Abstract
The paper presents the data on lipid fraction extraction from the raw fat of hibernating hunting animals. The processing of valuable raw materials must be maximized. For this purpose, various methods of rendering are used. As a result of temperature exposure, the protein part of raw fat undergoes significant changes. The protein denatures under the influence of temperature, and the dross formed during the rendering process absorbs and retains up to 30% of the fat. The authors propose using proteolytic enzyme preparations for a more complete extraction of fats, as the enzymes will hydrolyze the protein into compounds of lower molecular weight both before and during the rendering process. The experiment proved that the biocatalytic method allows achieving a fat yield of more than 95%. The best result can be obtained if the rendering is carried out at optimal parameters, which can be defined using a mathematical model. Mathematical modeling was carried out using an artificial neural network. During the study, a fully connected neural network was designed; it had eight hidden layers with 64 neurons in each, and its accuracy was measured by mean relative error, which amounted to 5.16%. With the help of the network, the optimal values of applied concentration, temperature and duration of rendering, at which a fat yield of more than 98% is achieved, were determined for each enzyme preparation. After that, the obtained values were confirmed experimentally. Thus, the study showed the efficiency of using artificial neural networks for modeling the biocatalytic method of lipid extraction.
1. Introduction
In the production of biologically active additives from hunting products, special attention should be paid to raw materials. The quality of raw fat significantly affects the quality of the finished product. The process of rendered fat production consists of several stages: preparation of raw materials for processing, rendering, separation of fat from the protein fraction, fat purification, cooling and supercooling, and packaging.
One of the most important stages is the process of fat rendering. The works of Vostrikova, N. [1,2], Gorbacheva M. [3,4,5,6], Zhdankina G. [7,8], and Slobodchikova, M. [9], are devoted to the development of methods of effective extraction of fats.The most widespread rendering method is the thermal method, which can be wet or dry. Depending on the technical solution and technological equipment, the rendering process is carried out using continuous machines and batch apparatuses. The rendering can be carried out at atmospheric pressure, overpressure and under vacuum.
The wet rendering method consists in putting raw fat in direct contact with water or live steam. When heated, adipose tissue proteins denature, and collagen undergoes hydrolysis. This leads to rupture of cell membranes, and the rendered fat migrates from the destroyed cells. If exposed to glutin, the rendered fat can emulsify and undergo hydrolysis with the formation of free fatty acids, which is undesirable. As a result of wet rendering, a three-phase system containing fat, broth, and dross is obtained. The main disadvantage of wet rendering is the high fat content in the dross: the fat is bound and difficult to extract. According to Cunha, A. [10], and Jenkins, B. [11], the use of enzyme preparations is one of the methods for destroying the cells of the pulp and releasing fat. Proteolytic enzymes allow increasing the yield of fat. The technical result of the proposed method is the increased yield of fat in the process of wet rendering, and the improved quality of the resulting fat with reduced acid and peroxide values.
Since fats are water-insoluble compounds, they can only be attacked by enzymes at the fat/water interface. Therefore, before introducing the lipase enzyme, the system is emulsified. In open-type boilers, the fat mass is heated to 180–200 °C; as a result, low-molecular fatty acids evaporate, while high-molecular fatty acids polymerize and the fat loses its useful properties. In steam baths, the raw fat mass is heated slowly, and, while its temperature rises from 50 °C to 80 °C, the lipase enzyme contained in the fat mass is activated, which quickly oxidizes the fat. As a result, the healing properties of the fat deteriorate. In addition, the finished product contains many remaining impurities (protein elements, and particles of connective tissue), which cannot be removed by subsequent separation.
Due to the high activity of the lipase enzyme, rendering the fat at 70–80 °C leads to fat oxidation during the process. When the fat of hibernating animals is rendered at 80–98 °C, the amount of metabolic bound water in the finished product exceeds the norm, and the resulting fat can be stored for no longer than one month, after which it acquires an unpleasant odor.
The enzyme hydrolyzes fats mainly in the sn-1 and sn-3 positions; therefore, the main products of hydrolysis are free fatty acids and 2-monoacylglycerols (β-monoacylglycerols). Molecules of 2-monoacylglycerols also have detergent properties and contribute to the emulsification of fat. Free fatty acids can rancidify, which worsens the quality of the product.
To inactivate the lipase enzyme, the pH of the medium should be decreased to less than 4.0, or inhibitors can be used. Lipase inhibitors are substances used to reduce the activity of lipases, thereby preventing the hydrolysis of triglycerides into monoglycerides and fatty acids. The main lipase inhibitors are proteases.
The efficiency of the fat rendering process depends on its technological parameters. In this case, efficiency can be understood as the percentage of rendered fat obtained from the total mass of fat. Optimal process parameters can be determined using mathematical modeling. One of the most popular present-day modeling methods is the use of artificial neural networks (ANNs). They have been extremely popular over the past decade, which is primarily due to the successful application of ANNs in computer vision and image identification. But ANNs are also quite accurate in forecasting and solving regression problems, to which the current study belongs. ANNs are used for modeling in various fields of science. In Food Science Technologies, neural networks are used relatively rarely; however, every year the number of publications is growing, and at the moment, much research has been carried out by authors from around the world [12,13,14,15,16,17,18,19,20,21,22].
In the work of Ali, A. [12], ANN, together with other methods of machine learning, is used for classifying different types of corn seeds. The model developed by An, T. [13], based on deep learning, provides a new idea for the intelligent detection of the withering process of black tea. Bhargava’s and Barisal’s [14] study that developed a system for classifying various types of fruits and determining the grade of fruits showed excellent results based on several machine learning algorithms, including neural networks. A fast, automatic, less expensive and accurate ANN disease detection method for rice was developed by Chen, J. D., Zhang, D. F., Nanehkaran, Y. A., and Li, D. L. [16]. Predicting the tissue composition of goat carcasses using a decision tree with the CHAID (DT) algorithm and the artificial neural network (ANN) method was studied by Ekiz, B., Baygul, O., Yalcintan, H., and Ozcan, M. [17]. Lu, A. N. [18], developed a model that relates changes in yogurt quality to transport distance based on the number of vibrations. To predict the rheological properties of microfluidized sugar cane juice, Tarafdar, A. [20] used an ANN with 12 layers. Torshizi, M. V. [21], used ANN to classify color changes in mushrooms under UV-A irradiation. Vasighi-Shojae’s, H. [22], model was used for nondestructive quality control of Golden Delicious apples. Such a variety of tasks for which ANNs are used proves the possibility of using networks to model a variety of technological processes in food science.
In the current work, the process of extracting the lipid fraction from the raw fat of hibernating hunting animals is being studied. Its efficiency varies depending on the values of technological parameters. It is not always possible to experimentally determine them so that the process proceeds with maximum efficiency or optimally. To identify the optimal parameters of technological processes, mathematical modeling methods are used, which include artificial neural networks.This study aims to apply an ANN for mathematical modeling of the biocatalytic method of obtaining rendered game animal fat. The study objectives include:
- -
- designing the ANN for predicting the output values of the process;
- -
- evaluating the ANN accuracy;
- -
- process optimization.
2. Material and Methods
2.1. Experiment
The experiment was carried out using the fat of game animals: bear, beaver, marmot and badger. The animals were hunted and the fat was recovered in Kemerovo Region, Siberia, Russia.
After hunting and gutting, the subcutaneous fat of bear, beaver, marmot and badger was frozen and delivered to the laboratory within 3 days. After being stored in the freezer for 12 h, the fat was thawed at 18–20 °C and ground in a meat grinder. After that, the fat was packaged in containers, and some of the processed raw material was used to obtain rendered fat (Figure 1) [23,24,25,26,27,28,29,30,31,32,33].
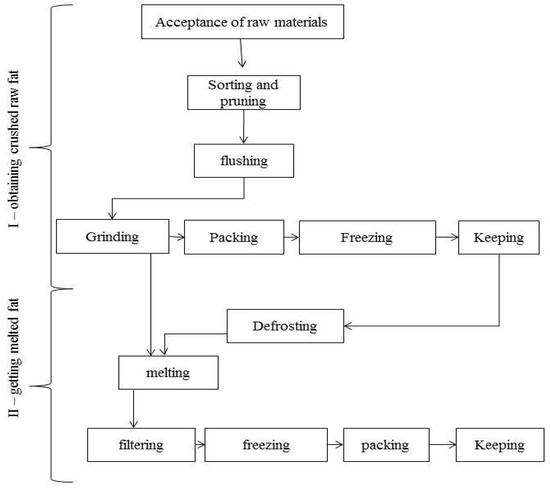
Figure 1.
General scheme of processing raw fat of hibernating game animals.
To inactivate lipase in the fat and prevent the formation of free fatty acids, proteolytic enzyme preparations were introduced: Neutrase, Protozym B, Fan Boost, and Ondea Pro [34].
Neutrase is a high-quality, broad-spectrum endoprotease, which provides mild hydrolysis. It is often used alone in the hydrolysis process, but can also be combined with exoprotease for superior palatability. It is involved in the generation of peptides. Its operating pH range is 6.0–9.0, and the operating temperature range is 30–65 °C.
Protozym B is a dry enzyme preparation of bacterial protease obtained by directed fermentation of a selection strain of Bacillus licheniformis, followed by purification and concentration. The enzyme preparation catalyzes the hydrolysis of high molecular weight proteins with the formation of low molecular weight peptides. The enzyme activity is 50,000 U/g. The enzyme is most active at the temperature of 55–65 °C and the pH of 6.0–10.0. The operating temperature range is 25–70 °C, and the operating pH range is 5.5–11.0.
Fan Boost hydrolyzes internal peptide bonds, which supports peptidases. Its optimum pH range is 6.0–9.0; the operating temperature range is 40–60 °C.
Ondea Pro is a mixture of α-amylase, cellulase, xylanase, protease and lipase. It works with endogenous proteases and β-amylases. The enzyme has a wide operating temperature of 30–120 °C, but the greatest activity is reached at temperatures above 100 °C.
Prepared samples of raw fat in the amount of 50 g were mixed with water in a ratio of 1:1 by weight. After that, an enzyme preparation was introduced into the water–fat mixture. The dosage of the enzyme preparation was calculated depending on the activity of the preparation, the heating temperature and the duration of rendering.
2.2. Parameter Measurement Procedure
In the course of the experiment, the yield of rendered fat was evaluated. The mass of raw fat was measured before rendering, and after the completion of the rendering process, the mass of the resulting dross and that of the rendered fat were evaluated. Based on the data on the change in mass, the yield of rendered fat in the experimental and control samples was evaluated. The indicator was measured as a percentage of the mass of raw fat.
2.3. Experimental Data Preparation
Based on the experimental data, a dataset of 147 marked-up records was prepared. The input variables were the temperature of rendering (T), the duration of rendering (D), the enzyme preparation (F) and its concentration introduced during the rendering of fat (C). The output variable was the yielded volume of rendered fat in percent (V).
T took values of 40 °C to 120 °C with a step size of 10 °C; D varied from 30 to 120 min with a step size of 30 min; C varied from 0 to 0.25% with a step size of 0.1%.
F was a categorical variable taking the values of “Neutrase”, “Protozym B”, “Fan Boost”, and “Ondea Pro”. For the study, only quantitative variables were used, so F was replaced by F1, F2, F3, and F4 variables, which took the value of 0 or 1, depending on the category (for the “Neutrase” value: F1 = 1, F2 = 0, F3 = 0, and F4 = 0; for the “Protozym B”: value F1 = 0, F2 = 1, F3 = 0, and F4 = 0; for the “Fan Boost” value: F1 = 0, F2 = 0, F3 = 1, and F4 = 0; and for the “Ondea Pro” value: F1 = 0, F2 = 0, F3 = 0, and F4 = 1).
The output V variable took values in the range of 5–99%.
2.4. Research Tools
The ANN was written in Python in the Google Colaboratory development environment (https://colab.research.google.com, accessed on 1 July 2023). The freely distributed PyTorch library was used in the work (https://pytorch.org/, accessed on 1 July 2023).
3. Results
3.1. Experiment Results
The introduction of enzyme preparations in the production of rendered fats revealed a positive trend (Figure 2, Figure 3, Figure 4 and Figure 5).
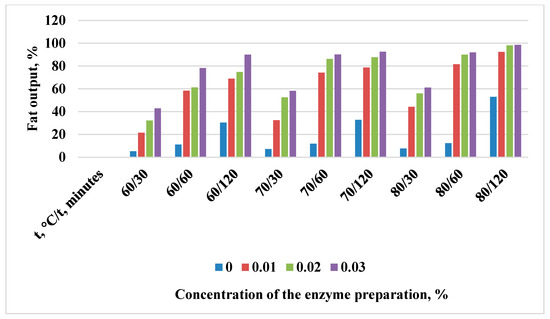
Figure 2.
Change in the yield of rendered fat with the Neutrase enzyme preparation.
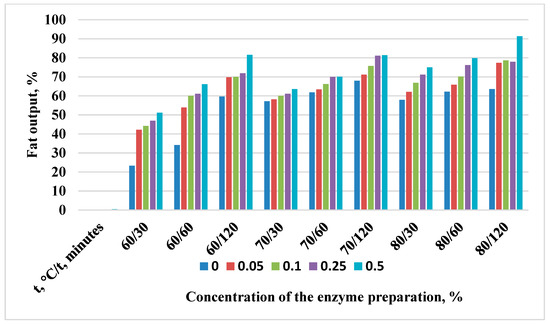
Figure 3.
Change in the yield of rendered beaver fat with the Protozym B enzyme preparation.
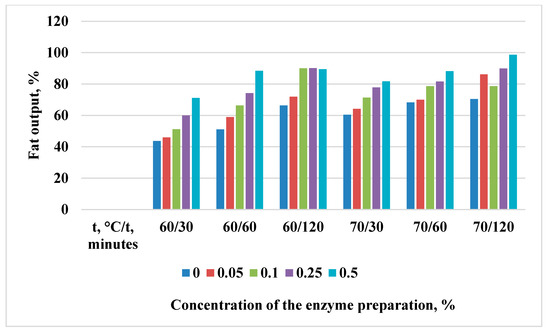
Figure 4.
Change in the yield of rendered marmot fat with the Fan Boost enzyme preparation.
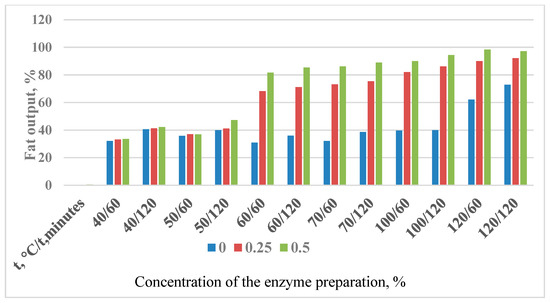
Figure 5.
Change in the yield of rendered badger fat with the Ondea Pro enzyme preparation.
The efficiency of the introduced enzyme preparation is determined by their concentration, the duration of exposure, and the temperature of the medium.
In the experimental samples, the yield of rendered fat was higher than in the control. The experimental output values exceed the control values by almost 2 times. The efficiency of the enzyme preparation increases with an increase in the duration of exposure, as well as in the concentration of the introduced enzyme preparation.
However, it is worth noting that when using Neutrase to render bear fat at 80 °C for 120 min, increasing the concentration of the preparation to 0.03% does not give a significant increase compared to the concentration of 0.02% (Figure 2). Therefore, it is advisable to add Neutrase with the concentration of 0.02% in order to reduce its consumption.
Enzyme activity is the most important characteristic of an enzyme preparation. It is expressed in units of activity per 1 g or 1 mL of the enzyme preparation (U/g or U/mL). The activity of each enzyme can reach its highest value, after which a decrease in activity is observed. Thus, the activity of Protozym B to render beaver fat with a concentration of 0.25% at 80 °C begins to decrease slightly if the duration of exposure exceeds 60 min (Figure 3).
The Fan Boost preparation with a concentration of 0.1% and 0.25% shows the highest activity at 60 °C, and increasing the temperature further is not feasible at the given concentrations.
3.2. ANN Design
The dataset consisted of seven input and one output variables. The architecture of a fully connected ANN was selected for modeling.
In the course of the study, the following parameters of the ANN were determined:
- -
- the number of hidden layers;
- -
- the number of neurons in the hidden layers;
- -
- the activation function;
- -
- the loss function;
- -
- the step;
- -
- the optimizer;
- -
- regularization;
- -
- the size and number of batches;
- -
- the number of epochs.
Out of the 147 dataset records, 98 were put in the training set (67%), and 49 were put in the test set (33%); a validation set was not used.
The resulting ANN had seven input neurons, and one output neuron. The input values were normalized using the minimum and maximum values by the formula:
where is the normalized value, xi is the original value, xmin is the minimum value, and xmax is the maximum value.
Thus, all the input values lay within the range from zero to one. Such normalization showed better results compared to mean normalization and standard deviation, and to the results obtained without normalization because most input values equaled only zero or one (F1, F2, F3, F4), and a smaller number of the variables had significantly higher values. Thus, the normalization made all the input data homogenous. The output values were normalized using the same method.
The network architecture was identified by enumeration of the possible values of the following parameters: the number of hidden layers, the number of neurons in the hidden layers (accepted the same number of neurons in each layer), the activation functions, the learning rate, the optimizer, regularization, the size and number of batches, the number of epochs. All the boundaries of the enumeration of options were chosen empirically, going beyond these boundaries did not give an acceptable accuracy of the ANN. The number of hidden layers varied from two to 10; the number of neurons in the hidden layers—from five to 150; the learning rate from 0.1 to 0.000001 step × 10−1; regularization from 0.1 to 0.000001 step × 10−1; the size and number of batches selected from one, two, seven, 14, 49, and 98; the number of epochs was chosen before the overfitting of the network; the activation function was selected from ELU, Hardsigmoid, Hardtanh, LeakyReLU, PReLU, ReLU, ReLU6, RReLU, SELU, CELU, GELU, Sigmoid, SiLU, and Tanh; and the optimizer was selected from Adadelta, Adam, Adagrad, ASGD, RMSProp, Rprop, and SGD. The parameters of the most accurate ANN are presented in Table 1.

Table 1.
ANN Parameters.
The ANN comprised eight hidden layers with 64 neurons in each. The activation function was ReLU with the formula:
where x is the input value.
Since BCELoss was used as the loss function, the Sigmoid activation function was used in the output layer. It has the formula:
BCELoss (Binary Cross Entropy) was selected as the loss function, it has the formula:
where y is the vector of output values from the dataset, is the vector of model output values, and N is the number of data.
Binary Cross Entropy is mainly used for classification problems with two classes, so it generates the probability of an object belonging to any class in the range from zero to one. It is also suitable for regression problems if the output neuron generates a value from zero to one, which is why the Sigmoid function was added to the output neuron in this ANN.
Adam (adaptive moment estimation) algorithm was used for optimization. It has the following formula for adjusting weights:
where Wt + 1 is new network parameters, Wt is current network parameters, α is the learning rate, is the exponential moving average of the gradient, β1 and β2 are the parameters of the exponential moving average, and ε is the smoothing parameter excluding division by zero.
The learning rate equaled 0.001, and Tikhonov regularization (L2 or weight decay) was used to avoid overtraining (its value was 0.00001). The ANN was trained in 49 batches with two records in each. The best results were obtained after 226 training epochs. The research was conducted on a computer platform with the following characteristics: Intel Core i7-11700F processor, RAM—32 Gb DDR4-3200, video card—GeForce RTX 2060 Super, and SSD (NVM Express m.2) 250 GB, HDD 1 TB. The network training time was 304,589 milliseconds.
4. Discussion
4.1. Evaluating the ANN Accuracy
In this study, the ANN accuracy was defined using mean relative error (MRE):
where MRE is the mean relative error (%), yi is the experimental value, is the model value, and N is the sample size.
For technological systems, the MRE criterion is one of the most common. The designed ANN showed the best MRE value of 5.16% after 409 training epochs (Figure 6). A model is usually considered adequate if the MRE lies within 10%; thus, the designed ANN can be used to optimize the studied process.
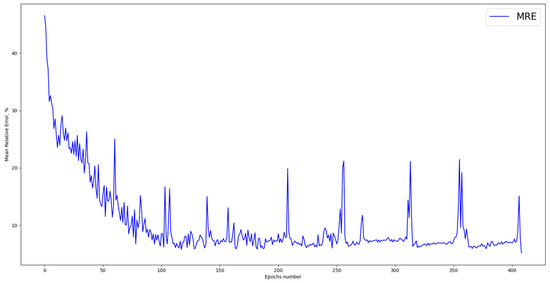
Figure 6.
Mean relative error.
4.2. Process Optimization
The practical significance of the study consists in defining the parameters of the technological process of obtaining rendered fat, at which the fat yield will be the highest; such parameters will be optimal, and the process of their definition is optimization. To determine the optimal parameters, the ANN with sufficient accuracy was designed. Next, a dataset was prepared with a large number of input parameter values and all possible combinations of them. The dataset allowed solving the optimization problem by exhaustive search.
The dataset comprised the following combinations of parameters: T took values from 70 to 150 °C with a step of 5 °C, D changed from 1 to 121 min with a step of 5 min, and C varied from 0 to 1% with a step of 0.01%; and the F1, F2, F3, and F4 variables took the value of one alternately. As a result, the volume of the dataset amounted to more than 214,000 lines.
The lower limit of the temperature range is determined by the activity of the lipase enzyme found in raw fat: rendering at temperatures below 70 °C will be accompanied with active hydrolytic decomposition of fats, which will significantly reduce the quality of the obtained product, while at a temperature of 70 °C and above lipase is inactivated. The upper temperature limit is determined by the varying technical properties of the apparatuses used for rendering: if continuous thin layer rendering is possible, the process temperature can reach values of more than 120–130 °C, while the duration of raw materials exposure can be decreased. This also explains the time intervals.
The concentration of enzyme preparations significantly depends on the enzyme activity; in practice it does not exceed 1% of the raw material mass. However, enzyme preparations are known to be expensive, and their excessive concentration is not economically advisable.
The dataset was fed to the input of the developed ANN, after which the simulated V values were obtained. For visualization and analysis of the obtained values, four point cloud graphs were built for the four enzymes used (Figure 7, Figure 8, Figure 9 and Figure 10). The axes show the enumerated values of temperature, rendering duration, and concentration of the introduced enzyme. The point clouds were formed for the predicted output values of V > 98%. Thus, each graph shows the optimal technological parameters of the fat rendering process for a particular enzyme, at which the largest volume of rendered fat can be obtained.
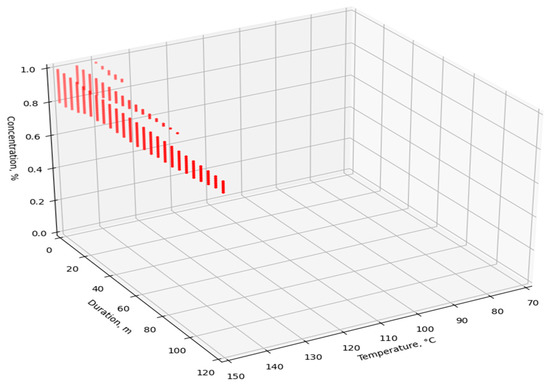
Figure 7.
Optimal technological parameters of fat rendering for the Fan Boost enzyme.
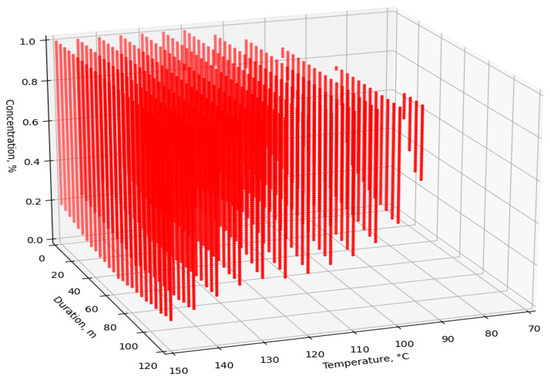
Figure 8.
Optimal technological parameters of fat rendering for the Neutrase enzyme.
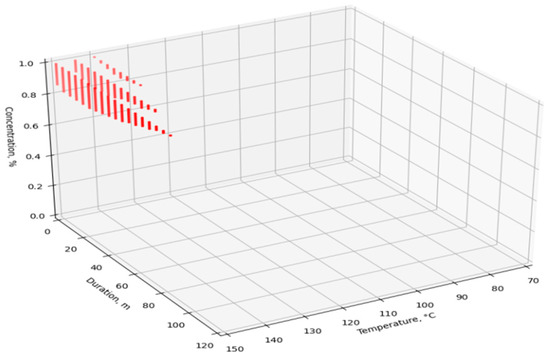
Figure 9.
Optimal technological parameters of fat rendering for the Ondea Pro enzyme.
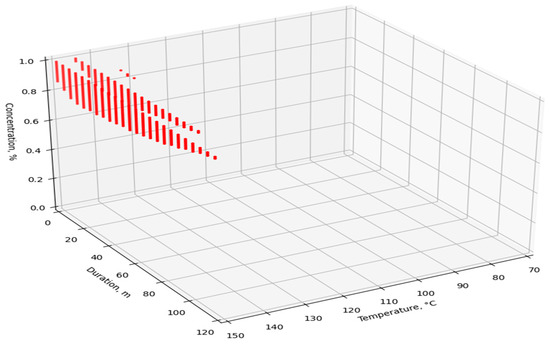
Figure 10.
Optimal technological parameters of fat rendering for the Protozym B enzyme.
The obtained optimal technological parameters were tested in further experiments. It was established that the use of the Fan Boost enzyme preparation is efficient at the temperatures of 120–150 °C, a rendering process duration of 5–120 min, and a rather high enzyme concentration of 0.8–1.0%.
Neutrase allows working in a wider range of variable technological parameters, but the highest efficiency is achieved at the temperatures of 100–150 °C, a rendering process duration of 1–20 min, and an enzyme preparation concentration of 0.2–0.8%.
The optimal parameters for using Ondea Pro and Protozym B are the temperatures of 120–150 °C, a rendering duration of 5–90 min, and a high enzyme concentration of 0.9–1.0%.
The use of a mixture of enzymes in optimization was not considered because the use of a mixture of enzymes for hydrolysis is possible provided a more detailed study of the structure of protein compounds of raw fat. However, this is difficult when implementing a biocatalytic method for extracting fats of hunting animals on an industrial scale due to the instability of qualitative and quantitative physico-chemical parameters of raw materials, including protein compounds of raw fat. To implement such a method, a large array of experimental data obtained from the study of raw fat isolated from different types of hunting animals is needed, taking into account their age characteristics, habitat, trapping conditions, nutrition, season, etc. Carrying out such studies requires large resources and, possibly, will be carried out in the future.
5. Conclusions
The fully connected artificial neural network was designed for predicting the percentage of rendered fat output in wet rendering, its mean relative error amounting to 5.16%. Next, a data set was prepared to determine the optimal technological values of the rendering process. As a result of optimization, the data on the effect of temperature, process duration, introduced enzyme preparation and its concentration on the yield of rendered fat were obtained. Based on the data, experimental studies were carried out, their results confirming that the process optimization is correct, and the obtained parameter dependences are feasible in practice. As can be seen from the data on the parameter selection optimization, the use of neural networks allows modeling the process of biocatalysis with a wide variation in the ranges of concentrations, temperatures, and durations, while obtaining preset values for the yield of the finished product, taking into account the technical and technological features of specific lines and industries.
Thus, the study showed the efficiency of using artificial neural networks for modeling the biocatalytic method of lipid extraction.
Author Contributions
A.V.S.—creation of neural networks, writing the manuscript. A.Y.P.—research organization, final approval of the version to be submitted. research methodology, design of the study, E.A.V.—analytical review of the literature, organization of experimental research, obtaining factual material. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kurzova, A.A.; Knyazeva, A.S.; Vostrikova, N.L. New standards for test methods of meat products. Vsyo Myase 2018, 3, 28–31. (In Russian) [Google Scholar] [CrossRef]
- Zhdankin, G.V.; Novikova, G.V. Development of microwave installer for heat treatment of inedible slaughter waste. Perm Agrar. J. 2017, 20, 23–29. [Google Scholar]
- Gorbacheva, M.V.; Tarasov, V.E.; Kalmanovich, S.A.; Sapozhnikova, A.I. Electrochemical activation as a fat rendering technology. Foods Raw Mater. 2021, 9, 32–42. [Google Scholar] [CrossRef]
- Gorbacheva, M.V.; Tarasov, V.E.; Kalmanovich, S.A.; Sapozhnikova, A.I. Ostrich fat production using electrolyzed fluid. Food Process. Tech. Technol. 2020, 50, 21–31. [Google Scholar] [CrossRef]
- Gorbacheva, M.V.; Tarasov, V.E.; Sapozhnikova, A.I.; Gordienko, I.M.; Strepetova, O.A. Method of Obtaining Ostrich Melted Fat. Russia Patent RU 2683559C1, 28 March 2019. [Google Scholar]
- Gorbacheva, M.V.; Tarasov, V.E.; Sapozhnikova, A.I. New technical solutions for the intensification of the process of fat extraction. In Innovations in the Food Industry: Education, Science, Production: Materials of the 4th All-Russian Scientific and Practical Conference; Far Eastern State Agrarian University: Blagoveshchensk, Russia, 2020; pp. 34–38. [Google Scholar]
- Zhdankin, G.V.; Samodelkin, A.G.; Novikova, G.V.; Belova, M.V.; Mikhajlova, E.D. Multi-Module Centrifugal Ultrahighfrequency Plant for Heat Treatment of Raw Material of Animal Origin and Separation of Liquid Fraction. Russia Patent RU 2694179C2, 9 July 2019. [Google Scholar]
- Zimina, M.I.; Sukhih, S.A.; Babich, O.O.; Noskova, S.Y.; Abrashina, A.A.; Prosekov, A.Y. Investigating antibiotic activity of the genus bacillus strains and properties of their bacteriocins in order to develop next-generation pharmaceuticals. Foods Raw Mater. 2016, 4, 92–100. [Google Scholar] [CrossRef]
- Smirnov, S.O.; Fazullina, O.F. Formula and technology development for obtaining biologically active natural food additives. Food Process. Tech. Technol. 2018, 48, 105–114. (In Russian) [Google Scholar] [CrossRef]
- Cunha, A.F.; Caetano, N.S.; Ramalho, E.; Crispim, A. Fat extraction from fleshings—Optimization of operating conditions. Energy Rep. 2020, 6, 381–390. [Google Scholar] [CrossRef]
- Jenkins, B.; Ronis, M.; Koulman, A. LC–MS lipidomics: Exploiting a simple high-throughput method for the comprehensive extraction of lipids in a ruminant fat dose-response study. Metabolites 2020, 10, 296. [Google Scholar] [CrossRef]
- Ali, A.; Qadri, S.; Mashwani, W.K.; Brahim Belhaouari, S.; Naeem, S.; Rafique, S.; Jamal, F.; Chesneau, C.; Anam, S. Machine learning approach for the classification of corn seed using hybrid features. Int. J. Food Prop. 2020, 23, 1097–1111. [Google Scholar] [CrossRef]
- An, T.; Yu, H.; Yang, C.; Liang, G.; Chen, J.; Hu, Z.; Hu, B.; Dong, C. Black tea withering moisture detection method based on convolution neural network confidence. J. Food Process Eng. 2020, 43, e13428. [Google Scholar] [CrossRef]
- Bhargava, A.; Barisal, A. Automatic Detection and Grading of Multiple Fruits by Machine Learning. Food Anal. Methods 2020, 13, 751–761. [Google Scholar] [CrossRef]
- Borodulin, D.M.; Shafrai, A.V.; Maximenko, A.A. Neural Network and Home Hydroponics. Food Process. Tech. Technol. 2023, 53, 384–395. (In Russian) [Google Scholar] [CrossRef]
- Chen, J.D.; Zhang, D.F.; Nanehkaran, Y.A.; Li, D.L. Detection of rice plant diseases based on deep transfer learning. J. Sci. Food Agric. 2020, 100, 3246–3256. [Google Scholar] [CrossRef]
- Ekiz, B.; Baygul, O.; Yalcintan, H.; Ozcan, M. Comparison of the decision tree, artificial neural network and multiple regression methods for prediction of carcass tissues composition of goat kids. Meat Sci. 2020, 161, 108011. [Google Scholar] [CrossRef]
- Lu, A.; Wei, X.; Cai, R.; Xiao, S.; Yuan, H.; Gong, J.; Chu, B.; Xiao, G. Modeling the effect of vibration on the quality of stirred yogurt during transportation. Food Sci. Biotechnol. 2020, 29, 889–896. [Google Scholar] [CrossRef]
- Shafrai, A.V.; Permyakova, L.V.; Borodulin, D.M.; Sergeeva, I.Y. Modeling the Physiological Parameters of Brewer’s Yeast during Storage with Natural Zeolite-Containing Tuffs Using Artificial Neural Networks. Information 2022, 13, 529. [Google Scholar] [CrossRef]
- Tarafdar, A.; Kaur, B.P.; Nema, P.K.; Babar, O.A.; Kumar, D. Using a combined neural network—Genetic algorithm approach for predicting the complex rheological characteristics of microfluidized sugarcane juice. LWT-Food Sci. Technol. 2020, 123, 109058. [Google Scholar] [CrossRef]
- Torshizi, M.V.; Asghari, A.; Tabarsa, F.; Danesh, P.; Akbarzadeh, A. Classification by artificial neural network for mushroom color changing under effect uv—A irradiation. Carpathian J. Food Sci. Technol. 2020, 12, 157–167. [Google Scholar] [CrossRef]
- Vasighi-Shojae, H.; Gholami-Parashkouhi, M.; Mohammadzamani, D.; Soheili, A. Predicting Mechanical Properties of Golden Delicious Apple Using Ultrasound Technique and Artificial Neural Network. Food Anal. Methods 2020, 13, 699–705. [Google Scholar] [CrossRef]
- Khachaturyan, L.R. Expertize of the quality of rendered animal fats. In Bulletin of Scientific Works of Young Scientists, Graduate Students, Undergraduates and Students of Gorsk State Agrarian University; Temiraev, V.K.h., Kudzaev, A.B., Eds.; Gorsky State Agrarian University: Vladikavkaz, Russia, 2018; pp. 365–367. (In Russian) [Google Scholar]
- Li, C.; Wang, B.; Qin, P.; Ge, W.; Zhang, M.; Yue, B.; Chen, H. Enzymatic centrifugation extraction of goose fat liver oil and its quality evaluation. Food Res. Dev. 2018, 39, 72–81. [Google Scholar]
- Novikov, A.M.; Semenov, A.V. Principles of rendering animal fat parameters in a high-frequency electromagnetic field. In Scientific and Practical Ways to Improve Environmental Sustainability and Socio-Economic Support of Agricultural Production: Proceedings of the International Scientific and Practical Conference Dedicated to the Year of Ecology in Russia; Caspian Research Institute of Arid Agricultural: Solenoe Zaymische, Russia, 2017; pp. 1278–1281. (In Russian) [Google Scholar]
- Poruchikov, D.; Samarin, G.; Vasilyev, A.; Ershova, I.; Normova, T.; Aleksandrova, G.A.; Filippova, I.V. UHF device introduction for animal raw material processing. Helix 2020, 10, 64–68. [Google Scholar] [CrossRef]
- Sander, A.; Antonije Košćak, M.; Kosir, D.; Milosavljević, N.; Parlov Vuković, J.; Magić, L. The influence of animal fat type and purification conditions on biodiesel quality. Renew. Energy 2018, 118, 752–760. [Google Scholar] [CrossRef]
- Slobodchikova, M.N.; Vasilyeva, V.T.; Ivanov, R.V.; Lebedeva, U.M. New aspects of non-waste use of secondary raw materials of horse breeding in Yakutia. Probl. Nutr. 2018, 87, 87–92. (In Russian) [Google Scholar] [CrossRef]
- Vasilevich, F.I.; Gorbacheva, M.V.; Sapozhnikova, A.I.; Gordienko, I.M. Integrated, environmentally safe disposal (recycling) of secondary products and animal waste: Innovative technical solutions. In Actual Problems of Veterinary Medicine, Zootechnics and Biotechnology: Collection of Scientific Papers of the International Educational-Methodical and Scientific-Practical Conference Dedicated to the 100th Anniversary of the Founding of Moscow State Academy of Veterinary Medicine and Biotechnology—MVA by K.I. Skryabin; Moscow State Academy of Veterinary Medicine and Biotechnology: Moscow, Russia, 2019; pp. 394–396. [Google Scholar]
- Vasilevich, F.I.; Gorbacheva, M.V.; Tarasov, V.E.; Sapozhnikova, A.I.; Gordienko, I.M. Electro-activated ostrich fat melting: An innovative solution. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 1615–1623. [Google Scholar]
- Volkov, V.V.; Mezenova OYa Hölling, A.; Grimm, T. Promising developments of processing technologies for byproducts of animal and plant origin using hydrolysis. In Baltic Maritime Forum: Materials of the VI International Baltic Maritime Forum; Kaliningrad State Technical University: Kaliningrad, Russia, 2018; pp. 24–30. [Google Scholar]
- Vostrikova, N.L.; Kuznetsova, O.A.; Kulikovskii, A.V. Methodological aspects of lipid extraction from biological matrices. Theory Pract. Meat Process. 2018, 3, 4–21. [Google Scholar] [CrossRef]
- Zhdankin, G.V.; Samodelkin, A.G.; Novikova, G.V.; Belova, M.V.; Gorbunov, B.I. Microwave Technology for Extracting Fat from Fat-Containing Raw Materials. Russia Patent RU 2636155C1, 21 November 2017. [Google Scholar]
- Dyshlyuk, L.; Pavsky, V.; Ivanova, S.; Babich, O.; Prosekov, A.; Chaplygina, T. The effect of postharvest ultraviolet irradiation on the content of antioxidant compounds and the activity of antioxidant enzymes in tomato. Heliyon 2020, 6, e03288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).