Evaluation of the In Vivo Biocompatibility of Amorphous Calcium Phosphate-Containing Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Biomaterials
2.2. Spheres Preparation
2.3. Characterization of Biomaterials
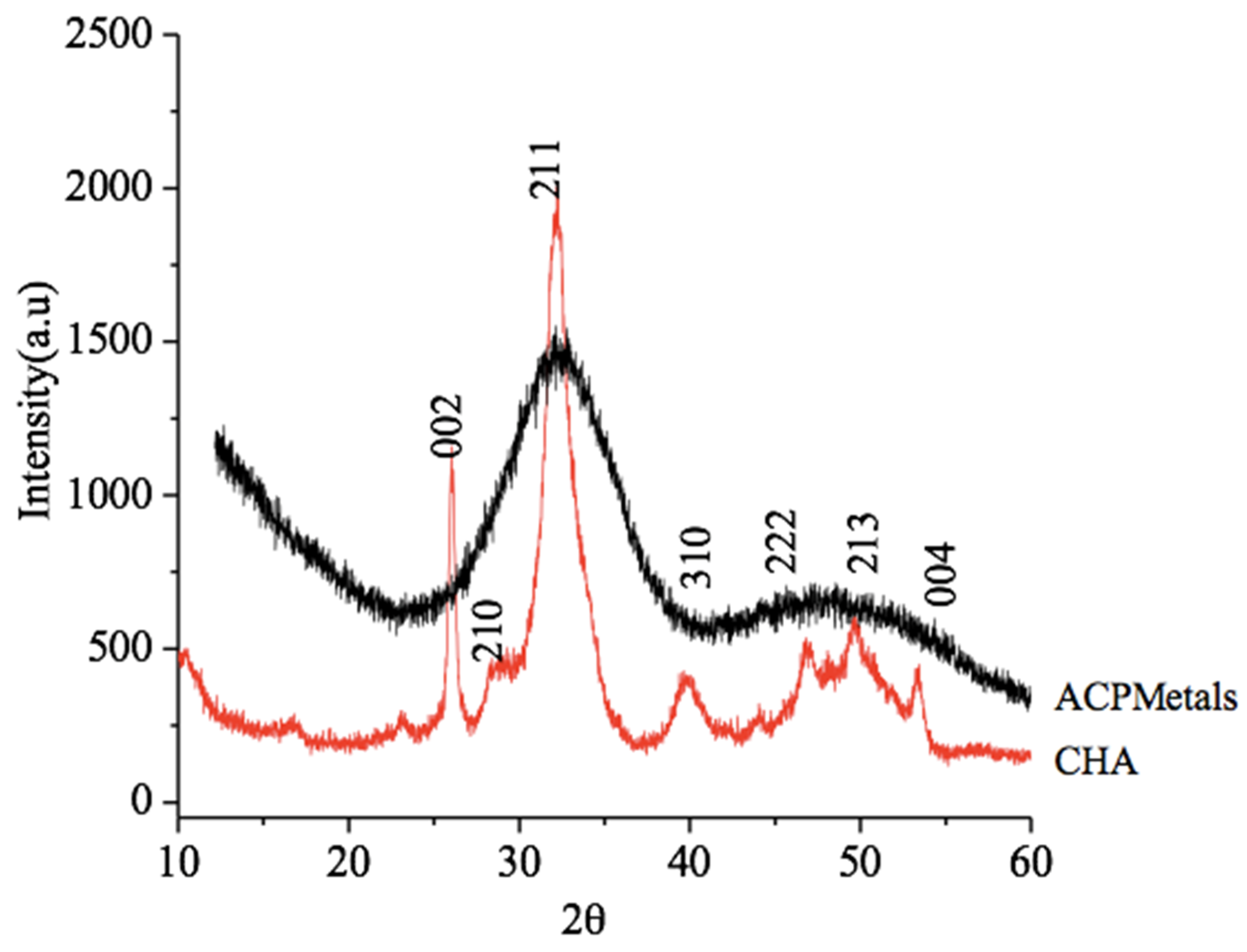
2.3.1. X-Ray Diffraction (XRD)
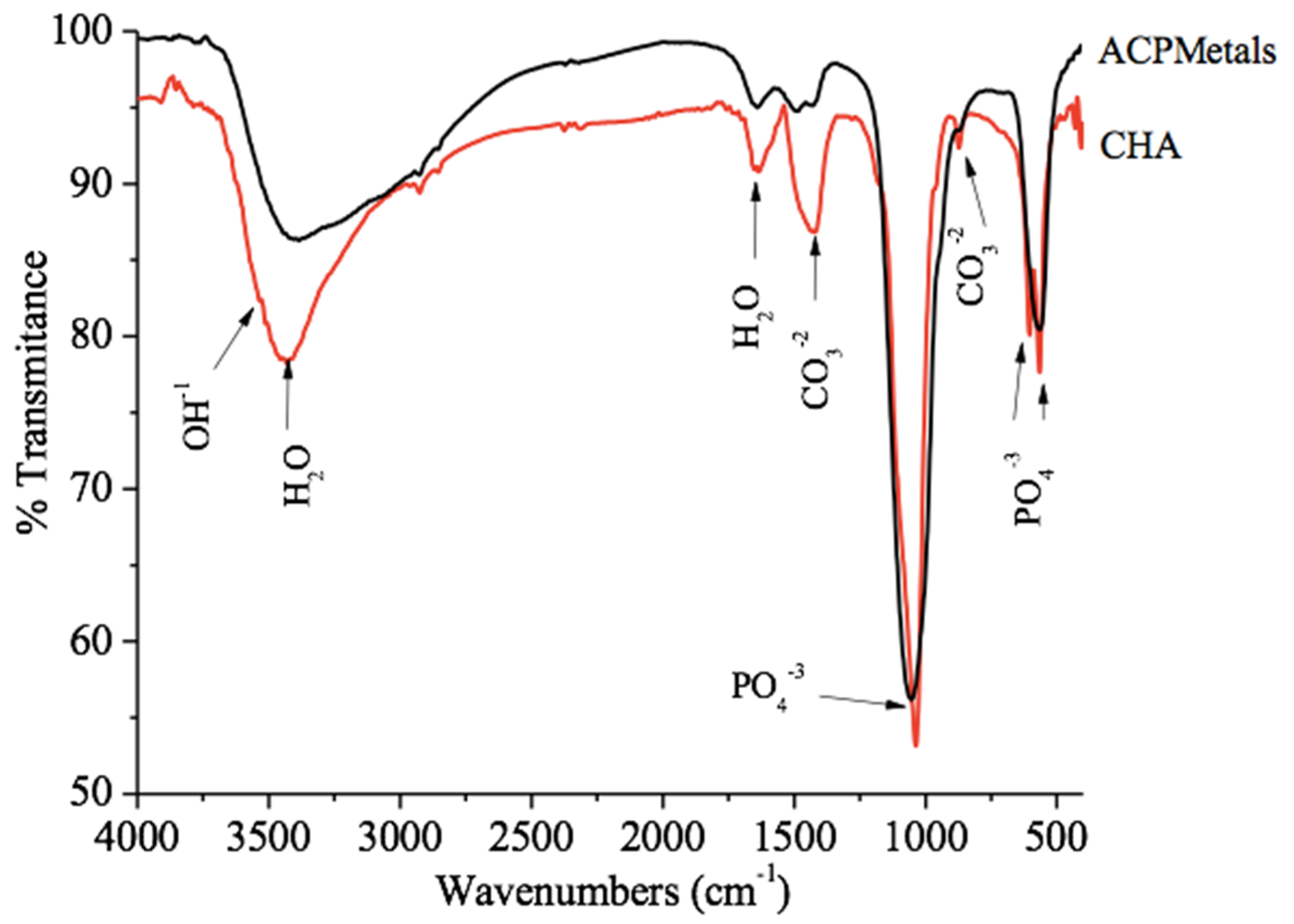
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Atomic Absorption Spectroscopy (AAS)
2.4. In Vivo Analysis
2.5. Animal Characterization
2.6. Anesthesia and Surgery Procedures
2.7. Obtaining the Samples
2.8. Sample Processing
2.9. Descriptive Histological Analysis
2.10. Analysis of Results According to ISO 10993-6: 2016
2.11. Statistical Analysis
3. Results
3.1. X-Ray Diffraction
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. Atomic Absorption Spectroscopy
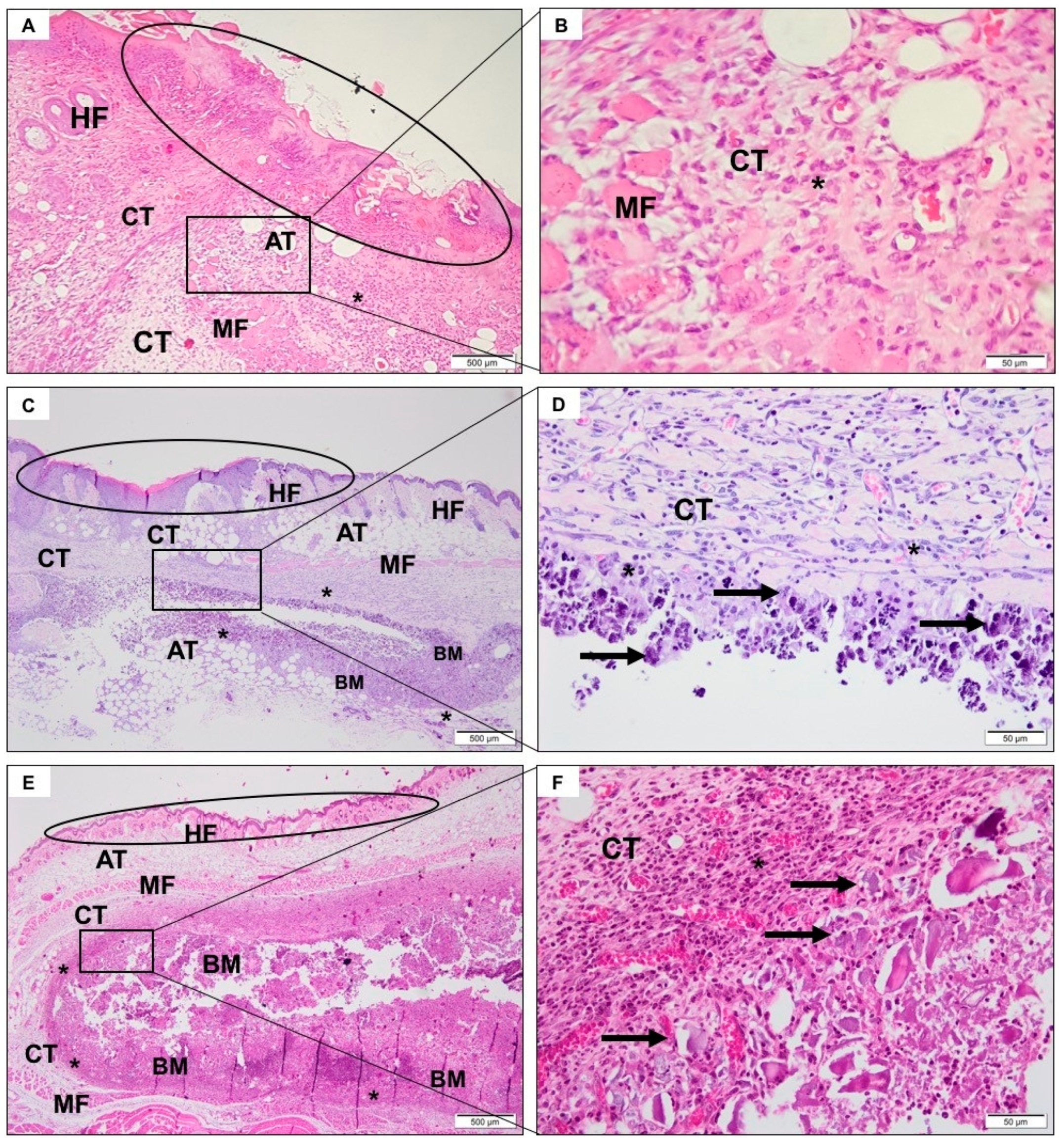
3.4. Descriptive Histological Analysis
3.4.1. SHAM Group
3.4.2. CHA Group
3.4.3. ACPMetals
3.5. Degree of Irritation of Biomaterials According to ISO 10993-6: 2016
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Electron. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Ono, K.; Yamamurmo, T.; Nakamura, T.; Kokubo, T. Quantitative study on osteoconduction of apatite-wollastonite containing glass ceramic granules, hydroxyapatite granules and alumina ganules. Biomaterials 1990, 11, 265–271. [Google Scholar] [PubMed]
- Mpc, F.; Ma, B.; Mc, M.; Ds, V. Biological Principles of Nanostructured Hydroxyapatite Associated with Metals: A Literature Review. Insights Biomed. 2019, 4, 13. [Google Scholar] [CrossRef]
- Resende, R.F.B.; Sartoretto, S.C.; Uzeda, M.J.; Alves, A.T.N.N.; Calasans-Maia, J.A.; Rossi, A.M.; Granjeiro, J.M.; Calasans-Maia, M. Randomized Controlled Clinical Trial of Nanostructured Carbonated Hydroxyapatite for Alveolar Bone Repair. Materials 2019, 12, 3645. [Google Scholar] [CrossRef] [PubMed]
- Mourão, C.F.; Lourenço, E.S.; Nascimento, J.R.B.; Machado, R.C.M.; Rossi, A.M.; Leite, P.E.C.; Granjeiro, J.M.; Alves, G.G.; Calasans-Maia, M.D. Does the association of blood-derived growth factors to nanostructured carbonated hydroxyapatite contributes to the maxillary sinus floor elevation? A randomized clinical trial. Clin. Oral Investig. 2018, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Calasans-Maia, M.; Junior, C.A.B.B.; Soriano-Souza, C.A.; Alves, A.T.N.N.; Uzeda, M.J.; Zelaya, V.M.; Mavropoulos, E.; Leão, M.H.R.; De Santana, R.B.; Granjeiro, J.M.; et al. Microspheres of alginate encapsulated minocycline-loaded nanocrystalline carbonated hydroxyapatite: Therapeutic potential and effects on bone regeneration. Int. J. Nanomed. 2019, 14, 4559–4571. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.; Baradari, H.; Champion, E.; Damia, C.; Viana-Trecant, M. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: A review of the parameters affecting the loading and release of the therapeutic substance. J. Control. Release 2017, 252, 1–17. [Google Scholar] [CrossRef]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Martin, V.; Bettencourt, A.F. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater. Sci. Eng. C 2018, 82, 363–371. [Google Scholar] [CrossRef]
- Resende, R.F.; Fernandes, G.V.O.; Santos, S.R.A.; Rossi, A.M.; Lima, I.; Granjeiro, J.M.; Calasans-Maia, M.D. Long-term biocompatibility evaluation of 0.5 § zinc containing hydroxyapatite in rabbits. J. Mater. Sci. Mater. Med. 2013, 24, 1455–1463. [Google Scholar] [CrossRef]
- Saijo, H.; Chung, U.-I.; Igawa, K.; Mori, Y.; Chikazu, D.; Iino, M.; Takato, T. Clinical application of artificial bone in the maxillofacial region. J. Artif. Organs 2008, 11, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Sprio, S.; Sandri, M.; Celotti, G.; Tampieri, A. Development of Sr and CO3 co-substituted hydroxyapatites for biomedical applications. Acta Biomater. 2008, 4, 656–663. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Habibovic, P.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; Van Blitterswijk, C. Comparison of two carbonated apatite ceramics in vivo. Acta Biomater. 2010, 6, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Calasans-Maia, M.; Fernandes, G.; Rossi, A.M.; Dias, E.P.; Almeida, G.; Mitri, F.; Granjeiro, J.M. Effect of Hydroxyapatite and Zinc-Containing Hydroxyapatite on Osseous Repair of Critical Size Defect in the Rat Calvaria. Key Eng. Mater. 2007, 361, 1273–1276. [Google Scholar] [CrossRef]
- Fernandes, G.; Calasans-Maia, M.; Mitri, F.; Bernardo, V.G.; Rossi, A.M.; Almeida, G.; Granjeiro, J.M. Histomorphometric Analysis of Bone Repair in Critical Size Defect in Rats Calvaria Treated with Hydroxyapatite and Zinc-Containing Hydroxyapatite 5%. Key Eng. Mater. 2008, 396, 15–18. [Google Scholar] [CrossRef]
- Nascimento, L.; Medeiros, M.; Calasans-Maia, J.A.; Alves, A.; Rossi, A.M.; Alves, G.G.; Granjeiro, J.M.; Calasans-Maia, M. Osseoinduction Evaluation of Hydroxyapatite and Zinc Containing Hydroxyapatite Granules in Rabbits. Key Eng. Mater. 2011, 493, 252–257. [Google Scholar] [CrossRef]
- Mayer, I.; Apfelbaum, F.; Featherstone, J. Zinc ions in synthetic carbonated hydroxyapatites. Arch. Oral Boil. 1994, 39, 87–90. [Google Scholar] [CrossRef]
- Ribeiro, S.; Sartoretto, S.C.; Resende, R.; Uzeda, M.; Alves, A.T.; Santos, S.; Pesce, G.; Rossi, A.M.; Granjeiro, J.M.; Miguel, F.; et al. In Vivo Evaluation of Zinc-Containing Nanostructured Carbonated Hydroxyapatite. Key Eng. Mater. 2016, 696, 223–229. [Google Scholar] [CrossRef]
- Zhao, S.-F.; Dong, W.-J.; Jiang, Q.-H.; He, F.-M.; Wang, X.-X.; Yang, G.-L. Effects of zinc-substituted nano-hydroxyapatite coatings on bone integration with implant surfaces. J. Zhejiang Univ. Sci. B 2013, 14, 518–525. [Google Scholar] [CrossRef]
- Chandran, S.; Vs, H.K.; Varma, H.; John, A. Osteogenic efficacy of strontium hydroxyapatite micro-granules in osteoporotic rat model. J. Biomater. Appl. 2016, 31, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 1996, 18, 517–523. [Google Scholar] [CrossRef]
- Valiense, H.; Barreto, M.; Resende, R.F.; Alves, A.; Rossi, A.M.; Mavropoulos, E.; Granjeiro, J.M.; Calasans-Maia, M.D. In vitro and in vivo evaluation of strontium-containing nanostructured carbonated hydroxyapatite/sodium alginate for sinus lift in rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 274–282. [Google Scholar] [CrossRef]
- Carmo, A.B.X.D.; Sartoretto, S.C.; Alves, A.T.N.N.; Granjeiro, J.M.; Miguel, F.B.; Calasans-Maia, M.; Calasans-Maia, M.D. Alveolar bone repair with strontium- containing nanostructured carbonated hydroxyapatite. J. Appl. Oral Sci. 2018, 26, 20170084. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, K.; Zhao, X.; Han, Y. Development of a strontium-containing hydroxyapatite bone cement. Biomaterials 2005, 26, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Hao, Y.Z.; Fang, C.Q.; Sun, L.; Ni, P.F.; Xu, K.; Li, H.Y.; Zhu, H.; Wang, J.; Huang, X.F. Influences of Sr dose on the crystal structure parameters and Sr distributions of Sr?incorporated hydroxyapatite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1275–1283. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Zelaya, V.M.; Zarranz, L.; Herrera, E.Z.; Alves, A.T.N.N.; Uzeda, M.J.; Mavropoulos, E.; Rossi, A.; Mello, A.; Granjeiro, J.M.; Calasans-Maia, M.; et al. In vitro and in vivo evaluations of nanocrystalline Zn-doped carbonated hydroxyapatite/alginate microspheres: Zinc and calcium bioavailability and bone regeneration. Int. J. Nanomed. 2019, 14, 3471–3490. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Syntesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, U.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2017, 52, 135–141. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Calasans-Maia, M.D.; Alves, T.N.N.; Resende, R.F.B.; Fernandes, C.J.C.; Padilha, P.M.; Rossi, A.M.; Teti, A.; Granjeiro, J.M.; Zambuzzi, W.F. The role of apoptosis associated speck-like protein containing a caspase-1 recruitment domain (ASC) in response to bone substitutes. Mater. Sci. Eng. C 2020, 112, 110965. [Google Scholar] [CrossRef]
- Michał, W.; Ewa, D.; Tomasz, C. Lecithin-based wet chemical precipitation of hydroxyapatite nanoparticles. Colloid Polym. Sci. 2015, 293, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.; Costa, A.M.; Bettini, J.; Ramirez, A.; Da Silva, M.H.P.; Rossi, A.M.; Da Silva, M.H.P.; Rossi, A.M. Structural Properties of Nanostructured Carbonate Apatites. Key Eng. Mater. 2008, 396, 611–614. [Google Scholar] [CrossRef]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Damien, E.; Revell, P.A. A comparative study on the in vivo behaviour of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744, 657–661. [Google Scholar] [CrossRef]
- Narasaraju, T.S.B.; Phebe, D.E. Some physico-chemical aspects of hydroxylapatite. J. Mater. Sci. 1996, 31, 1–21. [Google Scholar] [CrossRef]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.D.A.; Menezes, R.R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nazarian, H.; Pourbaghi-Masouleh, M.; Borhan, S. Comparative study of mesenchymal stem cells osteogenic differentiation on low-temperature biomineralized nanocrystalline carbonated hydroxyapatite and sintered hydroxyapatite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, M.; Zhang, X. Effects of sintering temperature on structure of hydroxyapatite studied with Rietveld method. J. Mater. Sci. Mater. Electron. 2003, 14, 817–822. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.J.; Rao, D.S.P.; Battese, G.E. Metafrontier frameworks for the study of firm-level efficiencies and technology ratios. Empir. Econ. 2007, 34, 231–255. [Google Scholar] [CrossRef]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, M.; Orly, I.; Menanteau, J. The influence of calcium phosphate biomaterials on human bone cell activities. Anin vitroapproach. J. Biomed. Mater. Res. 1990, 24, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Rigo, E.C.S.; Gehrke, S.A.; Carbonari, M. Síntese e caracterização de hidroxiapatita obtida pelo método de precipitação. Rev. Dent. Press Periodontia Implantol. 2007, 1, 39–50. [Google Scholar]
- Santos, M.L.; Florentino, A.O.; Saeki, M.J.; Aparecida, A.H.; Fook, M.V.L.; Guastaldi, A.C. Síntese de hidroxiapatita pelo método sol-gel utilizando precursores alternativos: Nitrato de cálcio e ácido fosfórico. Eclética Química J. 2005, 30, 29–35. [Google Scholar] [CrossRef][Green Version]
- Hao, Y.; Yan, H.; Wang, X.; Zhu, B.; Ning, C.; Ge, S. Evaluation of osteoinduction and proliferation on nano-Sr-HAP: A novel orthopedic biomaterial for bone tissue regeneration. J. Nanosci. Nanotechnol. 2012, 12, 207–212. [Google Scholar] [CrossRef]
- Costa, N.M.; Yassuda, D.H.; Sader, M.S.; Fernandes, G.V.D.O.; Soares, G.D.; Granjeiro, J.M. Osteogenic effect of tricalcium phosphate substituted by magnesium associated with Genderm® membrane in rat calvarial defect model. Mater. Sci. Eng. C 2016, 61, 63–71. [Google Scholar] [CrossRef]
- Bigi, A.; Foresti, E.; Gregorini, R.; Ripamonti, A.; Roveri, N.; Shah, J.S. The role of magnesium on the structure of biological apatites. Calcif. Tissue Int. 1992, 50, 439–444. [Google Scholar] [CrossRef]
- Cai, Y.L.; Zhang, J.J.; Zhang, S.; Venkatraman, S.; Zeng, X.T.; Du, H.J.; Mondal, D. Osteoblastic cell response on fluoridated hydroxyapatite coatings: The effect of magnesium incorporation. Biomed. Mater. 2010, 5, 54114. [Google Scholar] [CrossRef]
- Cruz, R.; Calasans-Maia, J.A.; Sartoretto, S.; Moraschini, V.; Rossi, A.M.; Louro, R.S.; Granjeiro, J.M.; Calasans-Maia, M.D. Does the incorporation of zinc into calcium phosphate improve bone repair? A systematic review. Ceram. Int. 2018, 44, 1240–1249. [Google Scholar] [CrossRef]
- Zuo, K.-H.; Zeng, Y.-P.; Jiang, D. Synthesis and magnetic property of iron ions-doped hydroxyapatite. J. Nanosci. Nanotechnol. 2012, 12, 7096–7100. [Google Scholar] [CrossRef]
- Pon-On, W.; Meejoo, S.; Tang, M. Incorporation of iron into nano hydroxyapatite particles synthesized by the microwave proces. Int. J. Nanosci. 2007, 6, 9–16. [Google Scholar] [CrossRef]
- Mayer, I.; Cuisinier, F.J.G.; Popov, I.; Schleich, Y.; Gdalya, S.; Burghaus, O.; Reinen, D. Phase Relations Between β-Tricalcium Phosphate and Hydroxyapatite with Manganese(II): Structural and Spectroscopic Properties. Eur. J. Inorg. Chem. 2006, 2006, 1460–1465. [Google Scholar] [CrossRef]
- Zambuzzi, W.; Oliveira, R.C.; Pereira, F.L.; Cestari, T.M.; Taga, R.; Granjeiro, J.M. Rat subcutaneous tissue response to macrogranular porous anorganic bovine bone graft. Braz. Dent. J. 2006, 17, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, H.; Shim, J.-S.; Chung, M.-K.; Park, Y.-B. Comparative study of recombinant human bone morphogenetic protein-2 carriers in rat subcutaneous tissues: Pilot study. Tissue Eng. Regen. Med. 2015, 12, 138–146. [Google Scholar] [CrossRef]
- Avula, M.N.; Rao, A.N.; Mcgill, L.D.; Grainger, D.W.; Solzbacher, F. Foreign body response to subcutaneous biomaterial implants in a mast cel deficient Kit murine model. Acta Biomater. 2014, 10, 1856–1863. [Google Scholar] [CrossRef]




| Biomaterial | Ca (%) | Mol Ca | P (%) | Mol P | Metals (%) |
|---|---|---|---|---|---|
| CP | 40.46 | 1.007 | 17.72 | 0.57 | - |
| ACPMetals | 28.75 | 0.72 | 17.84 | 0.58 | 3.52 (Zn) 2.46 (Sr) 0.78 (Mg) 2.65 (Mn) 4.45 (Fe) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moerbeck-Filho, P.; Sartoretto, S.C.; Uzeda, M.J.; Barreto, M.; Medrado, A.; Alves, A.; Calasans-Maia, M.D. Evaluation of the In Vivo Biocompatibility of Amorphous Calcium Phosphate-Containing Metals. J. Funct. Biomater. 2020, 11, 45. https://doi.org/10.3390/jfb11020045
Moerbeck-Filho P, Sartoretto SC, Uzeda MJ, Barreto M, Medrado A, Alves A, Calasans-Maia MD. Evaluation of the In Vivo Biocompatibility of Amorphous Calcium Phosphate-Containing Metals. Journal of Functional Biomaterials. 2020; 11(2):45. https://doi.org/10.3390/jfb11020045
Chicago/Turabian StyleMoerbeck-Filho, Pio, Suelen C. Sartoretto, Marcelo J. Uzeda, Maurício Barreto, Alena Medrado, Adriana Alves, and Mônica D. Calasans-Maia. 2020. "Evaluation of the In Vivo Biocompatibility of Amorphous Calcium Phosphate-Containing Metals" Journal of Functional Biomaterials 11, no. 2: 45. https://doi.org/10.3390/jfb11020045
APA StyleMoerbeck-Filho, P., Sartoretto, S. C., Uzeda, M. J., Barreto, M., Medrado, A., Alves, A., & Calasans-Maia, M. D. (2020). Evaluation of the In Vivo Biocompatibility of Amorphous Calcium Phosphate-Containing Metals. Journal of Functional Biomaterials, 11(2), 45. https://doi.org/10.3390/jfb11020045






