% barium boroaluminosilicate | 1000 | SDS removal + BCA Assay | [76] |
| PEG | Self-assembled PEG lysozyme | N/A | 8 | QCM | [53] |
| 33% trimethylamine N-oxide Zwitterionic Hydrogel | N/A | N/A | 3 * | SPR | [80] |
| 9% Poly(carboxybetaine acrylamide) Zwitterionic Hydrogel | N/A | N/A | 4.3 * | SPR | [49] |
6. Limitations of Existing Technologies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Sugars and Dental Caries. Available online: https://www.who.int/news-room/fact-sheets/detail/sugars-and-dental-caries (accessed on 15 June 2020).
- Venhoven, B.A.M.; de Gee, A.J.; Davidson, C.L. Polymerization contraction and conversion of light-curing BisGMA-based methacrylate resins. Biomaterials 1993, 14, 871–875. [Google Scholar] [CrossRef]
- O’Donnell, J.N.R.; Skrtic, D. Degree of Vinyl Conversion, Polymerization Shrinkage and Stress Development in Experimental Endodontic Composite. J. Biomim. Biomater. Tissue Eng. 2009, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Yu, J.; Sun, Y.; Xie, W. Study of POSS on the Properties of Novel Inorganic Dental Composite Resin. Polymers 2020, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Sun, Y.; Xie, W. POSS Dental Nanocomposite Resin: Synthesis, Shrinkage, Double Bond Conversion, Hardness, and Resistance Properties. Polymers 2018, 10, 369. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J. Res. Natl. Inst. Stand. Technol. 2003, 108, 167. [Google Scholar] [CrossRef]
- Tanaka, J.; Inoue, K.; Masamura, H.; Matsumura, K.; Najai, H.; Inoue, K. The Application of Fluorinated Aromatic Dimethacrylates to Experimental Light-cured Radiopaque Composite Resin, Containing Barium-Borosilicate Glass Filler—A Progress in Nonwaterdegradable Properties. Dent. Mater. J. 1993, 12, 1–11. [Google Scholar] [CrossRef]
- Arcís, R.W.; López-Macipe, A.; Toledano, M.; Osorio, E.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.A.; Pascual, C.D. Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dent. Mater. 2002, 18, 49–57. [Google Scholar] [CrossRef]
- Kuper, N.K.; van de Sande, F.H.; Opdam, N.J.M.; Bronkhorst, E.M.; de Soet, J.J.; Cenci, M.S.; Huysmans, M.C.D.J.N.M. Restoration Materials and Secondary Caries Using an In Vitro Biofilm Model. J. Dent. Res. 2015, 94, 62–68. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef]
- Imazato, S.; Torri, M.; Tsuchitani, Y. Immobilization of an antibacterial component in composite resin. Dent. Jpn. 1993, 30, 63–68. [Google Scholar]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R.B. Incorporation of Bacterial Inhibitor into Resin Composite. J. Dent. Res. 1994. [Google Scholar] [CrossRef]
- Gottenbos, B.; van der Mei, H.C.; Klatter, F.; Nieuwenhuis, P.; Busscher, H.J. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar] [CrossRef]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on Antibacterial Bonding Agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef]
- Gozzelino, G.; Lisanti, C.; Beneventi, S. Quaternary ammonium monomers for UV crosslinked antibacterial surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 21–28. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of Methacrylate Monomers with Antibacterial Effects Against S. Mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef]
- Bienek, D.R.; Giuseppetti, A.A.; Okeke, U.C.; Frukhtbeyn, S.A.; Dupree, P.J.; Khajotia, S.S.; Florez, F.L.E.; Hiers, R.D.; Skrtic, D. Antimicrobial, biocompatibility, and physicochemical properties of novel adhesive methacrylate dental monomers. J. Bioact. Compat. Polym. 2020. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.; Frukhtbeyn, S.; Giuseppetti, A.; Okeke, U.; Skrtic, D. Antimicrobial Monomers for Polymeric Dental Restoratives: Cytotoxicity and Physicochemical Properties. JFB 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Parolia, A.; Elkezza, A.; Yiu, C.K.; Abbott, P.; Matinlinna, J.P.; Fawzy, A.S. An in vitro study of a novel quaternary ammonium silane endodontic irrigant. Dent. Mater. 2019, 35, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.R.; Giuseppetti, A.A.; Frukhtbeyn, S.A.; Hiers, R.D.; Esteban Florez, F.L.; Khajotia, S.S.; Skrtic, D. Physicochemical, Mechanical, and Antimicrobial Properties of Novel Dental Polymers Containing Quaternary Ammonium and Trimethoxysilyl Functionalities. JFB 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Matinlinna, J.P.; Pichika, M.R.; Mak, K.-K.; Nagendrababu, V.; Fawzy, A.S. A quaternary ammonium silane antimicrobial triggers bacterial membrane and biofilm destruction. Sci. Rep. 2020, 10, 10970. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef]
- Ten Cate, J.M. Biofilms, a new approach to the microbiology of dental plaque. Odontology 2006, 94, 1–9. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71. [Google Scholar] [CrossRef]
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial Interactions in Dental Biofilm Development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Hay, D.I. Adsorbed Salivary Acidic Proline-rich Proteins Contribute to the Adhesion of Streptococcus mutans JBP to Apatitic Surfaces. J. Dent. Res. 1989, 68, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.E.P.; Laurence, J.S. Proteins, Pathogens, and Failure at the Composite-Tooth Interface. J. Dent. Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofim in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. [Google Scholar]
- Castagnola, M.; Scarano, E.; Passali, G.C.; Messana, I.; Cabras, T.; Iavarone, F.; Cintio, G.D.; Fiorita, A.; Corso, E.D.; Paludetti, G. Salivary biomarkers and proteomics: Future diagnostic and clinical utilities. Acta Otorhinolaryngolog. Italica 2017, 37, 94. [Google Scholar]
- McPherson, T.B.; Lee, S.J.; Park, K. Analysis of the Prevention of Protein Adsorption by Steric Repulsion Theory. In Proteins at Interfaces II; Horbett, T.A., Brash, J.L., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1995; Volume 602, pp. 395–404. ISBN 978-0-8412-3304-1. [Google Scholar]
- Müller, C.; Wald, J.; Hoth-Hannig, W.; Umanskaya, N.; Scholz, D.; Hannig, M.; Ziegler, C. Protein adhesion on dental surfaces—A combined surface analytical approach. Anal. Bioanal. Chem. 2011, 400, 679–689. [Google Scholar] [CrossRef]
- Tilton, R.D.; Robertson, C.R.; Gast, A.P. Manipulation of hydrophobic interactions in protein adsorption. Langmuir 1991, 7, 2710–2718. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- KL Prime; G Whitesides Self-assembled organic monolayers: Model systems for studying adsorption of proteins at surfaces. Science 1991, 252, 1164–1167. [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Chapman, R.G.; Ostuni, E.; Takayama, S.; Holmlin, R.E.; Yan, L.; Whitesides, G.M. Surveying for Surfaces that Resist the Adsorption of Proteins. J. Am. Chem. Soc. 2000, 122, 8303–8304. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that Resist Nonspecific Adsorption of Protein from Aqueous Buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Bernhard, C.; Roeters, S.J.; Franz, J.; Weidner, T.; Bonn, M.; Gonella, G. Repelling and ordering: The influence of poly(ethylene glycol) on protein adsorption. Phys. Chem. Chem. Phys. 2017, 19, 28182–28188. [Google Scholar] [CrossRef]
- Baggerman, J.; Smulders, M.M.J.; Zuilhof, H. Romantic Surfaces: A Systematic Overview of Stable, Biospecific, and Antifouling Zwitterionic Surfaces. Langmuir 2019, 35, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Hung, H.-C.; Lin, X.; Ma, J.; Zhang, P.; Sun, F.; Wu, K.; Jiang, S. Poly(ectoine) Hydrogels Resist Nonspecific Protein Adsorption. Langmuir 2017, 33, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-N.; Sun, F.; Hung, H.-C.; Jain, P.; Sinclair, A.; Zhang, P.; Bai, T.; Chang, Y.; Wen, T.-C.; Yu, Q.; et al. Ultra-low fouling and high antibody loading zwitterionic hydrogel coatings for sensing and detection in complex media. Acta Biomater. 2016, 40, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sabaté del Río, J.; Henry, O.Y.F.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef]
- Migliorini, E.; Weidenhaupt, M.; Picart, C. Practical guide to characterize biomolecule adsorption on solid surfaces (Review). Biointerphases 2018, 13, 06D303. [Google Scholar] [CrossRef]
- Prabowo, B.; Purwidyantri, A.; Liu, K.-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef]
- Li, C.; Lu, D.; Deng, J.; Zhang, X.; Yang, P. Amyloid-Like Rapid Surface Modification for Antifouling and In-Depth Remineralization of Dentine Tubules to Treat Dental Hypersensitivity. Adv. Mater. 2019, 31, 1903973. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, S.A.; Evans, E.; Benavidez, T.E.; Garcia, C.D. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta 2015, 872, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B Biointerfaces 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Ash, A.; Mulholland, F.; Burnett, G.R.; Wilde, P.J. Structural and compositional changes in the salivary pellicle induced upon exposure to SDS and STP. Biofouling 2014, 30, 1183–1197. [Google Scholar] [CrossRef]
- Xu, Z.; Coriand, L.; Loeffler, R.; Geis-Gerstorfer, J.; Zhou, Y.; Scheideler, L.; Fleischer, M.; Gehring, F.K.; Rupp, F. Saliva-coated titanium biosensor detects specific bacterial adhesion and bactericide caused mass loading upon cell death. Biosens. Bioelectron. 2019, 129, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, J.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Grass, S.; Umanskaya, N.; Scheibe, C.; Müller-Renno, C.; Davoudi, N.; Hannig, M.; Ziegler, C. Cleaning of biomaterial surfaces: Protein removal by different solvents. Colloids Surf. B Biointerfaces 2015, 128, 28–35. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Lee, M.-J.; Kim, J.-Y.; Kim, D.; Ryu, J.-H.; Jang, S.; Kim, K.-M.; Hwang, C.-J.; Choi, S.-H. Novel anti-biofouling light-curable fluoride varnish containing 2-methacryloyloxyethyl phosphorylcholine to prevent enamel demineralization. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Lee, M.-J.; Kwon, J.-S.; Kim, J.-Y.; Ryu, J.-H.; Seo, J.-Y.; Jang, S.; Kim, K.-M.; Hwang, C.-J.; Choi, S.-H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Chen, X.; Davies, M.C.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M.; Davies, J.; Dawkes, A.C.; Edwards, J.C. Recognition of Protein Adsorption onto Polymer Surfaces by Scanning Force Microscopy and Probe−Surface Adhesion Measurements with Protein-Coated Probes. Langmuir 1997, 13, 4106–4111. [Google Scholar] [CrossRef]
- Cao, L.; Wu, J.; Zhang, Q.; Baras, B.; Bhadila, G.; Li, Y.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Zhang, N.; et al. Novel Protein-Repellent and Antibacterial Resins and Cements to Inhibit Lesions and Protect Teeth. Int. J. Polym. Sci. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Xie, X.; Dai, Z.; Zhao, Z.; Imazato, S.; Al-Dulaijan, Y.; Al-Qarni, F.; Weir, M.; Reynolds, M.; et al. Nanostructured Polymeric Materials with Protein-Repellent and Anti-Caries Properties for Dental Applications. Nanomaterials 2018, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, C.; Melo, M.A.; Bai, Y.-X.; Cheng, L.; Xu, H.H. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral Sci. 2015, 7, 103–109. [Google Scholar] [CrossRef]
- Zhang, N.; Weir, M.D.; Romberg, E.; Bai, Y.; Xu, H.H.K. Development of novel dental adhesive with double benefits of protein-repellent and antibacterial capabilities. Dent. Mater. 2015, 31, 845–854. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Chen, C.; Liu, J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Melo, M.; Weir, M.; Xu, D.; Bai, Y.; Xu, H. Effects of Long-Term Water-Aging on Novel Anti-Biofilm and Protein-Repellent Dental Composite. IJMS 2017, 18, 186. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Imazato, S.; Weir, M.D.; Reynolds, M.A.; Xu, H.H.K. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C 2016, 67, 702–710. [Google Scholar] [CrossRef]
- Zhang, L.; Weir, M.D.; Chow, L.C.; Antonucci, J.M.; Chen, J.; Xu, H.H.K. Novel rechargeable calcium phosphate dental nanocomposite. Dent. Mater. 2016, 32, 285–293. [Google Scholar] [CrossRef]
- Al-Qarni, F.D.; Tay, F.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Xie, X.; Xu, H.H.K. Protein-repelling adhesive resin containing calcium phosphate nanoparticles with repeated ion-recharge and re-releases. J. Dent. 2018, 78, 91–99. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Zhang, K.; Xu, H.H.K. Protein-repellent nanocomposite with rechargeable calcium and phosphate for long-term ion release. Dent. Mater. 2018, 34, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xie, X.; Wang, B.; Weir, M.D.; Oates, T.W.; Xu, H.H.K.; Zhang, N.; Bai, Y. Protein-repellent and antibacterial effects of a novel polymethyl methacrylate resin. J. Dent. 2018, 79, 39–45. [Google Scholar] [CrossRef]
- Choi, W.; Jin, J.; Park, S.; Kim, J.-Y.; Lee, M.-J.; Sun, H.; Kwon, J.-S.; Lee, H.; Choi, S.-H.; Hong, J. Quantitative Interpretation of Hydration Dynamics Enabled the Fabrication of a Zwitterionic Antifouling Surface. ACS Appl. Mater. Interfaces 2020, 12, 7951–7965. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.; Qi, M.; Weir, M.D.; Reynolds, M.A.; Li, C.; Zhou, C.; Xu, H.H.K. Effects of single species versus multispecies periodontal biofilms on the antibacterial efficacy of a novel bioactive Class-V nanocomposite. Dent. Mater. 2019, 35, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Y.; Weir, M.D.; Lei, L.; Masri, R.; Lynch, C.D.; Oates, T.W.; Zhang, K.; Hu, T.; Xu, H.H.K. Effects of S. mutans gene-modification and antibacterial calcium phosphate nanocomposite on secondary caries and marginal enamel hardness. RSC Adv. 2019, 9, 41672–41683. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Cheng, L.; Jiang, Y.; Melo, M.A.S.; Weir, M.D.; Oates, T.W.; Zhou, X.; Xu, H.H.K. Novel dental composite with capability to suppress cariogenic species and promote non-cariogenic species in oral biofilms. Mater. Sci. Eng. C 2019, 94, 587–596. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yumoto, H.; Miyamoto, K.; Hirota, K.; Nakae, H.; Tanaka, S.; Murakami, K.; Kudo, Y.; Ozaki, K.; Miyake, Y. 2-Methacryloyloxyethyl phosphorylcholine (MPC)-polymer suppresses an increase of oral bacteria: A single-blind, crossover clinical trial. Clin. Oral Investig. 2019, 23, 739–746. [Google Scholar] [CrossRef]
- Li, B.; Jain, P.; Ma, J.; Smith, J.K.; Yuan, Z.; Hung, H.-C.; He, Y.; Lin, X.; Wu, K.; Pfaendtner, J.; et al. Trimethylamine N-oxide–derived zwitterionic polymers: A new class of ultralow fouling bioinspired materials. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of Dental Resins Resistant to Enzymatic and Hydrolytic Degradation in Oral Environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef]
- Tone, S.; Hasegawa, M.; Puppulin, L.; Pezzotti, G.; Sudo, A. Surface modifications and oxidative degradation in MPC-grafted highly cross-linked polyethylene liners retrieved from short-term total hip arthroplasty. Acta Biomater. 2018, 66, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, E.; Lange, S.C.; Pujari, S.P.; Tijhaar, E.J.; Smulders, M.M.J.; Savelkoul, H.F.J.; Zuilhof, H. Systematic Comparison of Zwitterionic and Non-Zwitterionic Antifouling Polymer Brushes on a Bead-Based Platform. Langmuir 2019, 35, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Vegas, A.J.; Anderson, D.G.; Langer, R.S.; Kilduff, J.E.; Belfort, G. Combinatorial synthesis with high throughput discovery of protein-resistant membrane surfaces. Biomaterials 2013, 34, 6133–6138. [Google Scholar] [CrossRef] [PubMed]
- Imbrogno, J.; Williams, M.D.; Belfort, G. A New Combinatorial Method for Synthesizing, Screening, and Discovering Antifouling Surface Chemistries. ACS Appl. Mater. Interfaces 2015, 7, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Imbrogno, J.; Belfort, G.; Wang, X.-L. Making polymeric membranes antifouling via “grafting from” polymerization of zwitterions. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Tsao, C.; Liu, S.; Jain, P.; Sinclair, A.; Hung, H.-C.; Bai, T.; Wu, K.; Jiang, S. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl. Acad. Sci. USA 2015, 112, 12046–12051. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Jr, L.; Bienek, D.R. Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials. J. Funct. Biomater. 2020, 11, 54. https://doi.org/10.3390/jfb11030054
Torres Jr L, Bienek DR. Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials. Journal of Functional Biomaterials. 2020; 11(3):54. https://doi.org/10.3390/jfb11030054
Chicago/Turabian StyleTorres Jr, Leopoldo, and Diane R. Bienek. 2020. "Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials" Journal of Functional Biomaterials 11, no. 3: 54. https://doi.org/10.3390/jfb11030054
APA StyleTorres Jr, L., & Bienek, D. R. (2020). Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials. Journal of Functional Biomaterials, 11(3), 54. https://doi.org/10.3390/jfb11030054