Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
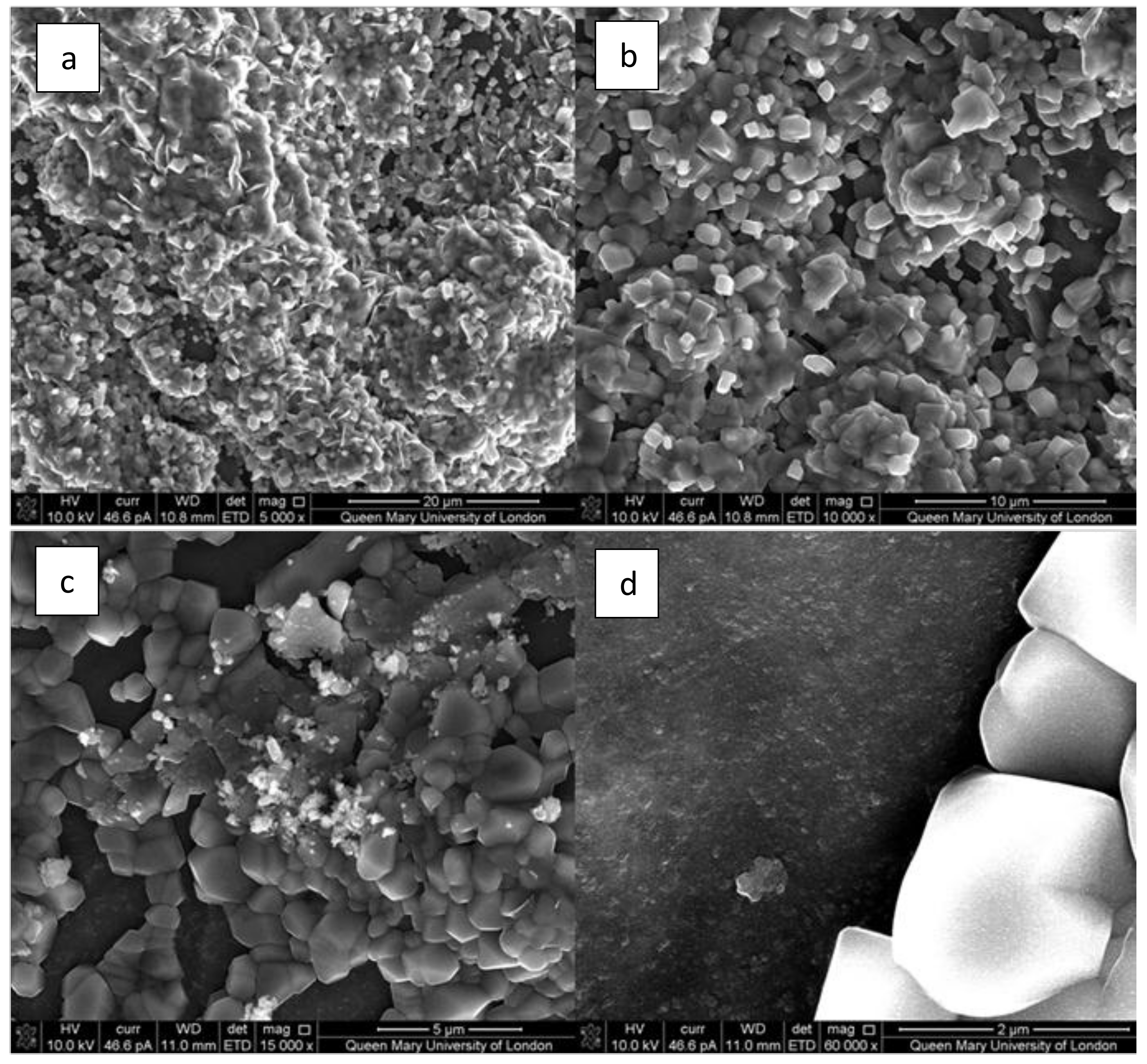
2.2.1. Glass Characterization
2.2.2. Fabrication of Composite Scaffold
2.2.3. Characterization of Scaffolds
2.2.4. Statistical Analysis
2.2.5. Evaluation of Bioactivity of Scaffolds in Simulated Body Fluid
3. Results and Discussion
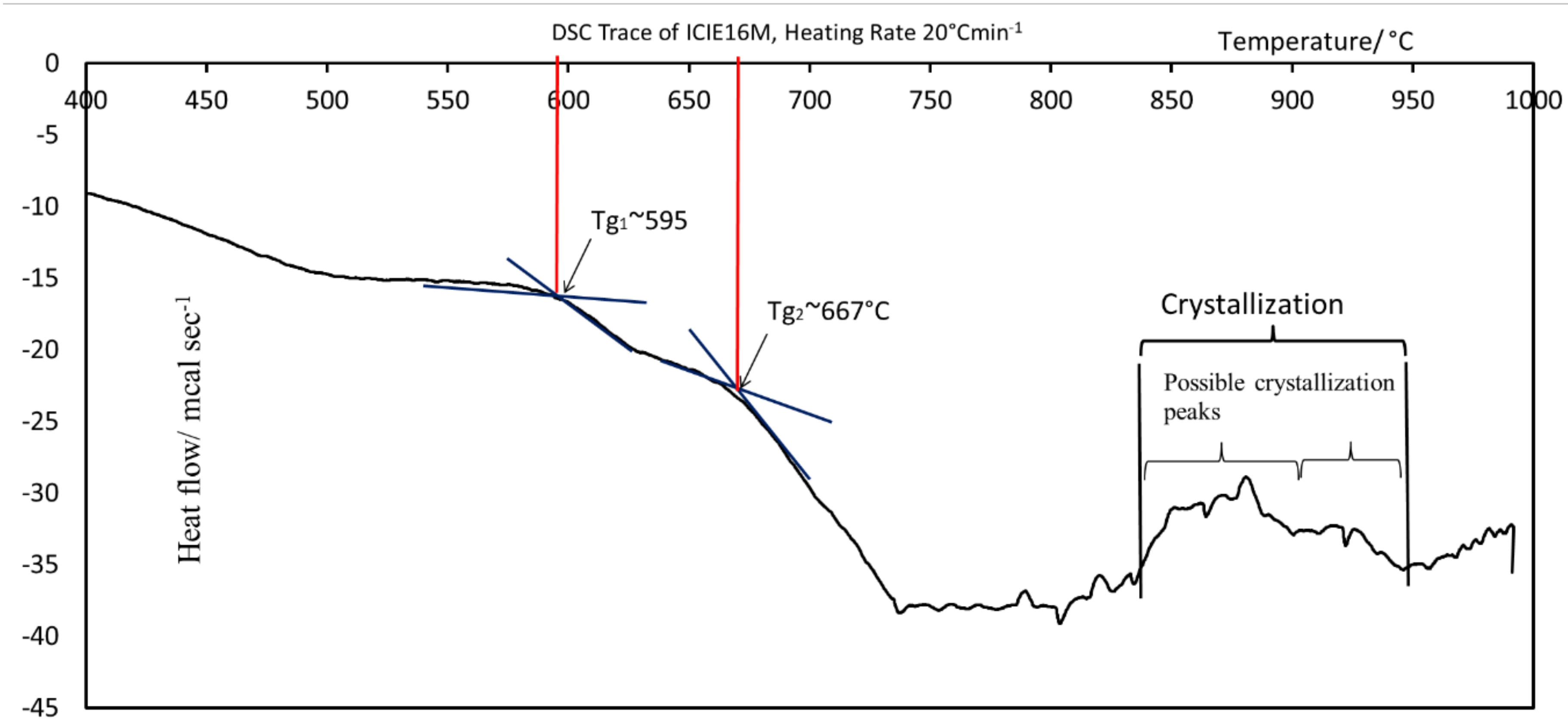
3.1. DSC Analysis of ICIE16M Glass Powder
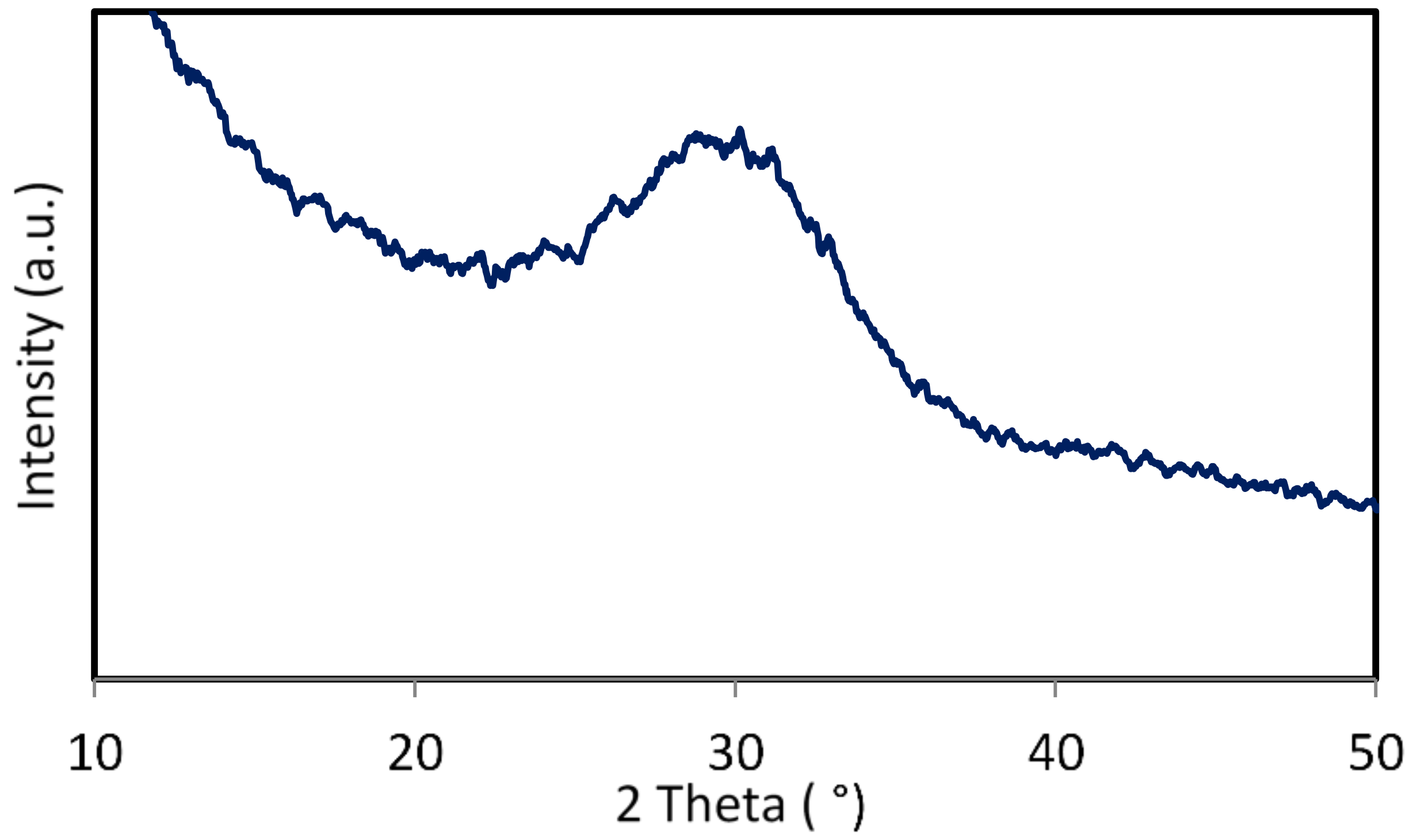
3.2. X-Ray Diffraction Analysis of ICIE16M Bioactive Glass
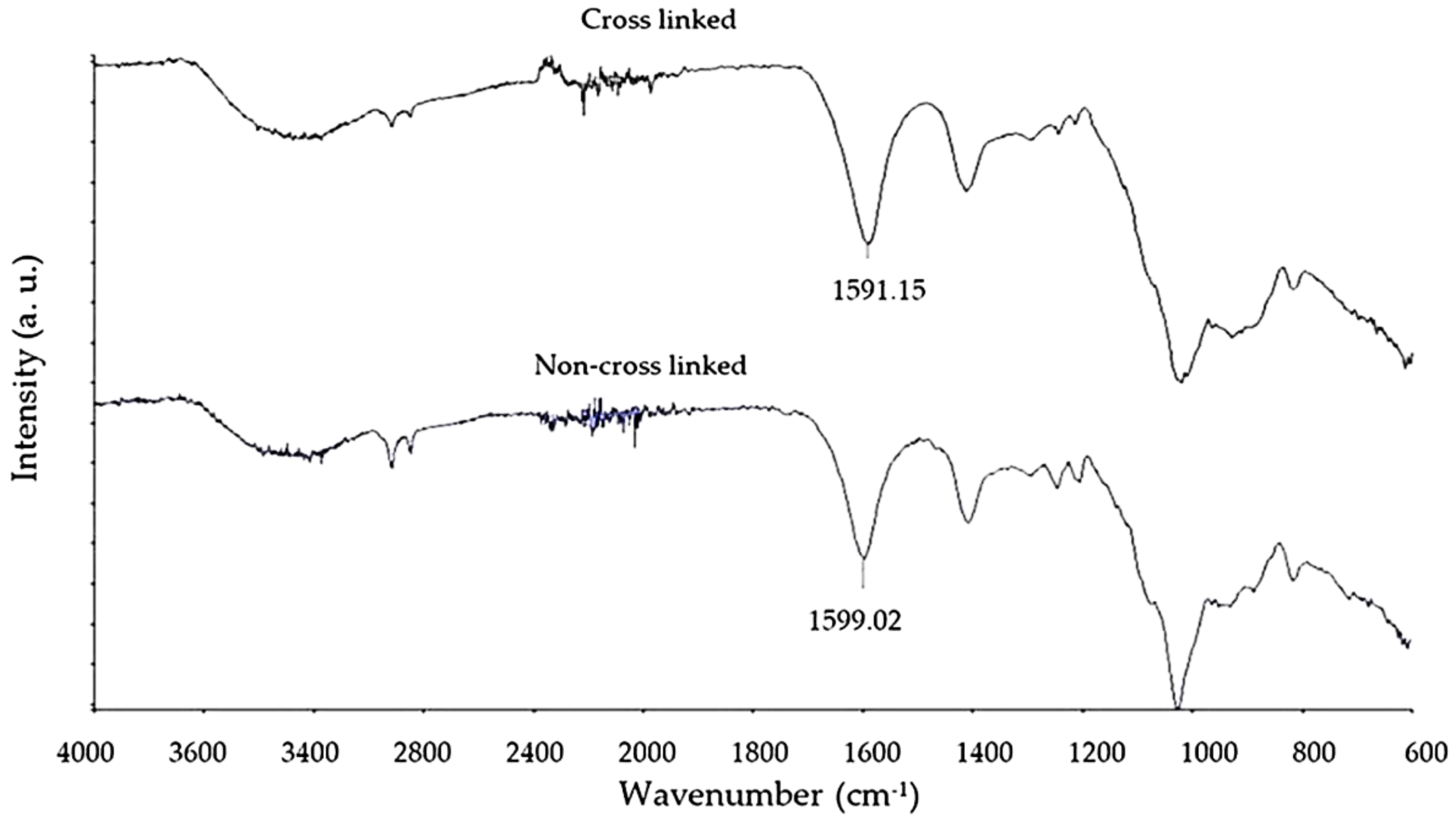
3.3. Fourier Transform Infrared Spectroscopy Analysis of Crosslinking
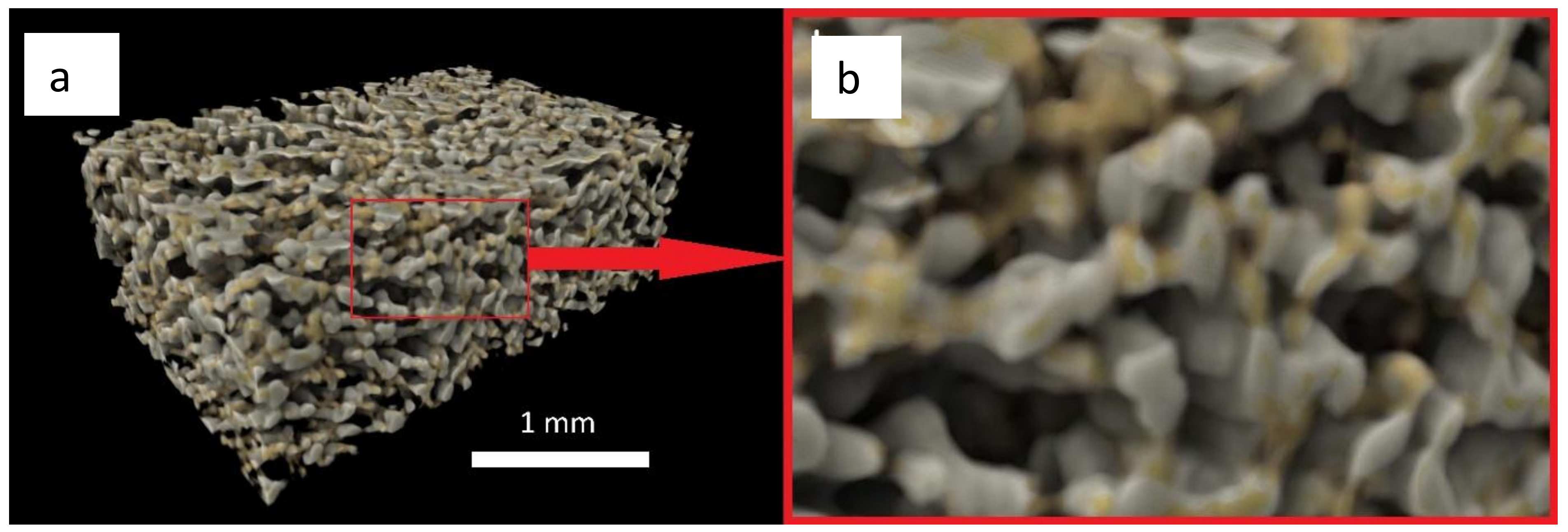
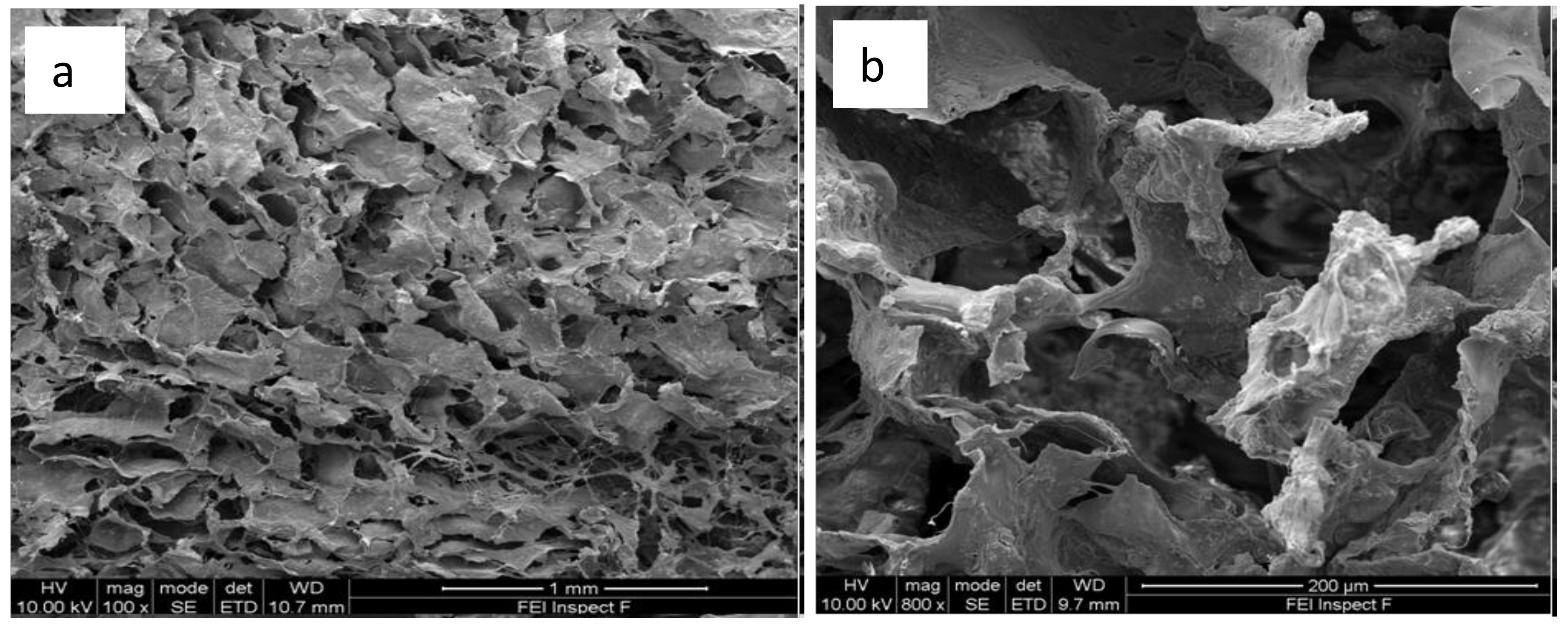
3.4. X-Ray Microtomography and Scanning Electron Microscopy Imaging of Scaffolds
3.5. Compression Testing of Scaffolds
3.6. Bioactivity of Composite Scaffolds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Romagnoli, C.; D’Asta, F.; Brandi, M.L. Drug delivery using composite scaffolds in the context of bone tissue engineering. Clin. Cases Miner. Bone Metab. 2013, 10, 155–161. [Google Scholar] [PubMed]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone graft substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Broggini, N.; Hjørting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and β-tricalcium phosphate: A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2006, 17, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.S.; Mobin, S.S.N.; Lypka, M.A.; Rommer, E.; Yen, S.; Urata, M.M.; Hammoudeh, J.A. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast. Reconstr. Surg. 2013, 131, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Tsesis, I.; Rosen, E.; Tamse, A.; Taschieri, S.; Del Fabbro, M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: A systematic review and meta-analysis. J. Endod. 2011, 37, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, H.; Song, X.; Gu, X.; Sun, C. Biomaterials for periodontal tissue regeneration. Rev. Adv. Mater. Sci. 2015, 40, 209–214. [Google Scholar]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Adams, D.J.; Laurencin, C.T.; Nukavarapu, S.P. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng. Part A 2012, 18, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Comparison of bioactive glass coated and hydroxyapatite coated titanium dental implants in the human jaw bone. Aust. Dent. J. 2011, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Nair, P.D. Novel porous, polysaccharide scaffolds for tissue engineering applications. Trends Biomater. Artif. Organs 2005, 18, 219–224. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials 1997, 18, 583–590. [Google Scholar] [CrossRef]
- Zmora, S.; Glicklis, R.; Cohen, S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials 2002, 23, 4087–4094. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The design of scaffolds for use in tissue engineering. Part, I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, S.-H.; Khang, D.; Choi, J.; Kim, H.; Kang, M. Biomimetic component coating on 3D scaffolds using high bioactivity of mesoporous bioactive ceramics. Int. J. Nanomedicine 2011, 6, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Yunos, D.M.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Taboas, J.M.; Maddox, R.D.; Krebsbach, P.H.; Hollister, S.J. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 2003, 24, 181–194. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Mano, J.F.; Reis, R.L. Nature-inspired calcium phosphate coatings: Present status and novel advances in the science of mimicry. Curr. Opin. Solid State Mater. Sci. 2003, 7, 309–318. [Google Scholar] [CrossRef]
- Chung, S.; Ingle, N.P.; Montero, G.A.; Kim, S.H.; King, M.W. Bioresorbable elastomeric vascular tissue engineering scaffolds via melt spinning and electrospinning. Acta Biomater. 2010, 6, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.; Wang, Y.; Zhu, Y.; Zhang, Y.; Gao, C. Poly (lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Pagliari, S.; Rinaldi, A.; Forte, G.; Fiaccavento, R.; Pagliari, F.; Franzese, O.; Minieri, M.; Di Nardo, P.; Licoccia, S. Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning. Acta Biomater. 2010, 6, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Knowles, J.C.; Day, R.M. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater. 2008, 4, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Budyanto, L.; Goh, Y.Q.; Ooi, C.P. Fabrication of porous poly (L-lactide)(PLLA) scaffolds for tissue engineering using liquid–liquid phase separation and freeze extraction. J. Mater. Sci. Mater. Med. 2009, 20, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Heijkants, R.G.J.C.; Van Tienen, T.G.; De Groot, J.H.; Pennings, A.J.; Buma, P.; Veth, R.P.H.; Schouten, A.J. Preparation of a polyurethane scaffold for tissue engineering made by a combination of salt leaching and freeze-drying of dioxane. J. Mater. Sci. 2006, 41, 2423–2428. [Google Scholar] [CrossRef]
- Sultana, N.; Wang, M. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: Surface modification and in vitro biological evaluation. Biofabrication 2012, 4, 015003. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure; gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Lin, Y.J.; Yen, C.N.; Hu, Y.C.; Wu, Y.C.; Liao, C.J.; Chu, I.M. Chondrocytes culture in three-dimensional porous alginate scaffolds enhanced cell proliferation; matrix synthesis and gene expression. J. Biomed. Mater. Res. A. 2009, 88, 23–33. [Google Scholar] [CrossRef] [PubMed]
- De Paula, F.L.; Barreto, I.C.; Rocha-Leão, M.H.; Borojevic, R.; Rossi, A.M.; Rosa, F.P.; Farina, M. Hydroxyapatite-alginate biocomposite promotes bone mineralization in different length scales in vivo. Front. Mater. Sci. China 2009, 3, 145–153. [Google Scholar] [CrossRef]
- Draget, K.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from algae. Biopolymers 2005, 1–30. [Google Scholar]
- Saurez-Gonzales, D.; Barnhart, K.; Saito, E.; Vanderby, R., Jr.; Hollister, S.J.; Murphy, W.L. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J. Biomed. Mater. Res. A. 2010, 95, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.H.; Yu, X.W.; Zeng, S.X.; Du, R.L.; Hu, Y.H.; Yuan, Z.; Lu, E.Y.; Dai, K.R.; Tang, T.T. Enhanced osteointegration of orthopaedic implant gradient coating composed of bioactive glass and nanohydroxyapatite. J. Mater. Sci. Mater. Med. 2010, 21, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomaterialia 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Hill, R.G.; Brauer, D.S. Predicting the bioactivity of glasses using the network connectivity or split network models. J. Non Cryst. Solids 2011, 357, 3884–3887. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Sriranganathan, D.; Kanwal, N.; Hing, K.A.; Hill, R.G. Strontium substituted bioactive glasses for tissue engineered scaffolds: The importance of octacalcium phosphate. J Mater Sci: Mater Med. 2016, 27, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F.; Day, D.E. Mechanical and in vitro performance of 13–93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008, 4, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Watts, S.; Hill, R.; Law, R. The effect of phosphate content on the bioactivity of soda-lime-phosphosilicate glasses. J. Mater Sci. 2009, 20, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Bretcanu, O.; Samaille, C.; Boccaccini, A.R. Simple methods to fabricate Bioglass-derived glass-ceramic scaffolds exhibiting porosity gradient. J. Mater Sci. Mater Med. 2008, 43, 4127–4134. [Google Scholar] [CrossRef]
- Vaz, F.M.; Canhão, H.; Fonseca, J.E. Bone: A Composite Natural Material. In Advances in Composite Materials—Analysis of Natural and Man-Made Materials; Tesinova, P., Ed.; In Tech: Lisbon, Portugal, 2011. [Google Scholar]
- Echezarreta-Lopez, M.M.; de Miguel, T.; Quintero, F.; Pou, J.; Landin, M. Fabrication of Zn-Sr–doped laser-spinning glass nanofibers with antibacterial properties. J. Biomater. Appl. 2017, 31, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Elgayar, I.; Aliev, A.E.; Boccaccini, A.R.; Hill, R.G. Structural analysis of bioactive glasses. J. Non Cryst. Solids. 2005, 351, 173–183. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Hill, R.G.; Yue, S.; Nightingale, D.; Lee, P.D.; Jones, J.R. Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater. 2011, 7, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2015, 32, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.S.; Zhou, W.S.; He, X.W.; Liu, W.; Bai, B.L.; Zhou, Q.; Huang, Z.L.; Tu, K.K.; Li, H.; Sun, T.; et al. A comparative study of zinc; magnesium; strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 62, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Strontium ranelate: A dual mode of action rebalancing bone turnover in favour of bone formation. Curr. Opin. Rheumatol. 2006, 18, S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction; osteoconduction and osseointegration. Eur. Spine J. 2001, 10, 96–101. [Google Scholar]
- Mourad, A.H.I. Thermo-Mechanical Characteristics of Thermally Aged Polyethylene/Polypropylene Blends. Mater. Des. 2010, 31, 918–929. [Google Scholar] [CrossRef]
- Mourad, A.H.I.; Fouad, M.H.; Elleithy, R. Impact of some environmental conditions on the tensile; creep-recovery; relaxation; melting and crystallinity behaviour of UHMWPE GUR410-medical grade. Mater. Des. 2009, 30, 4112–4119. [Google Scholar] [CrossRef]
- Mourad, A.H.I.; Akkad, R.O.; Soliman, A.A.; Madkour, T.M. Characterization of thermally treated and untreated polyethylene-polypropylene blends using DSC., TGA and IR techniques. Plast. Rubber. Compos. Macromol. Eng. 2009, 38, 265–278. [Google Scholar] [CrossRef]
- Mourad, A.H.I.; Beckry, A.M.; El-Maaddawy, T.; Grami, M.E. Effect of seawater and warm environment on glass/epoxy and glass/urethane composites. J. Appl. Compos. Mater. 2010, 17, 557–573. [Google Scholar] [CrossRef]
- Dehbi, A.; Mourad, A.H.I.; Djakhdane, K.; Hilal-Alnaqbi, A. Degradation of thermomechanical performance and lifetime estimation of multilayer greenhouse polyethylene films under simulated climatic conditions. Polym. Eng. Sci. 2015, 55, 243–484. [Google Scholar] [CrossRef]
- Davis, G.R.; Evershed, A.N.Z.; Mills, D. Quantitative high contrast X-ray microtomography for dental research. J. Dent. 2013, 41, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Macon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 1573–4838. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J. Biomed. Mater. Res. A 2009, 88, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, A.C.C.; Pochapski, M.T.; Tramonti, R.; da Silva, J.C.Z.; Antunes, A.C.; Pilatti, G.L.; Santos, F.A. Evaluation of physical-chemical properties and biocompatibility of a microrough and smooth bioactive glass particles. J. Mater. Sci. Mater. Med. 2008, 19, 2809–2817. [Google Scholar] [CrossRef] [PubMed]
- Loh, L.Q.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O'Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010, 4, 377. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J.; Ashby, M.F. The structure of cellular solids. In Cellular Solids: Structure and Properties (Cambridge Solid State Science Series, pp. 15-51); Cambridge University Press: Cambridge, UK, 1997; pp. 15–51. [Google Scholar]
- Jones, J.; Hill, R.G.; Zoe, Y.W. Process for producing porous scaffolds from sinterable. glass. Patent CA 2725253A1, 27 May 2008. Available online: http://brevets-patents.ic.gc.ca/opic-cipo/cpd/eng/patent/2725253/summary.html (accessed on 1 February 2019).
- Li, Y.; Li, J.; Zhu, S.; Luo, E.; Feng, G.; Chen, Q.; Hu, J. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 418, 725–730. [Google Scholar] [CrossRef] [PubMed]


| SiO2 | Na2O | CaO | SrO | K2O | MgO | ZnO | P2O5 | |
|---|---|---|---|---|---|---|---|---|
| ICIE16M | 49.46 | 6.60 | 27.27 | 3.00 | 6.60 | 3.00 | 3.00 | 1.07 |
| ICIE16 | 49.46 | 6.60 | 36.27 | 0 | 6.60 | 0 | 0 | 1.07 |
| Mean Trabecular Thickness (µm) | Max. Trabecular Thickness (µm) | Average Density (g µm−3) |
|---|---|---|
| 41.715 ± 11.37 | 108.165 | 9.63 × 10−14 |
| Mean pore Size (µm) | Max. pore Size (µm) | SA to weight ratio (m2g−1) |
| 109.8 ± 39.81 | 308.865 | 0.005 |
| Sample Type | Average* Young’s Modulus (MPa) | p-Value | Average Collapse Stress/Yield Stress (MPa) | p-Value |
|---|---|---|---|---|
| Alginate Scaffold | 1.82 ± 0.99 | 0.9 | 0.159 ± 0.01 | 0.022 |
| Composite Scaffold | 1.83 ± 0.66 | 0.175 ± 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatton, J.; Davis, G.R.; Mourad, A.-H.I.; Cherupurakal, N.; Hill, R.G.; Mohsin, S. Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass. J. Funct. Biomater. 2019, 10, 15. https://doi.org/10.3390/jfb10010015
Hatton J, Davis GR, Mourad A-HI, Cherupurakal N, Hill RG, Mohsin S. Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass. Journal of Functional Biomaterials. 2019; 10(1):15. https://doi.org/10.3390/jfb10010015
Chicago/Turabian StyleHatton, Jonathan, Graham Roy Davis, Abdel-Hamid I. Mourad, Nizamudeen Cherupurakal, Robert G. Hill, and Sahar Mohsin. 2019. "Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass" Journal of Functional Biomaterials 10, no. 1: 15. https://doi.org/10.3390/jfb10010015
APA StyleHatton, J., Davis, G. R., Mourad, A.-H. I., Cherupurakal, N., Hill, R. G., & Mohsin, S. (2019). Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass. Journal of Functional Biomaterials, 10(1), 15. https://doi.org/10.3390/jfb10010015







