1. Introduction
Digital health refers to the non-face-to-face care strategy defined by public health authorities as a natural evolution from previous paradigms: eHealth/mHealth, telehealth, telemedicine and telecare. Digital health experienced incredible growth thanks to the patient medical isolation during COVID-19 that lowered the cultural barriers from both sides. Thus, digital health expands the concept of eHealth understood as health data and process digitalization, to include digital consumers, and encompasses other uses of digital technologies for health such as the Internet of Medical Things (IoMT), advanced computing, big data analytics and artificial intelligence, among others. The purpose of this global strategy is to strengthen health systems for consumers, health professionals, healthcare providers and industry to empower patients [
1].
The Internet-of-Things (IoT) approach has been driven by the evolution of microelectronics during the last few decades and has led to the flourishing of a plethora of devices (e.g., wearables, home automation, automotive, industrial, etc.), connected to the cloud usually through some edge platforms (e.g., smartphones, home access points, communication gateways, etc.). Cloud back-end technologies allow for the application of advanced computation such as big data analytics and artificial intelligence (AI) on collected data, and front-end user interfaces provide the right exploitation related to every user profile. Furthermore, this device/edge/cloud model led to a complex cost model: (1) device costs are the cost per piece of hardware; (2) the edge cost, especially for smartphone-connected wearable devices, is related to the cost of developing and maintaining apps for android and IOS and (3) cloud has a complex cost model depending on the resources required such as storage, connectivity, analytics, etc. [
2].
The IoT has clearly been impacting health services in the sense that most devices measuring health-related parameters, from simple measurements such as temperature, pulse or oxygen saturation to complex instruments like computer tomography, automatically upload data to the cloud, linked to patients’ electronic records and ready to be exploited by all stakeholders. The continuous evolution of sensor devices being integrated in wearable devices causes a few of the devices to follow the path towards long-term and costly health validations and medical certifications, while their use has been rising among customers that believe in their measurements and computed health and wellbeing parameters (physical activity, quality of dream, etc.). These different paradigms for health and information and communication technologies (ICTs) lead to a complex health model, known as eHealth or mHealth, that has been successfully deployed during the COVID-19 pandemic in many places of the world. During the pandemic, the face-to-face care model was almost fully stopped and led to the rise of telehealth, telemedicine and telecare as a way for non-face-to-face interaction between clinicians and patients. Most solutions for this model are deployed as software applications for personal computers and smartphone platforms. These solutions are based on the use of ICT to store, retrieve and share digital health data for prevention, diagnosis, treatment and monitoring and educational and administrative activities by means of already standardized web and app services such as email, agendas, application viewers, etc. mHealth further extended that data exchange model for obtaining patient feedback using smartphone apps, usually in specialized domains (e.g., surgery follow-up, mental health, etc.).
Digital health expands the concept of eHealth/mHealth to include health data coming from digital consumers using connected health IoMT devices, whose data are elaborated by advanced computing methods (big data analytics and AI) to automatically provide relevant insights to all stakeholders, especially clinicians. This model can consider the everyday higher complexity of patient treatments (comorbidities, influence of multiple drugs, psychosocial aspects, etc.).
Health technology assessment (HTA) has been formulated by the WHO as a systematic and multidisciplinary evaluation of the properties of health technologies and interventions covering both their direct and indirect consequences [
3,
4]. The HTA Core Model [
5] structures the information contained in a comprehensive HTA into standardized pieces (assessment elements). It considers nine domains originally defined in the EUR-ASSESS project [
6]: (1) Health problems and current uses of the technology (implementation level); (2) Description and technical characteristics of technology; (3) Safety; (4) Clinical effectiveness; (5) Costs, economic evaluation; (6) Ethical analysis; (7) Organizational aspects; (8) Social aspects and (9) Legal aspects.
Technoeconomic analysis (TEA) [
7] uses software modeling as a cost/benefit analysis that simultaneously considers technical and economic factors to estimate capital cost, operating cost and revenues. TEA is then intended to provide a systematic approach for examining the inter-relationships between economic and technological aspects. Technoeconomic modeling (TEM) acts as a bridge, connecting the worlds of research and development (R&D), engineering and business. It conducts this by linking the technical details of a process (components, steps involved) to its financial viability. This allows organizations to clearly see which factors impact the profitability of their new technologies. TEM is valuable throughout the entire innovation journey. In the early stages, it helps assess the economic potential of new ideas. Scientists can then use TEM during experimentation to identify which aspects of the process have the biggest impact on making money. Finally, engineers can leverage TEM to compare different approaches and configurations, choosing the most cost-effective option. By considering insights from each development phase, TEM provides a solid foundation for making data-driven decisions [
8]. In the framework of health costs, we consider that cost/benefit, cost-effectiveness or cost/utility analysis could not be used for the evaluation since we do not have detailed values for the deployment of IoT solutions (e.g., how much the quality of life of patients is improved) since we are formulating it as the hypothesis.
In terms of research and economic models, we consider health and technology as two different well-established paradigms. On one side, health disciplines tend to be evidence-based. Related health technology development processes usually lead to long cycles to validate research and innovation before they enter the “health market”. They require a large amount of capital flow, and the relationship among health stakeholders follows a business-to-business (B2B) approach (tech companies to health service providers) that leads to the concentration of many health solutions in the hands of large corporations and industries (pharma, medical instruments, insurance companies, etc.). The long-term approach also facilitates the wide use of systematic reviews and meta-analysis as research tools. On the other side, the consumer electronics domain is much more dynamic, where the success of products is highly dependent on innovation and cost performance, driven by technology changes that have continuously evolved since the middle of the 20th century. This market is much more global than the health one due to the fewer regulations required to operate worldwide and is mostly linked to a B2C approach, where the impact of the consumers in buying technological goods is a key success factor. Both research and cost models are rapidly changing, sometimes due to big technological changes (paradigm shifts) as we have seen with the smartphone or wearable devices in the last decade.
This paper is oriented to assess the deployment of a new generation of IoMT solutions (including wearable devices, smartphones at the edge and the cloud) as health technologies. These are based on the use of inertial sensors for the analysis of human gait applied to three clinical cases: equilibrium assessment, fall prevention and surgery recovery in two different scenarios for IoMT data capture: (1) measurements supervised by clinicians in scheduled sessions and (2) unsupervised measurements during activities of daily life (ADLs) and cloud analysis to obtain relevant clinical parameters for each case.
This paper deals with three health technology assessment domains: (1) Health problems and current uses of the technology (implementation level); (2) Description and technical characteristics of technology and (5) Costs, economic evaluation. In our approach, we use technoeconomic analysis (TEA) and technoeconomic modeling (TEM) to account for the technical and economic factors to estimate capital and operating costs that will impact IoMT deployment. When it comes to IoMT deployment, some barriers arise to enter the existing health provision models. Most current IoMT devices are not easily considering the medical systems themselves or the data they collected that are connected to users’ personal health records.
The paper is organized as follows:
Section 2 introduces the subset of wearable IoMT devices that can be used for gait analysis and details the models for the clinical cases and scenarios considered in our analysis with the involved population.
Section 3 details how IoMT solutions can be integrated in digital health ecosystems and the proposal for our application specific IoMT platform. The methodology used for this study is detailed in
Section 4, including the population and healthcare figures for Catalonia related to the three clinical cases. The results are presented in
Section 5, after detailing the components and costs from the proposed IoMT solutions and its instances for the three clinical cases considered. The paper ends with a discussion of the results and the conclusions.
2. IoMT Devices for Gait Analysis
Health technologies for gait analysis have been a field of study for many researchers worldwide using different technologies.
Table 1 shows several examples of studies published in recent years, where different parameters are analyzed using different techniques and sensor types.
In these solutions, one type of sensor is responsible for the success of health measurements using wearable devices rather than fixed installations: inertial sensors. Even though they are not directly measuring the user’s electrical magnitudes like ECGs or impedimetric sensors, they do not require any surface contact interface (e.g., conductive gel) with the skin, thus reducing the set-up procedures to start working.
Accelerometers were the first inertial devices used to measure falls not only for humans but also for computer hard disks or any kind of instrument (e.g., lifts or cars). This use in health (the detection of human falls) led to a plethora of commercial alarm devices and services [
18]. Fall detection just requires sending an alarm when a fall event is locally generated by a sensor capturing the acceleration and a microcontroller computing the algorithm to identify the event. Later, the addition of gyroscopes or pressure sensors improved the quality of fall detection measurements and the reduction in false positives as they can infer the type of fall (e.g., forward, backward, etc.) and filter from activities in daily life (ADLs).
Since the end of the 20th century, inertial sensors have been manufactured using microelectronic chip technologies by adding further processing steps on specialized foundries, known as Micro-Electro-Mechanical System (MEMS) technological processes that integrate or hybridize tridimensional accelerometers, gyroscopes and magnetometers in a single chip together with basic computation and communication circuits. This ends up in the mass production of miniaturized devices that facilitates their use in many different applications, which helped to reduce prices and produce energy-efficient smart devices that have been key for their use in wearable devices.
These sensors are currently able to obtain either elementary sensor parameters (3D acceleration, angular rotation or magnetic field) or parameters characterizing complete trajectories such as yaw, pitch and roll or quaternions to be directly used in navigation systems (e.g., drones) or characterize gait (e.g., pedestrians). This is the reason why they are becoming ubiquitous for many different applications.
This paper deals with three different health assessments (equilibrium measurement, fall risk, rehabilitation monitoring) that use inertial sensors in wearable devices for two different scenarios (supervised and unsupervised measurements) that affect different populations above 65 years, as detailed in
Table 2.
The supervised scenario refers to specific sessions in which patients execute particular tests under the supervision of clinicians (e.g., nurses), during which some quantitative parameters are measured (e.g., step length, time, strength, etc.). In the unsupervised scenario, the same quantitative parameters are measured after identifying gait patters (e.g., walking straight, go upstairs, etc.) equivalent to those defined in the clinical tests but obtained from data captured by the wearable devices during activities of daily life (ADLs). The unsupervised continuous measurement has several advantages compared with the supervised one conducted in clinical premises or at home with clinical supervision: avoiding the psychologic disposition of patients and measuring different conditions (e.g., indoor vs. outdoor, time and weather variability, etc.).
Concerning the assessments, equilibrium evaluation is based on measuring balance, defined as the capacity to maintain stability with an equal distribution of weight on both sides of the body. It can be used as a preliminary indicator of fall risk among the elderly. This assessment is usually conducted once a year by applying some test currently conducted in primary care installations. We propose to obtain those measurements by monitoring users performing the tests while wearing our first IoMT device (detailed in the next sections).
The impact of falls on health has been extensively studied by many organizations due to its social and economic impact, especially in the elderly population [
19]. After the success of fall detection (e.g., as a business model especially in the US), some research and products appeared to address fall prevention to minimize the consequences of falls. Fall prevention can be computed looking at the evolution of the gait parameters along time whose derating can be viewed as an increase in fall risk. This requires not only managing the fall event but a continuous sending of gait parameters to be later analyzed in the cloud as a long-term analysis. Alerts about increasing fall risk are linked to the autonomy of elderly persons to live alone. This model fits perfectly with the IoMT paradigm.
The continuous monitoring of fall prevention requires a complex IoT solution: a better device, continuous connectivity, high bandwidth and storage between the device and the smartphone (edge) and specialized computational analysis to obtain an evaluation of the risk of fall. This analysis starts by identifying walking patterns (e.g., walking in a straight line, going up or down stairs, etc.) to produce clinically relevant parameters. Those can be easily obtained at the cloud level thanks to the parameter captures by the inertial sensors. Our IoMT solution with a second device (detailed in the next sections) has been adapted for fall risk assessment.
The monitoring of the evolution of the rehabilitation after a surgery corresponding to fractures that affect gait (e.g., femur, hip, knee, etc.) uses the same IoMT device selected for fall risk assessment. After the surgery, there is a recovery period whose duration depends on the health state of every person. These can either be short, such as intensive physical rehabilitation (e.g., for professional sport players), or long, when resting for long periods before returning to normal life. Health professionals use several gait-related parameters to measure the recovery evolution and act accordingly. Those are being conducted at hospital premises. We claim that instead, they can be measured by means of our IoMT solution either at home in a supervised manner or measured without supervision during the patient’s daily life.
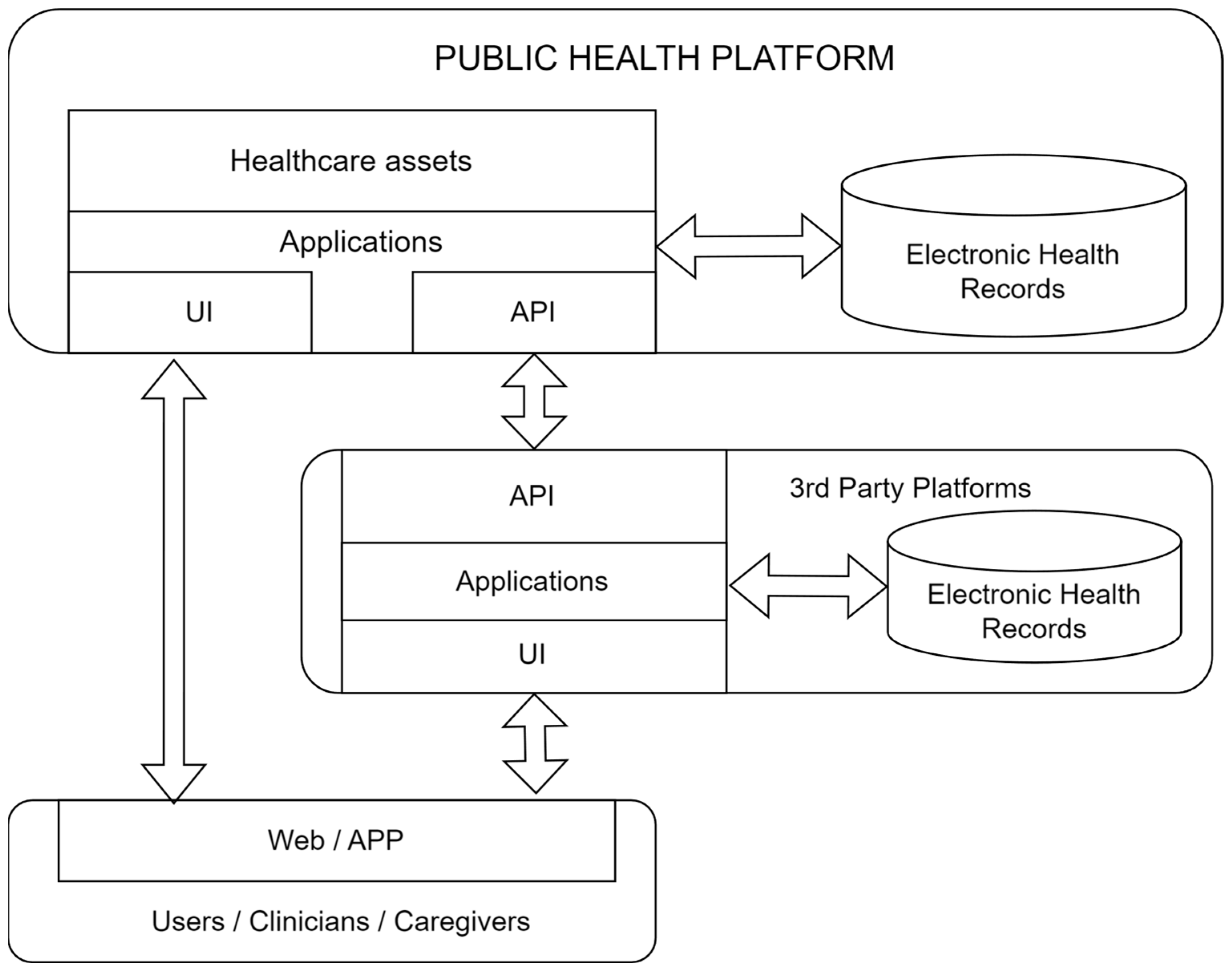
3. Digital Health Platforms and IoMT Solutions
This section focuses on the integration of the IoMT solution acquiring data from wearable devices and providing clinically relevant information to the patient records on the digital health platforms. Recent technological advancements, particularly in the realm of mobile devices and health applications, opened the possibility of implementing non-face-to-face care solutions and services on a broader scale. The EU eHealth Action Plan 2012–2020 [
20] underscored that eHealth tools and solutions hold the potential to alleviate current pressures on health systems, including constraints imposed by tight public budgets, shortages of professionals, a rising incidence of chronic diseases and the increasing demand and expectations of citizens for accessible and high-quality care. A new strategy was set up to monitor eHealth deployment across the EU and EEA countries associated with the program [
21].
In response to these challenges, countries with advanced economies are swiftly integrating non-face-to-face care services into their healthcare delivery models. As of 2018, the telemedicine and eHealth market were valued at USD 38.3 billion, with an estimated annual growth rate of 19.2%. This trajectory suggests an anticipated overall volume exceeding USD 130 billion by the year 2025 [
18].
In Catalonia, the public health system promoted the widespread adoption of the “La Meva Salut” (My Health) platform [
22] that includes a mobile app that can be used to interact with the health system and obtain relevant documents such as medical prescriptions and COVID-19 vaccination certifications. Additionally, a platform was introduced some years ago to exhibit health mobile apps, enabling clinicians to access the data and incorporate it into patients’ clinical records [
23].
Key components and features of an eHealth platform usually include a complete set of components, services and related technologies [
24]. Electronic health records (EHRs) rely on centralized systems for storing and managing patients’ health records digitally, allowing for easy access by authorized healthcare professionals. Health Information Exchange (HIE) platforms facilitate the secure exchange of health information among different healthcare entities, promoting interoperability and the continuity of care.
An Internet of Medical Things (IoMT) platform is a technology infrastructure specifically designed for the healthcare industry that leverages stakeholders’ access together with user interaction, IoT devices and sensors to collect, store, analyze and exchange medical data. Health data are considered highly sensitive by all stakeholders despite the different health data ownership models around the world. This main fact led to a plethora of digital health platforms (owned by different health organizations) to manage the electronic health records of their users/customers.
Furthermore, IT companies, from big players (e.g., Google, Apple, T-Systems, etc.) to new unicorn companies, become highly active in the health market. A complex ecosystem arises composed of many digital platforms that ideally should interact among them to provide better service, especially with a global worldwide health view. From the point of view of the patients, it is relevant to have simple access to their own data.
The method used to solve that problem has been the use of a whole set of standards for all data management aspects (coding, procedures, pharmacy, data bases, security, privacy, maintenance, interoperability, etc.). Unfortunately, the large number of different standards (and their evolution) are not homogeneously adopted. This leads to a lack of interoperability among providers, organizations, borders, etc. Few initiatives tackle this problem. An example is the proposal of the International Patient Summary [
25], a minimal set (not exhaustive) of a patient’s clinical data usable by all doctors and patients regardless of their country.
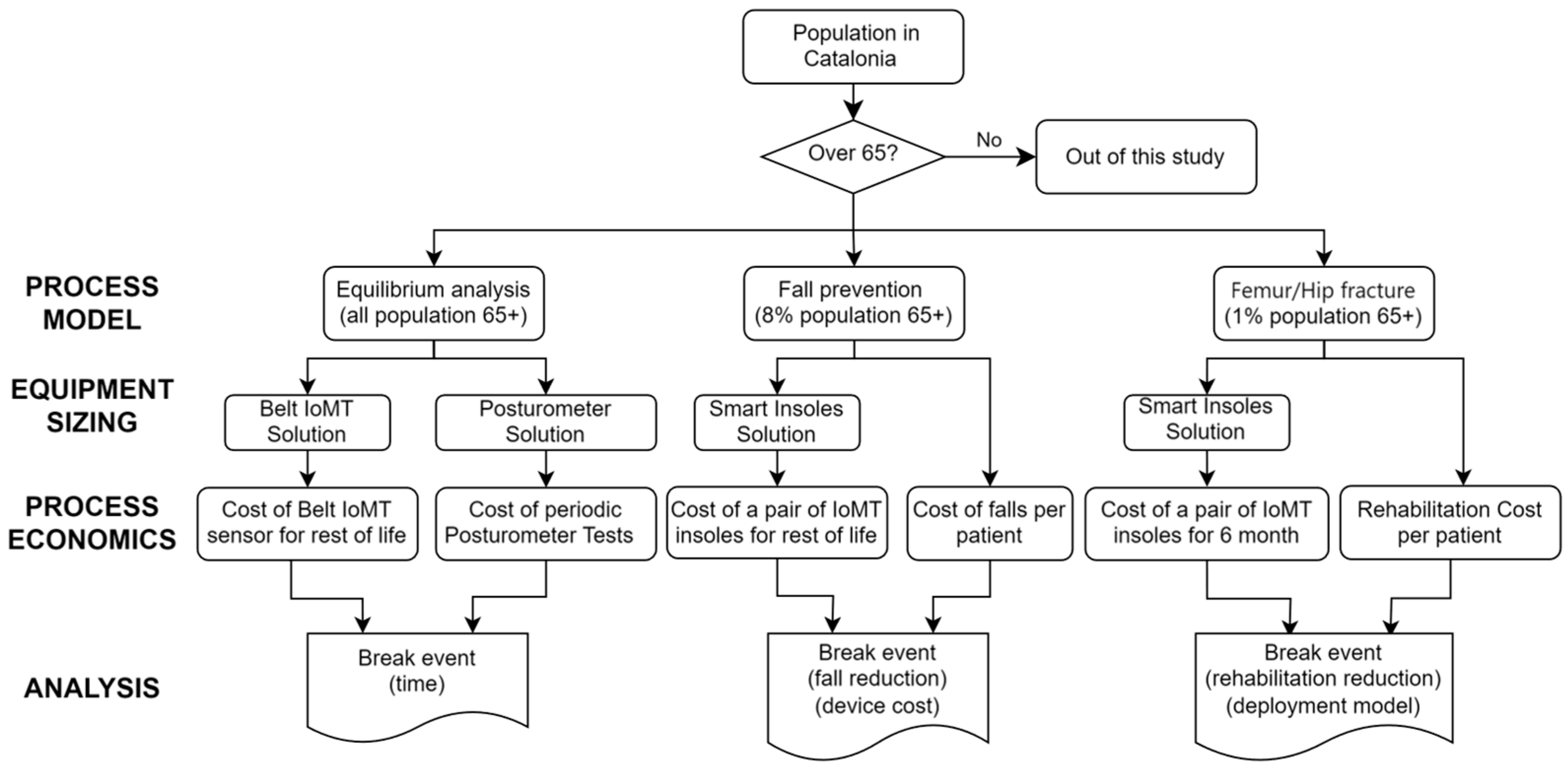
4. Methodology
The aim of our publication is to estimate the costs of using an IoMT solution to measure gait-related clinical parameters with wearable devices connected to a platform that provides relevant clinical data to the clinicians responsible for their healthcare. This will allow us to compare them with the costs of the current model being used and obtain some criteria to assess the profitability of its deployment.
To build a technoeconomic model (TEM), we will develop a model and calculate equipment sizing parameters, then estimate capital (CAPEX) and operating (OPEX) costs. The model allows us to generate a stream table. Information from that table is used in equipment calculations to derive parameters necessary for estimating CAPEX and OPEX costs. The UI consolidates inputs and results on a single sheet, facilitating model operation [
8]. The platform used for TEM is a spreadsheet model (Excel sheet) that does not require specialized software, so it is ideal for early-stage health technology assessment.
TME automates the correlations between financial metrics, processes and economic parameters. The metrics that we consider are shown in
Table 3: (i) IoMT costs; (ii) current health model costs; (iii) the percentage of the reduction in falls; (iv) the percentage of the reduction in rehabilitation duration due to the use of IoMT solutions and (v) the dependency of electronic device costs with mass production.
Dependencies for the cost of the IoMT solution contain both CAPEX and OPEX costs (devices, app, cloud, training and maintenance) and also the reduction in the number of hours spent by medical staff (OPEX cost). The current clinical costs are related to medical equipment (mostly for primary care) and staff costs. The realization of the tests at the patient’s home also reduces travel costs from patients to the health premises and the trips by the medical staff to the patients’ homes. However, this estimation is complex and will not be considered as a direct cost in our analysis.
Capital cost estimates are often divided into several classifications based on accuracy. In our case, we consider that our accuracy will be larger than study (±30%) or preliminary (±20%) estimates.
We deal with three different clinical cases (equilibrium measurement, fall risk assessment and rehabilitation monitoring) that use a similar IoMT platform but have different requirements in terms of usage and duration.
Table 4 indicates the population ranges for each of them (which impacts CAPEX and OPEX costs). Each case obtains population data (input) from different data sources (secondary data on patients’ statistics) and processes it into results for both the current health model and the IoMT one. The type, frequency and duration of measurements will have an impact on the OPEX costs, especially in the case of supervised measurements (in terms of clinician personnel costs).
The cost values depend on the specific (public) health service model. Our results refer to the Catalan region for which we collected the corresponding cost and patient data. IoMT costs will be estimated based on developed prototypes (linked to research projects). Furthermore, in the case of equilibrium assessment, the current health cost model will be based on data obtained through a clinical study.
We used Activity-Based Costing [
27] to account for the savings related to the cost of the savings given by the reduction in falls in fall prevention and the reduction in rehabilitation duration in surgery recovery.
5. Results
In this section, we detail the components and estimate the costs of the IoMT platform and generate the figures for each of the clinical cases considered. These figures will assess the impact and break event for each of them according to the criteria we selected according to the KPIs defined in
Table 3.
5.1. IoMT Platform Model
Currently, in the market, there are different business models in the IoT domain that account for different assets (devices, communication, smartphone apps, cloud storage and analytics, services, etc.). When restricted to the IoMT, there are mainly two main models that roughly depend on the public health model: private and public. In countries with mostly private health systems, companies offer services directly to consumers (patients). An example of those are panic buttons with fall detection that rely on monthly fees for an emergency service. In countries with mostly public health systems (like Catalonia), it is the administration who provides, directly or through a third party, the service to the users/customers. Most panic buttons are provided by social services, nowadays integrated in the health systems. There is also a rising private initiative linked to home security providers.
In both health system cases, those services include the IoT device, the communication (direct to the cloud or through another provider) and the services linked to the cloud infrastructure that contains data from users. As said, we focus on this last model as we will apply the analysis to our public health service.
In our analysis, we consider that the public health system purchases the IoMT platform for the selected set of patients through a public tender that includes the following: (i) providing devices and smartphone apps to users during the period of time required for every procedure; (ii) cloud data collection, storage, computation, access to users and clinicians and interoperability for the public health platforms and (iii) the information technology (IT) personnel costs related to the training for patients and professionals, as well as the telehealth service for patients, support for health centers, the maintenance of devices, etc. As already said, our cost model and estimates for aspects, such as training, maintenance and benefits for the service provider, come from a recent similar tender that has already been placed in Catalonia for the IoMT solution for diabetic patients [
44].
Security and privacy are key aspects provided by the ICT technologies implemented at all levels. The platform considers accessibility issues in different ways. There is still a significant part of the elderly population that does not use smartphones or has strong usage limitations. Fortunately, this barrier is being quickly reduced in our society. The impact of other accessibility issues related to visual or mechanical impairments can be greatly reduced thanks to the current design of devices and smartphone apps.
The three different cases will include two different devices according to the parameter monitoring requirements and usage model (how often they are used and where they are placed). Therefore, those devices will have different costs. The edge cost will be similar (device, app, communication service) in all cases since it implies a smartphone to monitor the device and bridge data from it to the cloud. Cloud costs are related to different factors such as connectivity, storage and computational requirements. The three clinical cases will have different requirements in terms of the number of patients being monitored, amount of data transferred and amount of computation required to produce clinically relevant parameters from relevant sensor data. According to the current model for health provisioning in Catalonia (which includes social services), the whole IoMT solution will be compensated by the government. The total annual costs for each of the IoMT solutions proposed consider a minimum of a 10-year duration for the wearable devices.
Table 5 shows the cost figures used for the components of the IoMT platform for the clinical cases studied. They were evaluated by implementing prototypes in R&D projects. We consider two Bluetooth-connected wearable devices (CAPEX cost): (1) belt-attached device and (2) wireless rechargeable insoles. The cloud cost (OPEX cost) was estimated according to the amount of data acquired and stored and the computation required to obtain the clinical indexes. Both aspects together with training and maintenance scale with the size of the population considered. Other OPEX costs, such as smartphone app and web access, are considered equal (per patient) for the three cases.
5.2. Equilibrium Measurement Case
Diving into the details of the conducted study, identified as the clinical method, to carry out this analysis, we consider that the health center had to acquire a posturograph, with an approximate cost of EUR 30,000 (CAPEX cost). Besides the price, which is unaffordable for the average person, one must also consider the size and complexity of the equipment, not designed for domestic use. Additionally, we account for the time dedicated by the medical staff to conduct the clinical test: one nurse with an hourly cost of around EUR 30 [
45] (OPEX cost).
We use a clinical study on equilibrium measurement with 70 patients as a basis for cost calculations. It turns out that each patient spent approximately 1 h undergoing the test. The cost per patient for the posturograph measurements does not include any app or cloud costs, and data are handled using USB sticks, while it required one hour of nurse support. Considering the cost of the posturograph, if we divide it by the number of patients who perform the test and add the cost per hour of a nurse, we obtain a cost per patient of EUR 468.
The IoMT platform contains one belt-attached IoMT device, Nordic Thingy52 DK [
46], which provides the necessary sensors and wireless BLE communication. The cost of the device is EUR 37.5, while, for the same 70 patients, the cost per patient for the IoMT is EUR 254 (mainly impacted by the app development cost).
At this stage, for only 70 people at one center, we found the savings (55%) of the IoMT to be too high compared with the clinical tests. When extrapolating this result to the entire population aged above 65 for our region, we have approximately 1.5 million individuals who will need to undergo the test.
Installing posturographs at every primary care center will cost around EUR 12.5 million, and the cost of clinical staff for one year is estimated at EUR 45.8 million. Therefore, the total cost will be EUR 58.5 million. The cost of the deployment of the proposed IoMT platform throughout the entire region, for executing the test for the same number of patients, is around EUR 60 million. Therefore, for only one year, the implementation of the two systems is similar.
The balance or equilibrium measurement clinical procedure, which impacts reducing the number of falls, must be in use for the whole life of patients while being autonomous. When extending the estimates to 5 years, the population above 65 years will increase by 400 thousand people, and for 10 years, the increase will be 1 million people, which is consequent with the population aging and the life expectation increase. The cost of performing one test per year per person using posturographs, in the 5th year, will be EUR 64 million and in the 10th year, 90 million. The accumulated cost for 11 years will be EUR 668 million.
For the IoMT platform, and considering 20% additional devices for repair issues, the cost breakdown is composed of the following: (i) a purchase cost of 3 million devices at EUR 37.5 per unit; (ii) the implementation of the mobile application and cloud system, and expenses for training and support, EUR 2 million; (iii) the cloud storage cost per patient (EUR 1/month) and (iv) the business benefit of 6% for the deployment company on the public tender. We have also considered a 2% cost inflation increase during that period in salaries.
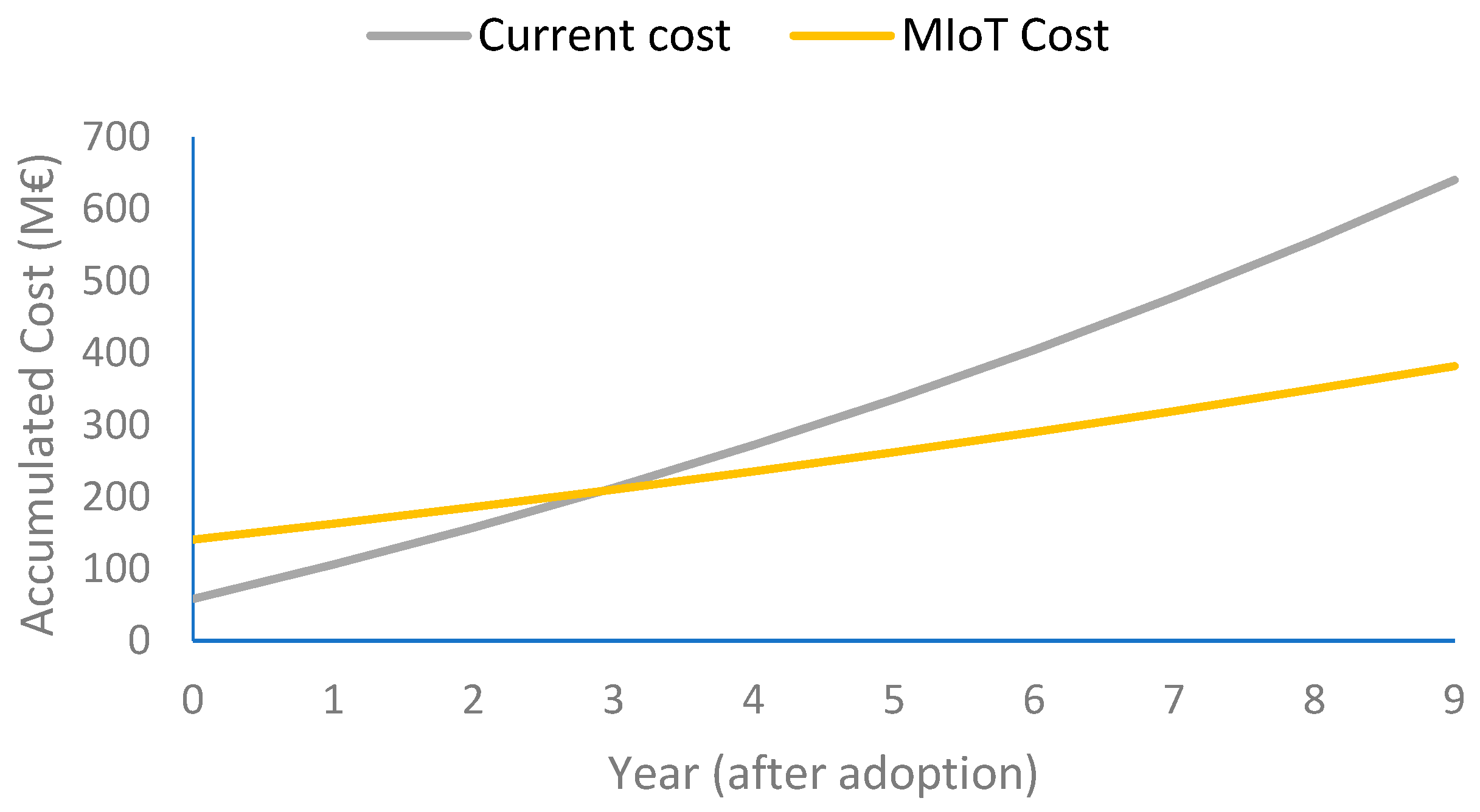
Figure 3 shows a comparison between the evolution of the costs of the current health model (using posturographs) and the IoMT solution for equilibrium assessment along the years for the 65+ population. The deployment of the posturograph system in the first year is nearly three times cheaper than the IoMT platform, due to the initial purchase of the devices. The break event is around the 3rd year. If we consider a cost reduction due to the economies of scale, which is heavily impacting the cost of electronic devices, we find that if the device would cost under EUR 11.5 per unit, the IoMT platform would be cheaper than the health model using posturographs in the first year. This is a reasonable assumption for a high volume of devices according to the current costs of activity band manufacturers, with a similar complexity to that of our device. This downscaling cannot be applied in the same way to posturographs since their number is much smaller.
Furthermore, with 420 posturographs and considering 240 days per year and 10 patients per day, all patients older than 65 can have one test every 1.5 years maximum, while in the IoMT case, they can have one on any given periodicity.
5.4. Surgery Recovery
A detailed study [
30] showed that by 2015, in the Catalan region, the average cost per patient in the 12 months following the fracture of the femur was EUR 11,721 compared to the EUR 3495 cost of the 12 months prior to the fracture. Thus, a femur fracture results in a cost increase of EUR 8226 per patient. It is also observed that 40% of the cost corresponds to hospital admissions, 43% to the use of social health resources (including primary care rehabilitation) and the remaining 17% includes different small factors such as the pharmacy, emergency department, nonurgent health transport and others. From those, 80% of the total cost is incurred in the first 6 months [
43].
According to the public statistics offered by Spanish National Statistics Institute (INE) for Catalonia, between 2015 and 2024, the Consumer Price Index increased by 20.0%. From the same report, we can conclude that the Health Department considers that 50% of the cost per patient corresponds to surgery and preliminary rehabilitation, while the other 50% corresponds to a set of rehabilitation sessions conducted at primary care premises. The rehabilitation cost after hospitalization considering the inflation rate from 2015 to 2023 is EUR 66 M.
The use of the IoMT platform will lead to different improvements with separate cost impacts: (1) a reduction in the mean time of rehabilitation, since the treatments will be scheduled according to the patients’ evolution rather than following a fixed period scheme; (2) an improvement in the final result of rehabilitation in terms of improving the independence degree in instrumental activities of daily life and (3) lower transportation costs for patients, while clinical costs can be similar since some of the monitoring sessions will be supervised, and clinicians will have to track the results of unsupervised measurements accessible through the cloud.
We consider that the patient will use the same IoMT platform as in the previous case, but only during the rehabilitation process, with a mean duration of 6 months. The cost per surgery rehabilitation of the IoMT platform considers that the patient holds one device during the whole recovery duration. A part of the duration, unitary costs of both IoMT solutions are of the same order as in the previous cases.
In this case, we can consider two different application models for monitoring the evolution of the rehabilitation. The first one is oriented to follow the same tests that could be conducted at the primary care center premises remotely supervised by a rehabilitation professional, like the equilibrium measurement case. The second one is similar to the gait analysis, and the patients are wearing their insoles during ADLs that continuously capture walking data, and the cloud software is identifying the test situation (e.g., walk in a straight line) and computing the rehabilitation recovery index that can be analyzed at any time by the clinicians. The differences among both models are (1) the time of the clinicians to supervise the test, which we consider equal for both solutions in the supervised case, while in the unsupervised one, we consider 50% of the total since after the first tests, clinicians will just monitor the parameters obtained by the IoMT platform and (2) the amount of data to be stored and computation required in the cloud. For the test-oriented supervised model, we consider a cloud cost of EUR 1 per month per patient, while for the ADL-oriented unsupervised one, this cost rises to EUR 3 per month and patient.
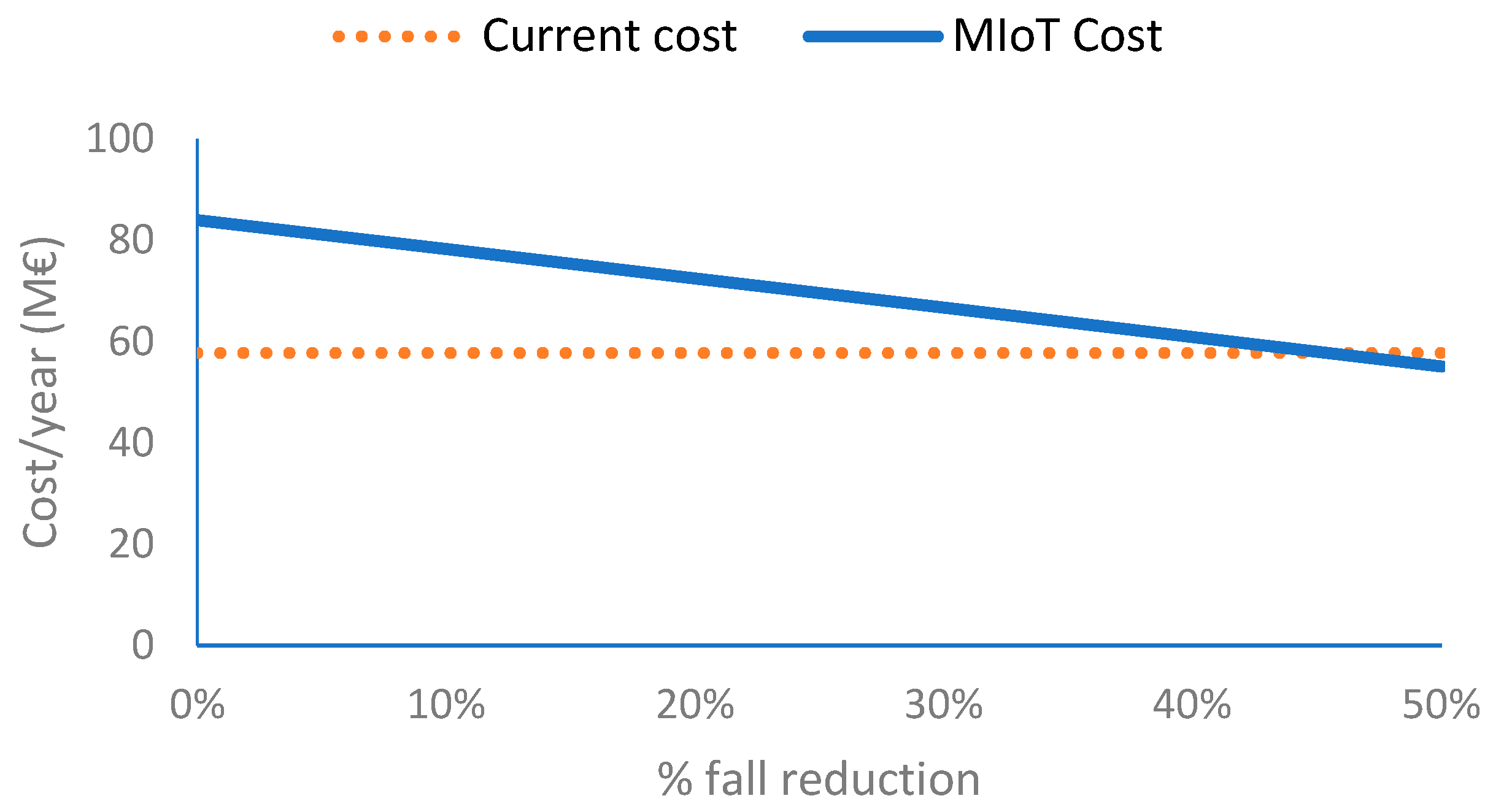
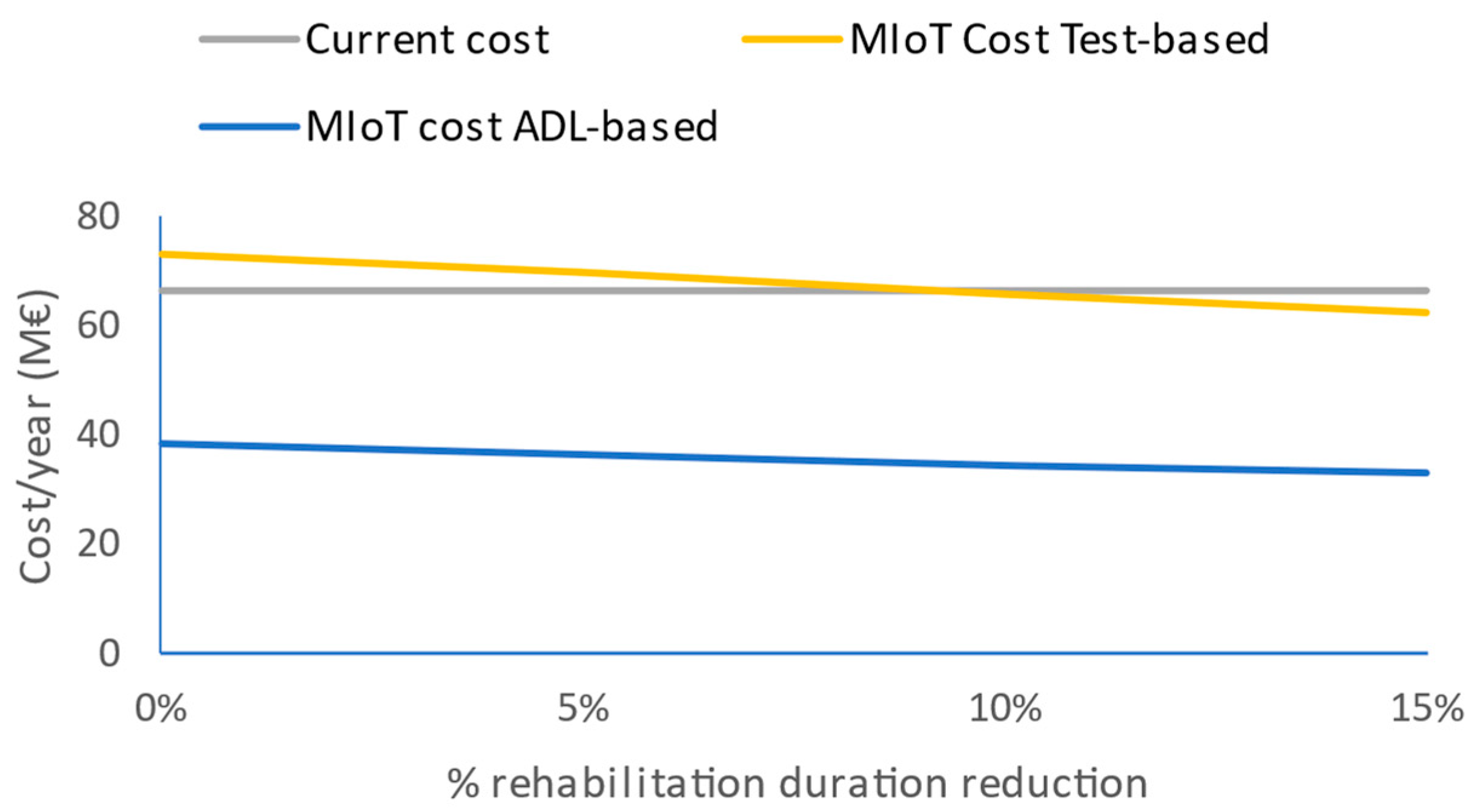
With these models, there will be a large difference between supervised and unsupervised models. The estimation of the costs of the deployment of the IoMT platform for this case is EUR 73.5 million for the test-oriented supervised model and EUR 38.4 million for the ADL-oriented unsupervised one, this one being much lower than the cost of the current rehabilitation model. In the supervised case, a 9.7% decrease in the duration of the mean rehabilitation compensates the IoMT system deployment costs.
Figure 6 depicts that dependency.
6. Discussion
The technoeconomic analysis of an Internet of Medical Things platform deployment requires considering a complex model that must include, in different ways, costs related to the intrinsic technology asset costs—device, edge, cloud and user interface—plus clinical and IT support costs as well as the procedures to move from the traditional model to the user-centric digital model for the health domain.
Table 4 shows the characteristics of the three cases (equilibrium measurement, fall prevention, rehabilitation recovery) addressed by this study based on the obtention of clinical indexes obtained from gait-related parameters. Concerning measurement, we considered two different deployment models: a scheduled test-based model supervised by clinicians and an unsupervised one from the continuous measurement of gait parameters during activities of daily life. The frequency of these measurements will be high in the case of continuous measurements during ADLs and low for scheduled tests at home when connected to a supervisor clinician. The duration of the measurement will usually be long (several years) when related to fall risk assessment for the elderly and short (few months) for monitoring rehabilitation.
The impact of the deployment for the different cases will have contributions in healthcare provision and cost. While this study is not analyzing healthcare procedures in depth, we claim that (1) automating measurements at home allows for a better organization of clinical resources and shortens transport dependencies and costs; (2) taking parameters directly from daily life activities provides more realistic insights into the analyzed parameters and promotes non-linear assessment between measurements and clinician monitoring, which also impacts the organization of clinical resources and (3) the continuous measurement of clinical parameters allows for an early detection of situations with high risk and therefore a fast reaction, as preventive methods, that should result in a high impact of the corresponding clinical decisions. According to the literature, we expect that the continuous measurement of clinical parameters provided by IoMT measurements will produce savings in fall-related injuries by avoiding falls and shortening the surgery rehabilitation duration. These factors have been analyzed in the cost versus expected savings of the IoMT deployment figures, together with the dependencies on the device cost scaling.
The technoeconomic analysis allows for detailing the dependencies of the IoMT proposal on the three studied cases. The first case studied is the equilibrium assessment, extrapolated from an existing clinical study that is developing a new model based on a posturograph as clinical assessment at primary care premises. Therefore, the analysis compares the cost of installing that system in all primary care centers, identified as a clinician method, along with the subsequent personnel costs for conducting the tests, with the IoMT method that allows patients to perform the assessment at home. At the level of all of Catalonia (7.8 million people and 420 primary care centers), the classical model would allow for one equilibrium assessment once every 1.5 years, while the IoMT one would allow for any given periodicity (e.g., once per month). Furthermore, when considering accumulated measurements along the years (also considering the increased elderly population), we found that after the 3rd year, the accumulated cost of the clinical model will be higher than the IoMT one, basically due to personnel costs. Additionally, our analysis did not consider the indirect costs associated with the travel to primary care centers that would be in favor of the IoMT solution.
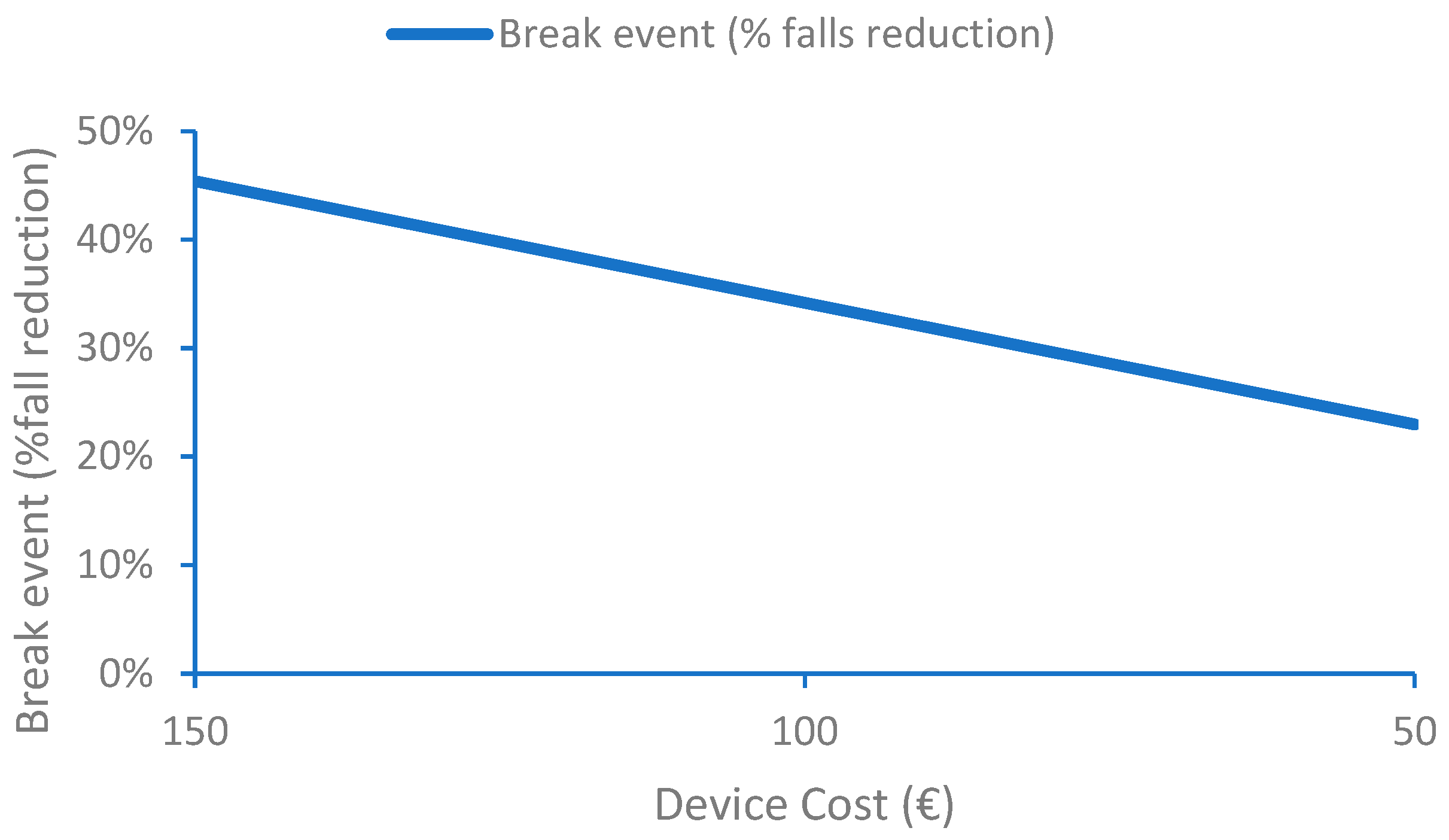
For the case of continuously monitoring fall risk during activities of daily life, the IoMT platform requires a more complex device to obtain gait parameters from which the patient-personalized risk indexes are computed. At the same time, cloud costs are also higher. The main advantage of this solution is the early detection of situations that can lead to a fall which are currently analyzed by a set of periodic tests during primary care visits (e.g., once a year). Identifying risky situations should lead to taking measures and therefore reducing the risk of falls and number of falls. Our analysis observes that the cost of the IoMT system compensates the cost of treatments related to falls when the reduction in the number of falls is 45%. This number is strongly dependent on the cost of the device that can profit from the economy of scale, which would have an impact on a huge cost reduction for large production volumes of electronic components. If the device cost could be reduced from EUR 150 to EUR 50, the break event would be reduced from 45% to 23%. From this economic point of view, one must also consider the social impact for the cases in which falls led to situations where the patient will require assistance or even die.
The last studied case focuses on rehabilitation from a surgery that affected gait and obtains the evaluation from a study of femur fractures in Catalonia. The study shows that hospitalization costs account for 50% of the total cost per person while the rest correspond to rehabilitation plus sociosanitary and pharmacy costs, accounting for the duration of the rehabilitation, which the study considers 6 months as the mean. In this case, for the same IoMT platform, we considered two different models: (i) a supervised rehabilitation session in which the clinician can conduct and monitor the tests conducted by patients at home and (ii) unsupervised monitoring during patient ADLs that allows for identifying clinically relevant gait parameters that can be monitored at any time by clinicians. As in the previous case, the analysis considers that, with the IoMT solution and the unsupervised model, the patient can be monitored more frequently which can allow for better rehabilitation by correcting unexpected effects and potentially reduce the duration of the rehabilitation. In this case, the analysis shows that IoMT deployment reduces the cost in approximately the same factor of the time reduced by clinicians to supervise rehabilitation (e.g., 43% in
Figure 6). For the supervised rehabilitation case, the deployment costs of the IoMT platform are compensated with a mean decrease in the duration of rehabilitation above 8.7%. This reduction is equivalent to 16 days in 6 months which indicates that the deployment of the IoMT solution will easily compensate the savings of the in-place costs. The dependency on the device costs is smaller than in the previous case when linked to rehabilitation duration (just a few days), and its impact on the total cost of deployment is much lower than in the previous cases since it is affecting less of the population. Once again, better rehabilitation will also have an impact on a better resulting health and wellbeing state of the patient.
The effort to deploy such IoMT devices in the daily routine of patients or in hospitals is considered in terms of the training of users and professionals, together with a user-centric approach for wearable device usability and the integration of clinical data in the health platform applications already being used. Device and app maintenance will be conducted by the technology supplier selected by the tender. Most of this effort will affect primary care or the related post-surgery follow-up service in hospitals, which now is mostly being conducted at the patient’s home using classical (non-IoMT) protocols. Therefore, we do not consider that there will be a high impact on the daily routine or clinical procedures.
Healthcare professionals using IoMT solutions will spend some additional time at the beginning of the process to train the users, and then their effort spent is expected (1) to be similar in the supervised model and (2) to decline in the unsupervised one since clinicians will focus on clinically relevant parameters rather than the procedures to obtain gait data. Continuous data acquisition will allow them to spend those time savings on the assessment of patients that should lead to improving their quality of life in terms of reducing the number of falls and the duration of rehabilitation.