Analysis of Sulfated Glycosaminoglycans in ECM Scaffolds for Tissue Engineering Applications: Modified Alcian Blue Method Development and Validation
Abstract
1. Introduction
2. Results
2.1. Optimization of the AB Method Conditions
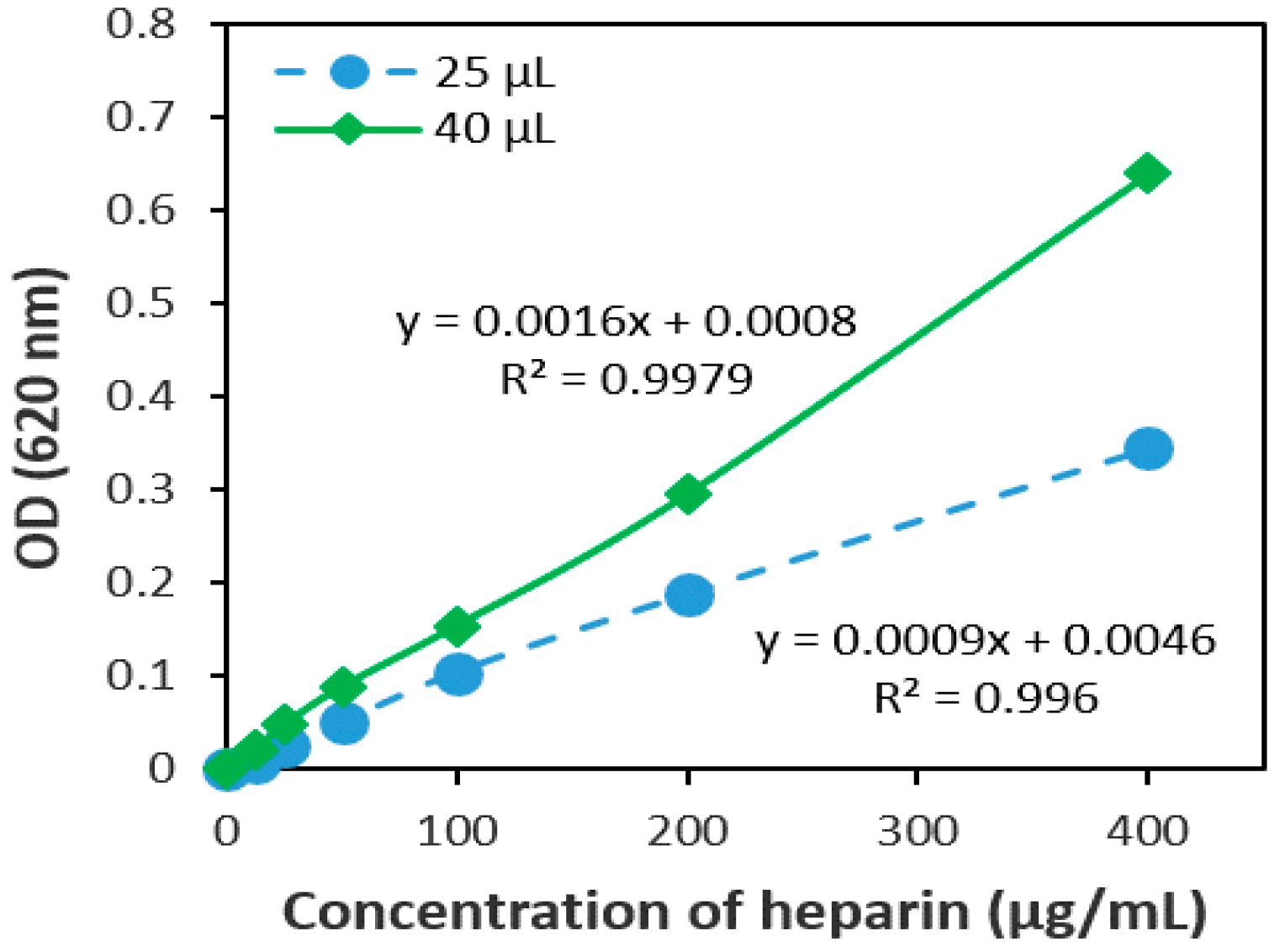
2.1.1. The Volume Ratio of the Sample and AB Dye
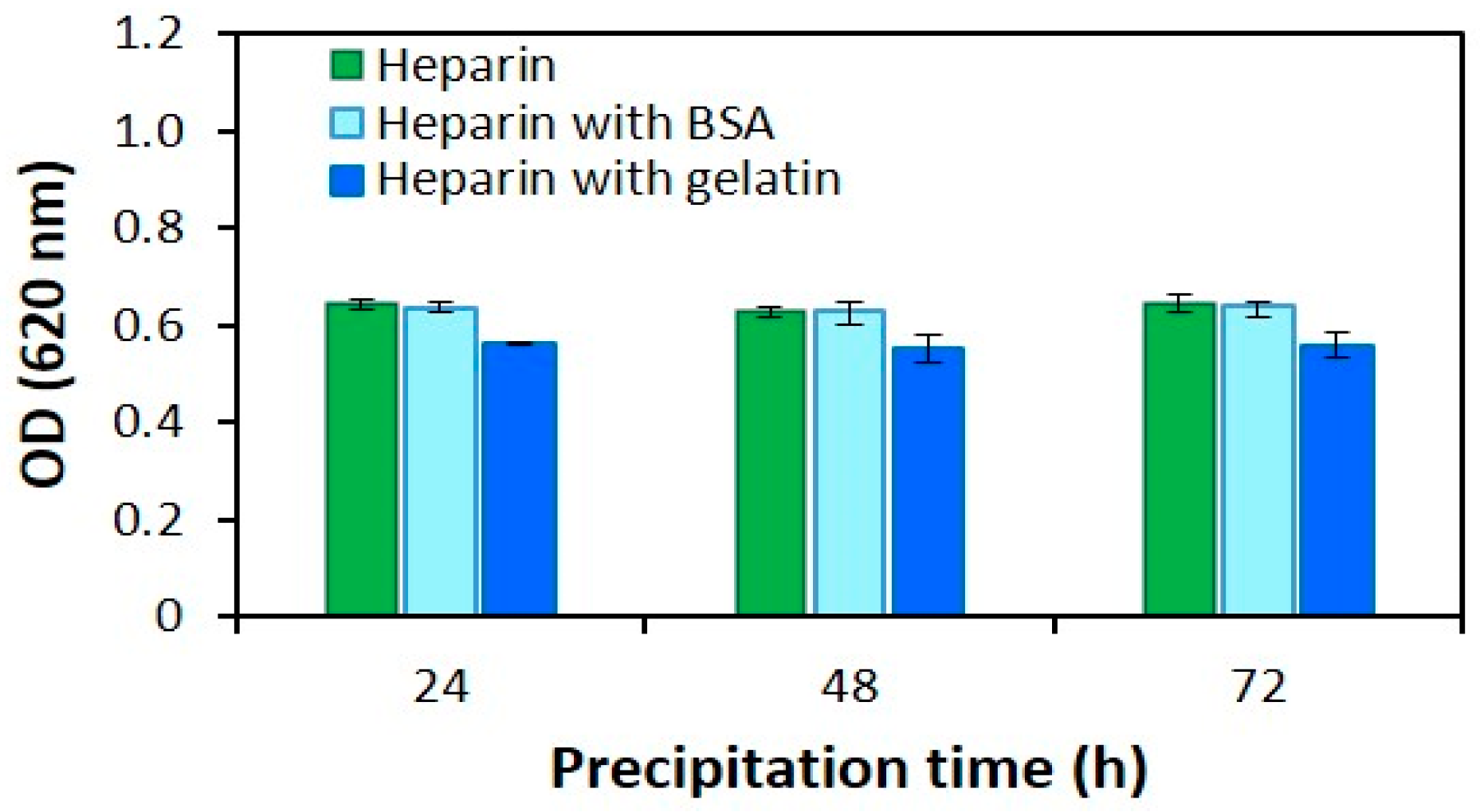
2.1.2. Precipitation Time
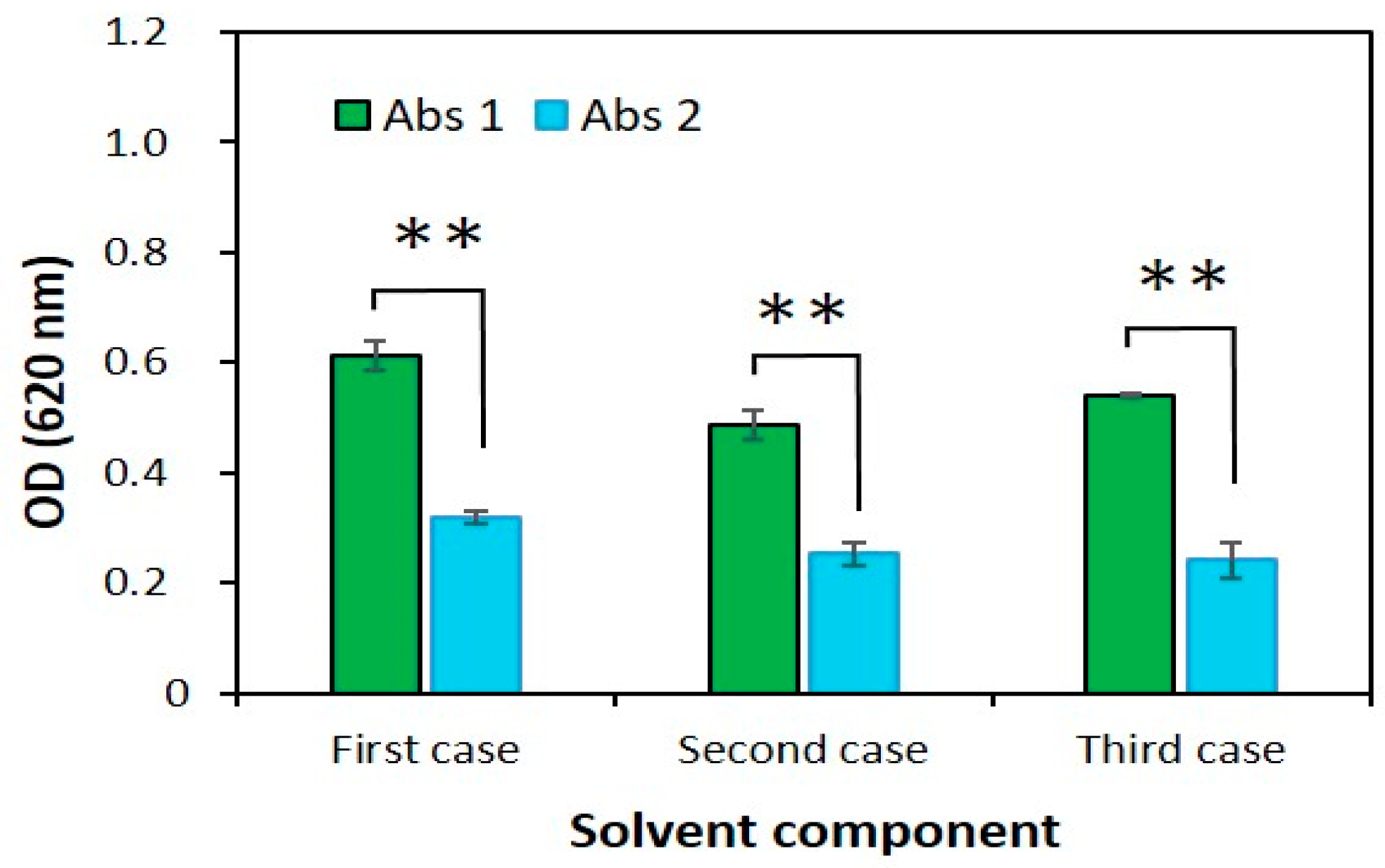
2.1.3. Solvent Component
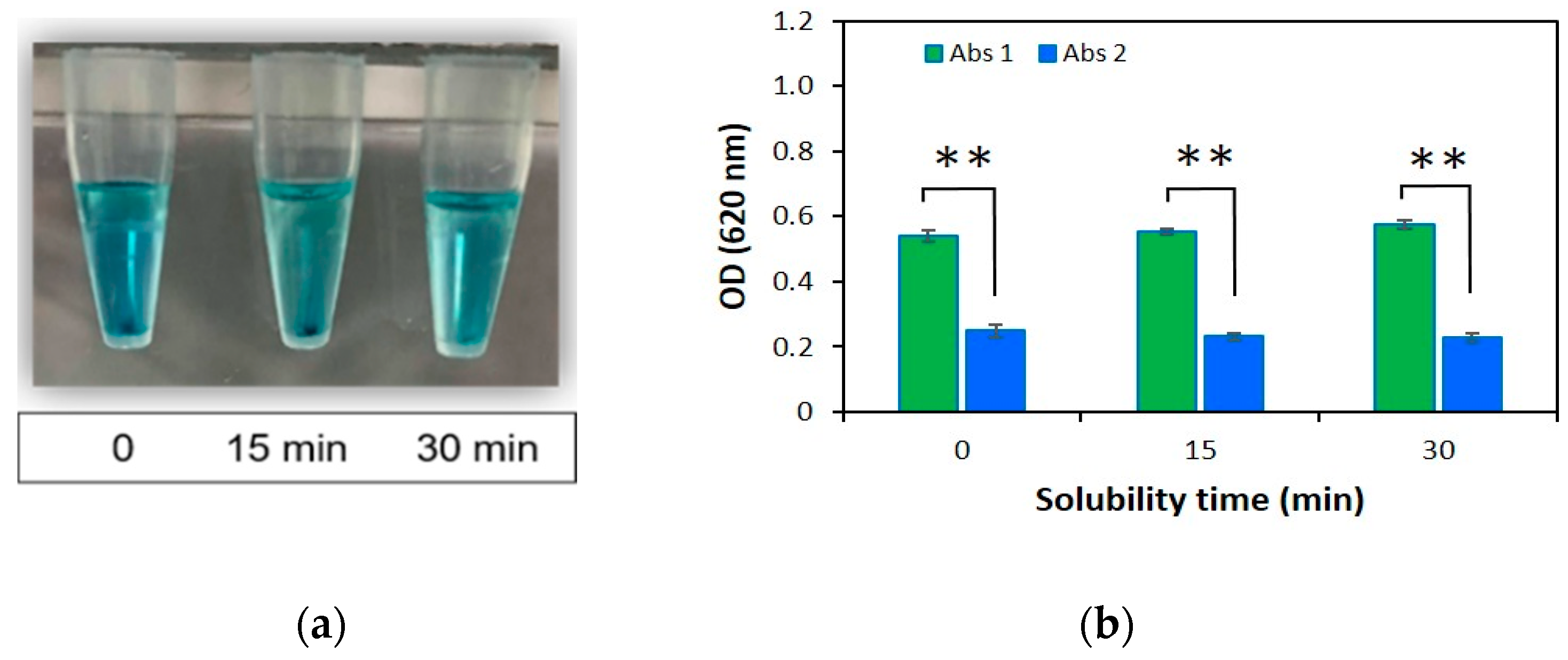
2.1.4. Solubility Time
2.2. Method Validation
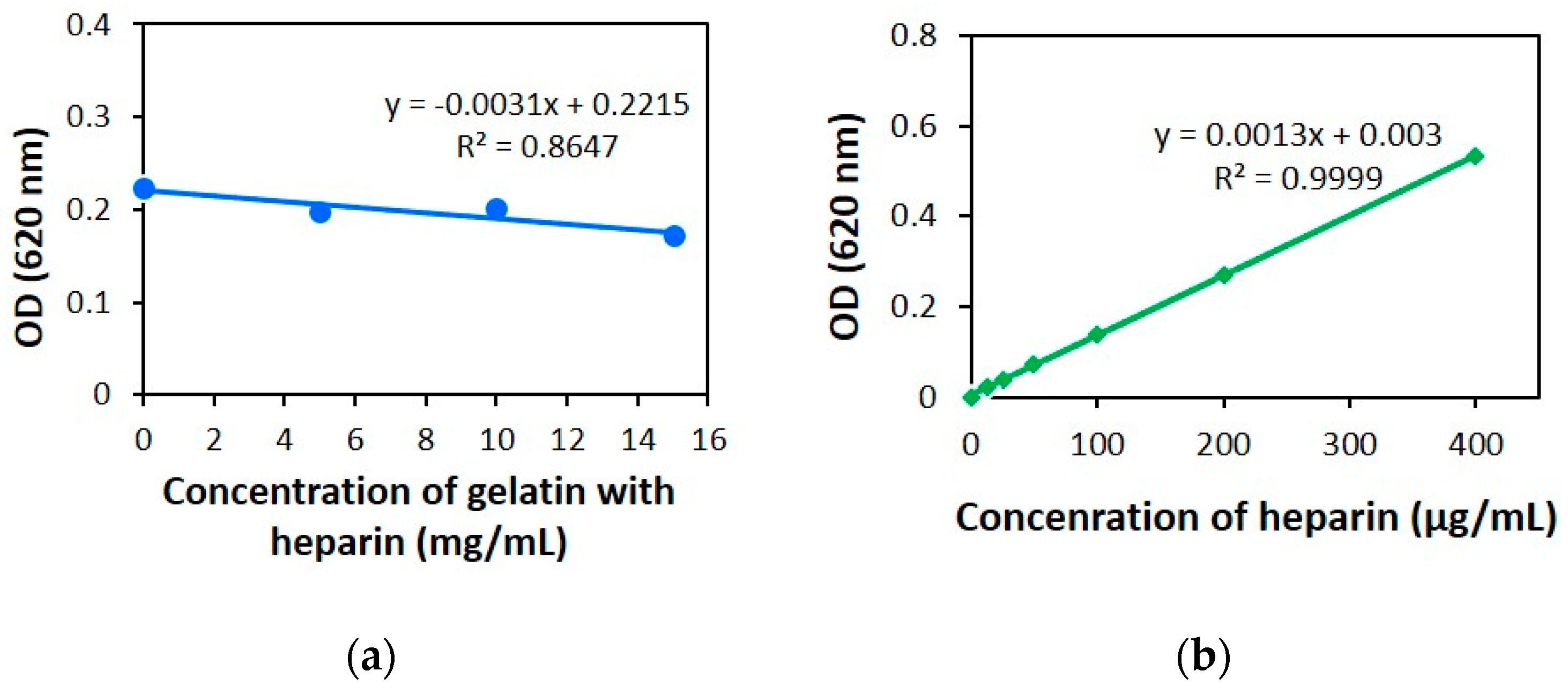
2.2.1. Linearity
2.2.2. Limit of Detection (LOD) and Quantification (LOQ)
2.2.3. Accuracy
2.2.4. Precision
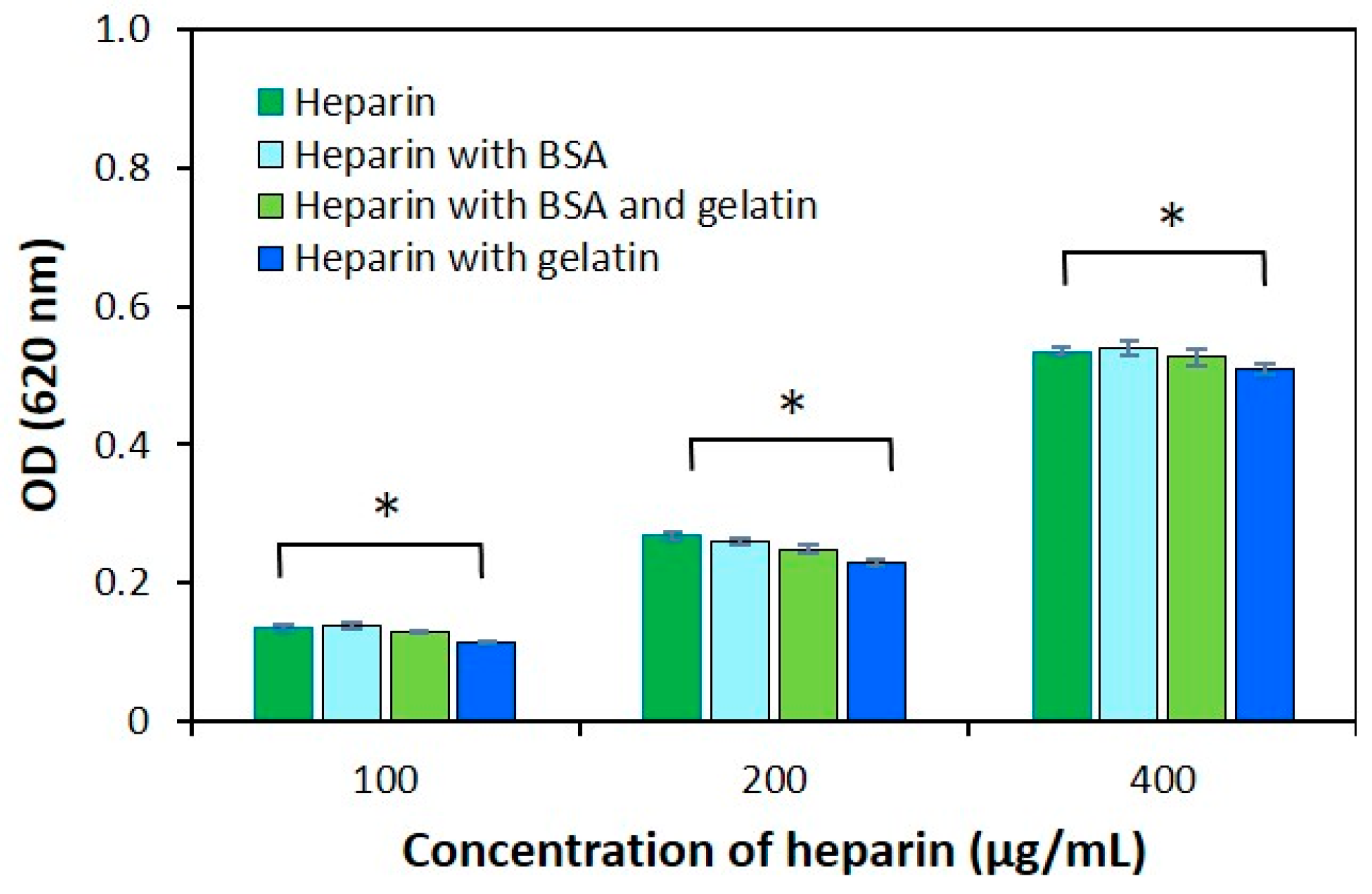
2.3. The Effect of Protein Structure on the Modified AB Method
2.4. Quantification of sGAGs in the L-ECM
3. Discussion
3.1. Assay Optimization
3.2. Method Validation
3.3. Protein Effect
3.4. Method Application
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Solutions Used in the Method Developing Process
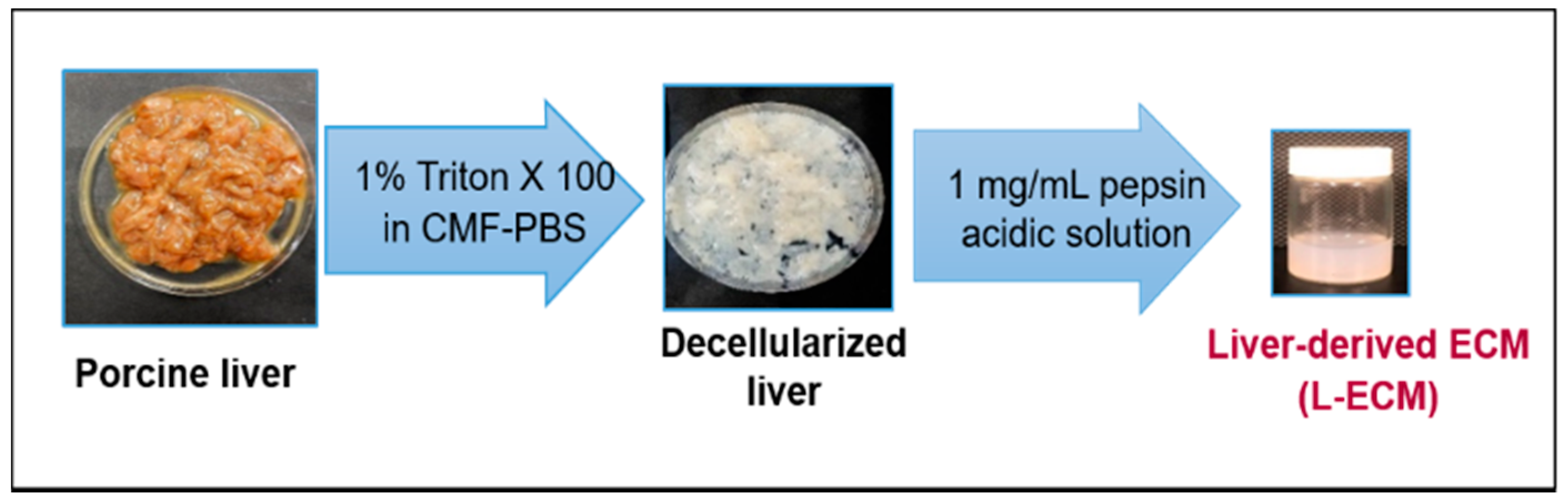
4.3. Decellularization and Solubility of the Porcine Liver
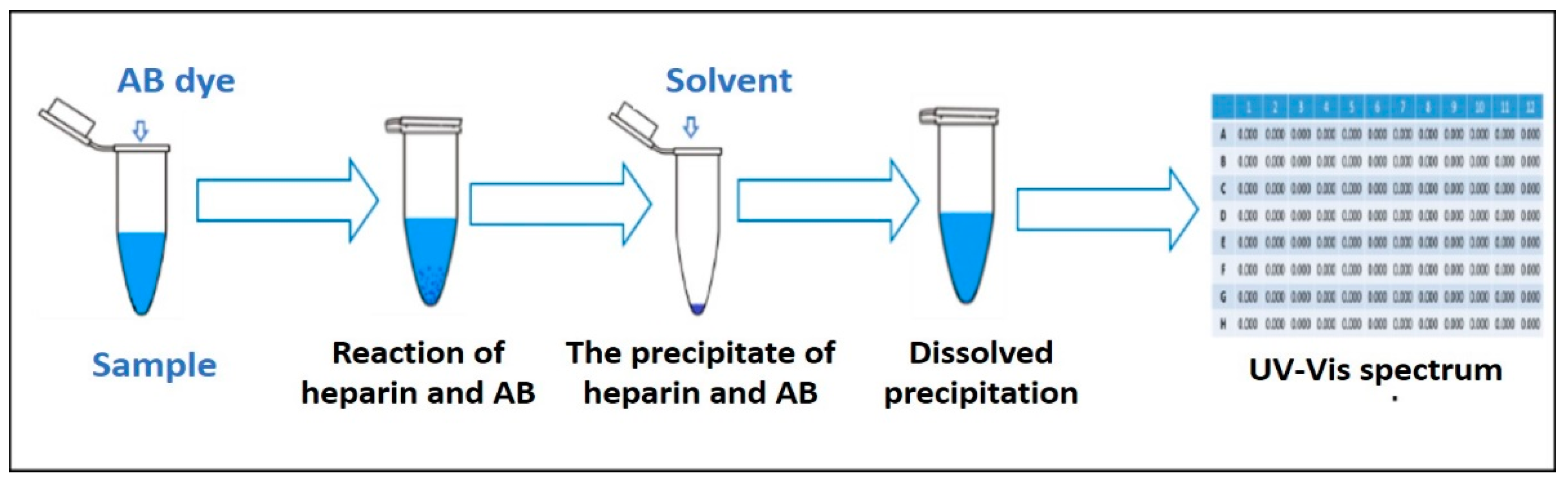
4.4. The Modified AB Method
4.5. Optimization of the Modified AB Method
4.6. Validation of the Modified AB Method
4.7. Determination of the Effect of Protein Structure on the Modified AB Method
4.8. The Amount of sGAG in Solubilized L-ECM
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Koide, N.; Shinji, T.; Tanabe, T.; Asano, K.; Kawaguchi, M.; Sakaguchi, K.; Koide, Y.; Mori, M.; Tsuji, T. Continued high albumin production by multicellular spheroids of adult rat hepatocytes formed in the presence of liver-derived proteoglycans. Biochem. Biophys. Commun. 1989, 161, 385–391. [Google Scholar] [CrossRef]
- Damania, A.; Kumar, A.; Teotia, A.K.; Kimura, H.; Kamihira, M.; Ijima, H.; Sarin, S.K.; Kumar, A. Decellularized liver matrix-modified cryogel scaffolds as potential hepatocyte carriers in bioartificial liver support systems and implantable liver constructs. ACS Appl. Mater. Interfaces 2018, 10, 114–126. [Google Scholar] [CrossRef]
- Schaefer, L.; Roland, M. Proteoglycans: From structural compounds to signaling molecules. Cell Tissue Res. 2010, 339, 237–246. [Google Scholar] [CrossRef]
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and sulfated glycosaminoglycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; Chapter 17. [Google Scholar]
- Sasisekharan, R.; Shriver, Z.; Venkataraman, G.; Narayanasami, U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat. Rev. Cancer 2002, 2, 521–528. [Google Scholar] [CrossRef]
- Dreyfuss, J.L.; Regatieri, C.V.; Jarrouge, T.R.; Cavalheiro, R.P.; Sampaio, L.O.; Nader, H.B. Heparan sulfate proteoglycans: Structure, protein interactions and cell signaling. Acad. Bras. Ciênc. 2009, 81, 409–429. [Google Scholar] [CrossRef]
- Meneghetti, M.C.Z.; Hughes, A.J.; Rudd, T.R.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef]
- Ishan, C.; Robert, J.L. Heparin-protein interactions. Angew. Chem. 2002, 41, 391–412. [Google Scholar]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar] [CrossRef]
- Studelska, D.R.; Giljum, K.; McDowell, L.M.; Zhang, L. Quantification of glycosaminoglycans by reversed-phase HPLC separation of fluorescent isoindole derivatives. Glycobiology 2006, 16, 65–72. [Google Scholar] [CrossRef]
- Guerrini, M.; Naggi, A.; Guglieri, S.; Santarsiero, R.; Torri, G. Complex glycosaminoglycans: Profiling substitution patterns by two-dimensional nuclear magnetic resonance spectroscopy. Anal. Biochem. 2005, 337, 35–47. [Google Scholar] [CrossRef]
- Yu, Y.; Sweeney, M.D.; Saad, O.M.; Crown, S.E.; Handel, T.M.; Leary, J.A. Chemokine-Glycosaminoglycan Binding: Specificity for CCR2 ligand binding to highly sulfated oligosaccharides using FTICR mass spectrometry. J. Boil. Chem. 2005, 280, 32200–32208. [Google Scholar] [CrossRef]
- Volpi, N.; Galeotti, F.; Yang, B.; Linhardt, R.J. Analysis of glycosaminoglycan-derived, precolumn, 2-aminoacridone–labeled disaccharides with LC-fluorescence and LC-MS detection. Nat. Protoc. 2014, 9, 541–558. [Google Scholar] [CrossRef]
- Price, K.N.; Tuinman, A.; Baker, D.C.; Chisena, C.; Cysyk, R.L. Isolation and characterization by electrospray-ionization mass spectrometry and high-performance anion-exchange chromatography of oligosaccharides derived from hyaluronic acid by hyaluronate lyase digestion: Observation of some heretofore unobserved oligo. Carbohydr Res. 1997, 303, 303–311. [Google Scholar] [CrossRef]
- Platzer, M.; Ozegowski, J.; Neubert, R. Quantification of hyaluronan in pharmaceutical formulations using high performance capillary electrophoresis and the modified uronic acid carbazole reaction. J. Pharm. Biomed. Anal. 1999, 21, 491–496. [Google Scholar] [CrossRef]
- Terry, D.; Chopra, R.; Ovenden, J.; Anastassiades, T. Differential Use of Alcian Blue and Toluidine Blue Dyes for the Quantification and Isolation of Anionic Glycoconjugates from Cell Cultures: Application to Proteoglycans and a High-Molecular-Weight Glycoprotein Synthesized by Articular Chondrocytes. Anal. Biochem. 2000, 285, 211–219. [Google Scholar] [CrossRef]
- Jiao, Q.; Liu, Q. Characterization of the interaction between methylene blue and glycosaminoglycans. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1999, 55, 1667–1673. [Google Scholar] [CrossRef]
- Hattori, M.; Arita, J.; Tabuchi, N.; Koike, S.; Nakamura, H. Fractional determination of sodium chondroitin sulfate/hydroxypropylmethyl cellulose mixture using Alucian Blue 8GX. Bunseki Kagaku. 2003, 52, 259–263. [Google Scholar] [CrossRef][Green Version]
- Frazier, S.B.; Roodhouse, K.A.; Hourcade, D.E.; Zhang, L. The Quantification of Glycosaminoglycans: A Comparison of HPLC, Carbazole, and Alcian Blue Methods. NIH Public Access. Open Glycosci. 2008, 1, 31–39. [Google Scholar] [CrossRef]
- Scott, J.E.; Quintarelli, G.; Dellovo, M.C. The chemical and histochemical properties of Alcian Blue—I. The mechanism of Alcian Blue staining. Histochemie 1964, 4, 73–85. [Google Scholar] [CrossRef]
- Alberto, L.; Anelis, Q.; Meily, S.; Elias, N.R.; Jose, C.; Julio, C.S. Rapid and sensitive anthrone e sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar]
- U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Veterinary Medicine (CVM). Guidance for Industry. Bioanalytical Method Validation. May 2018. Available online: http://www.labcompliance.de/documents/FDA/FDA-Others/Laboratory/f-507-bioanalytical-4252fnl.pdf (accessed on 30 April 2019).
- Behera, S.; Ghanty, S.; Ahmad, F.; Santra, S.; Banerjee, S. Analytical & Bioanalytical techniques UV-visible spectrophotometric method development and validation of assay of paracetamol tablet formulation. J. Anal. Bioanal. Tech. 2012, 3, 1656–1661. [Google Scholar]
- Ich. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology; European Medicines Agency: Amsterdam, The Netherlands, 2005; p. 17.
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Karlsson, M.; Björnsson, S. Quantitation of proteoglycans in biological fluids using Alcian blue. Methods Mol. Biol. 2001, 171, 159–173. [Google Scholar] [PubMed]
- Mulloy, B. The specificity of interactions between proteins and sulfated polysaccharides. Acad. Bras. Ciênc 2005, 77, 651–664. [Google Scholar] [CrossRef]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. Soc. B Boil. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Raspanti, M.; Viola, M.; Forlino, A.; Tenni, R.; Gruppi, C.; Tira, M.E. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J. Struct. Boil. 2008, 164, 134–139. [Google Scholar] [CrossRef]
- Peysselon, F.; Ricard-Blum, S. Heparin–protein interactions: From affinity and kinetics to biological roles. Application to an interaction network regulating angiogenesis. Matrix Biol. 2014, 35, 73–81. [Google Scholar] [CrossRef]
| Case No. | Chemicals | Ratio | Final Concentration of NaOH | The Dilution Ratio (OD.1/OD.2) |
|---|---|---|---|---|
| I | 5 N NaOH and 2-Aminoethanol | 1: 49 | 0.1 N | 1.9 |
| II | 5 N NaOH and 2-Aminoethanol | 1: 4.0 | 1.0 N | 1.9 |
| III | 25 N NaOH and 2-Aminoethanol | 1: 24 | 1.0 N | 2.2 |
| Concentration of Heparin (µg/mL) | 400 | 200 | 100 | 50 | 25 | 12.5 |
|---|---|---|---|---|---|---|
| Mean OD 1 (n = 3) | 0.534 | 0.268 | 0.136 | 0.071 | 0.036 | 0.021 |
| Standard deviation, SD | 0.017 | 0.007 | 0.006 | 0.004 | 0.002 | 0.002 |
| Parameter | Value |
|---|---|
| λmax (nm) | 620.0 |
| Linearity range (µg/mL) | 12.5–400 |
| Slope (b) | 0.0013 |
| Intercept (a) | 0.003 |
| Correlation coefficient, R2 (n = 3) | >0.996 |
| SE of intercept | 0.0005 |
| SD of intercept | 0.0013 |
| Limit of detection (µg/mL) | 2.95 |
| Limit of quantification (µg/mL) | 9.82 |
| Recovery (%) | 99.07 |
| Nominal Concentration of Heparin (µg/mL) | Recovery (%) | |||
|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Average | |
| 25 | 100.83 | 100.97 | 101.44 | 101.08 |
| 50 | 98.77 | 94.97 | 100.72 | 98.63 |
| 100 | 97.67 | 99.95 | 98.36 | 99.09 |
| Intraday Precision | |||||
| Nominal concentration (µg/mL) | Spiked concentration ± SD 1 mean value, (µg/mL) | CV2 (%) | RE3 (%) | ||
| No. 1 | No. 2 | No. 3 | |||
| 25 | 24.13 ± 0.89 | 24.87 ± 1.18 | 25.62 ± 1.54 | 4.80 | 0.515 |
| 50 | 51.82 ± 2.47 | 50.77 ± 2.31 | 47.92 ± 2.31 | 4.71 | −0.341 |
| 100 | 97.46 ± 2.04 | 101.28 ± 1.94 | 102.79 ± 2.47 | 2.14 | −0.51 |
| Interday precision | |||||
| Nominal concentration (µg/mL) | Spiked concentration ± SD 1 mean value, (µg/mL) | CV2 (%) | RE3 (%) | ||
| Day 1 | Day 2 | Day 3 | |||
| 25 | 24.87 ± 1.18 | 25.18 ± 2.47 | 24.59 ± 1.60 | 7.02 | 0.48 |
| 50 | 50.77 ± 2.31 | 47.49 ± 2.70 | 49.97 ± 4.37 | 6.33 | 1.19 |
| 100 | 101.28 ± 1.94 | 98.77 ± 2.31 | 97.67 ± 5.12 | 3.16 | 0.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iimaa, T.; Ikegami, Y.; Bual, R.; Shirakigawa, N.; Ijima, H. Analysis of Sulfated Glycosaminoglycans in ECM Scaffolds for Tissue Engineering Applications: Modified Alcian Blue Method Development and Validation. J. Funct. Biomater. 2019, 10, 19. https://doi.org/10.3390/jfb10020019
Iimaa T, Ikegami Y, Bual R, Shirakigawa N, Ijima H. Analysis of Sulfated Glycosaminoglycans in ECM Scaffolds for Tissue Engineering Applications: Modified Alcian Blue Method Development and Validation. Journal of Functional Biomaterials. 2019; 10(2):19. https://doi.org/10.3390/jfb10020019
Chicago/Turabian StyleIimaa, Tuyajargal, Yasuhiro Ikegami, Ronald Bual, Nana Shirakigawa, and Hiroyuki Ijima. 2019. "Analysis of Sulfated Glycosaminoglycans in ECM Scaffolds for Tissue Engineering Applications: Modified Alcian Blue Method Development and Validation" Journal of Functional Biomaterials 10, no. 2: 19. https://doi.org/10.3390/jfb10020019
APA StyleIimaa, T., Ikegami, Y., Bual, R., Shirakigawa, N., & Ijima, H. (2019). Analysis of Sulfated Glycosaminoglycans in ECM Scaffolds for Tissue Engineering Applications: Modified Alcian Blue Method Development and Validation. Journal of Functional Biomaterials, 10(2), 19. https://doi.org/10.3390/jfb10020019






