An Overview of the Sorption Studies of Contaminants on Poly(Ethylene Terephthalate) Microplastics in the Marine Environment
Abstract
1. Introduction
2. Poly(Ethylene Terephthalate) Microplastics
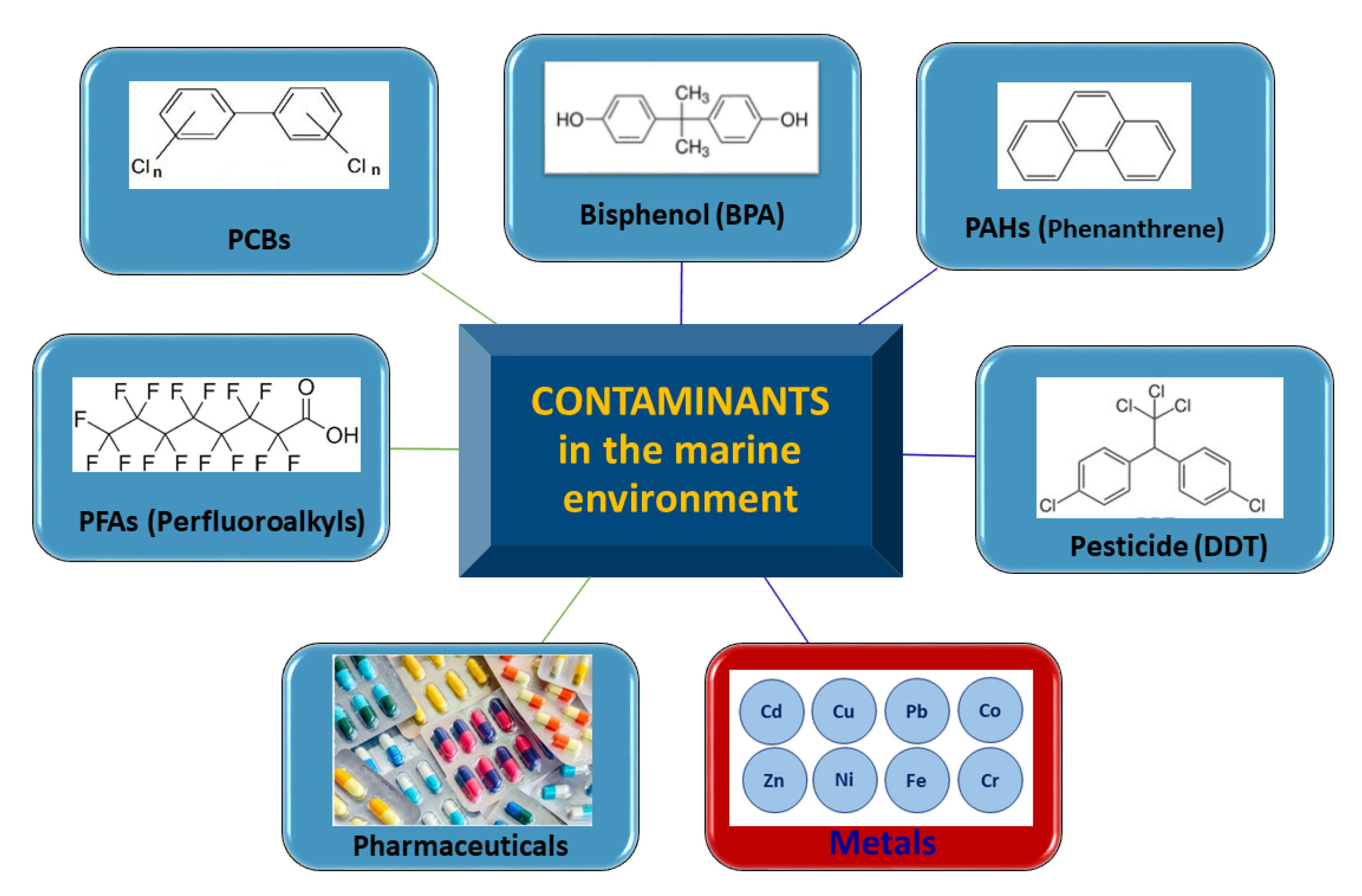
3. Contaminants in the Marine Environment
4. Sorption Mechanisms of Organic and Metal Contaminants to MPs/NPs
5. Adsorption Modelling
6. Adsorption Studies of Organic Pollutants on Poly(Ethylene Terephthalate) MPs/NPs
7. Adsorption Studies of Metals on Poly(Ethylene Terephthalate) MPs
8. Comparison between Marine and Laboratory Adsorption Studies on Poly(Ethylene Terephthalate) MPs
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferrari, F.; Striani, R.; Minosi, S.; De Fazio, R.; Visconti, P.; Patrono, L.; Catarinucci, L.; Corcione, C.E.; Greco, A. An innovative IoT-oriented prototype platform for the management and valorisation of the organic fraction of municipal solid waste. J. Clean. Prod. 2020, 247, 119618. [Google Scholar] [CrossRef]
- Ferrari, F.; Esposito Corcione, C.; Montagna, F.; Maffezzoli, A. 3D Printing of Polymer Waste for Improving People’s Awareness about Marine Litter. Polymers 2020, 12, 1738. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef]
- Beede, D.N.; Bloom, D.E. Economics of the Generation and Management of MSW; National Bureau of Economic Research Working Paper Series; National Bureau of Economic Research: Cambridge, MA, USA, 1995; Working Paper 5116. [Google Scholar]
- Ritchie, H.; Roser, M. Plastic Pollution. Available online: https://ourworldindata.org/plastic-pollution (accessed on 18 March 2021).
- Esposito Corcione, C.; Ferrari, F.; Striani, R.; Visconti, P.; Greco, A. An innovative green process for the stabilization and valorization of organic fraction of municipal solid waste (OFMSW): Optimization of the curing process II part. Appl. Sci. 2019, 9, 3702. [Google Scholar] [CrossRef]
- Ferrari, F.; Striani, R.; Visconti, P.; Esposito Corcione, C.; Greco, A. Durability analysis of formaldehyde/solid urban waste blends. Polymers 2019, 11, 1838. [Google Scholar] [CrossRef]
- Plastics—The Facts 2019. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 5 December 2020).
- Dewil, R.; Everaert, K.; Baeyens, J. The European plastic waste issue: Trends and toppers in its sustainable re-use. In Proceedings of the 17th International Congress of Chemical and Process Engineering, Prague, Czech Republic, 27–31 August 2006; pp. 27–31. [Google Scholar]
- Issifu, I.; Sumaila, U.R. A Review of the Production, Recycling and Management of Marine Plastic Pollution. J. Mar. Sci. Eng. 2020, 8, 945. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic pollutants from plastic waste—a review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Bjorksten, J. Polyesters and Their Applications; Reinhold Publishing Corporation: New York, NY, USA, 1956. [Google Scholar]
- Polyethylene Terephthalate (PET): Production, Price, Market and Its Properties. Available online: https://www.plasticsinsight.com/resin-intelligence/resin-prices/polyethylene-terephthalate/ (accessed on 7 April 2021).
- Tiseo, I. Global PET Bottle Production 2004–2021. Available online: https://www.statista.com/statistics/723191/production-of-polyethylene-terephthalate-bottles-worldwide/ (accessed on 28 March 2021).
- Dell’Anna, R.; Lionetto, F.; Montagna, F.; Maffezzoli, A. Lay-up and consolidation of a composite pipe by in situ ultrasonicwelding of a thermoplastic matrix composite tape. Materials 2018, 11, 786. [Google Scholar] [CrossRef]
- Greco, A.; Lionetto, F.; Maffezzoli, A. Processing and characterization of amorphous polyethylene terephthalate fibers for the alignment of carbon nanofillers in thermosetting resins. Polym. Compos. 2015, 36, 1096–1103. [Google Scholar] [CrossRef]
- Cascardi, A.; Dell’Anna, R.; Micelli, F.; Lionetto, F.; Aiello, M.A.; Maffezzoli, A. Reversible techniques for FRP-confinement of masonry columns. Constr. Build. Mater. 2019, 225, 415–428. [Google Scholar] [CrossRef]
- Lionetto, F.; Pappadà, S.; Buccoliero, G.; Maffezzoli, A. Finite element modeling of continuous induction welding of thermoplastic matrix composites. Mater. Des. 2017, 120, 212–221. [Google Scholar] [CrossRef]
- Lionetto, F.; Moscatello, A.; Totaro, G.; Raffone, M.; Maffezzoli, A. Experimental and numerical study of vacuum resin infusion of stiffened carbon fiber reinforced panels. Materials 2020, 13, 4800. [Google Scholar] [CrossRef]
- Lionetto, F.; Montagna, F.; Maffezzoli, A. Out-of-Plane Permeability Evaluation of Carbon Fiber Preforms by Ultrasonic Wave Propagation. Materials 2020, 13, 2684. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to Poly(Ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Pemberton, D.; Brothers, N.P.; Kirkwood, R. Entanglement of Australian fur seals in man-made debris in Tasmanian waters. Wildl. Res. 1992, 19, 151–159. [Google Scholar] [CrossRef]
- Sazima, I.; Gadig, O.B.F.; Namora, R.C.; Motta, F.S. Plastic debris collars on juvenile carcharhinid sharks (Rhizoprionodon lalandii) in southwest Atlantic. Mar. Pollut. Bull. 2002, 44, 1149–1151. [Google Scholar] [CrossRef]
- Gregory, M.R.; Andrady, A.L. Plastics in the marine environment. Plast. Environ. 2003, 379–401. [Google Scholar]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Azzarello, M.Y.; Van Vleet, E.S. Marine birds and plastic pollution. Mar. Ecol. Prog. Ser. 1987, 37, 295–303. [Google Scholar] [CrossRef]
- Blight, L.K.; Burger, A.E. Occurrence of plastic particles in seabirds from the eastern North Pacific. Mar. Pollut. Bull. 1997, 34, 323–325. [Google Scholar] [CrossRef]
- Barreiros, J.P.; Barcelos, J. Plastic ingestion by a leatherback turtle Dermochelys coriacea from the Azores (NE Atlantic). Mar. Pollut. Bull. 2001, 42, 1196–1197. [Google Scholar] [CrossRef]
- Baird, R.W.; Hooker, S.K. Ingestion of plastic and unusual prey by a juvenile harbour porpoise. Can. J. Fish. Aquat. Sci. 2000, 51, 172–178. [Google Scholar] [CrossRef]
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of plastic and plankton in the North Pacific central gyre. Mar. Pollut. Bull. 2001, 42, 1297–1300. [Google Scholar] [CrossRef]
- Zarfl, C.; Matthies, M. Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar. Pollut. Bull. 2010, 60, 1810–1814. [Google Scholar] [CrossRef]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. Wiley Interdiscip. Rev. Water 2018, 5, e1268. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; IVL Swedish Environmental Research Institute Report; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from synthetic textiles as a major source of microplastics in the environment: A review. Text. Res. J. 2021. [Google Scholar] [CrossRef]
- Zilla, G.; Marc, N. Briefing Note on Microplastics Literature Review. 2020. Available online: https://www.applia-europe.eu/images/Library/2020-10-28_APPLiA-RISE_Literature_Review_Final_for_release-3.pdf (accessed on 16 April 2021).
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Kirkham, M.B.; Halsband, C.; Nugegoda, D.; Ok, Y.S. Particulate Plastics in Terrestrial and Aquatic Environments; CRC Press: Boca Raton, FL, USA, 2020; ISBN 1000081419. [Google Scholar]
- Le, T.T.Y.; Grabner, D.; Nachev, M.; Peijnenburg, W.J.G.M.; Hendriks, A.J.; Sures, B. Modelling copper toxicokinetics in the zebra mussel, Dreissena polymorpha, under chronic exposures at various pH and sodium concentrations. Chemosphere 2021, 267, 129278. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, S.J.; Hwang, D.Y.; Seo, S. Recent Purification Technologies and Human Health Risk Assessment of Microplastics. Materials 2020, 13, 5196. [Google Scholar] [CrossRef]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.S. Current research trends on micro-and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2020, 409, 124967. [Google Scholar] [CrossRef]
- Gigault, J.; Pedrono, B.; Maxit, B.; Ter Halle, A. Marine plastic litter: The unanalyzed nano-fraction. Environ. Sci. nano 2016, 3, 346–350. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Galafassi, S.; Nizzetto, L.; Volta, P. Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 2019, 693, 133499. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Ali, T.; Mortula, M.M.; Attaelmanan, A.G. Evaluation of microplastics in beach sediments along the coast of Dubai, UAE. Mar. Pollut. Bull. 2020, 150, 110739. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, S.-S.; Zhang, J.-B.; Ou, Z.; Yang, Y.-Q.; Wang, M.-Y. Occurrence, Composition, and Relationships in Marine Plastic Debris on the First Long Beach Adjacent to the Land-Based Source, South China Sea. J. Mar. Sci. Eng. 2020, 8, 666. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Wagner, M.; Lambert, S. Freshwater Microplastics: Emerging Environmental Contaminants? Springer Nature: Heidelberg, Germany, 2018. [Google Scholar]
- Strungaru, S.-A.; Jijie, R.; Nicoara, M.; Plavan, G.; Faggio, C. Micro-(nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology. TrAC Trends Anal. Chem. 2019, 110, 116–128. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Guerranti, C.; Perra, G.; Martellini, T.; Giari, L.; Cincinelli, A. Knowledge about microplastic in Mediterranean tributary river ecosystems: Lack of data and research needs on such a crucial marine pollution source. J. Mar. Sci. Eng. 2020, 8, 216. [Google Scholar] [CrossRef]
- Zbyszewski, M.; Corcoran, P.L. Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut. 2011, 220, 365–372. [Google Scholar] [CrossRef]
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257, 113604. [Google Scholar] [CrossRef]
- De Leo, A.; Cutroneo, L.; Sous, D.; Stocchino, A. Settling Velocity of Microplastics Exposed to Wave Action. J. Mar. Sci. Eng. 2021, 9, 142. [Google Scholar] [CrossRef]
- Stocchino, A.; De Leo, F.; Besio, G. Sea Waves Transport of Inertial Micro-Plastics: Mathematical Model and Applications. J. Mar. Sci. Eng. 2019, 7, 467. [Google Scholar] [CrossRef]
- Bianco, A.; Passananti, M. Atmospheric micro and nanoplastics: An enormous microscopic problem. Sustainability 2020, 12, 7327. [Google Scholar] [CrossRef]
- Bianco, A.; Sordello, F.; Ehn, M.; Vione, D.; Passananti, M. Degradation of nanoplastics in the environment: Reactivity and impact on atmospheric and surface waters. Sci. Total Environ. 2020, 742, 140413. [Google Scholar] [CrossRef]
- Willis, K.; Hardesty, B.D.; Vince, J.; Wilcox, C. The success of water refill stations reducing single-use plastic bottle litter. Sustainability 2019, 11, 5232. [Google Scholar] [CrossRef]
- Scarr, S.; Hernandez, M. Drowning in Plastic. Available online: https://graphics.reuters.com/ENVIRONMENT-PLASTIC/0100B275155/index.html (accessed on 7 April 2021).
- Ocean Conservancy International Coastal Cleanup. Available online: https://oceanconservancy.org/wp-content/uploads/2020/10/FINAL_2020ICC_Report.pdf (accessed on 6 April 2021).
- Gjyli, L.; Vlachogianni, T.; Kolitari, J.; Matta, G.; Metalla, O.; Gjyli, S. Marine litter on the Albanian coastline: Baseline information for improved management. Ocean Coast. Manag. 2020, 187, 105108. [Google Scholar] [CrossRef]
- Vlachogianni, T.; Skocir, M.; Constantin, P.; Labbe, C.; Orthodoxou, D.; Pesmatzoglou, I.; Scannella, D.; Spika, M.; Zissimopoulos, V.; Scoullos, M. Plastic pollution on the Mediterranean coastline: Generating fit-for-purpose data to support decision-making via a participatory-science initiative. Sci. Total Environ. 2020, 711, 135058. [Google Scholar] [CrossRef]
- Simeonova, A.; Chuturkova, R. Marine litter accumulation along the Bulgarian Black Sea coast: Categories and predominance. Waste Manag. 2019, 84, 182–193. [Google Scholar] [CrossRef]
- Brouwer, R.; Hadzhiyska, D.; Ioakeimidis, C.; Ouderdorp, H. The social costs of marine litter along European coasts. Ocean Coast. Manag. 2017, 138, 38–49. [Google Scholar] [CrossRef]
- Botero, C.M.; Zielinski, S.; Pereira, C.I.; León, J.A.; Dueñas, L.F.; Puentes, V. The first report of deep-sea litter in the South-Western Caribbean Sea. Mar. Pollut. Bull. 2020, 157, 111327. [Google Scholar] [CrossRef]
- Scotti, G.; Esposito, V.; D’Alessandro, M.; Panti, C.; Vivona, P.; Consoli, P.; Figurella, F.; Romeo, T. Seafloor litter along the Italian coastal zone: An integrated approach to identify sources of marine litter. Waste Manag. 2021, 124, 203–212. [Google Scholar] [CrossRef]
- Gerigny, O.; Brun, M.; Fabri, M.-C.; Tomasino, C.; Le Moigne, M.; Jadaud, A.; Galgani, F. Seafloor litter from the continental shelf and canyons in French Mediterranean water: Distribution, typologies and trends. Mar. Pollut. Bull. 2019, 146, 653–666. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, C.; Xu, J.; Zhu, L.; Peng, G.; Li, D. Composition, spatial distribution and sources of plastic litter on the East China Sea floor. Sci. Total Environ. 2020, 742, 140525. [Google Scholar] [CrossRef]
- Duncan, E.M.; Davies, A.; Brooks, A.; Chowdhury, G.W.; Godley, B.J.; Jambeck, J.; Maddalene, T.; Napper, I.; Nelms, S.E.; Rackstraw, C. Message in a bottle: Open source technology to track the movement of plastic pollution. PLoS ONE 2020, 15, e0242459. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made—Supplementary Information. Sci. Adv. 2017, 3, 19–24. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Bianchi, S.; Ceccarini, A.; Manariti, A.; Vinciguerra, V. Quantification of Poly(Ethylene terephthalate) micro-and nanoparticle contaminants in marine sediments and other environmental matrices. J. Hazard. Mater. 2020, 385, 121517. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Xu, J.; Zhu, L.; Peng, G.; Xu, P.; Li, D. Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar. Pollut. Bull. 2019, 146, 173–182. [Google Scholar] [CrossRef]
- Gavigan, J.; Kefela, T.; Macadam-Somer, I.; Suh, S.; Geyer, R. Synthetic microfiber emissions to land rival those to waterbodies and are growing. PLoS ONE 2020, 15, e0237839. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.G.; Muñoz-Tabares, J.A.; Aguilar-Guzmán, J.C.; Vazquez-Duhalt, R. A novel and simple method for polyethylene terephthalate (PET) nanoparticle production. Environ. Sci. Nano 2019, 6, 2031–2036. [Google Scholar] [CrossRef]
- Contaminants in the Marine Environment. Available online: https://ec.europa.eu/environment/marine/good-environmental-status.html (accessed on 19 March 2021).
- Llorca, M.; Ábalos, M.; Vega-Herrera, A.; Adrados, M.A.; Abad, E.; Farré, M. Adsorption and Desorption Behaviour of Polychlorinated Biphenyls onto Microplastics’ Surfaces in Water/Sediment Systems. Toxics 2020, 8, 59. [Google Scholar] [CrossRef]
- Weber, R.; Herold, C.; Hollert, H.; Kamphues, J.; Ungemach, L.; Blepp, M.; Ballschmiter, K. Life cycle of PCBs and contamination of the environment and of food products from animal origin. Environ. Sci. Pollut. Res. 2018, 25, 16325–16343. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.P. Recent development in analysis of persistent organic pollutants under the Stockholm Convention. TrAC Trends Anal. Chem. 2013, 45, 48–66. [Google Scholar] [CrossRef]
- Annex, C. Of the Stockholm Convention on Persistent Organic Pollutants; United Nations Environment Programme: Geneva, Switzerland, 2008; Volume 29. [Google Scholar]
- Syberg, K.; Knudsen, C.M.H.; Tairova, Z.; Khan, F.R.; Shashoua, Y.; Geertz, T.; Pedersen, H.B.; Sick, C.; Mortensen, J.; Strand, J. Sorption of PCBs to environmental plastic pollution in the North Atlantic Ocean: Importance of size and polymer type. Case Stud. Chem. Environ. Eng. 2020, 2, 100062. [Google Scholar] [CrossRef]
- Anđelić, I.; Roje-Busatto, R.; Ujević, I.; Vuletić, N.; Matijević, S. Distribution of Bisphenol A in Sediment and Suspended Matter and Its Possible Impact on Marine Life in Kaštela Bay, Adriatic Sea, Croatia. J. Mar. Sci. Eng. 2020, 8, 480. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Xie, B.; Dionysiou, D.D.; Zhao, Y. Microplastics as both a sink and a source of bisphenol A in the marine environment. Environ. Sci. Technol. 2019, 53, 10188–10196. [Google Scholar] [CrossRef]
- Rochman, C.M.; Manzano, C.; Hentschel, B.T.; Simonich, S.L.M.; Hoh, E. Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environ. Sci. Technol. 2013, 47, 13976–13984. [Google Scholar] [CrossRef]
- Salloum, M.J.; Chefetz, B.; Hatcher, P.G. Phenanthrene sorption by aliphatic-rich natural organic matter. Environ. Sci. Technol. 2002, 36, 1953–1958. [Google Scholar] [CrossRef]
- Lima, A.L.C.; Farrington, J.W.; Reddy, C.M. Combustion-derived polycyclic aromatic hydrocarbons in the environment—A review. Environ. Forensics 2005, 6, 109–131. [Google Scholar] [CrossRef]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Surridge, B.; Hoover, D.; Grace, R.; Gobas, F.A.P.C. Perfluoroalkyl contaminants in an Arctic marine food web: Trophic magnification and wildlife exposure. Environ. Sci. Technol. 2009, 43, 4037–4043. [Google Scholar] [CrossRef]
- Ali, A.M.; Langberg, H.A.; Hale, S.E.; Kallenborn, R.; Hartz, W.F.; Mortensen, Å.-K.; Ciesielski, T.M.; McDonough, C.A.; Jenssen, B.M.; Breedveld, G.D. The fate of poly-and perfluoroalkyl substances in a marine food web influenced by land-based sources in the Norwegian Arctic. Environ. Sci. Process. Impacts 2021. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Carbonic Anhydrase Sensitivity to Pesticides: Perspectives for Biomarker Development. Int. J. Mol. Sci. 2020, 21, 3562. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Riascos-Flores, L.; Bruneel, S.; Van der Heyden, C.; Deknock, A.; Van Echelpoel, W.; Forio, M.A.E.; De Saeyer, N.; Berghe, W.V.; Spanoghe, P.; Bermudez, R. Polluted paradise: Occurrence of pesticide residues within the urban coastal zones of Santa Cruz and Isabela (Galapagos, Ecuador). Sci. Total Environ. 2021, 763, 142956. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Xie, Z.; Xu, J.; Guo, B. Synthesis, properties of biodegradable poly (butylene succinate-co-butylene 2-methylsuccinate) and application for sustainable release. Materials 2019, 12, 1507. [Google Scholar] [CrossRef]
- Pagano, M.; Stara, A.; Aliko, V.; Faggio, C. Impact of Neonicotinoids to Aquatic Invertebrates—In Vitro Studies on Mytilus galloprovincialis: A Review. J. Mar. Sci. Eng. 2020, 8, 801. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Gouda, M.H.; Ali, S.M.; Salerno, M.; Eldin, M.S. Effective Elimination of Contaminant Antibiotics Using High-Surface-Area Magnetic-Functionalized Graphene Nanocomposites Developed from Plastic Waste. Materials 2020, 13, 1517. [Google Scholar] [CrossRef]
- Mohammadi, L.; Rahdar, A.; Khaksefidi, R.; Ghamkhari, A.; Fytianos, G.; Kyzas, G.Z. Polystyrene Magnetic Nanocomposites as Antibiotic Adsorbents. Polymers 2020, 12, 1313. [Google Scholar] [CrossRef]
- Godoy, V.; Martín-Lara, M.A.; Calero, M.; Blázquez, G. The relevance of interaction of chemicals/pollutants and microplastic samples as route for transporting contaminants. Process Saf. Environ. Prot. 2020, 138, 312–323. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Dvorák, T.; Šimonová Baranyaiová, T.; Šimon, E.; Eckstein Andicsová, A.; Švajdlenková, H.; Opálek, A.; Krížik, P.; Nosko, M. Simple and Eco-Friendly Route from Agro-Food Waste to Water Pollutants Removal. Materials 2020, 13, 5424. [Google Scholar] [CrossRef]
- Mistewicz, K.; Kępińska, M.; Nowak, M.; Sasiela, A.; Zubko, M.; Stróż, D. Fast and Efficient Piezo/Photocatalytic Removal of Methyl Orange Using SbSI Nanowires. Materials 2020, 13, 4803. [Google Scholar] [CrossRef] [PubMed]
- Dąbek, L.; Picheta-Oleś, A.; Szeląg, B.; Szulżyk-Cieplak, J.; Łagód, G. Modeling and Optimization of Pollutants Removal during Simultaneous Adsorption onto Activated Carbon with Advanced Oxidation in Aqueous Environment. Materials 2020, 13, 4220. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Piras, F.; Santoro, O.; Pastore, T.; Pio, I.; De Dominicis, E.; Gritti, E.; Caricato, R.; Lionetto, M.G.; Mele, G.; Santoro, D. Controlling micropollutants in tertiary municipal wastewater by O3/H2O2, granular biofiltration and UV254/H2O2 for potable reuse applications. Chemosphere 2020, 239, 124635. [Google Scholar] [CrossRef]
- Mele, C.; Lionetto, F.; Bozzini, B. An erosion-corrosion investigation of coated steel for applications in the oil and gas field, based on bipolar electrochemistry. Coatings 2020, 10, 92. [Google Scholar] [CrossRef]
- Mele, C.; Boniardi, M.V.; Casaroli, A.; Degli Esposti, M.; Di Pietro, D.; Guastamacchia, P.; Bozzini, B. A comprehensive assessment of the performance of corrosion resistant alloys in hot acidic brines for application in oil and gas production. Corros. Eng. Sci. Technol. 2017, 52, 99–113. [Google Scholar] [CrossRef]
- Bozzini, B.; D’Urzo, L.; Romanello, V.; Mele, C. Electrodeposition of Cu from acidic sulfate solutions in the presence of bis-(3-sulfopropyl)-disulfide (SPS) and chloride ions. J. Electrochem. Soc. 2006, 153, C254. [Google Scholar] [CrossRef]
- Bozzini, B.; Gianoncelli, A.; Kourousias, G.; Boniardi, M.; Casaroli, A.; Dal Zilio, S.; Hussain, R.; Abyaneh, M.K.; Kiskinova, M.; Mele, C. The role of chromium in the corrosion performance of cobalt-and cobalt-nickel based hardmetal binders: A study centred on X-ray absorption microspectroscopy. Int. J. Refract. Met. Hard Mater. 2020, 92, 105320. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent advances in applications of hybrid graphene materials for metals removal from wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019, 1, 1–30. [Google Scholar] [CrossRef]
- Prunier, J.; Maurice, L.; Perez, E.; Gigault, J.; Wickmann, A.-C.P.; Davranche, M.; Ter Halle, A. Trace metals in polyethylene debris from the North Atlantic subtropical gyre. Environ. Pollut. 2019, 245, 371–379. [Google Scholar] [CrossRef]
- Wang, F.; Pan, Y.; Cai, P.; Guo, T.; Xiao, H. Single and binary adsorption of heavy metal ions from aqueous solutions using sugarcane cellulose-based adsorbent. Bioresour. Technol. 2017, 241, 482–490. [Google Scholar] [CrossRef]
- Spilarewicz-Stanek, K.; Jakimińska, A.; Kisielewska, A.; Batory, D.; Piwoński, I. Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials 2020, 13, 4653. [Google Scholar] [CrossRef]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Khajeahmadi, N.; Tajbakhsh, S.; Saeedi, R.; Mohammadi, M.J.; Keshtkar, M.; Khorsand, M.; Ghasemi, F.F. Characterization of plastic debris and association of metals with microplastics in coastline sediment along the Persian Gulf. Waste Manag. 2018, 78, 649–658. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Kedzierski, M.; d’Almeida, M.; Magueresse, A.; Le Grand, A.; Duval, H.; César, G.; Sire, O.; Bruzaud, S.; Le Tilly, V. Threat of plastic ageing in marine environment. Adsorption/desorption of micropollutants. Mar. Pollut. Bull. 2018, 127, 684–694. [Google Scholar] [CrossRef]
- Bozzini, B.; Mele, C.; D’Urzo, L. Electrodeposition of Cu from acidic sulphate solutions in the presence of PEG-Part II visible electroreflectance spectroscopy measurements during electrodeposition. J. Appl. Electrochem. 2006, 36, 87–96. [Google Scholar] [CrossRef]
- Bozzini, B.; D’Urzo, L.; Mele, C. Electrodeposition of Cu from cyanoalkaline solutions in the presence of CPC and PEG: An electrochemical and in situ SERS investigation. J. Electrochem. Soc. 2005, 152, C255. [Google Scholar] [CrossRef]
- Abbasi, S.; Moore, F.; Keshavarzi, B.; Hopke, P.K.; Naidu, R.; Rahman, M.M.; Oleszczuk, P.; Karimi, J. PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci. Total Environ. 2020, 744, 140984. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano-and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hazardous Substances in Marine Organisms. Available online: https://www.eea.europa.eu/data-and-maps/indicators/hazardous-substances-in-marine-organisms/hazardous-substances-in-marine-organisms-1 (accessed on 8 April 2021).
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption behavior and mechanisms of organic contaminants to nano and microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, B. Sorption behavior of p-nitrophenol on the interface between anion− cation organobentonite and water. Environ. Sci. Technol. 2000, 34, 2997–3002. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Mei, W.; Chen, G.; Bao, J.; Song, M.; Li, Y.; Luo, C. Interactions between microplastics and organic compounds in aquatic environments: A mini review. Sci. Total Environ. 2020, 736, 139472. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.; Koelmans, A.A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. 2016, 23, 8819–8826. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, Z.; Yang, L.; Liu, X. Effects of particle size and solution chemistry on Triclosan sorption on polystyrene microplastic. Chemosphere 2019, 231, 308–314. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.-H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470, 1545–1552. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Klontza, I. Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece). Mar. Environ. Res. 2008, 65, 283–290. [Google Scholar] [CrossRef]
- Wu, B.; Taylor, C.M.; Knappe, D.R.U.; Nanny, M.A.; Barlaz, M.A. Factors controlling alkylbenzene sorption to municipal solid waste. Environ. Sci. Technol. 2001, 35, 4569–4576. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.-D.; Hundal, L.S.; Thompson, M.L.; Schmidt-Rohr, K. Correlation of poly (methylene)-rich amorphous aliphatic domains in humic substances with sorption of a nonpolar organic contaminant, phenanthrene. Environ. Sci. Technol. 2002, 36, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, W.; Stefańska, J. Adsorption and biodegradation of antidiabetic pharmaceuticals in soils. Chemosphere 2014, 95, 281–288. [Google Scholar] [CrossRef]
- Torres, F.G.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-la-Torre, G.E. Sorption of chemical contaminants on degradable and non-degradable microplastics: Recent progress and research trends. Sci. Total Environ. 2020, 757, 143875. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- El-Khaiary, M.I.; Malash, G.F.; Ho, Y.-S. On the use of linearized pseudo-second-order kinetic equations for modeling adsorption systems. Desalination 2010, 257, 93–101. [Google Scholar] [CrossRef]
- Ho, Y.-S. Isotherms for the sorption of lead onto peat: Comparison of linear and non-linear methods. Polish J. Environ. Stud. 2006, 15, 81–86. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Kumar, K.V.; Sivanesan, S. Pseudo second order kinetics and pseudo isotherms for malachite green onto activated carbon: Comparison of linear and non-linear regression methods. J. Hazard. Mater. 2006, 136, 721–726. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. A general kinetic model for adsorption: Theoretical analysis and modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015, 12, 600–610. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Removal of lead ions from aqueous solution using sphagnum moss peat as adsorbent. Water SA 1996, 22, 219–224. [Google Scholar]
- Hu, J.-Q.; Yang, S.-Z.; Guo, L.; Xu, X.; Yao, T.; Xie, F. Microscopic investigation on the adsorption of lubrication oil on microplastics. J. Mol. Liq. 2017, 227, 351–355. [Google Scholar] [CrossRef]
- Xu, P.; Ge, W.; Chai, C.; Zhang, Y.; Jiang, T.; Xia, B. Sorption of polybrominated diphenyl ethers by microplastics. Mar. Pollut. Bull. 2019, 145, 260–269. [Google Scholar] [CrossRef]
- Zhang, J. Physical insights into kinetic models of adsorption. Sep. Purif. Technol. 2019, 229, 115832. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Ofomaja, A.E. Intraparticle diffusion process for lead (II) biosorption onto mansonia wood sawdust. Bioresour. Technol. 2010, 101, 5868–5876. [Google Scholar] [CrossRef]
- Ala’a, H.; Ibrahim, K.A.; Albadarin, A.B.; Ali-Khashman, O.; Walker, G.M.; Ahmad, M.N.M. Remediation of phenol-contaminated water by adsorption using poly (methyl methacrylate)(PMMA). Chem. Eng. J. 2011, 168, 691–699. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, X.; Chen, C.; Wang, J. Algal sorbent derived from Sargassum horneri for adsorption of cesium and strontium ions: Equilibrium, kinetics, and mass transfer. Appl. Microbiol. Biotechnol. 2019, 103, 2833–2843. [Google Scholar] [CrossRef] [PubMed]
- Vadi, M.; Mansoorabad, A.O.; Mohammadi, M.; Rostami, N. Investigation of Langmuir, Freundlich and Temkin adsorption isotherm of tramadol by multi-wall carbon nanotube. Asian J. Chem. 2013, 25, 5467. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Al-Jabari, M. Kinetic models for adsorption on mineral particles comparison between Langmuir kinetics and mass transfer. Environ. Technol. Innov. 2016, 6, 27–37. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Deryło-Marczewska, A.; Słota, A. Adsorption and desorption kinetics of benzene derivatives on mesoporous carbons. Adsorption 2013, 19, 391–406. [Google Scholar] [CrossRef]
- Marczewski, A.W. Extension of Langmuir kinetics in dilute solutions to include lateral interactions according to regular solution theory and the Kiselev association model. J. Colloid Interface Sci. 2011, 361, 603–611. [Google Scholar] [CrossRef]
- Hatch, C.D.; Wiese, J.S.; Crane, C.C.; Harris, K.J.; Kloss, H.G.; Baltrusaitis, J. Water adsorption on clay minerals as a function of relative humidity: Application of BET and Freundlich adsorption models. Langmuir 2012, 28, 1790–1803. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Q.; Hu, Z.; Yan, L.; Ieong, U.-I.; Xu, Y. Adsorption of chlorophenols on polyethylene terephthalate microplastics from aqueous environments: Kinetics, mechanisms and influencing factors. Environ. Pollut. 2020, 265, 114926. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Arroyo-Manzanares, N.; Rivas-Montoya, E.; Ochando-Pulido, J.M.; Guillamon, E.; García-Campaña, A.M.; Martinez-Ferez, A. Effects of different vehiculization strategies for the allium derivative propyl propane thiosulfonate during dynamic simulation of the pig gastrointestinal tract. Can. J. Anim. Sci. 2018, 99, 244–253. [Google Scholar] [CrossRef]
- Song, X.; Wu, X.; Song, X.; Shi, C.; Zhang, Z. Sorption and desorption of petroleum hydrocarbons on biodegradable and nondegradable microplastics. Chemosphere 2020, 128553. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, J.; Liu, K.; Liu, N.; Zhou, B. Adsorption of bisphenol A based on synergy between hydrogen bonding and hydrophobic interaction. Langmuir 2014, 30, 13861–13868. [Google Scholar] [CrossRef]
- Zuo, L.-Z.; Li, H.-X.; Lin, L.; Sun, Y.-X.; Diao, Z.-H.; Liu, S.; Zhang, Z.-Y.; Xu, X.-R. Sorption and desorption of phenanthrene on biodegradable poly (butylene adipate co-terephthalate) microplastics. Chemosphere 2019, 215, 25–32. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Llorca, M.; Schirinzi, G.; Martínez, M.; Barceló, D.; Farré, M. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 2018, 235, 680–691. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D. Elucidating the co-transport of bisphenol A with polyethylene terephthalate (PET) nanoplastics: A theoretical study of the adsorption mechanism. Environ. Pollut. 2021, 270, 116192. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hentschel, B.T.; Teh, S.J. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE 2014, 9, e85433. [Google Scholar] [CrossRef] [PubMed]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Villain, J.; Falcou-Préfol, M.; Kerros, M.E.; Henry, M.; Pedrotti, M.L.; Bruzaud, S. Microplastics in Mediterranean Sea: A protocol to robustly assess contamination characteristics. PLoS ONE 2019, 14, e0212088. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef]
- Johansen, M.P.; Prentice, E.; Cresswell, T.; Howell, N. Initial data on adsorption of Cs and Sr to the surfaces of microplastics with biofilm. J. Environ. Radioact. 2018, 190, 130–133. [Google Scholar] [CrossRef]
- Ateia, M.; Zheng, T.; Calace, S.; Tharayil, N.; Pilla, S.; Karanfil, T. Sorption behavior of real microplastics (MPs): Insights for organic micropollutants adsorption on a large set of well-characterized MPs. Sci. Total Environ. 2020, 720, 137634. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Zhang, L.; Wang, K.; Yu, X.; Zheng, Z.; Zheng, R. Sorption behaviors of phenanthrene on the microplastics identified in a mariculture farm in Xiangshan Bay, southeastern China. Sci. Total Environ. 2018, 628, 1617–1626. [Google Scholar] [CrossRef]
- Leslie, H.A.; Depledge, M.H. Where is the evidence that human exposure to microplastics is safe? Environ. Int. 2020, 142, 105807. [Google Scholar] [CrossRef]


| Model | Parameters | Ref. |
|---|---|---|
| Experimental data: qt, qe, t | qt and qe are the amount (mg g−1) of a target chemical adsorbed per unit mass of microplastics at time t (min) and at the equilibrium: where c0, ct, and ce are the initial concentration (mg L−1), concentration (mg L−1) at time t (min), and concentration (mg L−1) at the equilibrium of a target chemical in liquid phase; is the volume (L) of the solution; W is the mass (g) of microplastics | [1] |
| Pseudo-first-order | k1 is the rate constant (min−1) | [159] |
| Pseudo-second-order | k2 is the rate constant (g mg−1 min−1) | [160] |
| Boyd’s film diffusion | [4] |
| Model | Parameters | Ref. |
|---|---|---|
| Langmuir | qm is the maximum adsorption capacity (mg g−1) of microplastics under monolayer adsorption; kL is the surface adsorption equilibrium (Langmuir) constant (L mg−1); qe is the amount (mg g−1) of a target chemical adsorbed on per unit mass of microplastics at time t (min); ce is the concentration (mg L−1) at the equilibrium of a target chemical in liquid phase | [167] |
| Freundlich | qe is the amount (mg g−1) of a target chemical adsorbed on per unit mass of microplastics at time t (min); ce is the concentration (mg L−1) at the equilibrium of a target chemical in liquid phase; kF and are the Freundlich constants related to adsorption capacity (L mg−1) and adsorption intensity | [168] |
| Temkin | qe is the amount (mg g−1) of a target chemical adsorbed on per unit mass of microplastics at time t (min); ce is the concentration (mg L−1) at the equilibrium of a target chemical in liquid phase; aT and bT are the Temkin isotherm constant (L mg−1) and Temkin constant (mol−1) related to adsorption heat; R is the gas constant with a value of 8.314 J mol−1 K−1; T is the absolute temperature (K) | [169] |
| Dubinin–Radushkevich | qe is the amount (mg g−1) of a target chemical adsorbed on per unit mass of microplastics at time t (min); qD is the adsorption capacity (mg g−1); β is the Dubinin–Radushkevich model constant (mol2 J−2) related to adsorption energy; ε is the Polanyi potential calculated by | [170] |
| PET Source | Size (µm) | Water | Pollutant Type and Content | Model | Parameter | pH | Ref. |
|---|---|---|---|---|---|---|---|
| pure MPs | <150 | ultrapure sea lake | Chlorophenols: 4-chlorophenol (MCP) 2,4-dichlorophenol (DCP) 2,4,6-trichlorophenol (TCP) 5 mg/L | Langmuir | qm (mg/g) = 2.87 (MCP); 0.37 (DCP); 0.10 (TCP) kL(L/mg) = 3.64 (MCP); 81.60 (DCP); 92.10 (TCP) | 8 | [175] |
| Pseudo-second order | qe (mg/g) = 41.60 (MCP); 70.30 (DCP); 29.80 (TCP) k2*103( g/(µg⋅h) = 5.31(MCP); 9.78 (DCP); 125.0 (TCP) | ||||||
| bottles | 2700 | Milli-Q | amoxicillin (AMX), atrazine(ATZ), diuron (DIR), paracetamol (PAC) phenol (PHN), vancomycin (VAC) 1 mg/L (adsorption) | Langmuir | qm (mg/g) = 7.18 (AMX); 2.80 (PHN) kL(L/mg) = 0.30 (AMX); 3.19 (PHN) | 7 | [103] |
| Pseudo-second order | qe (mg/g) = 2.48 (AMX); 1.01 (PHN) k2 (mg/g ⋅day) = 0.005 (AMX); 0.056 (PHN) | ||||||
| pure MPs | 50–200 | ultrapure | No. 10 diesel oil/water solution 500 mg/L | Langmuir | qm (mg/g) = 1753 (adsorp.); 28.90 (desorp.) kL(L/mg) = 2.58. 15 (adsorp.); 3.37. 15 (desorp.) | - | [180] |
| Pseudo-second order | qe (mg/g)=51.90 k2 (g/(mg⋅h))=0.09 | ||||||
| pure MPs | 100–150 | deionized | sulfamethoxazole 2.4 mg/L | Freundlich | kF (L/kg) = 24.7 n = 1.05 | - | [104] |
| bottles | <5000 | Milli-Q sea urban waste irrigation | Cd, Co, Cr, Cu, Ni, Pb, Zn 1 mg/L | Langmuir | qm (mg/g) = 4.93 (Pb) kL (L/mg) = 0.16 (Pb) | 7 | [110] |
| pure MPs | 65 | Milli-Q | Cd 60 mg/L | Langmuir | qm (mg/g) = 0.25 kL (L/g) = 0.003 | 6 | [123] |
| Pseudo-second order | qe (mg/g) = 0.11 k2 (g/mg h) = 1.96 | ||||||
| bottles | - | distilled | Cu, Zn 5 mg/L | Langmuir | qm (mg/g) = 0.36 (Cu); 0.21 (Zn) kL(L/mg) = 0.18 (Cu); 0.14 (Zn) | - | [187] |
| pellets | 3000 | sea immersion, San Diego Bay | Mn, Co, Ni, Zn, Al, Cr, Fe, Pb | Pseudo-first order | qe (mg/g) = 0.16 (Mn); 0.09 (Al) k (g/mg h) = 0.09 (Mn); 0.25 (Al) | 8 | [188] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lionetto, F.; Esposito Corcione, C. An Overview of the Sorption Studies of Contaminants on Poly(Ethylene Terephthalate) Microplastics in the Marine Environment. J. Mar. Sci. Eng. 2021, 9, 445. https://doi.org/10.3390/jmse9040445
Lionetto F, Esposito Corcione C. An Overview of the Sorption Studies of Contaminants on Poly(Ethylene Terephthalate) Microplastics in the Marine Environment. Journal of Marine Science and Engineering. 2021; 9(4):445. https://doi.org/10.3390/jmse9040445
Chicago/Turabian StyleLionetto, Francesca, and Carola Esposito Corcione. 2021. "An Overview of the Sorption Studies of Contaminants on Poly(Ethylene Terephthalate) Microplastics in the Marine Environment" Journal of Marine Science and Engineering 9, no. 4: 445. https://doi.org/10.3390/jmse9040445
APA StyleLionetto, F., & Esposito Corcione, C. (2021). An Overview of the Sorption Studies of Contaminants on Poly(Ethylene Terephthalate) Microplastics in the Marine Environment. Journal of Marine Science and Engineering, 9(4), 445. https://doi.org/10.3390/jmse9040445







