Abstract
Grossberg’s classification of adaptive resonance mechanisms includes the cognitive-emotional resonances that support conscious feelings and recognition of them. In this regard, a relevant question concerns the processing of signals deriving from the internal body and their contribution to interpersonal synchronization. This study aims to assess hemodynamic inter-subject coherence in the prefrontal cortex (PFC) through functional near-infrared spectroscopy (fNIRS) hyperscan recording during dyadic synchronization tasks proposed with or without a social frame and performed in two distinct interoceptive conditions: focus and no focus on the breathing condition. Individuals’ hemodynamic data (oxygenated and de-oxygenated hemoglobin (O2Hb and HHb, respectively)) were recorded through fNIRS hyperscanning, and coherence analysis was performed. The findings showed a significantly higher O2Hb coherence in the left PFC when the dyads performed the synchronization tasks with a social frame compared with no social frame in the focus condition. Overall, the evidence suggests that the interoceptive focus and the presence of a social frame favor the manifestation of a left PFC interpersonal tuning during synchronization tasks.
1. Introduction
The term “social interoception” was introduced in the literature to describe the link and the potential influence of interoception (the process by which our brain receives and processes information derived from our body [1]) on a number of social processes, such as self-other differentiation [2], social cognition, social isolation and connectedness [3], and emotional experience [4,5,6].
The ability to intentionally focus attention on one’s body signal for a determined span of time is defined as “interoceptive attentiveness” (IA) [7,8]. Weng and colleagues [9] showed that it is possible to modulate IA to observe positive outcomes in an individual’s emotional and cognitive health. However, there has been little neuroscientific research conducted to date on how manipulating interoception can impact the interpersonal synchronization processes. Turn-taking, mimicry, and non-verbal social communication [10], as well as time and content synchronization [11], can be included in interpersonal synchronization processes.
Previously, single-brain studies were carried out to investigate the neural correlates of IA manipulation (operationalized as focused on breathing) on the inter-personal synchronization necessary for performing joint tasks [12,13,14,15]. In two recent studies, functional near-infrared spectroscopy (fNIRS) was used to record the oxygenated hemoglobin (O2Hb) changes during joint tasks involving motor and cognitive synchronization, while the participants were required to concentrate on their breathing to better understand the hemodynamic correlates of IA manipulation in interpersonal synchronization [12,14]. In the first study, the induction of explicit focus on breathing during a socially framed motor task requiring synchronization increased the responsiveness of the prefrontal cortex (PFC), which is involved in sustained attention, reorientation of attention, social responsiveness, and synchronization. In the absence of a broader and more explicit social frame, this effect was not significant in the motor task [14].
The PFC was shown to play a significant role in high-order functions, including social and cognitive functions, motor control, and attention. Grossberg [16] recently proposed an increasingly comprehensive attentive brain architecture in his predictive adaptive resonance theory (pART) and assigned to the PFC the control of high-order functions, including working memory, learned plans, predictions, and optimized action. As a part of a larger interoceptive network [17], the PFC is also crucial in initiating and maintaining focused attention on a target while regulating internal and external interferences [18]. Furthermore, the PFC supports sustained attention to breathing by increasing individuals’ awareness of the mind wandering and enables them to bring their attention back to breathing [19]. Additionally, it has been associated with social functions, such as fully aware motor control and the ability to adapt to shifting rhythmic patterns [20], and also interpersonal coordination and cooperative interactions [21,22].
In the second fNIRS study, hemispheric lateralization was reported with an increase in O2Hb in the right PFC when intentional attention toward breathing was induced during a cognitive synchronization task, namely a linguistic task [12]. According to previous studies, the right PFC appears to support the execution of IA tasks [6,23,24] and sustained and goal-directed attention [18].
Although the above-mentioned fNIRS studies were the first to describe the effect of IA manipulation on individuals completing motor and cognitive tasks in synchronization with another partner and aiding in identifying the role of the PFC in this phenomenon at the intraindividual level, one obvious shortcoming consisted of the lack of assessments of the interactional dynamics between the two members of the dyad.
With the development of the hyperscanning paradigm [25], numerous works have calculated inter-agent synchronization and inter-brain coupling metrics that reflect the degree of social attunement based on the simultaneous recording of behavioral and hemodynamic responses from various agents involved in a joint task or a social exchange [22,26]. For instance, this made it possible to investigate how dyads’ inter-individual brain synchronization changes depending on whether the participants are collaborating or competing [27,28].
In addition, hyperscanning studies were extensively used for deepening synchronization mechanisms during motor [29,30,31], linguistic, and cognitive tasks. Naturalistic paradigms including verbal collaboration and turn-taking have revealed a lateralization effect, with right-sided activations of the dorsolateral PFC and temporal areas [32].
However, it has also been established that the setting of the interaction affects inter-brain synchrony. For example, facing the interacting partner appears to improve inter-brain synchrony, as evidenced by more simultaneous increases in activity within the left inferior frontal cortex and the right temporal parietal junction (TPJ) in subjects who were singing [33] or playing interactive games while facing each other as opposed to a wall [34]. Additionally, face-to-face interactions showed increased inter-brain synchrony, but back-to-back ones did not [35].
Furthermore, inter-brain synchronization in the left PFC was discovered to predict the effectiveness of teaching, highlighting the significance of shared attention for the accomplishment of shared objectives [36]. Greater inter-brain synchrony in the left frontopolar region was also found in dyads with different social experience [37]. In regions connected to the social alignment loop, such as the left inferior frontal cortex, behavioral alignment was found to be mediated by inter-brain synchrony [38].
Nonetheless, these hyperscanning studies did not manipulate IA when individuals performed the tasks in synchrony. Moreover, the social framing was not explicitly emphasized. Therefore, it is also interesting to determine the impact of these two variables on intercerebral coherence in terms of lateralization.
With reference to the influence of a social frame on synchronization performance, before it was shown how, during a real-person, joint-tapping hyperscanning experiment, interpersonal sensorimotor performance and interbrain synchrony in the left TPJ was greater in a bidirectional than in a unidirectional condition, indicating the social effect of a more cooperative condition [39] or suggesting a potential neural mechanism for selective tuning in to a target speaker while tuning out others [40]. Additionally, as stated above, the presence of an explicit social frame during a motor synchronization task, executed while paying attention to breathing, augmented the O2Hb values in the PFCs of individuals [14]. Thus, it may be argued that even basic exercises of synchronization, if explicitly socially framed, may differently impact an individual’s inter-brain coherence. This research used neural coherence indices to explore the hemodynamic correlates of between-brain interconnectivity by using a two-person neuroscience paradigm. In former fNIRS hyperscanning experiments, coherence indices were used to examine the synchronization of brain rhythms during cooperative and competitive joint activities [22,26,41,42], social exchanges [43], and gesture observation and reproduction [44].
Therefore, the primary aim of the present study is to assess hemodynamic inter-subject coherence through fNIRS hyperscan recording during dyadic synchronization tasks, proposed with and without a social frame and performed in two distinct interoceptive conditions. Specifically, the experimental design examines two distinct conditions of presence and absence of interoceptive focus (i.e., when the attention of the participants is focused on breathing versus not focused on breathing), the specific synchronization task performed by the participants (cognitive versus motor), as well as the social frame applied at the beginning of the synchronization task (that could be socially framed or not).
Given previous evidence, we hypothesized observed higher inter-brain coherence in the PFC of the dyads during focusing on breathing compared with not focusing on the breathing condition for both synchronization tasks [12,14].
Secondly, regarding social frame manipulation, we expect to observe an increase in the inter-brain coherence effect for socially framed synchronization compared with non-socially framed synchronization. It is also supposed that an increased effect in response to the motor compared with the linguistic synchronization task will be present, given the effect of the social frame we previously observed specifically for the motor task in our previous research [14].
Thirdly, as indicated in the literature reported above, we aim to observe a potential lateralization effect even in terms of inter-brain coherence, with a potential right hemisphere lateralization effect connected to interoceptive focus [12,23,24] and a left hemispheric activation predominance for positive emotions derived from the synchronization.
Finally, taking into account that coherence indices were used before in fNIRS hyperscanning experiments to investigate PFC synchrony, we also intend to deepen whether inter-subject hemodynamic coherence indices can be exploited as a reliable indicator of dyads’ neural synchronization in this context (i.e., when the interoceptive attention to breathing is manipulated).
2. Materials and Methods
2.1. Participants
A total of 32 university students were enlisted for the current fNIRS experiment using a non-probabilistic convenience sampling technique (14 females; age mean = 27.1; standard deviation = 3.19). Each dyad was composed of two individuals of the same sex matched for age, and they did not meet before the experiment. We have previously estimated the adequate sample size to detect medium effects via inferential statistics (f = 0.25), with the α error probability set at 0.05 and with 0.80 power (G*Power 3.1 software [45]). The analysis suggested that a total of 15 observations (i.e., in our case, dyads) would be sufficient. All participants were right-handed and had normal or corrected-to-normal eyesight. The criteria for exclusion included pregnancy, past meditative experience, severe physical and chronic illnesses, convulsions, persistent pain, and any mental or neurological disorders. They signed written informed consent forms and willingly agreed to participate in the study after being advised they would not receive payment for their contributions. The Ethics Committee of the Department of Psychology (Catholic University of the Sacred Heart in Milan, Italy) gave its approval for this study (2020 TD-a.a.2020–2021), which was conducted in conformity with the Declaration of Helsinki.
2.2. Joint Synchronization Tasks Description
Two basic motor and cognitive synchronization tasks were adopted as joint tasks in the current study.
The participants in the motor synchronization task had to synchronize and coordinate their finger-tapping motion for three minutes with the other person in their dyad. The participants were instructed to sit in a chair with their elbows resting on a table and their dominant hand’s fingers spread about a centimeter apart. They were instructed to use all of the fingers on their dominant hand to tap the table. They were not told to move at a certain speed or to extend their fingers as wide as they could. All they had to do was ensure their finger movements matched those of the participant sitting in front of them. The average number of loops—measured as the total number of times a finger-tapping pattern was repeated—was 60.
A modified version of the human-to-human alternating speech task was utilized for the cognitive synchronization test, requiring the participants to syllabicate in unison with the other participant in the dyad for a total of three minutes. The four syllables of “LA”, “BA”, “CA”, and “DA” were to be spoken consecutively and alternately by the participants. To pronounce a syllable at the same time, for example, when one member of the dyad said “LA”, the other member should have paired the syllable by saying “LA”, and so on. The speech patterns were not chosen in advance. Without any breaks, each language synchronization task session lasted three minutes. The number of repetitions from “LA” to “DA” in each loop throughout the course of the three minutes was at least 45.
These tasks were employed in prior single-brain investigations [12,14] and were used for this hyperscanning study to ensure consistency in the experimental design.
2.3. Procedure and Experimental Manipulations
Each dyad was positioned such that the participants could comfortably interact with each other face to face. The participants received procedural instructions before the experiment started. They were informed that they were required to execute two joint synchronization tasks following different experimental conditions in which IA was manipulated.
In the first condition, IA was purposefully controlled by instructing the participants to concentrate on their breathing. The following directions were given in this focus on breathing: “During this task, we ask you to concentrate on your breathing. Try to pay attention to how you feel and whether your breathing changes as you complete the activity.” The participants were not instructed to breathe at a certain pace. In contrast, no specific instructions were given in the no attention to breathing condition, which was regarded as the control condition, in which interoception was not manipulated, and the participants were only instructed to complete the joint tasks. The same interoceptive manipulation was used in earlier investigations to preserve the procedure’s reliability, and it was shown to have an impact on the hemodynamic neural correlates [12,46].
For the social framing manipulation, we asked the participants to perform the same motor and cognitive synchronization tasks previously described, but they were socially framed by specifying that they needed to synchronize in order to develop greater teamwork skills. In this way, the absence of a social frame resulted from not emphasizing the sharing of intention, whereas stressing the shared intentionality explicitly served to introduce the social frame [14].
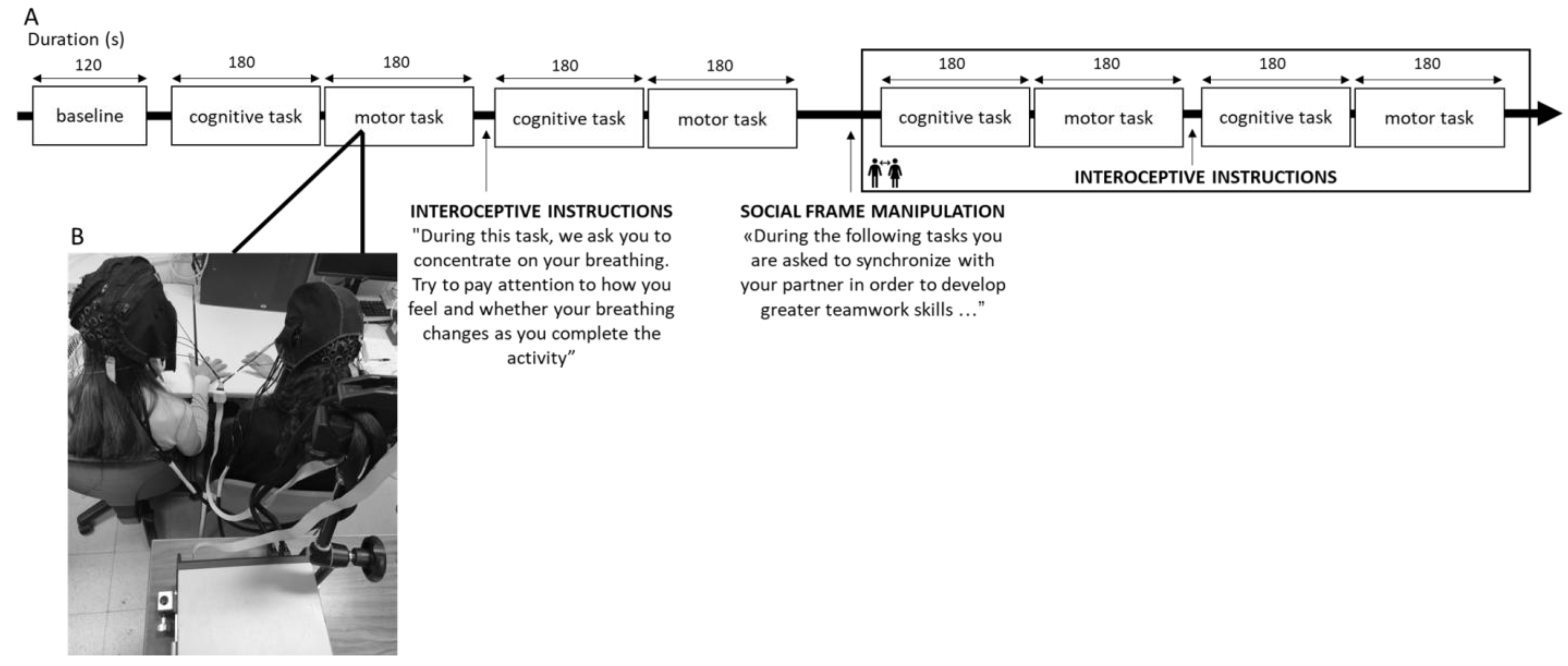
A 120 s baseline of each dyad member’s hemodynamic resting state was gathered before the synchronization tasks began. The order in which the condition and the synchronization tasks were performed were randomized and counterbalanced to prevent any potential biases brought on by sequence effects. After completing the activities, there was a debriefing phase in which the participants rated their attention to their breathing, the other person, and the task on a scale of 0 to 10. The entire experimental procedure took one hour to be completed (Figure 1A,B).
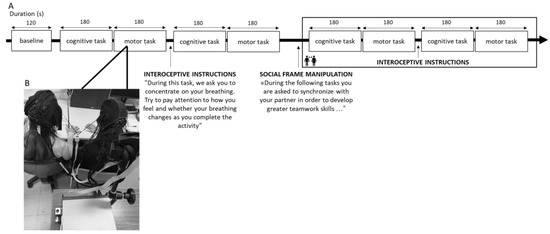
Figure 1.
(A) Experimental procedure representing the setting for the joint task and (B) the fNIRS hyperscanning acquisition from the dyad. To avoid an order effect, the task execution was randomized and counterbalanced for the type of the task and the condition.
2.4. fNIRS Data Recording and Biosignal Data Analysis
The hemodynamic signal was recorded using a six-channel optode matrix of an NIRScout System (NIRx Medical Technologies, LLC, Los Angeles, CA, USA). This system measures fluctuations in the concentrations of oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb). Using an fNIRS cap, four light sources or emitters and four detectors were placed over the scalp in line with the worldwide standard 10/5 system [47].
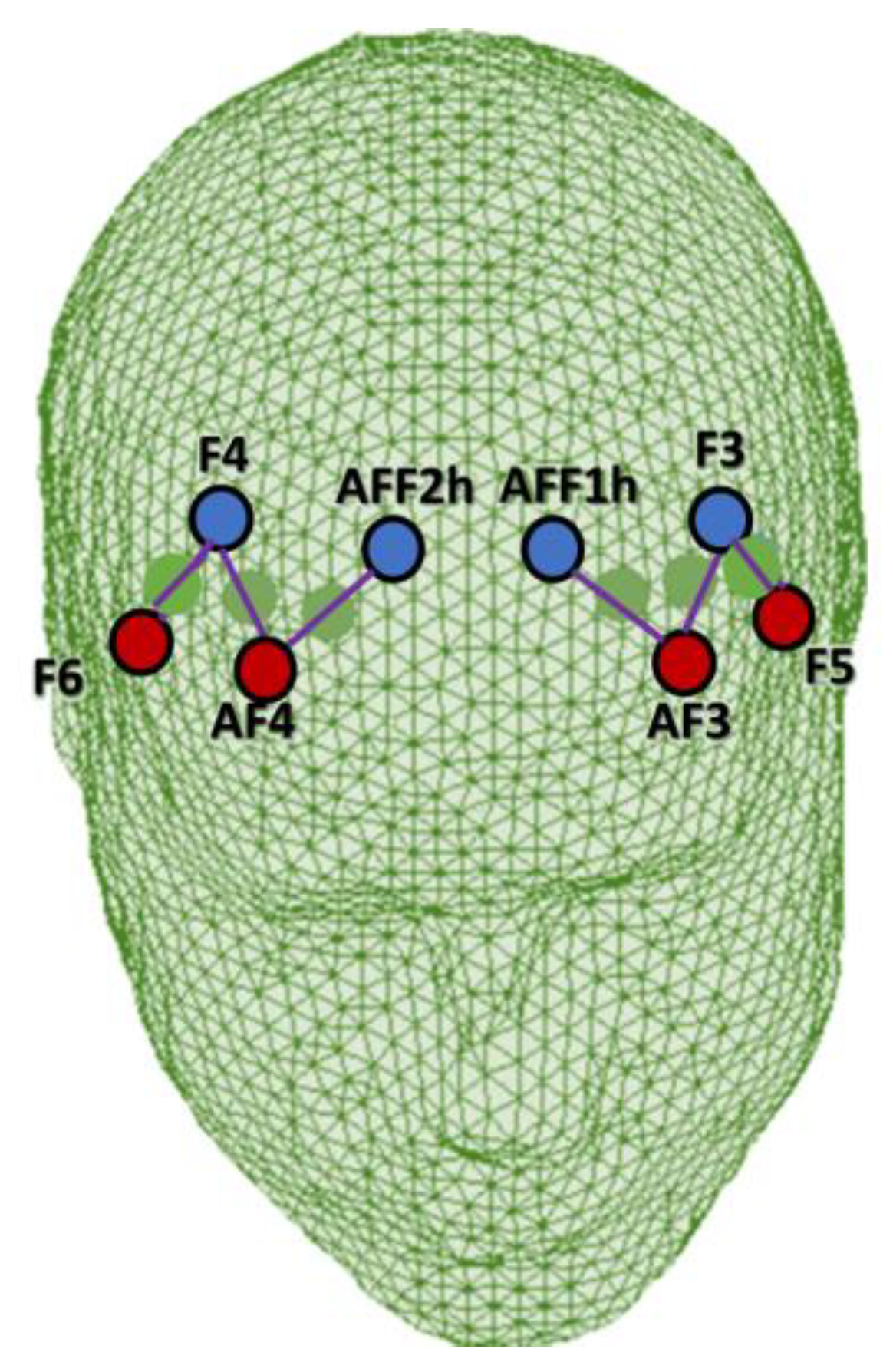
For the montage, four emitters were installed at AF3, AF4, F5, and F6, and four detectors were installed at AFF1h, AFF2h, F3, and F4. The emitter-detector distance for consecutive optodes was kept at 30 mm, and two wavelengths of near-infrared light were used (760 and 850 nm). Six channels were acquired using this optode configuration: Ch1 (AF3-F3), Ch2 (AF3-AFF1h), and Ch3 (F5-F3), which corresponded to the left PFC, and Ch4 (AF4-F4), Ch5 (AF4-AFF2h), and Ch6 (F6-F4), which corresponded to the right PFC [6,48] (Figure 2). The sources, detectors, and space between them were placed in relation to the underlying functional region and the most appropriate Brodmann area according to online atlases [49,50].
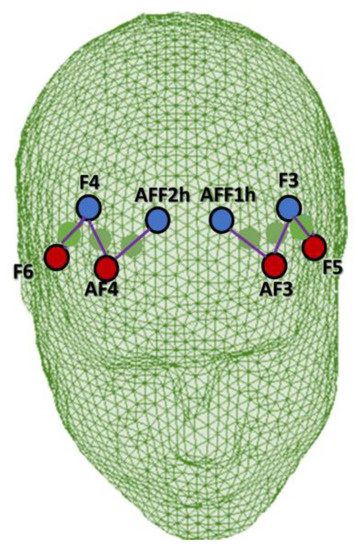
Figure 2.
Head rendering with the fNIRS montage layout. Emitters and detectors are indicated in red and blue, respectively.
2.5. Hemodynamic Data Reduction
NIRStar Acquisition Software (NIRx Medical Technologies LLC, 15 Cherry Lane, Glen Head, NY, USA) was used to continuously record the fluctuations in O2Hb and HHb concentrations during an initial 120 s resting baseline and the tasks. The signals from the six channels were collected at a sample rate of 6.25 Hz and then extracted and converted with nirsLAB software (v2014.05; NIRx Medical Technologies LLC, 15 Cherry Lane, Glen Head, NY, USA) based on their wavelengths and positions, producing mmol mm values that corresponded to the variations in the concentration of O2Hb and HHb per channel. Each channel’s acquired raw O2Hb and HHb data were digitally band-pass filtered at 0.01–0.3 Hz [41,43].
Both during the experimental phase and the signal analysis, raw time series were visually reviewed subject by subject to identify noisy channels caused by motion artifacts or amplitude variations (criterion for rejection: amplitude of hemoglobin (Hb) signal above or below ±5 SD; visual inspection). Artifacts caused 3% of the data to be removed. Channels with poor optical coupling and lack of heartbeat oscillations at 1 Hz were disregarded during this visual evaluation. Additionally, a linear-phase FIR filter on respiration was applied (0.3 Hz), which produced a symmetric impulse response [51,52].
Both during the experimental phase and the signal analysis, raw time series were visually inspected subject by subject to identify noisy channels caused by motion artifacts or amplitude variations. Here, 3% of the data were removed for artifacts.
The mean concentration of each channel for the tasks was determined following biosignal analysis. Based on the mean concentrations in the time series for each channel and subject, the effect size in each condition was calculated.
The effect sizes (Cohen’s d) were calculated by dividing the difference between the baseline and trial means by the baseline standard deviation (SD) such that D = (m1 m2)/s, where m1 and m2 are the mean concentration levels for the baseline and trial, respectively, and s is the baseline SD. The effect sizes from the six channels were averaged in order to increase the signal-to-noise ratio. While raw fNIRS data were initially relative values that could not be directly averaged across people or channels, and the normalized effect sizes were averaged regardless of the unit, since the effect size is unaffected by the differential pathlength factor (DPF).
For the statistical analysis of the fNIRS data, the channels were grouped to compose the lateralization factor for the left (Ch1, Ch2, and Ch3) and right (Ch4, Ch5, and Ch6) hemispheres, corresponding to the left and right PFCs.
2.6. Coherence Value Analysis
A first analysis was conducted to obtain the inter-brain coherence by computing the partial correlation coefficient Πij for each dyad, applied to each channel and to the lateralization factor for both the O2Hb and the HHb. These indices were obtained by normalizing the covariance matrix’s inverse:
Γ = Σ − 1
Γ = (Γij) = Σ − 1 inverse of the covariance matrix
This analysis permits evaluating the relationship between two signals (i, j) independent of one another [53], and it was previously applied often in earlier fNIRS hyperscanning research [22,54].
2.7. Statistical Analysis
A second step of analysis was applied to the coherence values, considered as dependent measures of a repeated measures ANOVA with independent within the factors of condition (two: focus on breathing or no focus on breathing) × task (two: motor or cognitive) × lateralization (two: left or right) × frame (two: not social or social). For this analysis, IBM SPSS Statistics (version 25) was used. For all ANOVA tests, in case of significant effects, pairwise comparisons were conducted to explore the significant interactions between simple effects, and the Bonferroni correction was applied to lessen the possible bias of repeated comparisons. The degrees of freedom for all ANOVA tests were adjusted using the Greenhouse–Geisser epsilon where required. Using partial eta squared (η2) indices, the magnitudes of the statistically significant effects were calculated.
3. Results
Two sets of results corresponding to the two analyses performed on the hemodynamic dependent measures will be described below. The first step of analysis included the application of coherence analysis for each dyad. The second step concerned the application of an inferential statistical ANOVA test to the coherence values considered dependent measures.
3.1. First Step: Coherence Results
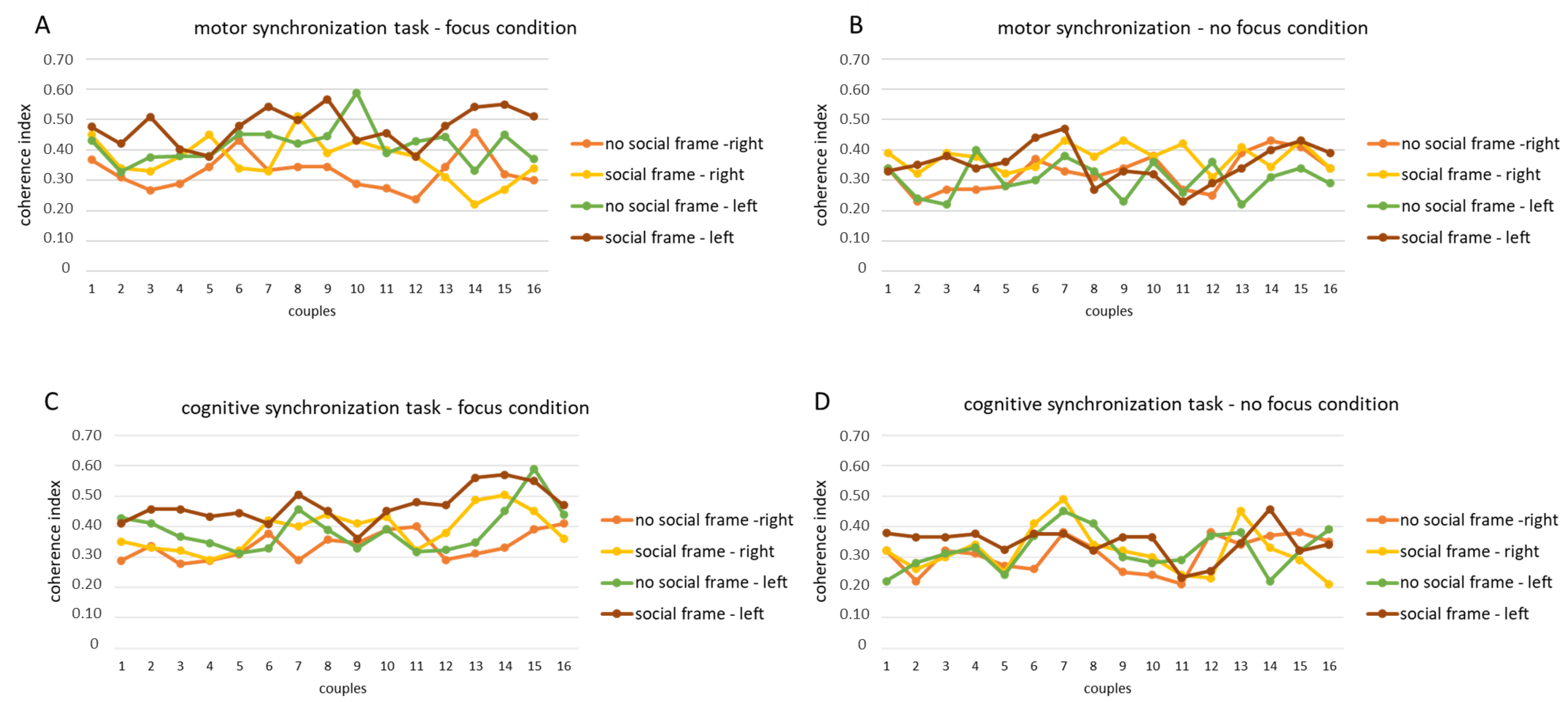
For the first step of analysis, we found the computed coherence values for each fNIRS channel in each experimental condition for both the O2Hb and HHb. A successive coherence analysis was applied to the lateralization factor for the left and right hemispheres, which were calculated as the average of the homologous channels for both the O2Hb and HHb, respectively. However, due to the limited significant values of coherence for the HHb, only the results for the O2Hb were considered and reported. In the graphs below, we have reported for this first step the mean trend of the coherence index for each dyad of participants (Figure 3A–D).
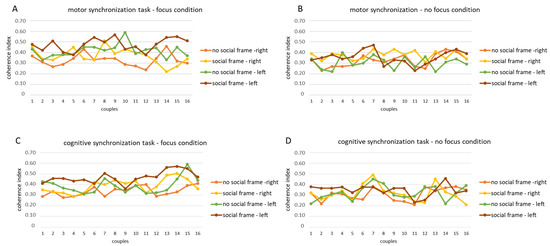
Figure 3.
fNIRS coherence values for O2Hb. Trend of the coherence indices for the motor synchronization task in the focus (A) and no focus conditions (B) and for the cognitive synchronization task in the focus (C) and no focus conditions (D) in each dyad.
3.2. Second Step: ANOVA Results
The ANOVAs applied to the inter-brain coherence indices as dependent variables for each dyad revealed significant effects for the D values of the O2Hb hemoglobin. The following paragraphs report the significant results obtained for the ANOVAs.
A first significant main effect was observed for the lateralization (F [1, 15] = 7.09, p = 0.01, η2 = 0.421), for which higher coherence for the O2Hb values was observed in the left compared with the right hemisphere.
Secondly, an interaction effect was detected for condition × task × lateralization (F [1, 43] = 8.09, p = 0. 01, η2 = 0.498). The pairwise comparison revealed an increase in coherence of the O2Hb values in the focus condition for both tasks (motor and cognitive) in the left compared with the right hemisphere (motor: F [1, 15] = 8.56, p = 0.01, η2 = 0.442; linguistic; F [1, 15] = 8.11, p = 0.01, η2 = 0.432).
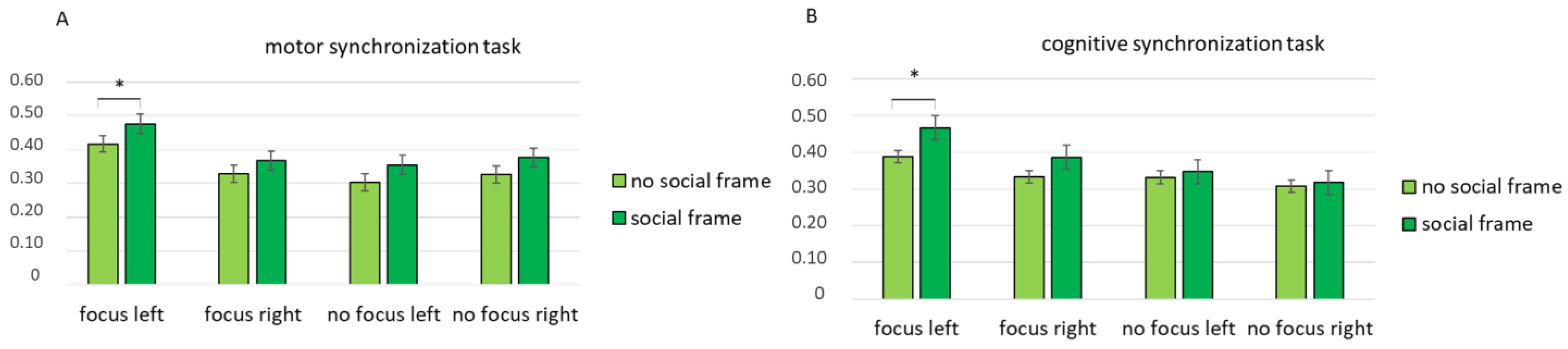
Then, a third interaction effect was found for condition × task × lateralization × frame (F [1, 82] = 7.89, p = 0.01, η2 = 0.432). A pairwise comparison revealed an increase in coherence in the left hemisphere when the participants in the focus condition performed the motor synchronization task with a social frame compared with no social frame (F [1, 15] = 8.98, p = 0.01, η2 = 0.408) (Figure 4A). According to the pairwise comparison, greater coherence was observed in the left hemisphere when the participants in the focus condition performed the cognitive synchronization task with a social frame compared with no social frame (F [1, 15] = 8.04, p = 0.01, η2 = 0.391) (Figure 4B).
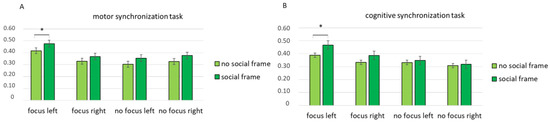
Figure 4.
Mean coherence indices for the cognitive and motor synchronization tasks. All asterisks (*) mark statistically significant differences, with p ≤ 0.05.
4. Discussion
In the present work, the hemodynamic interpersonal attunement of healthy participants during synchronization tasks with and without a social frame was explored and executed in two different experimental conditions. In particular, these tasks were executed in the presence or absence of explicit focus on breathing as interoceptive (IA) manipulation conditions. A social neuroscientific hyperscanning approach by fNIRS was applied to allow the recording of participants’ hemodynamic responses related to the motor and cognitive synchronization tasks presented with or without a social frame. For the fNIRS signal, an analysis of the coherence indices and a comparison of the fNIRS coherence’s strength for the conditions, tasks, and homologous PFC channels were performed.
The coherence analysis was computed to check the inter-subject neural hemodynamic coherence between the dyads for the left and right hemispheres considered in each experimental condition.
First, it was chosen to report the main significance results in graphs to describe the trend of synchronization within the dyads. In former fNIRS hyperscanning experiments, coherence indices were used to examine the synchronization of the hemodynamic signal during cooperative and competitive joint activities [22,26,41,42], social exchanges [43], and gesture observation and reproduction [44]. At the methodological level, the results of the present study proved that inter-subject hemodynamic coherence indices can be exploited as a reliable indicator of dyads’ PFC tuning in this context, specifically when there is a focus on breathing and the social frame is manipulated.
Moreover, some relevant and significant outcomes were detected. In general, higher coherence for the O2Hb values was observed in the left compared with the right hemisphere when the participants were executing both synchronization tasks (motor and cognitive). This effect was especially observed during the condition of focusing on breathing (i.e., the interoceptive condition). Additionally, greater coherence for the O2Hb values was observed in the left hemisphere when the participants in the focus condition performed both synchronization tasks with a social frame compared with when they executed the same tasks without an explicit social frame.
The higher inter-brain coherence in the left compared with the right PFC observed for both synchronization tasks in the interoceptive condition can be explained by taking into consideration the role of the left PFC in synchronization. In fact, former studies reported a right hemisphere lateralization effect connected to interoceptive focus [12,23,24]. However, they did not apply IA to social synchronization tasks. Therefore, a possible explanation for this apparently counterintuitive result may consider the left PFC’s role in relation to the positive impact of synchronization.
Past hyperscanning research on cooperation exploited fNIRS to assess subjects’ brain activity during a cooperative dual task [41] which adopted the same computation for neural coupling. The fNIRS results revealed increased brain PFC activity and higher synchronization over the left PFC after feedback, in line with the perception of a positive dynamic of social synchronization and compatible with positive emotions and approach-related motivations. This finding can be explained by considering the left PFC’s “emotional relevance” in comparison with the right PFC. As was previously noted, approach motivation, the capacity to control negative emotions, and general well-being are all correlated with the frontal cortical asymmetry favoring the left hemisphere [55,56,57,58]. According to converging data from evolutionary psychology and developmental research, cooperation is psychologically gratifying for the individual, and as a result, during cooperative conditions, frontal reward-processing regions may be more active than other cortical areas.
Therefore, it may be plausible that the synchronization per se, together with IA, may promote the left PFC’s neural synchrony in dyads. Nonetheless, the effect of interoception on PFC lateralization, particularly during social processes, needs to be further explored in future studies.
Furthermore, this PFC left-lateralized synchrony was not only associated with the interoceptive condition but also with the social framework (compared with no social framework) for both synchronization tasks (motor and cognitive).
The PFC, together with the TPJ, has been previously considered a neuroanatomical region belonging to the so-called “mutual attention system”, whose main characteristic is the mutual and synchronized activation aspect [40]. In particular, inter-brain synchrony in the left PFC was also found in several previous hyerscanning studies during simultaneous singing [33], face-to-face conversations [34], shared attention for the achievement of mutual goals [36], and also during a real-person, joint-tapping hyperscanning experiment in a bidirectional rather than unidirectional condition, indicating the social effect of a more cooperative condition [39] or suggesting a potential neural mechanism for selective tuning in to a target speaker while tuning out others [40]. Therefore, the present study adds to the existing body of knowledge evidence that even basic exercises of synchronization, which are also explicitly socially framed, may increase an individual’s inter-brain coherence in the left PFC.
Interestingly, different from what was hypothesized in our second hypothesis, no differences were observed between the motor and cognitive synchronization tasks, since the increased effect of coherence in the left PFC was detected for both tasks in the condition of focusing on breathing when the tasks were socially framed compared with not being socially framed. This result suggests that the effect of the synchronization and the social frame affects both the motor [14] and cognitive synchronization.
Such evidence is also crucial from a neuroanatomical perspective, since the involvement of the left prefrontal areas has been associated with interoception during social synchronization.
While this study demonstrated the relevance of coherence indices as a marker of the focus on breathing as an interoceptive condition and of social frame manipulation in synchronization tasks, it also presents some limitations. In fact, given the focus of this study on the PFC, fNIRS channels were applied on the frontal locations only without covering the whole scalp, which would have included somatosensory cortical regions and other relevant structures, such as the TPJ. Future research should focus on these structures’ roles in interoceptive processing [7] and social synchronization [39], respectively.
Furthermore, this basic research adopted some simplistic synchronization tasks that just required finger tapping or alternate syllable pronunciation. Future studies could (1) utilize more ecological and complex joint activities, such as dancing, playing an instrument, or communicating in a live interactive speech, as previously performed in prior hyperscanning studies, and (2) integrate multi-level measurements of the empathic resonance mechanisms, such as facial behavior analysis (to explore participants’ facial feedback) [59] and self-reported measures, such as a scale to measure empathic behavior (e.g., the Balanced Emotional Empathy Scale) and individual differences in approach or avoidance motivational tendencies (e.g., the Behavioral Inhibition and Activation Scale) [60], to gather the complexity of this phenomenon. Moreover, an impfigureortant effect may be suggested for the gender factor in relation to the social synchronization. Indeed, as shown by Cheng, Li, and Hu (2015), “the presence of a homogender of a heterogender dyad” may modulate interbrain activity, and future enquiries could be formulated about the role of gender on the joint cognitive and brain strategies.
Finally, to increase the ecological validity of the findings, participants could receive less artificial and more contextually related instructions for the social frame.
5. Conclusions
Overall, this hyperscanning research shows how manipulating IA, which is attained by concentrating on breathing, and the social frame in a dyadic condition enhances the emergence of hemodynamic indicators of interpersonal tuning in the left PFC during basic synchronization tasks. The increase in coherence in the left PFC can be considered a neuroanatomic marker of the combination of IA and social synchronization. This evidence opens the way to also considering in Grossberg’s pART model the contribution of interoception on high-level cognitive and emotional functions and on resonance mechanisms.
To the best of our knowledge, this is the first time that the effect of IA and the social frame on inter-brain neural synchrony has been explored during an interactive social dynamic involving two individuals. This experiment contributes to increasing the knowledge relating to those studies within the category of “social interoception”, which intends to investigate the impact of interoception on social dynamics and processes.
Author Contributions
Conceptualization, M.B. and L.A.; methodology, M.B. and L.A.; software, L.A.; validation, M.B. formal analysis, M.B.; investigation, M.B.; resources, M.B.; data curation, M.B. and L.A.; writing—original draft preparation, L.A.; writing—review and editing, M.B. and L.A.; visualization, M.B.; supervision, M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets generated and analyzed for this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge Carlotta Acconito and Katia Rovelli for their assistance with data curation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khalsa, S.S.; Adolphs, R.; Cameron, O.G.; Critchley, H.D.; Davenport, P.W.; Feinstein, J.S.; Feusner, J.D.; Garfinkel, S.N.; Lane, R.D.; Mehling, W.E.; et al. Interoception and Mental Health: A Roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.E.; Tsakiris, M. Going at the heart of social cognition: Is there a role for interoception in self-other distinction? Curr. Opin. Psychol. 2018, 24, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.J.; Winkielman, P.; Dobkins, K. Interoception and Social Connection. Front. Psychol. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burleson, M.H.; Quigley, K.S. Social interoception and social allostasis through touch: Legacy of the Somatovisceral Afference Model of Emotion. Soc. Neurosci. 2021, 16, 92–102. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L. One’s Interoception Affects the Representation of Seeing Others’ Pain: A Randomized Controlled qEEG Study. Pain Res. Manag. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L. Interoception as a social alarm amplification system. What multimethod (EEG-fNIRS) integrated measures can tell us about interoception and empathy for pain? Neuropsychol. Trends 2021, 29, 39–64. [Google Scholar] [CrossRef]
- Schulz, S.M. Neural correlates of heart-focused interoception: A functional magnetic resonance imaging meta-analysis. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160018. [Google Scholar] [CrossRef]
- Tsakiris, M.; De Preester, H. The interoceptive Mind: From Homeostasis to Awareness; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Weng, H.Y.; Feldman, J.L.; Leggio, L.; Napadow, V.; Park, J.; Price, C.J. Interventions and Manipulations of Interoception. Trends Neurosci. 2021, 44, 52–62. [Google Scholar] [CrossRef]
- Charman, T. Commentary: Glass half full or half empty? Testing social communication interventions for young children with autism - Reflections on Landa, Holman, O’Neill, and Stuart (2011). J. Child Psychol. Psychiatry Allied Discip. 2011, 52, 22–23. [Google Scholar] [CrossRef]
- Delaherche, E.; Chetouani, M.; Mahdhaoui, A.; Saint-Georges, C.; Viaux, S.; Cohen, D. Interpersonal Synchrony: A Survey Of Evaluation Methods Across Disciplines. IEEE Trans. Affect. Comput. 2012, 3, 349–365. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L. Interoceptive attentiveness induces significantly more PFC activation during a synchronized linguistic task compared to a motor task as revealed by functional Near-Infrared Spectroscopy. Brain Sci. 2022, 12, 301. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. Delta-Alpha EEG pattern reflects the interoceptive focus effect on interpersonal motor synchronization. Front. Neuroergonomics 2022, 1–10. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. The Increasing Effect of Interoception on Brain Frontal Responsiveness During a Socially Framed Motor Synchronization Task. Front. Hum. Neurosci. 2022, 16, 1–9. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. EEG brain oscillations are modulated by interoception in response to a synchronized motor vs. cognitive task. Front. Neuroanat. 2022, 16. [Google Scholar] [CrossRef]
- Grossberg, S. Attention: Multiple types, brain resonances, psychological functions, and conscious states. J. Integr. Neurosci. 2021, 20, 197–232. [Google Scholar] [CrossRef]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef]
- Kondo, H.; Osaka, N.; Osaka, M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage 2004, 23, 670–679. [Google Scholar] [CrossRef]
- Dickenson, J.; Berkman, E.T.; Arch, J.; Lieberman, M.D. Neural correlates of focused attention during a brief mindfulness induction. Soc. Cogn. Affect. Neurosci. 2013, 8, 40–47. [Google Scholar] [CrossRef]
- Stephan, K.M.; Thaut, M.H.; Wunderlich, G.; Schicks, W.; Tian, B.; Tellmann, L.; Schmitz, T.; Herzog, H.; McIntosh, G.C.; Seitz, R.J.; et al. Conscious and subconscious sensorimotor synchronization-Prefrontal cortex and the influence of awareness. Neuroimage 2002, 15, 345–352. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Song, B.; Pan, Y.; Cheng, X.; Zhu, Y.; Hu, Y. How to calculate and validate inter-brain synchronization in a fnirs hyperscanning study. J. Vis. Exp. 2021, 175, 1–16. [Google Scholar]
- Balconi, M.; Pezard, L.; Nandrino, J.-L.; Vanutelli, M.E. Two is better than one: The effects of strategic cooperation on intra- and inter-brain connectivity by fNIRS. PLoS ONE 2017, 12, e0187652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Wang, D.X.; Zhang, Y.R.; Tang, Y.Y. Enhancing Attention by Synchronizing Respiration and Fingertip Pressure: A Pilot Study Using Functional Near-Infrared Spectroscopy. Front. Neurosci. 2019, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Olszewska-Guizzo, A.; Husain, S.F.; Bose, J.; Choi, J.; Tan, W.; Wang, J.; Tran, B.X.; Wang, B.; Jin, Y.; et al. Brief relaxation practice induces significantly more prefrontal cortex activation during arithmetic tasks comparing to viewing greenery images as revealed by functional near-infrared spectroscopy (fNIRS). Int. J. Environ. Res. Public Health 2020, 17, 8366. [Google Scholar] [CrossRef] [PubMed]
- Montague, P.R.; Berns, G.S.; Cohen, J.D.; McClure, S.M.; Pagnoni, G.; Dhamala, M.; Wiest, M.C.; Karpov, I.; King, R.D.; Apple, N.; et al. Hyperscanning: Simultaneous fMRI during linked social interactions. Neuroimage 2002, 16, 1159–1164. [Google Scholar] [CrossRef]
- Crivelli, D.; Balconi, M. Near-infrared spectroscopy applied to complex systems and human hyperscanning networking. Appl. Sci. 2017, 7, 922. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Cooperation and competition with hyperscanning methods: Review and future application to emotion domain. Front. Comput. Neurosci. 2017, 11, 1–21. [Google Scholar] [CrossRef]
- Angioletti, L.; Vanutelli, M.E.; Fronda, G.; Balconi, M. Exploring the Connected Brain by fNIRS: Human-to-Human Interactions Engineering. Appl. Mech. Mater. 2019, 893, 13–19. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Hu, Y. Synchronous brain activity during cooperative exchange depends on gender of partner: A fNIRS-based hyperscanning study. Hum. Brain Mapp. 2015, 36, 2039–2048. [Google Scholar] [CrossRef]
- Baker, J.M.; Liu, N.; Cui, X.; Vrticka, P.; Saggar, M.; Hosseini, S.M.H.; Reiss, A.L. Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, X.; Zhang, Z.; Li, X.; Hu, Y. Cooperation in lovers: An fNIRS-based hyperscanning study. Hum. Brain Mapp. 2017, 38, 831–841. [Google Scholar] [CrossRef]
- Kelsen, B.A.; Sumich, A.; Kasabov, N.; Liang, S.H.Y.; Wang, G.Y. What has social neuroscience learned from hyperscanning studies of spoken communication? A systematic review. Neurosci. Biobehav. Rev. 2022, 132, 1249–1262. [Google Scholar] [CrossRef]
- Osaka, N.; Minamoto, T.; Yaoi, K.; Azuma, M.; Shimada, Y.M.; Osaka, M. How two brains make one synchronized mind in the inferior frontal cortex: FNIRS-based hyperscanning during cooperative singing. Front. Psychol. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Tang, H.; Mai, X.; Wang, S.; Zhu, C.; Krueger, F.; Liu, C. Interpersonal brain synchronization in the right temporo-parietal junction during face-to-face economic exchange. Soc. Cogn. Affect. Neurosci. 2015, 11, 23–32. [Google Scholar] [CrossRef]
- Jiang, J.; Dai, B.; Peng, D.; Zhu, C.; Liu, L.; Lu, C. Neural synchronization during face-to-face communication. J. Neurosci. 2012, 32, 16064–16069. [Google Scholar] [CrossRef]
- Davidesco, I.; Laurent, E.; Valk, H.; West, T.; Dikker, S.; Milne, C.; Poeppel, D. Brain-to-brain synchrony predicts long-term memory retention more accurately than individual brain measures. bioRxiv 2019, 644047. [Google Scholar]
- Sun, B.; Xiao, W.; Lin, S.; Shao, Y.; Li, W. Brain and Cognition Cooperation with partners of differing social experience: An fNIRS-based hyperscanning study. Brain Cogn. 2021, 154, 105803. [Google Scholar] [CrossRef]
- Shamay-tsoory, S.G.; Saporta, N.; Marton-alper, I.Z.; Gvirts, H.Z. Herding Brains: A Core Neural Mechanism for Social Alignment. Trends Cogn. Sci. 2019, 23, 174–186. [Google Scholar] [CrossRef]
- Dai, R.; Liu, R.; Liu, T.; Zhang, Z.; Xiao, X.; Sun, P.; Yu, X.; Wang, D.; Zhu, C. Holistic cognitive and neural processes: A fNIRS-hyperscanning study on interpersonal sensorimotor synchronization. Soc. Cogn. Affect. Neurosci. 2018, 13, 1141–1154. [Google Scholar] [CrossRef]
- Gvirts, H.Z.; Perlmutter, R. What Guides Us to Neurally and Behaviorally Align With Anyone Specific? A Neurobiological Model Based on fNIRS Hyperscanning Studies. Neuroscientist 2020, 26, 108–116. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Interbrains cooperation: Hyperscanning and self-perception in joint actions. J. Clin. Exp. Neuropsychol. 2017, 39, 607–620. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Brains in competition: Improved cognitive performance and inter-brain coupling by hyperscanning paradigm with functional near-infrared spectroscopy. Front. Behav. Neurosci. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Fronda, G.; Vanutelli, M.E. Donate or receive? Social hyperscanning application with fNIRS. Curr. Psychol. 2019, 38, 991–1002. [Google Scholar] [CrossRef]
- Balconi, M.; Fronda, G.; Bartolo, A. Affective, Social, and Informative Gestures Reproduction in Human Interaction: Hyperscanning and Brain Connectivity. J. Mot. Behav. 2021, 53, 296–315. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. J. Mater. Environ. Sci. 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Angioletti, L. Aching face and hand: The interoceptive attentiveness and social context in relation to empathy for pain. J. Integr. Neurosci. 2022, 21, 1–13. [Google Scholar] [CrossRef]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Empathy in negative and positive interpersonal interactions. What is the relationship between central (EEG, fNIRS) and peripheral (autonomic) neurophysiological responses? Adv. Cogn. Psychol. 2017, 13, 105–120. [Google Scholar] [CrossRef]
- Giacometti, P.; Perdue, K.L.; Diamond, S.G. Algorithm to find high density EEG scalp coordinates and analysis of their correspondence to structural and functional regions of the brain. J. Neurosci. Methods 2014, 229, 84–96. [Google Scholar] [CrossRef]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10-10 system. Neuroimage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Naseer, N.; Hong, M.J.; Hong, K.S. Online binary decision decoding using functional near-infrared spectroscopy for the development of brain-computer interface. Exp. Brain Res. 2014, 232, 555–564. [Google Scholar] [CrossRef]
- Naseer, N.; Hong, K.S. Classification of functional near-infrared spectroscopy signals corresponding to the right- and left-wrist motor imagery for development of a brain-computer interface. Neurosci. Lett. 2013, 553, 84–89. [Google Scholar] [CrossRef]
- Wheland, D.; Joshi, A.; McMahon, K.; Hansell, N.; Martin, N.; Wright, M.; Thompson, P.; Shattuck, D.; Leahy, R. Robust identification of partial-correlation based networks with applications to cortical thickness data. Proc.—Int. Symp. Biomed. Imaging 2012, 1551–1554. [Google Scholar]
- Balconi, M.; Vanutelli, M.E.; Gatti, L. Functional brain connectivity when cooperation fails. Brain Cogn. 2018, 123, 65–73. [Google Scholar] [CrossRef]
- Balconi, M.; Mazza, G. Lateralisation effect in comprehension of emotional facial expression: A comparison between EEG alpha band power and behavioural inhibition (BIS) and activation (BAS) systems. Laterality Asymmetries Body Brain Cogn. 2010, 15, 361–384. [Google Scholar] [CrossRef]
- Davidson, R.J. Anterior Cerebral Asymmetry and the Nature of Emotion. Brain Cogn. 1992, 20, 125–151. [Google Scholar] [CrossRef]
- Koslov, K.; Mendes, W.B.; Pajtas, P.E.; Pizzagalli, D.A. Asymmetry in resting intracortical activity as a buffer to social threat. Psychol. Sci. 2011, 22, 641–649. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biol. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef]
- Balconi, M.; Canavesio, Y. Is empathy necessary to comprehend the emotional faces? The empathic effect on attentional mechanisms (eye movements), cortical correlates (N200 event-related potentials) and facial behaviour (electromyography) in face processing. Cogn. Emot. 2016, 30, 210–224. [Google Scholar] [CrossRef]
- Balconi, M.; Bortolotti, A. Resonance mechanism in empathic behavior. BEES, BIS/BAS and psychophysiological contribution. Physiol. Behav. 2012, 105, 298–304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).