Design of Silk-Elastin-Like Protein Nanoparticle Systems with Mucoadhesive Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of ANS-Loaded SELP Nanoparticles
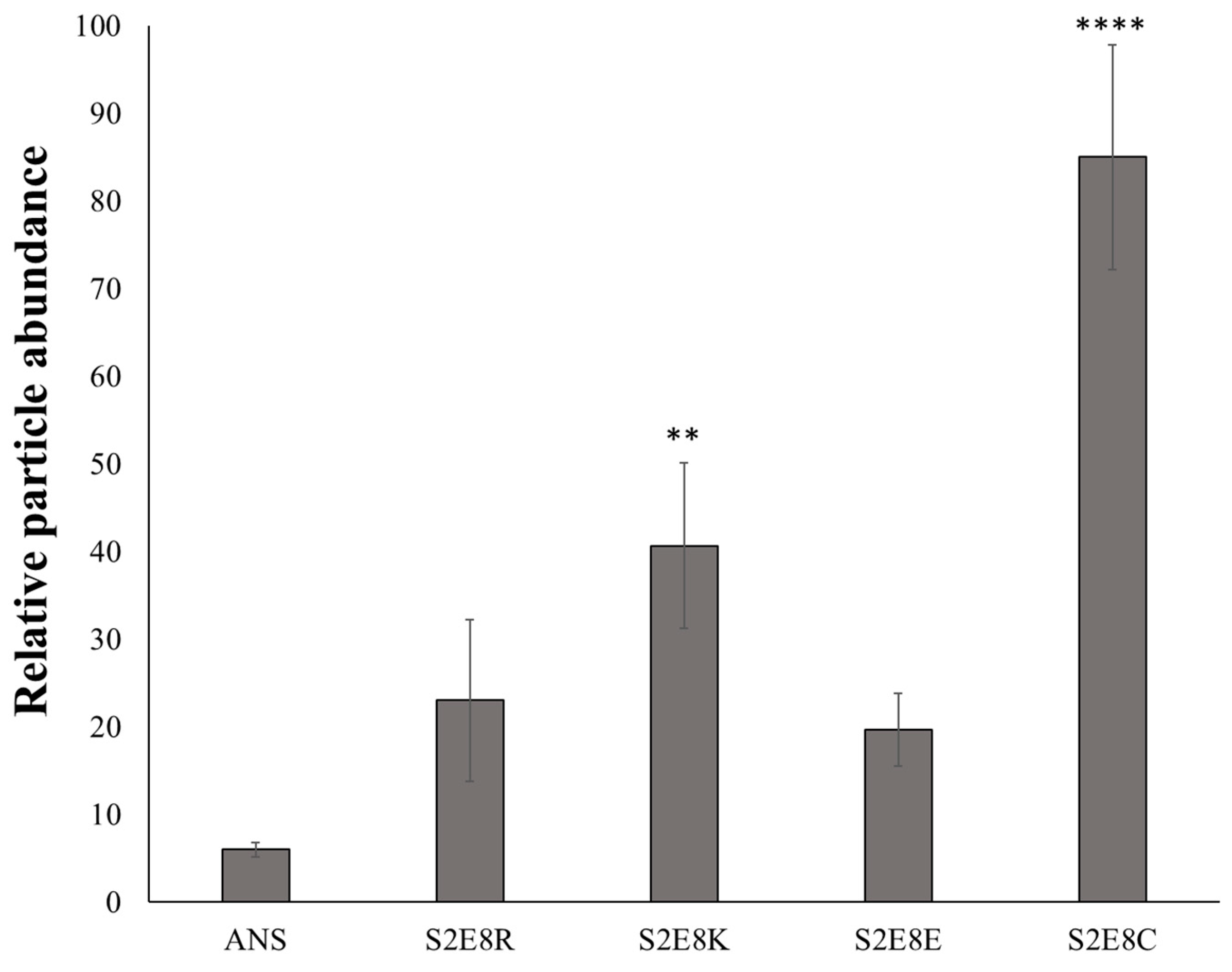
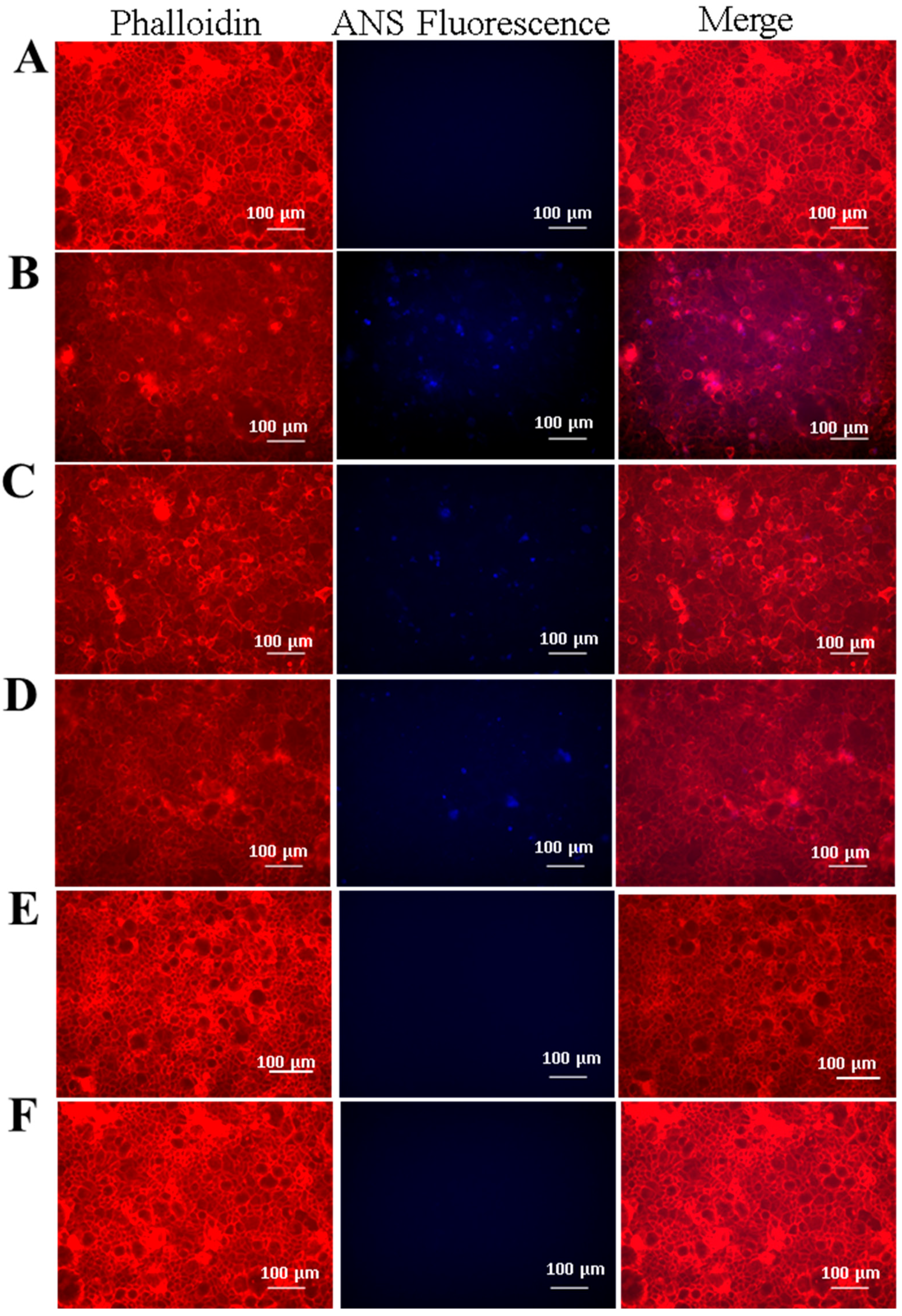
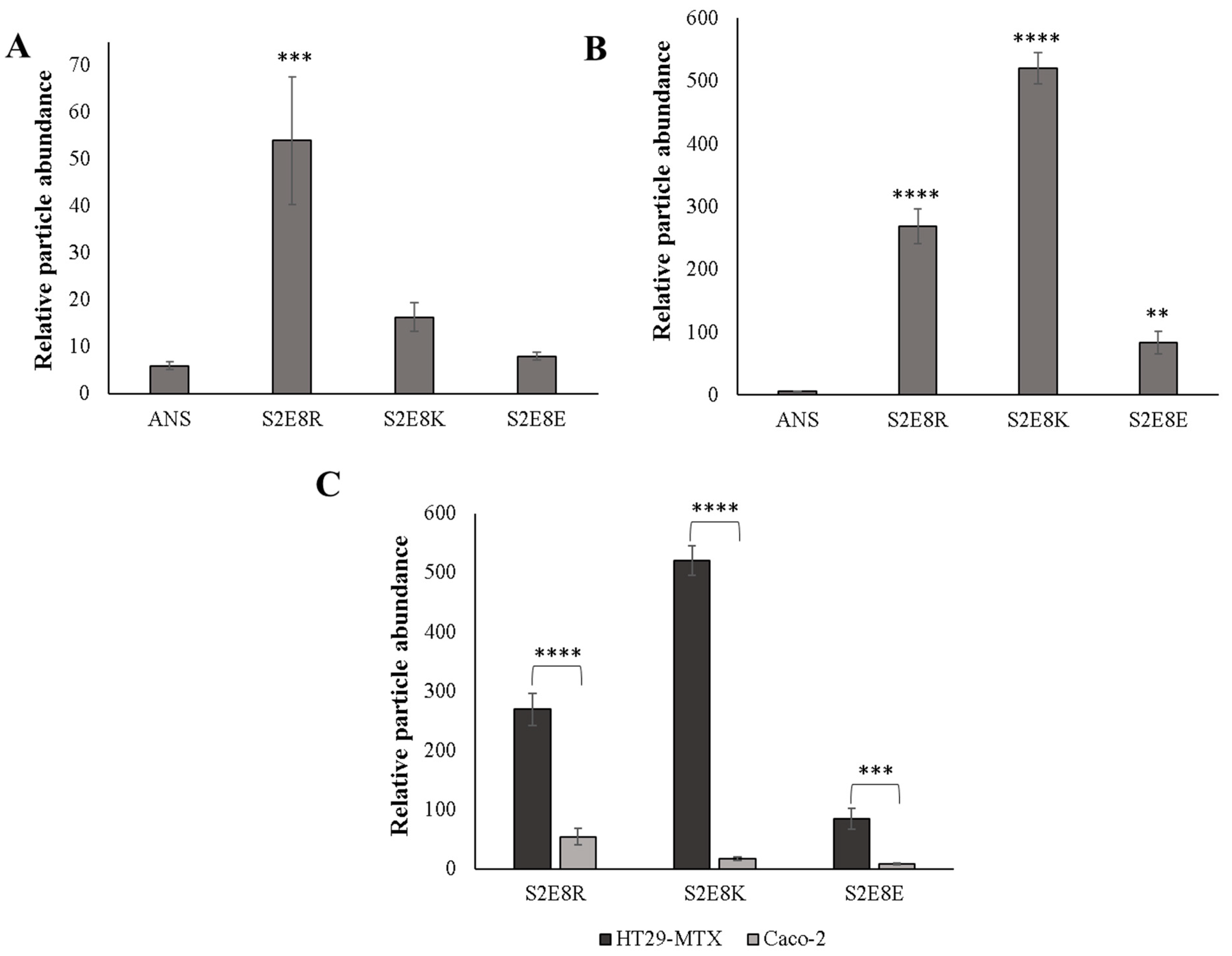
2.2. Evaluation of SELP Nanoparticle Mucoadhesive Properties
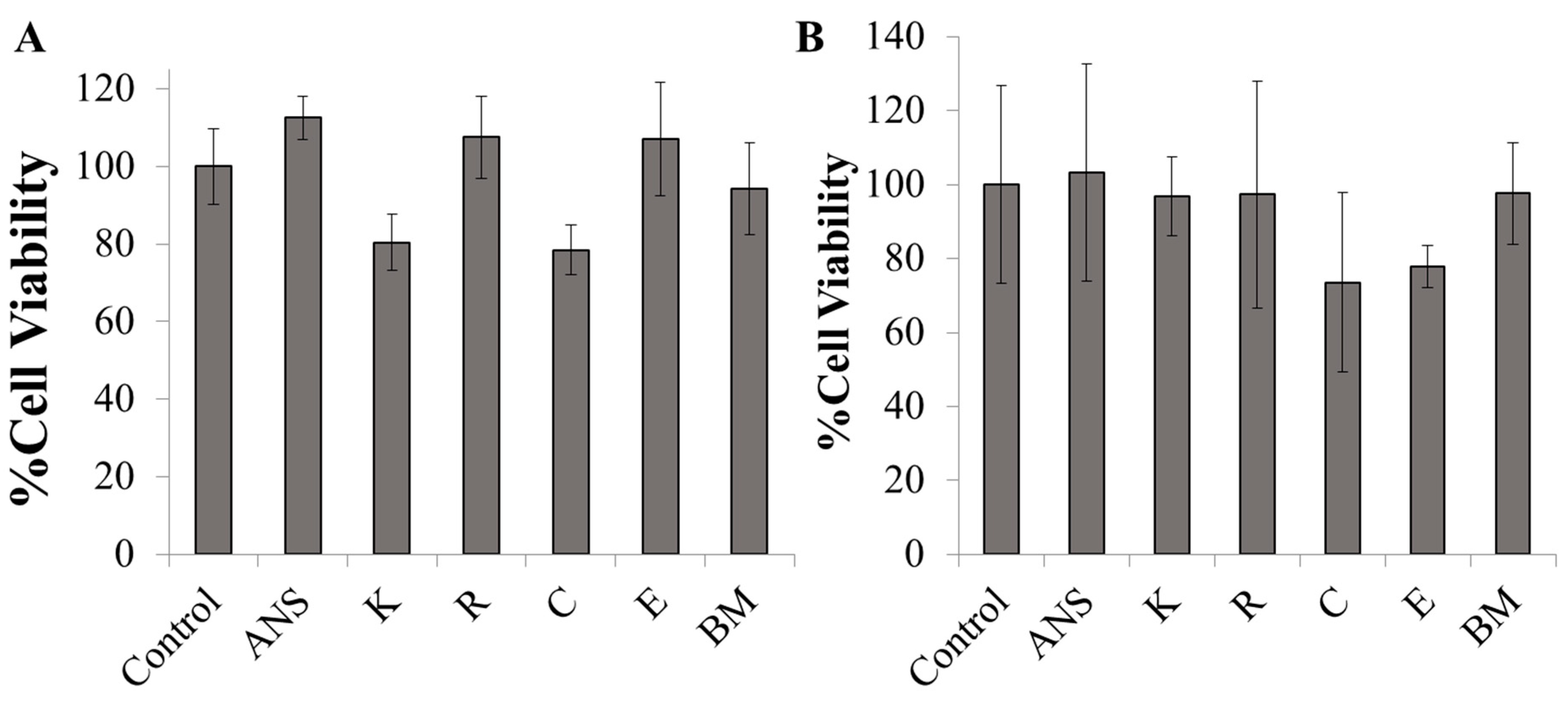
2.3. In Vitro Cytotoxicity of ANS-Loaded Nanoparticles
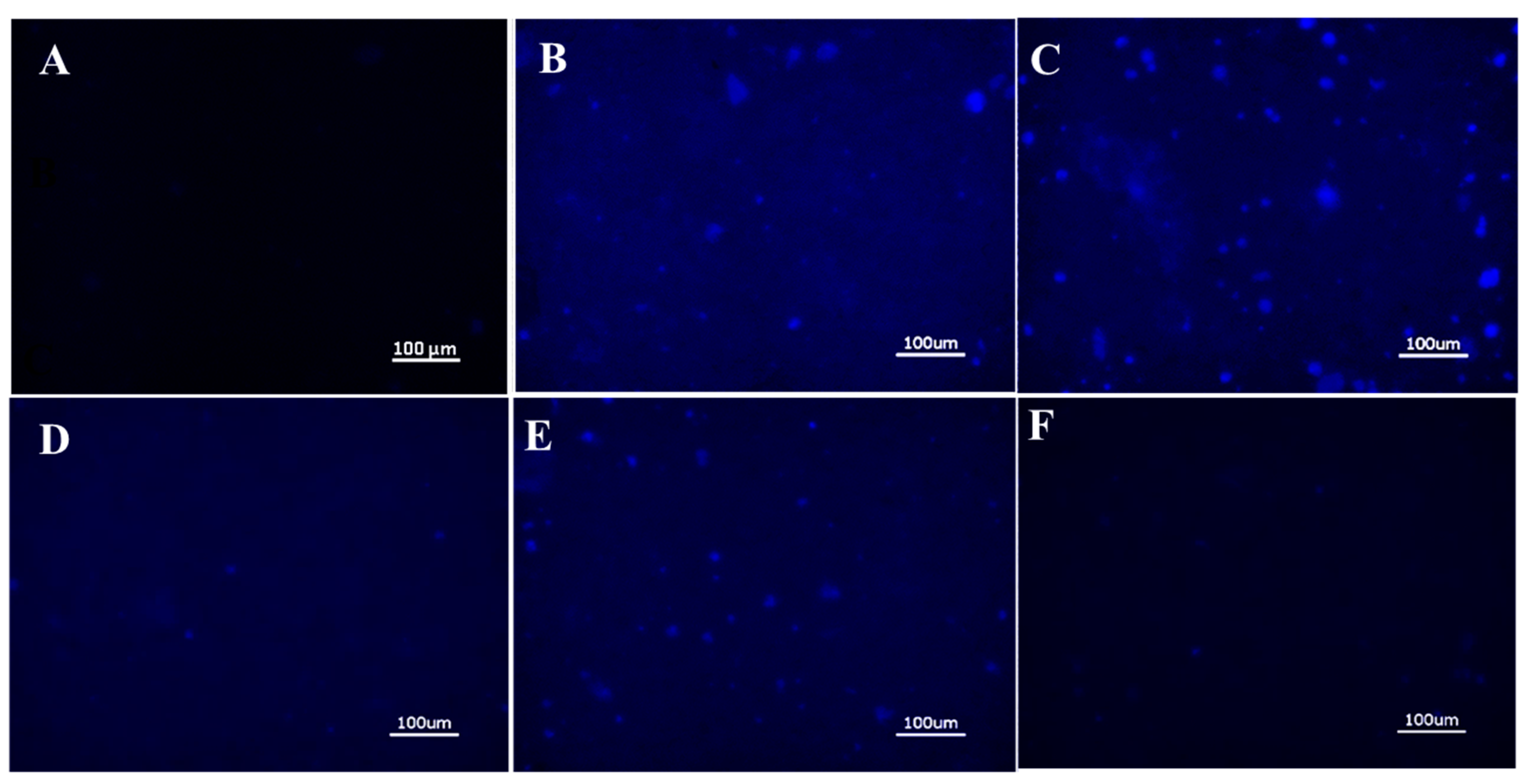
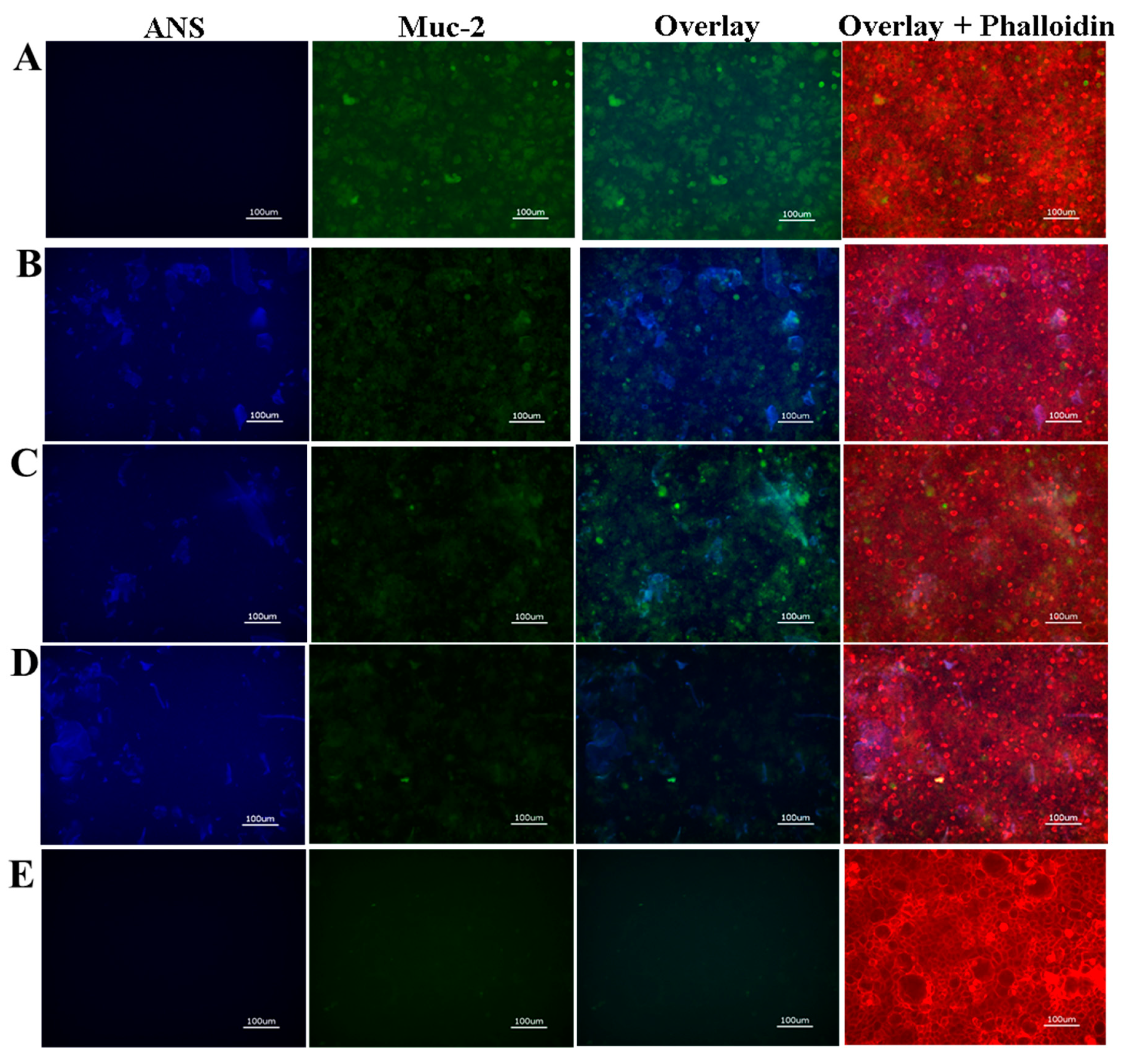
2.4. Cellular Adhesion of ANS-Loaded SELP Nanoparticles
3. Materials and Methods
3.1. Biosynthesis of SELP Proteins
3.2. Assembly of 8-Anilinonaphthalene-1-Sulfonic Acid (ANS)-Loaded Micellar-Like SELP Nanoparticles
3.3. Dynamic Light Scattering (DLS)
3.4. Fluorescence Spectroscopy
3.5. Cell Culture-Caco-2 and HT29-MTX Epithelial Cells and hCSSCs, and ANS-Loaded SELP Nanoparticle Adhesion to Epithelial Cell Monolayers
3.6. Immunohistochemistry
3.7. Fluorescence Microscopy
3.8. Preparation of Biosimilar Mucus and Adhesion of ANS-Loaded SELP Nanoparticles
3.9. Cytotoxicity of SELP Nanoparticles and Biosimilar Mucus in Cell Culture Assays
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.; Krauland, E.; Wirtz, D.; Hanes, J. Transport of Polymeric Nanoparticle Gene Carriers in Gastric Mucus. Biotechnol. Prog. 2004, 20, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Gasco, M.R.; Chetoni, P.; Burgalassi, S.; Saettone, M.F. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int. J. Pharm. 2002, 238, 241–245. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Buenestado, A.; Naline, E.; Faisy, C.; Blouquit-Laye, S.; Couderc, L.J.; Le Guen, M.; Fischler, M.; Devillier, P. Intranasal drug delivery: An efficient and non-invasive route for systemic administration: Focus on opioids. Pharmacol. Ther. 2012, 134, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Nielsen, H.M. Mucus as a Barrier to Drug Delivery—Understanding and Mimicking the Barrier Properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Petrou, G.; Jansson, R.; Högqvist, M.; Erlandsson, J.; Wagberg, L.; Hedhammar, M.; Crouzier, T. Genetically Engineered Mucoadhesive Spider Silk. Biomacromolecules 2018, 19, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.H.; Moghadam, A.; Ijaz, M.; Mahmood, A.; Götz, R.X.; Matuszczak, B.; Bernkop-Schnürch, A. S-protected thiolated cyclodextrins as mucoadhesive oligomers for drug delivery. J. Colloid Interface Sci. 2018, 531, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; Miziara, L.; Lorenzón, E.N.; De Souza, A.L.R.; Kiill, C.P.; Fangueiro, J.F.; Garcia, M.L.; Gremião, P.D.; Silva, A.M.; Souto, E.B. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: Interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015, 93, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N-Trimethyl Chitosan Chloride-Coated PLGA Nanoparticles Overcoming Multiple Barriers to Oral Insulin Absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Schäfer, U.; Lehr, C. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Pena, A.I.V.; Alonso, M.J.; Kissel, T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: The effect of mucus on particle adsorption and transport. Pharm. Res. 2002, 19, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Halpert, G.; Babu, T.; Ortiz, J.; Kanvinde, P.; Dimitrion, P.; Narayan, J.; Zierden, H.; Betageri, K.; Musmanno, O.; et al. Mucus-penetrating budesonide nanosuspension enema for local treatment of inflammatory bowel disease. Biomaterials 2018, 185, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.L.; Yildiz, H.; Dar, Z.; Lock, J.Y.; Carrier, R.L. Lipids alter microbial transport through intestinal mucus. PLoS ONE 2018, 13, e0209151. [Google Scholar] [CrossRef] [PubMed]
- Adebisi, A.O.; Conway, B.R.; Adebisi, D.A.O. Lectin-conjugated microspheres for eradication of Helicobacter pylori infection and interaction with mucus. Int. J. Pharm. 2014, 470, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; He, H.; Han, L.; Qin, J.; Chen, S.; Ru, G.; Li, R.; Yang, P.; Wang, J.; Yang, V.C. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J. Control. Release 2016, 233, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Salimi, E.; Le-Vinh, B.; Zahir-Jouzdani, F.; Matuszczak, B.; Ghaee, A.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems changing their zeta potential via a flip-flop mechanism. Int. J. Pharm. 2018, 550, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, H.; Li, J.; Qiu, J.-N.; Huang, J.-M.; Li, M.-C.; Guan, Y.-Q. Preparation and characterization of layer-by-layer hypoglycemic nanoparticles with pH-sensitivity for oral insulin delivery. J. Mater. Chem. B 2018, 6, 7451–7461. [Google Scholar] [CrossRef]
- Gavin, A.; Pham, J.T.; Wang, D.; Brownlow, B.; Elbayoumi, T.A. Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics. Int. J. Nanomed. 2015, 10, 1569–1584. [Google Scholar]
- Hejjaji, E.M.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Boil. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Synthesis and evaluation of mucoadhesive acryloyl-quaternized PDMAEMA nanogels for ocular drug delivery. Colloids Surf. B Biointerfaces 2017, 155, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, M.H.; Conway, B.R.; Smith, A.M. Development of mucoadhesive sprayable gellan gum fluid gels. Int. J. Pharm. 2015, 488, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tarakanova, A.; Dinjaski, N.; Wang, Q.; Xia, X.; Chen, Y.; Wong, J.Y.; Buehler, M.J.; Kaplan, D.L. Design of Multistimuli Responsive Hydrogels Using Integrated Modeling and Genetically Engineered Silk–Elastin-Like Proteins. Adv. Funct. Mater. 2016, 26, 4113–4123. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Machado, R.; Da Costa, A.; Ribeiro, A.; Collins, T.; Gomes, A.C.; Leonor, I.B.; Kaplan, D.L.; Reis, R.L.; Casal, M. Silk-based biomaterials functionalized with fibronectin type II promotes cell adhesion. Acta Biomater. 2017, 47, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.G.; Rim, N.G.; Huang, W.; Tarakanova, A.; Yeo, J.; Buehler, M.J.; Kaplan, D.L.; Wong, J.Y. Fabrication and Characterization of Recombinant Silk-Elastin-Like-Protein (SELP) Fiber. Macromol. Biosci. 2018, 18, e1800265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-L.; Qian, Z.-G.; Chen, L.; Kaplan, D.L.; Xia, X.-X. Rationally Designed Redox-Sensitive Protein Hydrogels with Tunable Mechanical Properties. Biomacromolecules 2016, 17, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.X.; Wang, M.; Lin, Y.; Xu, Q.; Kaplan, D.L. Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery. Biomacromolecules 2014, 15, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Megeed, Z.; Cappello, J.; Ghandehari, H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv. Drug Deliv. Rev. 2002, 54, 1075–1091. [Google Scholar] [CrossRef]
- Price, R.; Poursaid, A.; Cappello, J.; Ghandehari, H. In vivo evaluation of matrix metalloproteinase responsive silk-elastinlike protein polymers for cancer gene therapy. J. Control. Release 2015, 213, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.X.; Xu, Q.; Hu, X.; Qin, G.; Kaplan, D.L. Tunable self-assembly of genetically engineered silk--elastin-like protein polymers. Biomacromolecules 2011, 12, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xia, X.; Huang, W.; Lin, Y.; Xu, Q.; Kaplan, D.L. High Throughput Screening of Dynamic Silk-Elastin-Like Protein Biomaterials. Adv. Funct. Mater. 2014, 24, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moldes, Z.; Ebrahimi, D.; Plowright, R.; Dinjaski, N.; Perry, C.C.; Buehler, M.J.; Kaplan, D.L. Intracellular Pathways Involved in Bone Regeneration Triggered by Recombinant Silk-silica Chimeras. Adv. Funct. Mater. 2018, 28, 1702570. [Google Scholar] [CrossRef] [PubMed]
- Dinjaski, N.; Plowright, R.; Zhou, S.; Belton, D.J.; Perry, C.C.; Kaplan, D.L. Osteoinductive recombinant silk fusion proteins for bone regeneration. Acta Biomater. 2017, 49, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Bushmarina, N.A.; Kuznetsova, I.M.; Biktashev, A.G.; Turoverov, K.K.; Uversky, V.N. Partially folded conformations in the folding pathway of bovine carbonic anhydrase II: A fluorescence spectroscopic analysis. ChemBioChem 2001, 2, 813–821. [Google Scholar] [CrossRef]
- Kim, W.; Thévenot, J.; Ibarboure, E.; Lecommandoux, S.; Chaikof, E.L. Self-Assembly of Thermally Responsive Amphiphilic Diblock Copolypeptides into Spherical Micellar Nanoparticles. Angew. Chem. 2010, 122, 4353–4356. [Google Scholar] [CrossRef]
- Fujita, Y.; Mie, M.; Kobatake, E. Construction of nanoscale protein particle using temperature-sensitive elastin-like peptide and polyaspartic acid chain. Biomaterials 2009, 30, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.; Diedrichsen, R.G.; Christophersen, P.C.; Mu, H.; Nielsen, H.M. Evaluation of drug permeation under fed state conditions using mucus-covered Caco-2 cell epithelium. Eur. J. Pharm. Sci. 2018, 118, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Baldursdóttir, S.G.; Müllertz, A.; Nielsen, H.M. Property profiling of biosimilar mucus in a novel mucus-containing in vitro model for assessment of intestinal drug absorption. Eur. J. Pharm. Biopharm. 2014, 87, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Quaresma, P.; Lobo, J.M.S.; Amaral, M.H. Development of mucoadhesive and thermosensitive eyedrops to improve the ophthalmic bioavailability of ibuprofen. J. Drug Deliv. Sci. Technol. 2016, 35, 69–80. [Google Scholar] [CrossRef]
- Jain, A.; Hurkat, P.; Jain, A.; Jain, A.; Jain, A.; Jain, S.K. Thiolated Polymers: Pharmaceutical Tool in Nasal Drug Delivery of Proteins and Peptides. Int. J. Pept. Res. Ther. 2019, 25, 15–26. [Google Scholar] [CrossRef]
- Duan, H.; Lü, S.; Gao, C.; Bai, X.; Qin, H.; Wei, Y.; Wu, X.; Liu, M. Mucoadhesive microparticulates based on polysaccharide for target dual drug delivery of 5-aminosalicylic acid and curcumin to inflamed colon. Colloids Surf. B Biointerfaces 2016, 145, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin. Drug Deliv. 2015, 12, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Hutton, D.A.; Pearson, J.P. The MUC2 gene product: A human intestinal mucin. Int. J. Biochem. Cell Boil. 1998, 30, 797–801. [Google Scholar] [CrossRef]
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E.; et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, 266ra172. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, Y.; Mann, M.M.; Funderburgh, J.L.; Wagner, W.R. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp. Eye Res. 2014, 120, 71–81. [Google Scholar] [CrossRef] [PubMed]
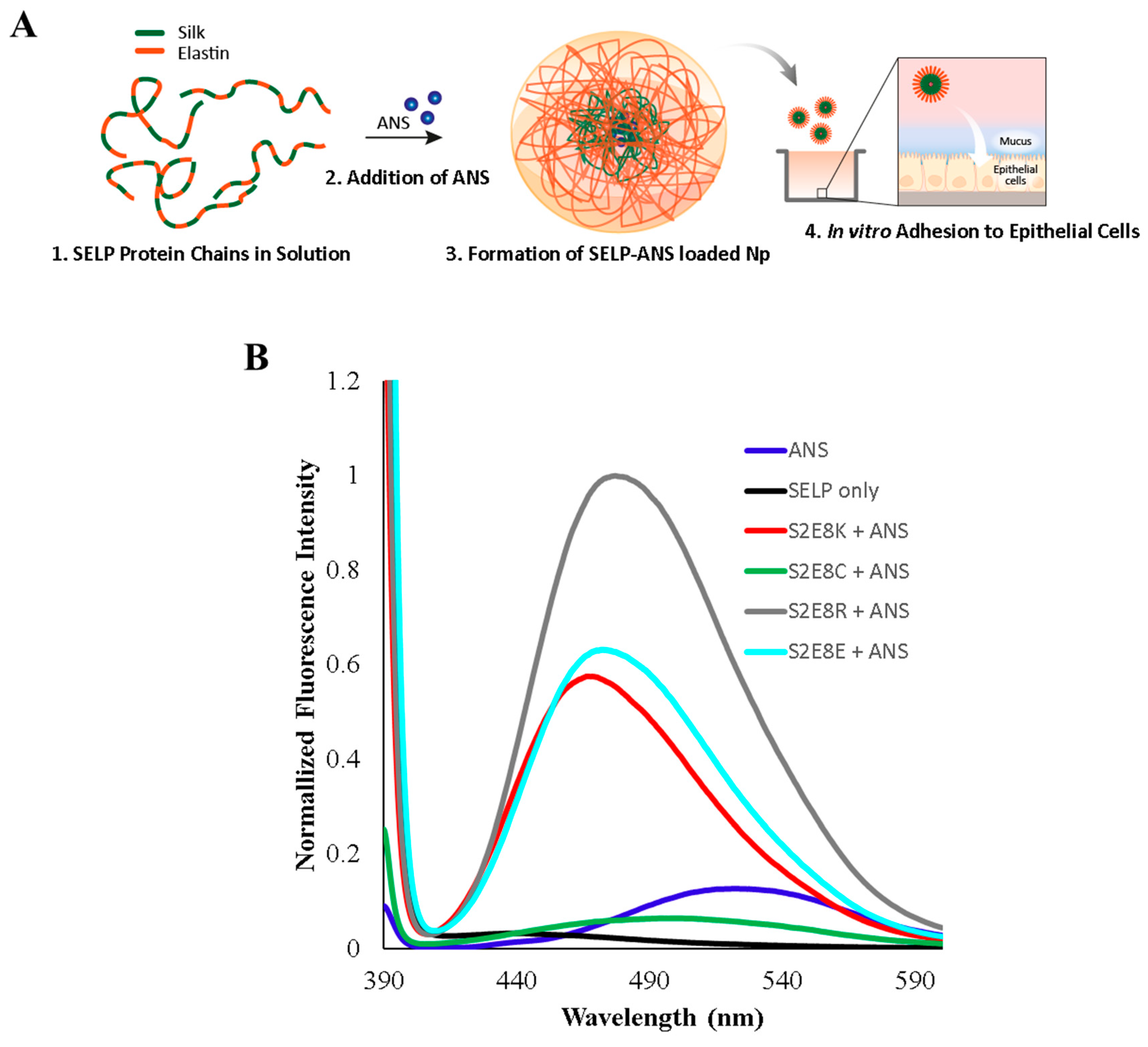
| Name | Sequence | MW (kDa) | Np Size (nm) | Np PDI | Fluorescence λmax (nm) |
|---|---|---|---|---|---|
| S2E8R | GAGAGSGAGAGSGVGVPGVGVPGVGVP GVGVPGRGVPGVGVPGVGVPGVGVP | 63 | 78 ± 1 | 0.2 | 479 ± 3 |
| S2E8K | GAGAGSGAGAGSGVGVPGVGVPGVGVP GVGVPGKGVPGVGVPGVGVPGVGVP | 57 | 102 ± 3 | 0.3 | 470 ± 2 |
| S2E8E | GAGAGSGAGAGSGVGVPGVGVPGVGVP GVGVPGEGVPGVGVPGVGVPGVGVP | 65 | 206 ± 3 | 0.2 | 477 ± 2 |
| S2E8C | GAGAGSGAGAGSGVGVPGVGVPGVGVP GVGVPGCGVPGVGVPGVGVPGVGVP | 58 | 73 ± 4 | 0.4 | 500 ± 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, R.N.; Wu, W.A.; McKay, T.B.; Xu, Q.; Kaplan, D.L. Design of Silk-Elastin-Like Protein Nanoparticle Systems with Mucoadhesive Properties. J. Funct. Biomater. 2019, 10, 49. https://doi.org/10.3390/jfb10040049
Parker RN, Wu WA, McKay TB, Xu Q, Kaplan DL. Design of Silk-Elastin-Like Protein Nanoparticle Systems with Mucoadhesive Properties. Journal of Functional Biomaterials. 2019; 10(4):49. https://doi.org/10.3390/jfb10040049
Chicago/Turabian StyleParker, Rachael N., Wenyao A. Wu, Tina B. McKay, Qiaobing Xu, and David L. Kaplan. 2019. "Design of Silk-Elastin-Like Protein Nanoparticle Systems with Mucoadhesive Properties" Journal of Functional Biomaterials 10, no. 4: 49. https://doi.org/10.3390/jfb10040049
APA StyleParker, R. N., Wu, W. A., McKay, T. B., Xu, Q., & Kaplan, D. L. (2019). Design of Silk-Elastin-Like Protein Nanoparticle Systems with Mucoadhesive Properties. Journal of Functional Biomaterials, 10(4), 49. https://doi.org/10.3390/jfb10040049





