Abstract
Fish is a good source of Animal Source Proteins (ASP). Families from low-income countries with limited access to other animal source proteins can utilize it to improve the nutrition status of infants and young children. The objective of the study was to assess if fish fed during the early complementary feeding period had an effect on improved head circumference (HC) and mid-upper arm circumference (MUAC) among infants aged 6–7 months. A randomised controlled trial was conducted from April 2019 to January 2020 in the Samfya district, Luapula Province, Zambia. The infants (238) were randomised to either the fish group (intervention) or the sorghum group (control). Every week for a period of 6 months, infants received seven sachets of fish powder and sorghum powder, respectively. Compliance was also monitored during the fish powder distribution. The head circumference measurements were conducted at baseline and once each follow-up month for a period of six months while the MUAC measurements were conducted twice (at baseline and endline). Using statistical software for data science (STATA) (version 16), a linear mixed effect model was used to analyse the data. The results showed that fish improved head circumference for age z score (HCZ) by 0.53 (95% CI: 0.23–0.82), p-value < 0.001, and MUAC by 0.36 (95% CI: 0.13–0.59) p-value < 0.002. Therefore, fish can be used as the main source of protein in complementary foods for infants and young children in low-income communities with limited access to meat.
1. Introduction
Protein energy malnutrition has been associated with poor head circumference (HC) and brain development in children below the age of five [1]. There is a rapid increase in the circumference growth in the first thousand days of a child’s life. Therefore, routine measurement of the HC (frontal occipital circumference) should be a component of nutritional assessment. However, even though HC is a marker of neurodevelopment, most low- and middle-income countries do not conduct HC measurements regularly [2]. A community-based birth cohort study that was conducted in Vellore India found that small low HC at the age of 2 was negatively associated with cognitive development at both 2 and 5 years of age [3].
In Zambia, despite having under-five clinics where children below the age of five go for health and nutrition assessments monthly, HC measurements are not routinely conducted. The clinics routinely focus on weight and height due to the high prevalence of stunting (35%) and underweight (11.8%) in the children below the age of five [4]. However, HC circumference measurements are routinely conducted twice (in June and December) during the child health week.
The mid-upper arm circumference (MUAC) is a simple reliable measurement for the screening of children’s nutrition statuses [5]. A pilot study on animal source proteins (ASPs) derived from eggs showed an improved MUAC in children aged 6–9 years in a school feeding program in Uganda [6]. Children who received from one to two eggs per day for 6 months had improved MUAC compared to those who received none. MUAC is used as admission and discharge criteria within community-based programs and for the management of acute malnutrition. A study conducted by World Fish in Zambia found small dried fish to be a nutrient dense food source that is important for the first 1000 days [7]. It is also important to note that Chisense, the fish source used in this study, is rich in essential long-chain polyunsaturated fatty acids eicosapentaenoic acid (EPA) and docosohexaenoic acid (DHA), which are crucial for brain development and cognition of the child in the first 1000 days of life [8]. The nutrient content of the small fish (Chisense) used in the current study is reported in Table 2 of the main study, which has been published [9]. This current study reporting on HC and MUAC formed part of the main study that investigated the efficacy of fish as an early complementary food on the linear growth of infants aged 6–7 months and found that fish consumption improved linear growth [9]. To our knowledge, there is no study that looked at fish and improved HC and MUAC among children. Therefore, it was useful to investigate the effect of fish powder provided in early complimentary feeding on improved HC and MUAC.
2. Materials and Methods
2.1. Study Area
The study was conducted at Shikamushile Rural Health Centre (RHC) from April 2019 to January 2020 in the Samfya district of Luapula Province, Zambia. The community, which consists of low-income families, is situated near lake Bangweulu and lake Chifunabuli. The main livelihood for the occupants in this community is substance farming and fishing. Fish is readily available throughout the year in this community as it is preserved using drying and smocking methods to last long. The main complementary food fed to children in this district, however, is plain cassava or maize.
2.2. Participants and Study Design
The study was a 6-month single-blind, two-arm-randomised controlled trial (RCT). Fish powder was provided to the intervention group while a placebo in the form of sorghum powder was provided to the control group. As mentioned earlier, this was part of the larger study that was conducted in the Samfya District, Luapula Province, to assess the efficacy of fish early in the complementary feeding period to improve infant’s growth (length) [9].
2.3. Sample Size Determination
The sample size calculation was based on the main study that looked at linear growth [9]. Figure 1 summarizes the total number of infants recruited, including those that dropped out and those that completed the study. Similar to the main study [9], the population consisted of 235 participants at baseline (intervention: 118 and control: 117). Of the 235, the number of participants who withdrew from the study was 16 and those who dropped out at subsequent follow-up was 33. Therefore, a total number of 100 participants from the intervention group and 86 from the control group completed the 6 months’ intervention (Figure 1).
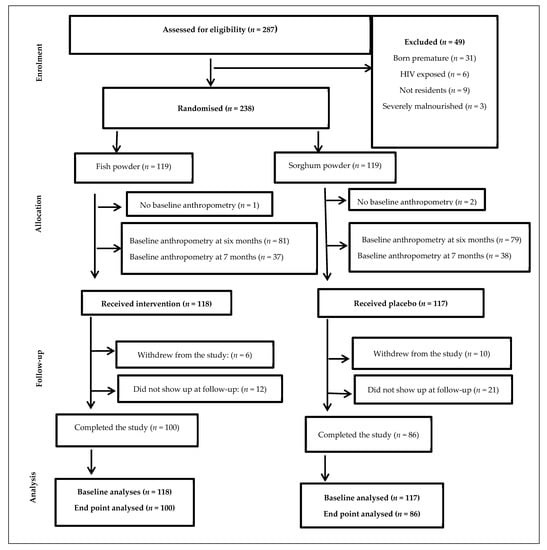
Figure 1.
Flow chart of the study participants.
2.4. Participant Recruitment
2.4.1. Randomisation
The randomisation schedule was prepared by an independent statistician. The infants who met the inclusion criteria were randomly assigned to either the fish powder group or sorghum powder group at 6–7 months. A 1:1 ratio using a computerised random number generator and block randomisation in Microsoft Excel was used to randomise the infants into two groups of equal size. To enable gender balance in both groups, the randomisation schedule was stratified by gender. The allocation of concealment was performed using a central randomisation service through a computer algorithm to protect the randomisation schedule. Both groups (fish and sorghum) had 119 infants randomly assigned out of the total 238, Figure 1.
2.4.2. Inclusion Criteria
- Infants aged 6–7 months who attended the under-five clinic at the sampled RHC.
- Infants whose mothers provided informed written consent.
- Infants whose mothers had no plans of moving away from the study area during the study period.
2.4.3. Exclusion Criteria
- Infants with chronic or congenital diseases/disorders that affected the growth of the children.
- These included Down syndrome, cerebral palsy, spina bifida, and any other related condition.
- Premature infants.
- HIV exposed/infected infants.
- Infants who tested positive to the fish allergy skin prick test.
2.5. Preparation of the Powders (Fish and Sorghum) and Daily Consumption by Infants
The small dried fish (Chisense)/sorghum was roasted and grinded into a powder. The preparation of the powders was carried out in the food service laboratory, in the Department of Nutritional Sciences at Mukuba University. The lab assistants who were not part of the research team carried out the preparations under the supervision of the principle investigator. Standard food safety and hygienic measures were ensured. The methodology of the preparation of the fish and sorghum powders have been published and reporting on the large/main study [9]. The fish powder was then packed in small (12 g fish and 7 g sorghum) sachets. Infants in the fish powder group were given one sachet per day to be added to any food provided by the mother. A similar procedure and provision were made for the infants in the sorghum powder group (one sachet of sorghum powder per day).
2.6. Measurements
All the measurements took place at Shikamushile RHC. The anthropometric measurements (HC and MUAC) were taken and dietary intake assessments were performed by means of multiple interviewer-led 24 h dietary recalls that were performed at 4 time points (at baseline, second month of the intervention, fourth month of the intervention, and at endline). The mothers of infants were also visited in their homes once a week by research assistants to collect information on illnesses experienced and adherence to the intervention.
2.6.1. Socio-Demographic Information
The social demographic data were collected at baseline using a questionnaire. The data collected included age of parents, occupation, level of education, source of income, family size, mother’s number of births, and marital status. The data on breastfeeding and the type and history of complementary food the infant was provided with were also collected.
2.6.2. Anthropometry
Head circumference (HC)
The head circumference was measured at baseline and once a month throughout the study duration. The mothers assisted the research assistants to perform the measurements.
The procedure to determine the HC of the infant was as follows [10,11]:
A SECA non-stretchable measuring tape was used. The infants were measured whilst lying down on a bed. Any covering from the head was removed. The tape was positioned at the supraorbital ridges and occiput. An average of two measurements were taken. If the second measurement differs from the first measurement by 0.5 cm, then a third measurement was taken. The measurements were read to the nearest mm. The head circumference measurements of the infant were entered into WHO anthro Version 3.2.2. Software [12]. The HC of the infants were used to compute z-scores for the head-circumference-for-age (HCZ).
Mid-upper arm circumference (MUAC)
The MUAC was taken from the infants at baseline and at the end of the intervention in both groups.
The procedure to determine the MUAC of the infant were as follows [10,11]:
A non-stretchable WHO, MUAC tape S0145620 was used [13]. The measurements were taken at the mid-point of the infant’s right arm, which was marked with a pen. The infant laid on the bed. The measuring tape was placed perpendicular to the long axis of the arm. An average of two measurements were taken. If the second measurement differs from the first measurement by 0.5 cm, then a third measurement was taken. The measurements were recorded to the nearest mm.
The MUAC was classified according to World Health Organization (WHO) thresholds [14,15]. The WHO thresholds for MUAC are: normal, 12.5 cm and above; moderate wasting 11.5–<12.5 cm; and severe wasting 0–11.5 cm [14,15].
2.7. Assessment of Fish Allergy
Fish allergy was assessed by means of a skin prick test performed on the forearm, using a drop of histamine dihydrochloride 10 mg/mL as a positive and water as a negative control. A fish allergy test was conducted on infants in the form of a skin prick test (SPT) at baseline. The sorghum powder also had a small quantity of fish powder added in order have a similar fish aroma (3/100 g of sorghum) as the fish product. A skin reaction of more than 3 mm was considered a positive result. This was to ensure that all infants who participated in the study were not allergic to fish.
2.8. Study Adherence
The details of adherence related to the consumption of the intervention products have been described in detail in the published article that looked at fish consumption and linear growth [9]. In summary, it is important to note that, noncompliance to the intervention by the mothers for two (2) consecutive weeks resulted in infants exiting the study.
2.9. Statistical Analysis
The completed questionnaires were checked for completeness, coded, and entered into (excel 2016). The data were then checked for missing values and exported to STATA (version 16) for analysis. Descriptive statistics were used to compute the means, standard deviation’s (SD), frequencies, and percentages.
A linear mixed effects model was used for the analysis of the primary outcomes of the HC and MUAC with the participants as the random effects in the model to account for the repeated measures in time. The model includes an indicator variable for the intervention, a discrete time variable, and the interaction between the intervention and time variable. The test for a significant interaction effect between the intervention and time was the overall test for an intervention effect. The estimation for the model parameters was performed via the full maximum likelihood; this was the imputation approach used for handling the missing data at the time points post randomisation and for facilitating the intention to treat analysis. The estimated intervention effects at each month and at 12 months considered the baseline difference at randomisation and used the difference of differences approach to estimate the mean difference as the intervention effect as well as the 95% confidence interval.
3. Results
3.1. Demographic Characteristics
The demographic characteristics of the infants in the study are presented in Table 1. The number of male participants was 125 (53.4%) and of females was 110 (46.7%), making a total of 235 infants included in the study. The demographic characteristics were similar for both the fish and sorghum groups. The infants in both groups (100%) were still breastfeeding, and the majority of them were introduced to solid foods at 6 months. The mean age of infants was 6.64 (±0.54) and 6.63 (±0.50) months for the fish powder and sorghum powder group, respectively. The mean HC (41.4 ± 1.8) in the fish powder group was lower compared to the sorghum powder group (44.3 ± 34.9) at baseline while the mean MUAC was similar in both groups (fish powder 13.2 ± 0.6 and sorghum powder 13.1 ± 0.6). However, there was no statistical difference in both the intervention and control group MUAC and HC at baseline.

Table 1.
Demographic characteristics of participating infants in the study (n = 235).
3.2. Head Circumference Z-Scores (HCZ)
The effect of fish powder on the head circumference z-score (HCZ) is shown in Table 2. The overall linear mixed effect shows that the mean HCZs were greater for the fish group compared to the Sorghum (p < 0.05). However, in the second month of follow-up visits, there was no significant difference between the fish powder group and sorghum group (p > 0.05).

Table 2.
The intervention effect between fish powder and sorghum powder on head circumference z-scores (HCZs) from the first (1) month of follow-up to the last month (6) of follow-up.
Figure 2 shows the locally weighted scatterplot smoothing of HCZs for the fish and sorghum groups. The HCZ in the fish group improved throughout all the six months’ follow-up (180 days) compared to the sorghum group. The fish powder group had a faster growth from baseline.
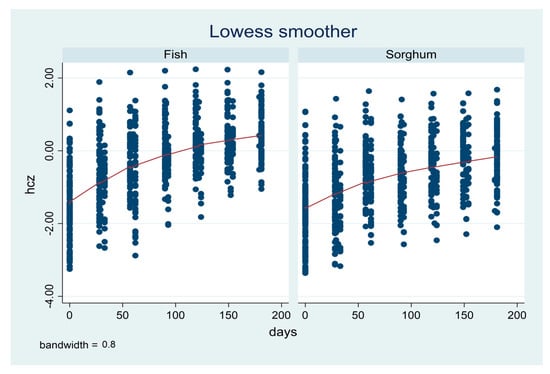
Figure 2.
Lowess smoother comparing fish and sorghum powder HCZs.
3.3. Mid-Upper Arm Circumference (MUAC)
Table 3 shows the effect of the fish powder on the MUAC. The measurements were conducted twice, at the baseline and at the end point. The MUAC thresholds were used to determine whether the child was wasted or not (refer to section the foot note of Table 3). After the six months intervention, there was a significant intervention effect of 0.36 (95% CI 0.13–0.59) between the fish group and the sorghum group. The fish led to an increased MUAC in the fish group compared to the sorghum group.

Table 3.
The intervention effect between fish powder and sorghum powder on mid-upper arm circumference (MUAC) z-scores from the first (1) month of follow-up to the last month (6) of follow-u.
Figure 3 shows the intervention effect of fish and sorghum in box plots on the MUAC. Both the fish powder group and sorghum powder group had increased MUACs. However, the fish powder group had higher values compared to the sorghum powder group at the end of the six months’ intervention. These results are an indication that fish powder improved the MUAC among the infants in the study compared with sorghum powder.
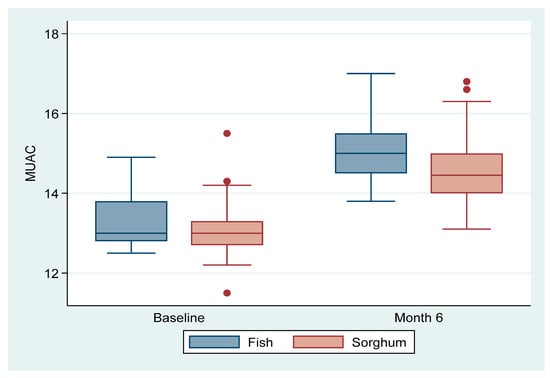
Figure 3.
Effect of fish compared to sorghum on MUAC (cm).
4. Discussion
The consumption of animal source foods by infants has been positively associated with better HC and cognitive development (child language, motor, personal, and social skills) [17]. A longitudinal study in rural Nepal found that children (from 6 months to 8 years) who consumed animal source protein had better head circumferences compared to those who did not consume it [18]. The consumption of animal source food has also been association with better MUACs [19]. In Kenya, school-going children who were provided with meat and milk supplements for 23 months were found to have better MUACs compared to those without animal source food supplements [19].
4.1. Effect of the Fish Powder on HCZ
This study found a mean effect of 0.53 (95% CI, 0.23–0.82) on the HCZs between the fish powder and sorghum powder groups. Fish powder consumption increased the HCZs among the infants. This was similar to what was found with the length-for-age z-scores [9]. Due to a paucity of comparative studies using similar intervention types, we looked at other studies that assessed the relationship between animal source protein (ASP) and HCZs. An observational study on head growth that evaluated livestock agriculture interventions (animal source food consumption ASFs) and its relationship with HCZs among children aged 6 months–8 years found that children below the age of three (3) who had consumed meat twice or more 24 hrs prior to the interview had better height-for-age z-scores (HAZ) compared to those who had eaten none [18]. In Malawi, children aged 6–9 months who were provided with an egg per day for a period of 6 months showed an increase in the HCZ compared to the ones who were not [20].
The current study indicates that the intervention (fish) group had a rapid increase in HCZs in the first three months; however, in the last three months, there was a slow increase despite the fish powder group having improved HCZs over the sorghum powder group. The finding that there was a rapid increase in HCZs in the first three months of the intervention and slow increase in the last three months is difficult to explain. It may be that the food consumed with protein supplements from fish was not sufficient when the infant’s absolute energy and nutrient requirements increased with age.
Furthermore, this could be due to the seasonality of food. At the beginning of the intervention, the first three months coincided with the post-harvest season while the last few months of the study took place during the planting season. A sachet of fish powder contributed 12 g (7.6 g of protein) and a sachet of sorghum powder contributed 7 g (0.9 g of protein) to the daily food consumption of infants. It is therefore possible that the foods to which the powders were added in both groups might have reduced in quantity (thus, there was less nutrient intake apart from the nutrient supplements from the fish/sorghum powders), contributing to the slow increase in HCZs. However, a study conducted in Peru found that the seasonality of food was not associated with head circumference [21].
The effect of fish powder on the HCZs in the current study is an indication that if children consume fish in their early life, they are likely to have good brain development, thus improving their psychomotor development and school performance in later life [18].
4.2. Effect of the Fish on MUAC
The estimated mean MUAC difference in the fish on MUACs compared to sorghum was 0.36 (95% CI, 0.13–0.59) improvement. Due to the absence of comparative studies, research on ASP (eggs) have shown a large increase in MUACs among school-going children age 2–10 years [6,22]. The results of a school feeding program conducted in Uganda on children aged 6–9 years showed an increase in MUACs to children who were fed two eggs per day (5 days a week) for 6 months compared to those who did not [6]. In Kelantan, Malaysia, the MUAC was found to increase by double among infants who were in the intervention arm (fed meat and milk) compared to the control group in a six months intervention conducted on malnourished children (2–10 years) from food insecure households [22]. The increase in MUAC reported in our study conforms with the literature that ASP consumption increases lean body mass [23].
5. Conclusions
This study showed that fish consumption as an early complementary food is positively associated with improved HC and MUAC among infants aged 6–7 months. These results are in agreement with the efficacy impact on linear growth reported for this trial [9]. Fish can be utilised as a cheaper and affordable ASP for complementary feeding in infants and young children from countries with access to fish.
Author Contributions
Conceptualization, G.C., A.V.G. and E.V.N.; methodology, G.C., A.V.G. and E.V.N.; formal analysis, C.J.L.; investigation, G.C.; resources, G.C.; data curation, G.C.; writing—original draft preparation, G.C.; writing—critical review and editing, A.V.G. and E.V.N.; supervision, A.V.G. and E.V.N.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mukuba University, Kitwe, Zambia.
Institutional Review Board Statement
Ethical approval for the study was obtained from Stellenbosch Human Research Ethics Committee (HREC: M18/10/037), Tropical Disease Research Centre (TDRC: STC 2019/03) Ndola, Zambia and the National Health Research Authority Lusaka, Zambia.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments
The authors would like to thank the staff at Samfya district health office for their support throughout data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tiwari, K.; Goyal, S.; Malvia, S.; Sanadhya, A.; Suman, R.L.; Jain, R. Impact of malnutrition on head size and development quotient. Int. J. Res. Med Sci. 2017, 5, 3003–3006. [Google Scholar] [CrossRef]
- Nicolaou, L.; Ahmed, T.; Bhutta, Z.A.; Bessong, P.; Kosek, M.; Lima, A.A.M.; Shrestha, S.; Chandyo, R.; Mduma, E.R.; Murray-Kolb, L.; et al. Factors associated with head circumference and indices of cognitive development in early childhood. BMJ Glob. Health 2020, 5, e003427. [Google Scholar] [CrossRef] [PubMed]
- Koshy, B.; Srinivasan, M.; Murugan, T.P.; Bose, A.; Christudoss, P.; Mohan, V.R.; John, S.; Roshan, R.; Kang, G. Association between head circumference at two years and second and fifth year cognition. BMC Pediatr. 2021, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Zambia Statistics Agency; Ministry of Health; University Teaching Hospital Virology Laboratory; The DHS Program ICF. Zambia Demographic and Health Survey 2018. Lusaka, Zambia, Rockville, Maryland, USA. 2019. Available online: https://dhsprogram.com/pubs/pdf/FR361/FR361.pdf (accessed on 20 January 2020).
- Haq, I.U.; Mehmood, Z.; Khan, N.; Khan, M.N.; Israr, M.; Khan, E.A.; Ahmad, M.I.; Ali, M. Risk Factors of Mid-upper Arm Circumference (MUAC) Based Child Malnutrition in the Flood-affected Areas of Pakistan: A Cross-sectional Study. Ecol. Food Nutr. 2021, 60, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Miller, J.D.; Gaines, B.L. The effect of egg supplementation on growth parameters in children participating in a school feeding program in rural Uganda: A pilot study. Food Nutr. Res. 2017, 61, 1330097. [Google Scholar] [CrossRef] [PubMed]
- Byrd, K.A.; Pincus, L.; Pasqualino, M.M.; Muzofa, F.; Cole, S.M. Dried small fish provide nutrient densities important for the first 1000 days. Matern. Child Nutr. 2021, 17, e13192. [Google Scholar] [CrossRef] [PubMed]
- Longley, C.; Thilsted, S.H.; Beveridge, M.; Cole, S.; Nyirenda, D.B.; Heck, S.; Hother, A.L. The role of fish in the first 1,000 days in Zambia. Inst. Dev. Stud. 2014, 9, 27–35. [Google Scholar]
- Chipili, G.; Van Graan, A.; Lombard, C.J.; Van Niekerk, E. The Efficacy of Fish as an Early Complementary Food on the Linear Growth of Infants Aged 6–7 Months: A Randomised Controlled Trial. Nutrients 2022, 14, 2191. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Walton, S.; Wells, J. Life Study Standard Operating Procedures: Infant Anthropometry. In Standard Operating Procedures: Infant Anthropometry; University College London: London, UK, 2016; pp. 5–27. Available online: https://discovery.ucl.ac.uk/id/eprint/1485685/9/Life%20Study%20Infant%20Anthropometry%20Standard%20Operating%20Procedures%201485685.pdf (accessed on 16 February 2018).
- Cape Town Metropole Paediatric Interest Group. Anthropometriy Guidelines Paediatrics; Screening: South Africa, 2009; pp. 19–68. Available online: https://pdf4pro.com/view/anthropometry-guideline-paediatrics-adsa-75db.html (accessed on 28 January 2018).
- Anthro, H.O. WHO Anthro for Personal Computers, Version 3.1. Software for Assessing Growth and Development of the World’s Children. 2010. Available online: https://who-anthro.software.informer.com/3.2/ (accessed on 12 February 2018).
- UNICEF Bulletin. Mid-Upper Arm Circumference (Muac) Measuring Tapes. 2009, 13, 1–2. Available online: https://www.unicef.org/supply/files/Mid_Upper_Arm_Circumference_Measuring_Tapes.pdf (accessed on 12 February 2018).
- Onyango, A.W.; De Onis, M. Who Child Growth Standards: Training Course on Child Growth Assessment; World Health Organization: Geneva, Switzerland, 2008; Volume 7, pp. 1–7. [Google Scholar]
- WHO/UNICEF. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. Geneva, Switzerland. 2009. Available online: http://apps.who.int/iris/bitstream/10665/44129/1/9789241598163_eng.pdf?ua=1 (accessed on 10 February 2018).
- World Health Organization; Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO: Geneva, Switzerland, 2006; 339p. [Google Scholar]
- Balehegn, M.; Mekuriaw, Z.; Miller, L.; McKune, S.; Adesogan, A.T. Animal-sourced foods for improved cognitive development. Anim. Front. 2019, 9, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.C.; Joshi, N.; Lohani, M.; Singh, R.; Bhatta, N. Head growth of undernourished children in rural Nepal: Association with demographics, health and diet. Paediatr. Int. Child Health 2016, 36, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Grillenberger, M.; Neumann, C.G.; Murphy, S.P.; Bwibo, N.O.; van’t Veer, P.; Hautvast, J.G.A.J.; West, C.E. Food Supplements Have a Positive Impact on Weight Gain and the Addition of Animal Source Foods Increases Lean Body Mass of Kenyan Schoolchildren. J. Nutr. 2003, 133 (Suppl. S2), 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Caswell, B.; Iannotti, L.; Lutter, C.; Arnold, C.D.; Chipatala, R. The effect of eggs on early child growth in rural Malawi: The Mazira Project randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, E.; Wells, J.C.; Stanojevic, S.; Miranda, J.J.; Cole, T.J.; Stock, J.T. Birth month associations with height, head circumference, and limb lengths among peruvian children. Am. J. Phys. Anthr. 2014, 154, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ihab, A.N.; Rohana, A.J.; Wan Manan, W.M.; Wan Suriati, W.N.; Zalilah, M.S.; Mohamed Rusli, A. The Impact of Animal Source food (ASF) on the Growth of Malnourished Children in Bachok, Kelantan: Randomized Controlled Intervention Trial. J. Nutr. Food Sci. 2014, 4, 321. [Google Scholar] [CrossRef]
- Whaley, S.E.; Sigman, M.; Neumann, C.; Bwibo, N.; Guthrie, D.; Weiss, R.E.; Alber, S.; Murphy, S.P. The Impact of Dietary Intervention on the Cognitive Development of Kenyan School Children. J. Nutr. 2003, 133, 3965–3971. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).