Hemocyte Health Status Based on Four Biomarkers to Assess Recovery Capacity in American Lobster (Homarus americanus) after Exposure to Marine Diesel and Diluted Bitumen
Abstract
1. Introduction
2. Materials and Methods
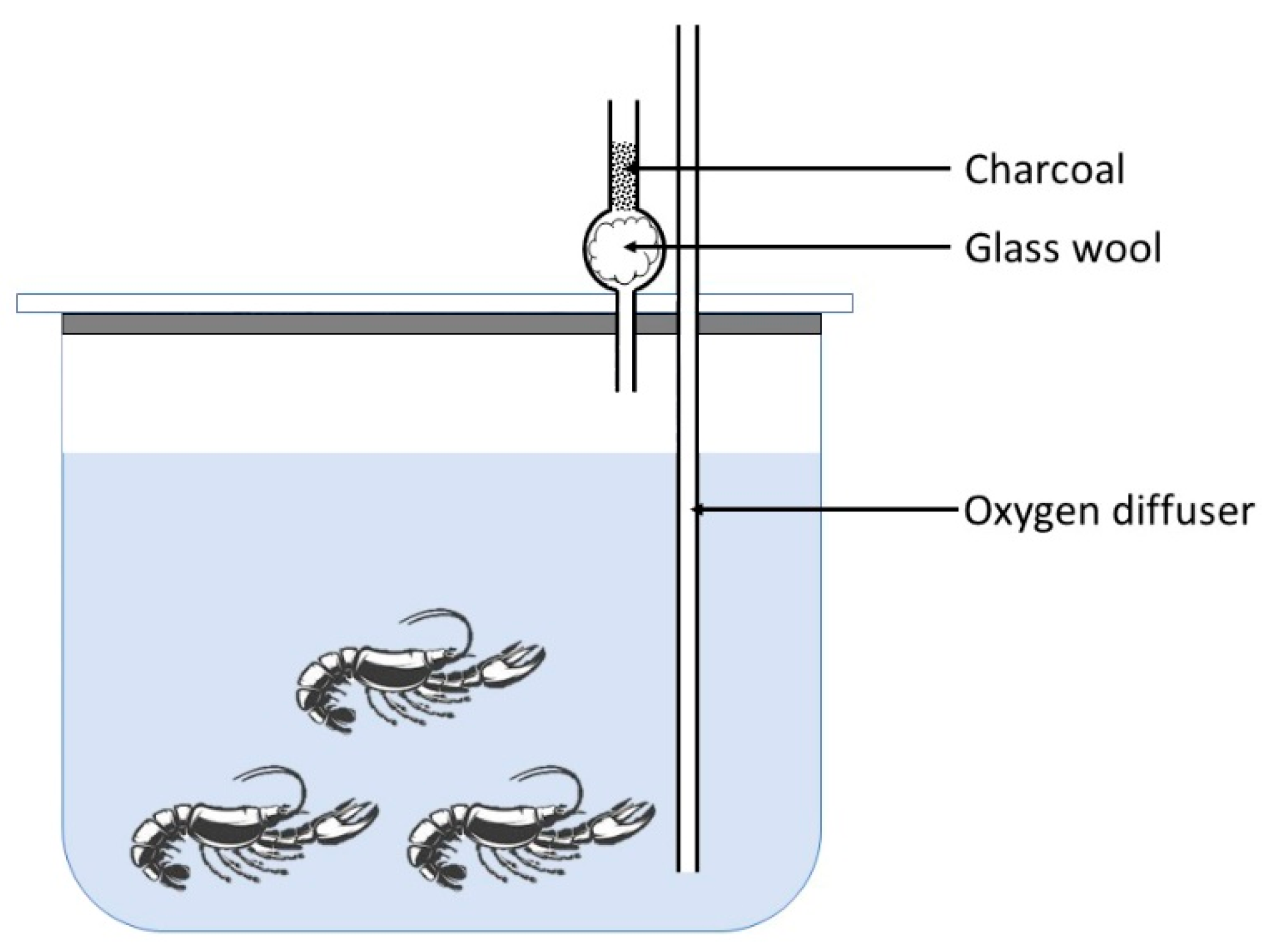
2.1. Conditions of Oil Spill Simulation
2.1.1. Oil Products
2.1.2. Water Accommodated Fraction
2.1.3. Monitoring of Dissolved Aromatic Hydrocarbons by Fluorescence
2.2. Animals
2.3. Exposure and Recovery
2.4. Hemolymph Sampling
2.5. Biotests
2.5.1. Brix Index
2.5.2. Cellular Viability
2.5.3. Lysosomal Membrane Destabilization Index
2.5.4. EROD
2.6. Tainting Assay
2.7. Tissues Analysis for Polycyclic Aromatic Hydrocarbons (PAHs)
2.7.1. PAHs Extraction
2.7.2. PAHs Detection and Quantification
2.8. Statistical Treatment of Biomarker Responses
3. Results
3.1. Exposure
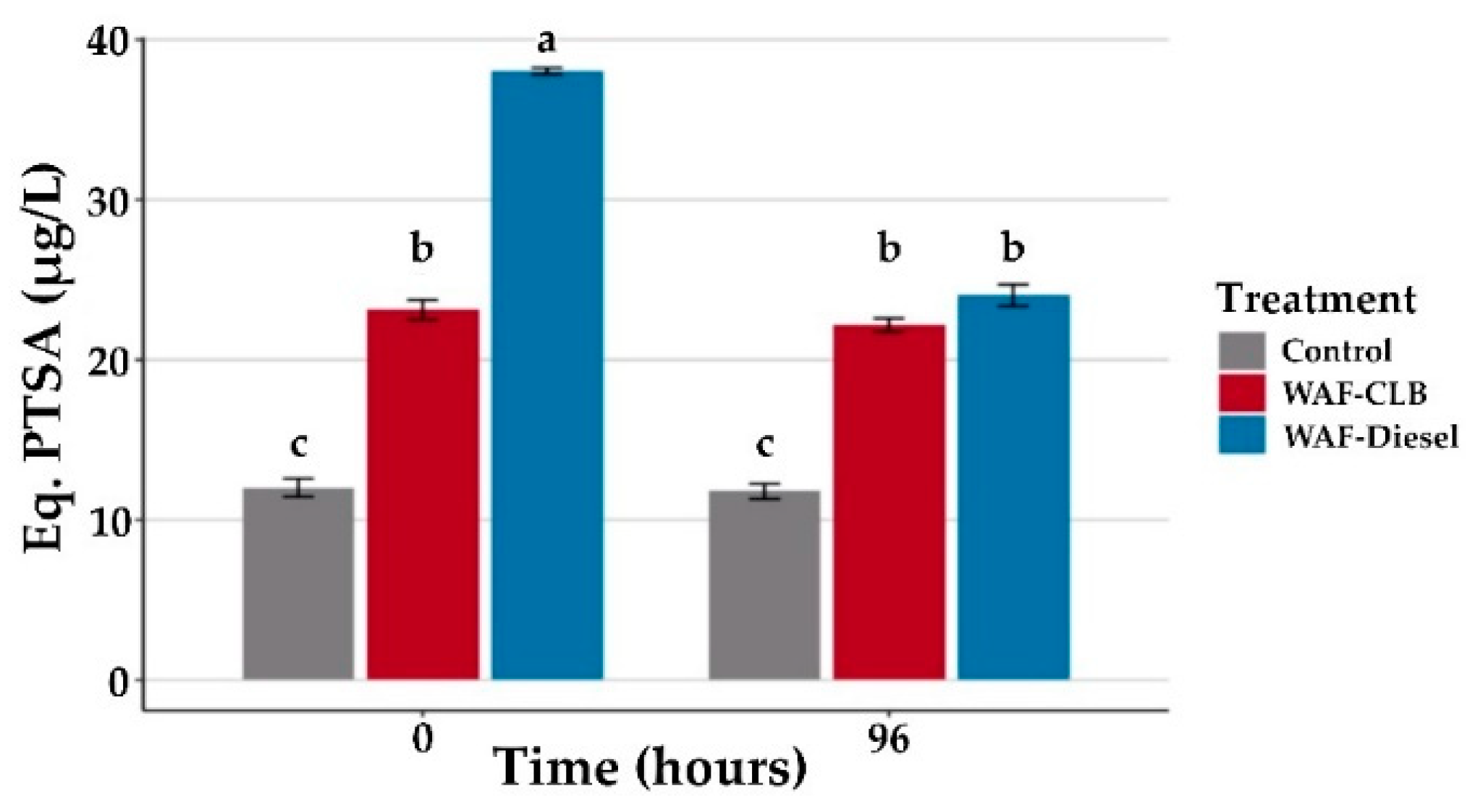
3.1.1. Monitoring of Dissolved Aromatic Hydrocarbons in Experimental Units
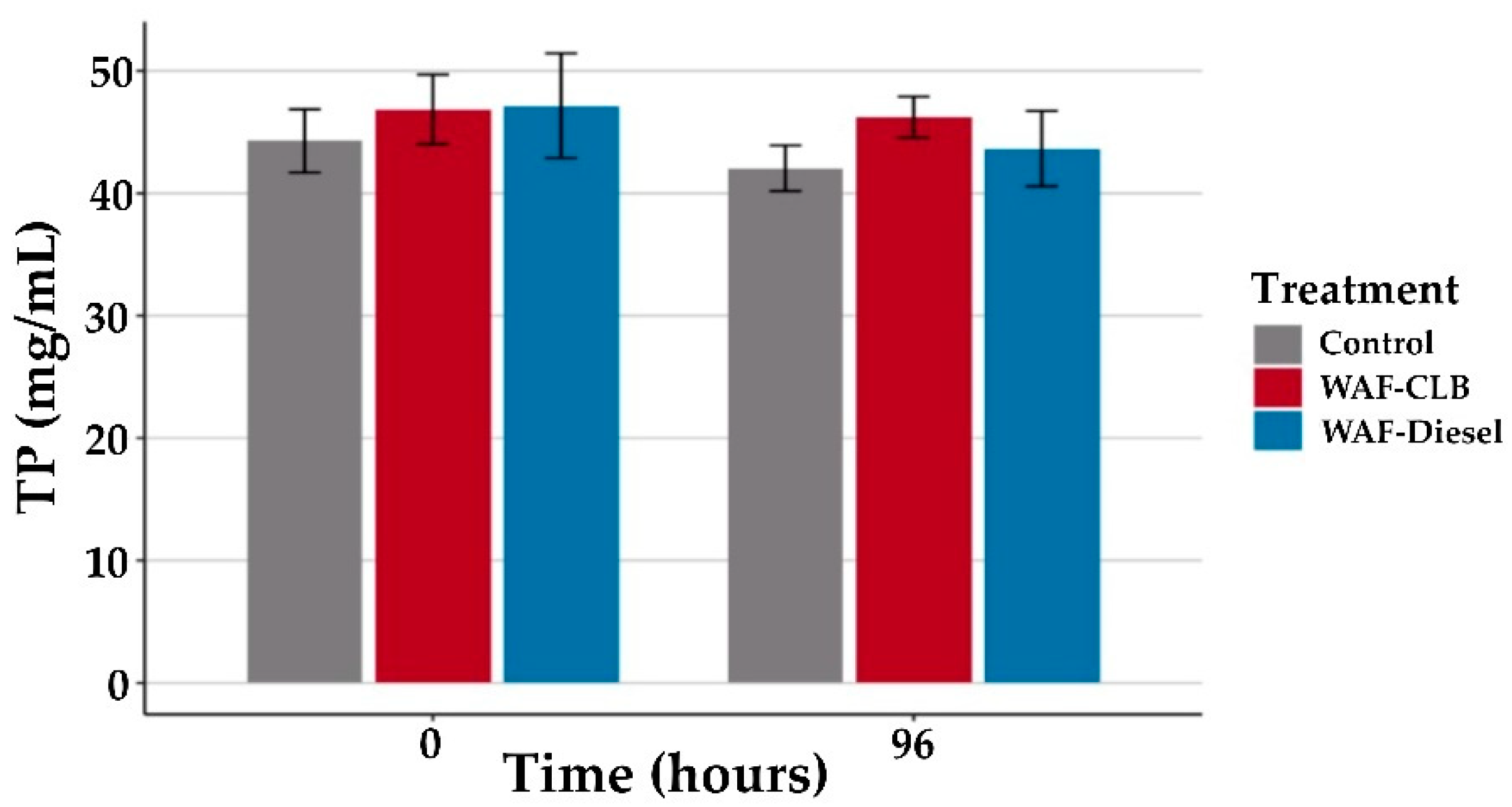
3.1.2. Sublethal Impacts after 96 h Exposure
3.2. Recovery Capacity
3.2.1. Brix Index
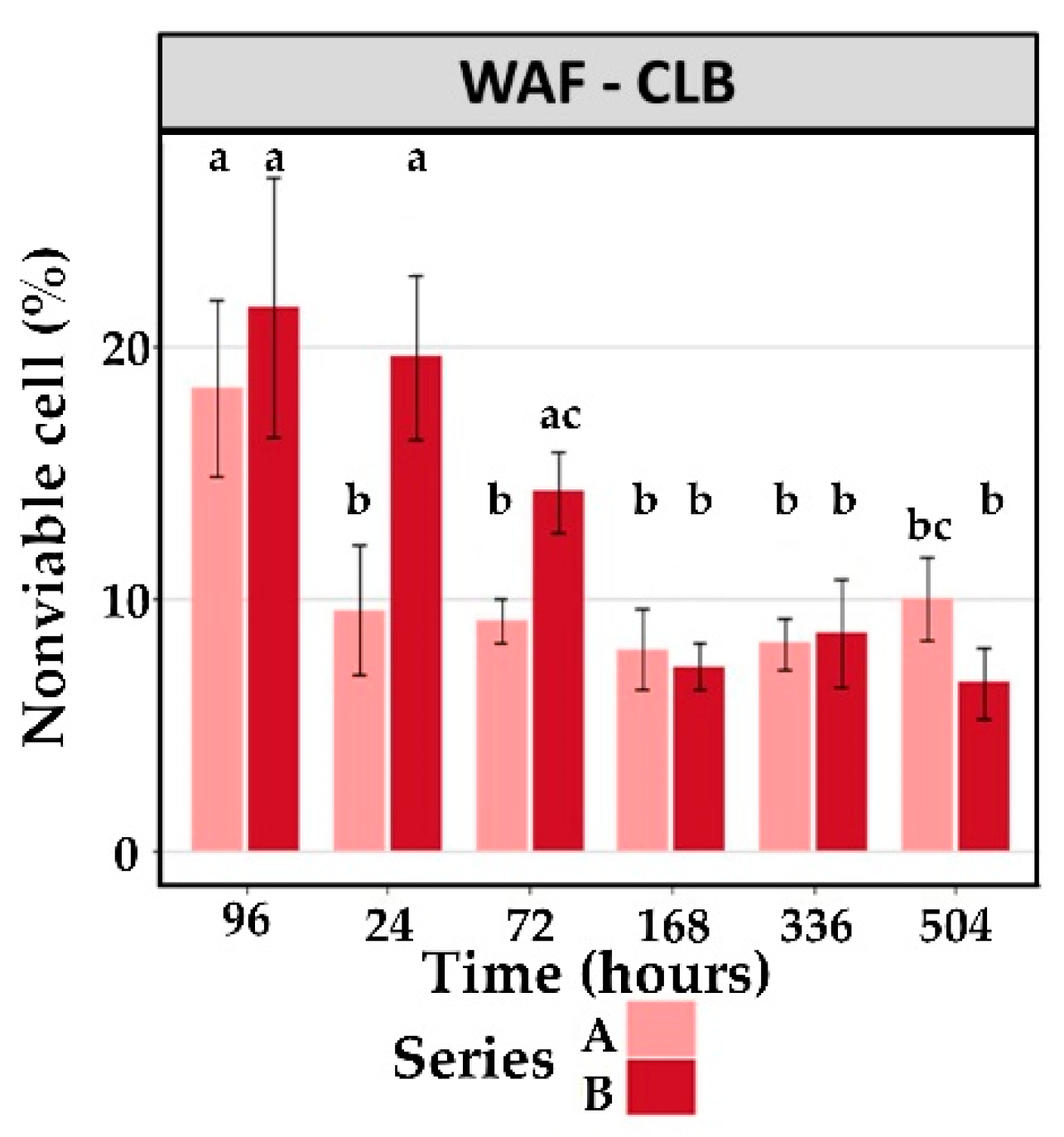
3.2.2. Cellular Viability
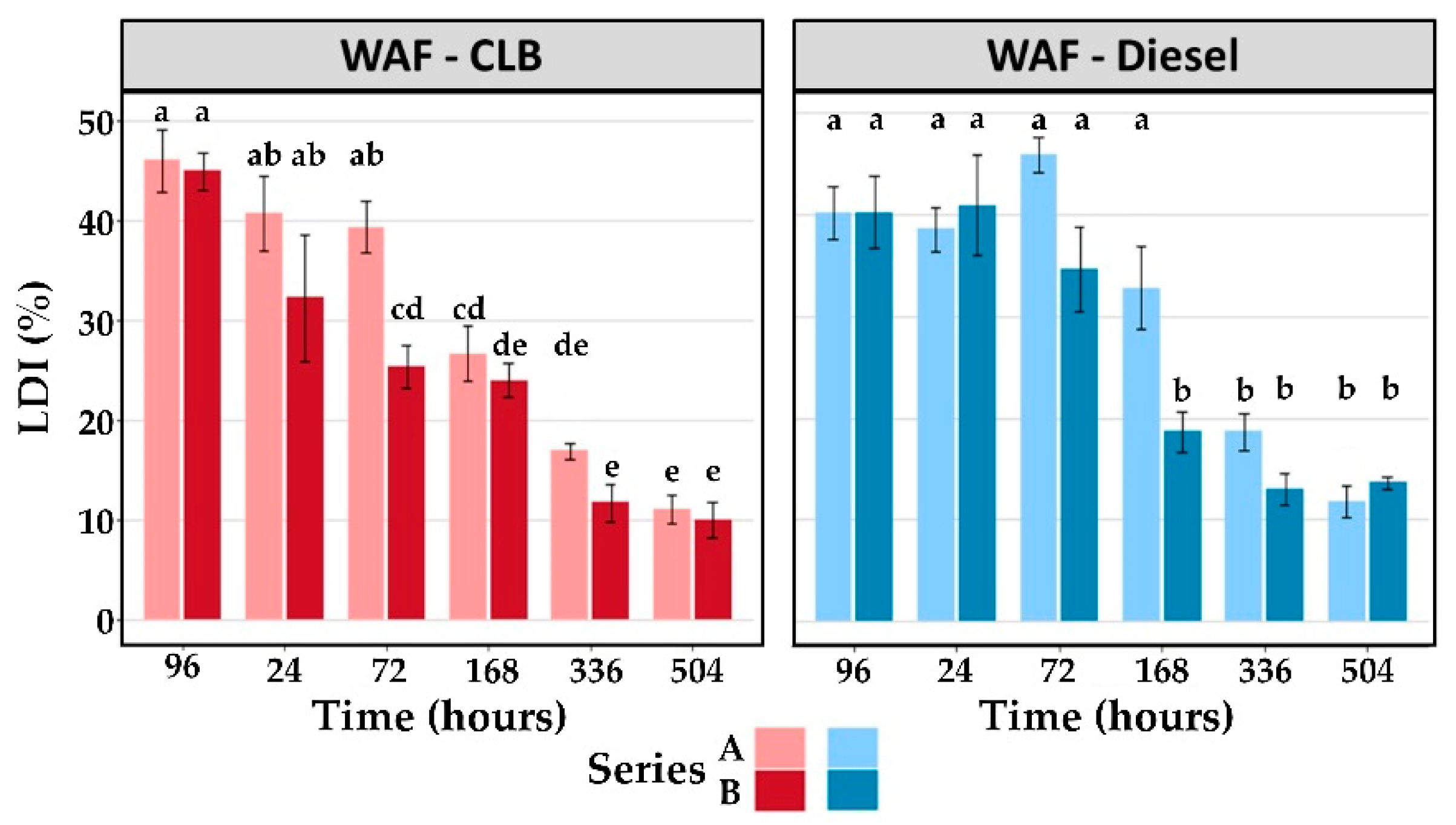
3.2.3. Lysosomal Membrane Destabilization Index
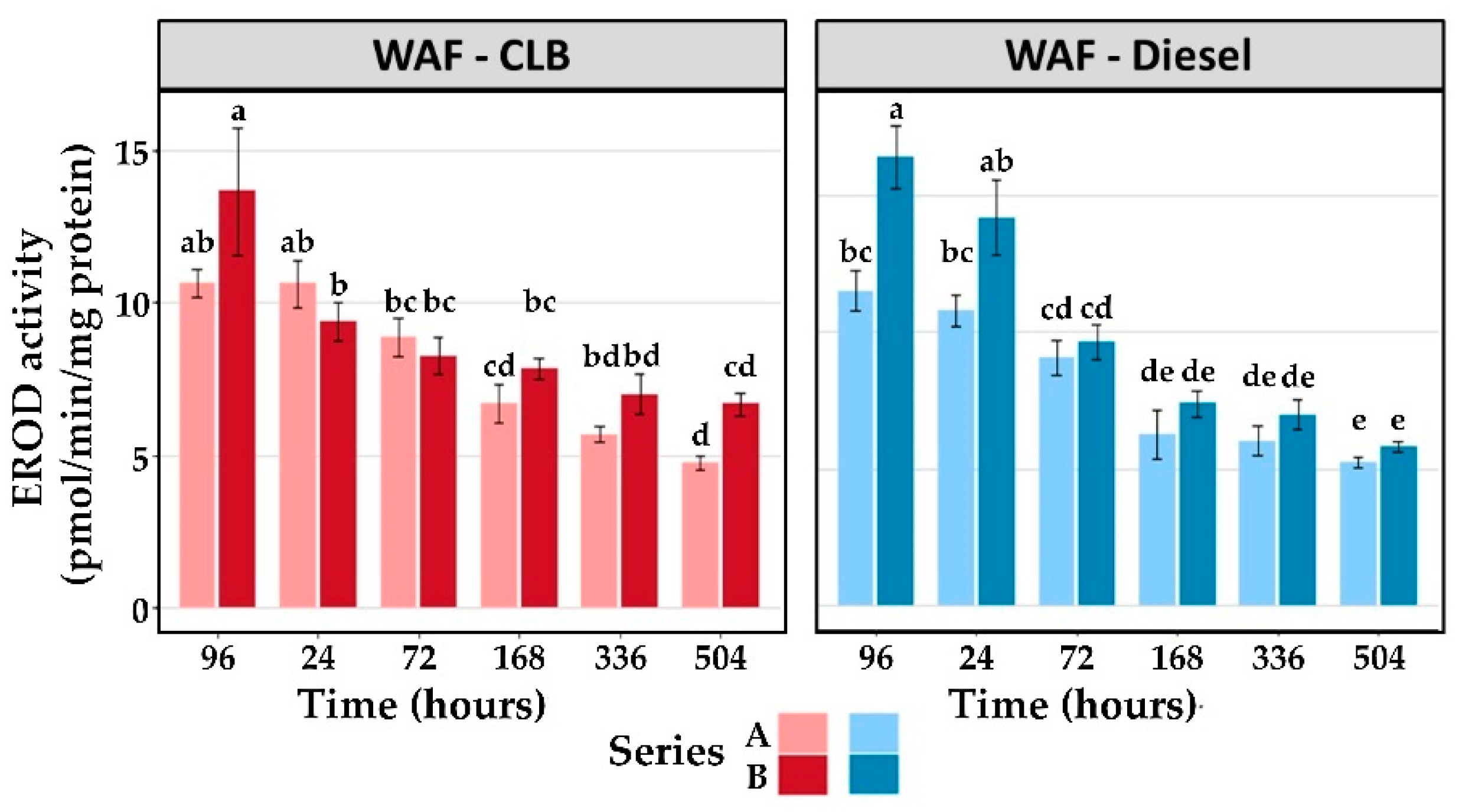
3.2.4. EROD
3.3. Tainting Assay
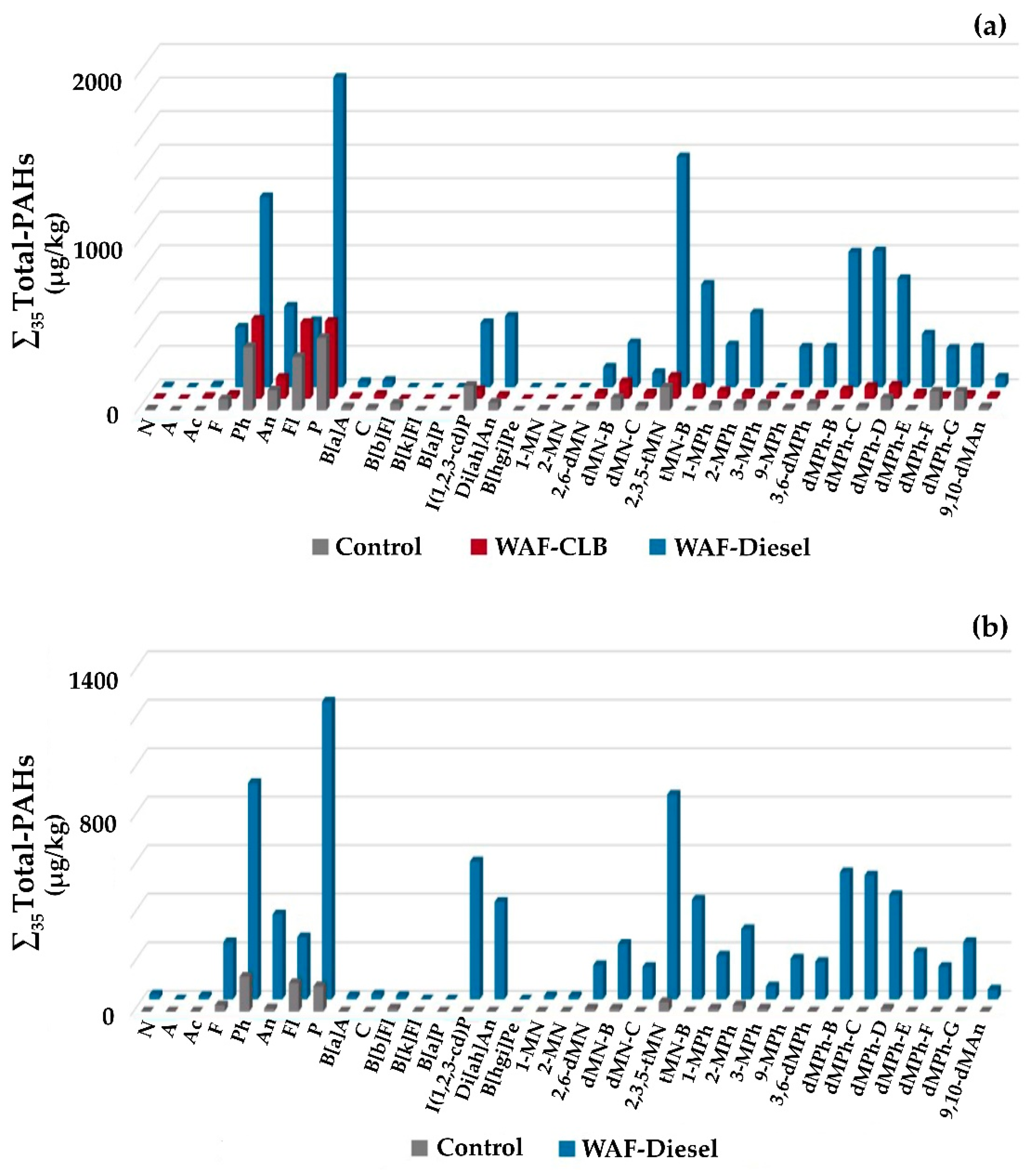
3.4. Chemical Analysis
4. Discussion
4.1. Biomarkers and Industry
4.2. Impact of Hydrocarbon Exposure
4.3. Recovery Capacity in Case of Hydrocarbon Exposure
4.4. Consumption After an Oil Spill
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Department of Fisheries and Oceans. Seafisheries Landed Quantity by Region. 2017. Available online: https://www.dfo-mpo.gc.ca/stats/commercial/land-debarq/sea-maritimes/s2017aq-eng.htm (accessed on 1 September 2020).
- Fishwatch, U.S. American Lobster Homarus Americanus. Available online: https://www.fishwatch.gov/profiles/american-lobster (accessed on 1 September 2020).
- Department of Fisheries and Oceans. Seafisheries Landed Value by Region. 2018. Available online: http://dfo-mpo.gc.ca/stats/commercial/land-debarq/sea-maritimes/s2018av-eng.htm (accessed on 1 September 2020).
- Natural Resources Canada. Crude Oil Facts. Available online: https://www.nrcan.gc.ca/science-data/data-analysis/energy-data-analysis/energy-facts/crude-oil-facts/20064 (accessed on 1 September 2020).
- Natural Resources Canada. Petroleum Products Facts. Available online: https://www.nrcan.gc.ca/science-data/data-analysis/energy-data-analysis/energy-facts/petroleum-products-facts/20065 (accessed on 1 September 2020).
- U.S. Energy Information Administration (EIA). Annual Energy Outlook 2012: With Projections to 2035; EIA-US, Government Printing Office: Washington, DC, USA, 2012.
- Lee, K.; Boufadel, M.; Chen, B.; Foght, J.; Hodson, P.; Swanson, S.; Venosa, A. Expert Panel Report on the Behaviour and Environmental Impacts of Crude Oil Released into Aqueous Environments; Royal Society of Canada: Ottawa, ON, Canada, 2015; ISBN 978-1-928140-02-3. [Google Scholar]
- Marty, J.; Potter, S. Risk Assessment for Marine Spills in Canadian Waters, Phase 1: Oil Spills South of the 60th Parallel; WSP: Montreal, QC, Canada, 2014; p. 172. [Google Scholar]
- O’Sullivan, A. The Amoco Cadiz oil spill. Mar. Pollut. Bull. 1978, 9, 123–128. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhu, Q.; Wu, W. The weathering of oil after the Deepwater Horizon oil spill: Insights from the chemical composition of the oil from the sea surface, salt marshes and sediments. Environ. Res. Lett. 2012, 7, 035302. [Google Scholar] [CrossRef]
- Michel, J.; Fingas, M. Oil Spills: Causes, Consequences, Prevention, and Countermeasures. In Fossil Fuels; World Scientific Series in Current Energy Issues; Gerard M Crawley, Marcus Enterprise LLC, USA & University of South Carolina: Columbia, SC, USA, 2016; Volume 1, pp. 159–201. [Google Scholar]
- Rumney, H.S.; Laruelle, F.; Potter, K.; Mellor, P.K.; Law, R.J. Polycyclic aromatic hydrocarbons in commercial fish and lobsters from the coastal waters of Madagascar following an oil spill in August 2009. Mar. Pollut. Bull. 2011, 62, 2859–2862. [Google Scholar] [CrossRef] [PubMed]
- Kingston, P. Recovery of the marine environment following the Braer spill, Shetland. In Proceedings of the International Oil Spill Conference, Seattle, WA, USA, 8–11 March 1999; pp. 103–109. [Google Scholar]
- Law, R.J.; Kelly, C.; Baker, K.; Jones, J.; McIntosh, A.D.; Moffat, C.F. Toxic equivalency factors for PAH and their applicability in shellfish pollution monitoring studies. J. Environ. Monit. 2002, 4, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Williams, U.P.; Kiceniuk, J.W.; Fancey, L.L.; Botta, J.R. Tainting and Depuration of Taint by Lobsters (Homarus americanus) Exposed to water Contaminated with a No. 2 Fuel Oil: Relationship with Aromatic Hydrocarbon Content in Tissue. J. Food Sci. 1989, 54, 240–243. [Google Scholar] [CrossRef]
- Sánchez, F.; Velasco, F.; Cartes, J.; Olaso, I.; Preciado, I.; Fanelli, E.; Serrano, A.; Gutierrez-Zabala, J. Monitoring the Prestige oil spill impacts on some key species of the Northern Iberian shelf. Mar. Pollut. Bull. 2006, 53, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, M.L. Recent spill experiences: The North Cape spill. Presented at the Arctic and Marine Oil Spill Program Technical Seminar (AMOP), Calgary, AB, Canada, 12–14 June 1996. [Google Scholar]
- Cobb, J.S.; Clancy, M. North Cape Oil Spill: An Assessment of the Impact on Lobster Populations; University of Rhode Island: Kingston, RI, USA, 5 January 1998; p. 205. [Google Scholar]
- Cobb, J.S.; Clancy, M.; Wähle, R.A. Habitat-based assessment of lobster abundance: A case study of an oil spill. Am. Fish. Soc. 1999, 22, 285–298. [Google Scholar]
- French, D.P. North Cape Oil Spill: Synthesis of Injury Quantification and Restoration Scaling for Lobsters; Report to NOAA Damage Assessment Center: Silver Spring, MD, USA, 1999; p. 21. [Google Scholar]
- Gibson, M.R.; Angell, T.E.; Lazar, N.B. Estimation of Lobster Strandings Following the North Cape Oil Spill in Block Island Sound. Research Reference Document 97/1. Available online: https://www.ser-rrc.org/project/usa-rhode-island-the-north-cape-american-lobster-homarus-americanus-restoration-project-block-island-sound/ (accessed on 1 July 2020).
- Felder, D.L.; Thoma, B.P.; Schmidt, W.E.; Sauvage, T.; Self-Krayesky, S.L.; Chistoserdov, A.; Bracken-Grissom, H.D.; Fredericq, S. Seaweeds and Decapod Crustaceans on Gulf Deep Banks after the Macondo Oil Spill. Bioscience 2014, 64, 808–819. [Google Scholar] [CrossRef]
- Atema, J.; Stein, L. Sublethal Effects of Crude Oil on Lobster (Homarus Americanus) Behavior; Tech. Rep. WHOI-72-74; Oceanographic Institution: Woods Hole, MA, USA, 1972; 81p. [Google Scholar]
- Atema, J.; Stein, L.S. Effects of crude oil on the feeding behaviour of the lobster Homarus Americanus. Environ. Pollut. (1970) 1974, 6, 77–86. [Google Scholar] [CrossRef]
- Biedron, I.; Evans, S. Time for Action: Six Years after Deepwater Horizon; OCEANA: Washington, DC, USA, 2016; Available online: https://usa.oceana.org/publications/reports/time-action-six-years-after-deepwater-horizon (accessed on 1 September 2020).
- Blumer, M.; Hunt, J.M.; Atema, J.; Stein, L. Interaction between Marine Organisms and Oil Pollution; USEPA Ecological Research Series R3-73-042; United States Environmental Protection Agency: Washington, DC, USA, May 1973.
- Richardson, C. Effects of drilling cuttings on the behaviour of the Norway lobster Nephrops norvegicus. Mar. Pollut. Bull. 1984, 15, 170–174. [Google Scholar] [CrossRef]
- Webby, A.; Ling, N. Acute sublethal toxicity of MV Rena contaminants (heavy fuel oil, oil dispersant and cryolite) to finfish and rock lobster. N. Z. J. Mar. Freshw. Res. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Payne, J.F.; Kiceniuk, J.; Misra, R.; Fletcher, G.; Thompson, R. Sublethal effects of petroleum hydrocarbons on adult American lobsters (Homarus americanus). Can. J. Fish. Aquat. Sci. 1983, 40, 705–717. [Google Scholar] [CrossRef]
- Michel, P.; Abarnou, J.L. Amoco Cadiz: Informations sur la Contamination des Espèces Exploitées; Centre National pour l’Exploitation des Oceans (CNEXO). Serie: Actes de Colloques, n°6. 1978, pp. 143–150. Available online: https://archimer.ifremer.fr/doc/00306/41724/ (accessed on 1 September 2020).
- Lagadic, L.; Caquet, T.; Amiard, J.C.; Ramade, F. Biomarqueurs en Écotoxicologie: Principes et définitions. In Biomarqueurs en Écotoxicologie: Aspects Fondamentaux; Elsevier Masson: Paris, France, 1997; pp. 1–9. [Google Scholar]
- McDonald, S.J.; Willett, K.L.; Thomsen, J.; Beatty, K.B.; Connor, K.; Narasimhan, T.R.; Erickson, C.M.; Safe, S.H. Sublethal detoxification responses to contaminant exposure associated with offshore production platforms. Can. J. Fish. Aquat. Sci. 1996, 53, 2606–2617. [Google Scholar] [CrossRef]
- Fournier, M.; Brousseau, P.; Tryphonas, S.; Cyr, D. Biomarkers of immunotoxicity: An evolutionary perspective. In Environmental Endocrine Disruptors: An Evolutionary Perspective; Taylor & Francis Group: Abingdon, UK, 2000; pp. 182–216. [Google Scholar]
- Callaway, R.; Shinn, A.P.; Grenfell, S.E.; Bron, J.E.; Burnell, G.; Cook, E.J.; Crumlish, M.; Culloty, S.; Davidson, K.; Ellis, R.P.; et al. Review of climate change impacts on marine aquaculture in the UK and Ireland. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 389–421. [Google Scholar] [CrossRef]
- Simon, C.J.; FitzGibbon, Q.P.; Battison, A.; Carter, C.G.; Battaglene, S.C. Bioenergetics of Nutrient Reserves and Metabolism in Spiny Lobster Juveniles Sagmariasus verreauxi: Predicting Nutritional Condition from Hemolymph Biochemistry. Physiol. Biochem. Zool. 2015, 88, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Mendo, T.; Simon, C.; Green, B.; Gardner, C. Spatial Variability in Condition of Southern Rock Lobsters (Jasus edwardsii) at the Start of the Tasmanian Fishing Season. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, G.; Riley, J.G. An analysis of refractometry as a method of determining blood total protein concentration in the American lobster Homarus americanus (Milne Edwards). Aquac. Res. 2002, 33, 557–562. [Google Scholar] [CrossRef]
- Paterson, B.D.; Davidson, G.W.; Spanoghe, P.T. Measuring total protein concentration in the blood of the western rock lobster (Panulirus cygnus George) by refractometry. In Proceedings of the International Symposium on Lobster Health Management, Adelaide, Australia, 19–21 September 1999. [Google Scholar]
- Lorenzon, S.; Giulianini, P.G.; Martinis, M.; Ferrero, E.A. Stress effect of different temperatures and air exposure during transport on physiological profiles in the American lobster Homarus americanus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 94–102. [Google Scholar] [CrossRef]
- Moisan, N.; Cauvier, C.; Coulombe, F. Conservation Prolongée du Homard. MAPAQ, DIT. Rapport de R-D, n°171. 2008, p. 8. Available online: http://www.mapaq.gouv.qc.ca/Fr/Peche/md/Publications/ (accessed on 1 September 2020).
- Battison, A.L. Use of the Brix value with cuticle indices to describe haemolymph biochemistry parameters in Homarus americanus H. Milne Edwards, 1837 (Decapoda: Malacostraca: Nephropidae). J. Crustac. Biol. 2018, 38, 625–634. [Google Scholar] [CrossRef]
- Martínez-Gómez, C.; Bignell, J.; Lowe, D. Lysosomal Membrane Stability in Mussels; ICES Techniques in Marine Environmental Sciences; International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2015; pp. 1–41. ISBN 9788774821. [Google Scholar]
- Rocha, A.J.D.S.; Gomes, V.; Passos, M.J.D.A.C.R.; Hasue, F.M.; Santos, T.D.C.A.D.; Bícego, M.C.; Taniguchi, S.; Van Ngan, P. EROD activity and genotoxicity in the seabob shrimp Xiphopenaeus kroyeri exposed to benzo[a]pyrene (BaP) concentrations. Environ. Toxicol. Pharmacol. 2012, 34, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Centre d’Expertise en Analyse Environnementale du Québec. Impact Chronique Associé aux Activités Régulières d’une Plate-Forme Pétrolière Pour les Écosystèmes Aquatiques du Golfe du Saint-Laurent, Étude GENV25, Évaluation Environnementale Stratégique Globale sur les Hydrocarbures; Ministère du Développement Durable, de l’Environnement et de la Lutte Contre les Changements Climatiques: Québec, QC, Canada, 2015; p. 75. [Google Scholar]
- Rewitz, K.F.; Styrishave, B.; Løbner-Olesen, A.; Andersen, O. Marine invertebrate cytochrome P450: Emerging insights from vertebrate and insect analogies. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D. The fate of organic xenobiotics in aquatic ecosystems: Quantitative and qualitative differences in biotransformation by invertebrates and fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 43–49. [Google Scholar] [CrossRef]
- Rewitz, K.; Styrishave, B.; Andersen, O. CYP330A1 and CYP4C39 enzymes in the shore crab Carcinus maenas: Sequence and expression regulation by ecdysteroids and xenobiotics. Biochem. Biophys. Res. Commun. 2003, 310, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Monari, M.; Foschi, J.; Matozzo, V.; Marin, M.; Fabbri, M.; Rosmini, R.; Serrazanetti, G. Investigation of EROD, CYP1A immunopositive proteins and SOD in haemocytes of Chamelea gallina and their role in response to B[a]P. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 382–392. [Google Scholar] [CrossRef]
- Little, P.J.; James, M.O.; Pritchard, J.B.; Bend, J.R. Temperature-dependent disposition of [14C]benzo(a)pyrene in the spiny lobster, Panulirus argus. Toxicol. Appl. Pharmacol. 1985, 77, 325–333. [Google Scholar] [CrossRef]
- Crude Monitor. Cold Lake. Available online: https://www.crudemonitor.ca/crudes/index.php?acr=CL&PHPSESSID=32b25d47a44bfecd3f6c8c5b9ff (accessed on 1 November 2020).
- Singer, M.; Aurand, D.; Bragin, G.; Clark, J.; Coelho, G.; Sowby, M.; Tjeerdema, R. Standardization of the Preparation and Quantitation of Water-accommodated Fractions of Petroleum for Toxicity Testing. Mar. Pollut. Bull. 2000, 40, 1007–1016. [Google Scholar] [CrossRef]
- Barron, M.G.; Ka’Aihue, L. Critical evaluation of CROSERF test methods for oil dispersant toxicity testing under subarctic conditions. Mar. Pollut. Bull. 2003, 46, 1191–1199. [Google Scholar] [CrossRef]
- Payne, S.J.; King, C.K.; Zamora, L.M.; Virtue, P. Temporal changes in the sensitivity of coastal Antarctic zooplankton communities to diesel fuel: A comparison between single- and multi-species toxicity tests. Environ. Toxicol. Chem. 2014, 33, 882–890. [Google Scholar] [CrossRef]
- Conmy, R.N.; Coble, P.G.; Farr, J.; Wood, A.M.; Lee, K.; Pegau, W.S.; Walsh, I.D.; Koch, C.R.; Abercrombie, M.I.; Miles, M.S.; et al. Submersible Optical Sensors Exposed to Chemically Dispersed Crude Oil: Wave Tank Simulations for Improved Oil Spill Monitoring. Environ. Sci. Technol. 2014, 48, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Bose, N.; Robinson, B.; Nguyen, H.D. Assessing hydrocarbon presence in the waters of port au port bay, newfoundland and labrador, for AUV oil spill delineation tests. J. Ocean Technol. 2020, 15, 102–112. [Google Scholar]
- Young-Lai, W.W.; Charmantier-Daures, M.; Charmantier, G. Effect of ammonia on survival and osmoregulation in different life stages of the lobsterHomarus americanus. Mar. Biol. 1991, 110, 293–300. [Google Scholar] [CrossRef]
- Leavitt, D.F.; Bayer, R.C. A refractometric method of determining serum protein concentration in the American lobster. Aquaculture 1977, 12, 169–171. [Google Scholar] [CrossRef]
- Song, L.; Li, X.; Clarke, S.; Wang, T.; Bott, K. The application of neutral red retention assay to evaluate the differences in stress responses to sexual maturation and spawning between different sizes of Pacific oyster, Crassostrea gigas (Thunberg). J. Shellfish Res. 2007, 26, 493–499. [Google Scholar] [CrossRef]
- Small, H.; Shields, J.; Neil, D.; Taylor, A.; Coombs, G. Differences in enzyme activities between two species of Hematodinium, parasitic dinoflagellates of crustaceans. J. Invertebr. Pathol. 2007, 94, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Burgeot, T.; Ménard, D. Mesure de L’activité Enzymatique: Éthoxyresorutine-o-Dééthylase (EROD) chez les Poissons Marins; Quae: Versailles, France, 2004. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- International Standards Organization. ISO5492:1992 Sensory Analysis–Vocabulary; International Organization for Standardization: Geneva, Switzerland, 1992. [Google Scholar]
- Reilly, T.; York, R.K. Guidance on Sensory Testing and Monitoring of Seafood for Presence of Petroleum Taint Following an Oil Spill. In Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris; N.O.A.A.; Technical Memorandum NOS OR&R 9; National Oceanic and Atmospheric Administration: Seattle, WA, USA, August 2001. [Google Scholar]
- United States Environmental Protection Agency (US-EPA). Polynuclear Hydrocarbons; US-EPA: Washington, DC, USA, 1984; Volume 10.
- R Core Development Team. A Language and Environment for Statistical Computing. Available online: http://www.R-project.org (accessed on 6 January 2020).
- McCay, D.F. Development and application of damage assessment modeling: Example assessment for the North Cape oil spill. Mar. Pollut. Bull. 2003, 47, 341–359. [Google Scholar] [CrossRef]
- Turnbull, A.; Malhi, N.; Seger, A.; Harwood, T.; Jolley, J.; Fitzgibbon, Q.; Hallegraeff, G. Paralytic shellfish toxin uptake, tissue distribution, and depuration in the Southern Rock Lobster Jasus edwardsii Hutton. Harmful Algae 2020, 95, 101818. [Google Scholar] [CrossRef]
- Yender, R.; Michel, J.; Lord, C. Managing Seafood Safety after an Oil Spill; Hazardous Materials Response Division, Office of Response and Restoration, National Oceanic and Atmospheric Administration: Seattle, WA, USA, 2010; p. 72. [Google Scholar]
- Ross, D. Antioxidant defence systems and oxydative stress. In Membrane Lipid Oxidation 2; CRC Press: Abingdon, UK, 1991; pp. 151–170. [Google Scholar]
- Barron, M.G.; Conmy, R.N.; Holder, E.L.; Meyer, P.; Wilson, G.J.; Principe, V.E.; Willming, M.M. Toxicity of Cold Lake Blend and Western Canadian Select dilbits to standard aquatic test species. Chemosphere 2018, 191, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.J.D.S.; Santos, T.C.A.; Gomes, V.; Bícego, M.C.; Barbosa, A.C.R.D.A.; Passos, M.J.D.A.C.R.; Hasue, F.M.; Van Ngan, P. Assessment of trophic transfer of benzo(a)pyrene genotoxicity from the post-larval pink shrimp F. brasiliensis to the juvenile Florida pompano T. carolinus. Environ. Toxicol. Pharmacol. 2012, 34, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M. Comparative toxicity of selected PAHs in rainbow trout hepatocytes: Genotoxicity, oxidative stress and cytotoxicity. Drug Chem. Toxicol. 2018, 43, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, A.; Tremblay, R.; Audet, C.; Gagné, J.-P.; Pelletier, É.; St-Louis, R. Under ice spills of conventional crude oil and diluted bitumen: Physiological resilience of the blue mussel and transgenerational effects. Sci. Total Environ. 2021, 779, 146316. [Google Scholar] [CrossRef]
- Fulford, R.S.; Griffit, R.J.; Brown-Peterson, N.J.; Perry, H.; Sanchez-Rubio, G. Impacts of the Deepwater Horizon oil spill on blue crab, Callinectes sapidus, larval settlement in Mississippi. In Impacts of Oil Spill Disasters on Marine Habitats and Fisheries in North America; Brian Alford, M.S.P.J., Christopher, C.G., Eds.; CRC Press: Abingdon, UK, 2014; p. 253. [Google Scholar]
- Williams, U.P.; Kiceniuk, J.W.; Ryder, J.E.; Botta, J.R. Effects of an Oil Spill on American Lobster Homarus Americanus from Placentia Bay, Newfoundland. Available online: https://antoanmoitruong.com/wp-content/uploads/2018/03/Effects-of-an-oil-spill-on-American-lobster-Homarus-americanus-from-Placentia-Bay-Newfoundland.pdf (accessed on 1 October 2020).
- Heras, H.; Zhou, S.; Ackman, R.G. Uptake and Depuration of Petroleum Hydrocarbons by Atlantic Salmon: Effect of Different Lipid Levels. In Proceedings of the Sixteenth Arctic and Marine Oil Spill Program (AMOP) Technical Seminar, Calgary, Alberta, 1 October 1997; pp. 343–351. [Google Scholar]
- Howgate, P.; Mackie, P.; Whittle, K.; Farmer, J. Petroleum tainting in fish. In Rapports et Procès-Verbaux des Réunions; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1977; pp. 143–146. [Google Scholar]
- Motohiro, T.; Iseya, Z. Effects of water polluted by oil on aquatic organisms. II. n-paraffins, aromatic hydrocarbons and crude oil concentration on taint in scallop (Pecten yessoensis). Bull. Fish. Sci. Hokkaido Univ. 1976, 26, 367–371. [Google Scholar]
- Phillips, D.H. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. Toxicol. Environ. Mutagen. 1999, 443, 139–147. [Google Scholar] [CrossRef]
- Kameda, Y.; Shirai, J.; Komai, T.; Nakanishi, J.; Masunaga, S. Atmospheric polycyclic aromatic hydrocarbons: Size distribution, estimation of their risk and their depositions to the human respiratory tract. Sci. Total Environ. 2005, 340, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Shim, G.A. Dietary Exposure Estimation of Benzo[a]pyrene and Cancer Risk Assessment. J. Toxicol. Environ. Health Part A 2007, 70, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Park, K.; Lee, H.; Yang, J.-H.; Lee, C. Estimation of Excess Cancer Risk on Time-Weighted Lifetime Average Daily Intake of PAHs from Food Ingestion. Hum. Ecol. Risk Assess. Int. J. 2007, 13, 669–680. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, S.; Shen, H.; Ma, J. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc. Natl. Acad. Sci. USA 2009, 106, 21063–21067. [Google Scholar] [CrossRef]
- Government of Canada. Health Canada’s Maximum Levels for Chemical Contaminants in Foods. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/maximum-levels-chemical-contaminants-foods.html#shr-pg0 (accessed on 1 December 2020).
- Agence Française de Sécurité Sanitaire des Aliments (AFSSA). Avis de l’Agence Française de Sécurité Sanitaire des Aliments Relatif à L’évaluation des Risques Sanitaires qui Pourraient Résulter de la Contamination des Produits de la mer Destinés à la Consommation Humaine, Suite au Naufrage du Pétrolier Prestige. n° 2003-SA-0007. 2003, pp. 1–6. Available online: https://www.com/DOCS/DOC79.pdf (accessed on 1 September 2020).
- Chao, C.; Engelward, B.P. Applications of CometChip for Environmental Health Studies. Chem. Res. Toxicol. 2020, 33, 1528–1538. [Google Scholar] [CrossRef] [PubMed]




| Cold Lake Blend (CLB) | Marine Diesel | |
|---|---|---|
| Origin | Alberta | New Brunswick |
| API (degree API) | 20.9 | 35 |
| Density at 15 °C (g/mL) | 0.9249 | 0.84 |
| Viscosity at 15 °C (c P) | 285 | 2 |
| Nickel (ppm) | 65 | N/A |
| Vanadium (ppm) | 168 | N/A |
| Saturated hydrocarbons (%) | 45 | 65–95 |
| Aromatic hydrocarbons (%) | 30 | 5–25 |
| Treatment | EROD Activity | Induction Rate | |
|---|---|---|---|
| 0 h | 96 h | ||
| Control | 5.8 ± 0.3 b | 5.5 ± 0.3 b | 0 |
| WAF-CLB | 6.2 ± 0.6 b | 12.3 ± 1.1 a | 2 |
| WAF-Diesel | 5.9 ± 0.2 b | 14.0 ± 0.9 a | 2.4 |
| Treatment | End of Exposure | Recovery Period | ||||
|---|---|---|---|---|---|---|
| 96 h | 24 h | 72 h | 168 h | 336 h | 504 h | |
| Series A | ||||||
| Control | 41.2 ± 2.5 | 39.4 ± 2.9 | 37.7 ± 2.2 | 35.7 ± 2.8 | 31.8 ± 3.3 | 29.7 ± 2.3 |
| WAF-CLB | 47.5 ± 3.3 | 47.4 ± 2.7 | 42.3 ± 2.1 | 39.1 ± 1.6 | 37.1 ± 2.6 | 37.2 ± 1.9 |
| WAF-Diesel | 42.4 ± 2.2 | 41.8 ± 2.0 | 37.1 ± 2.7 | 37.7 ± 1.7 | 32.3 ± 1.8 | 30.0 ± 1.4 |
| Series B | ||||||
| Control | 42.1 ± 4.7 | 44.3 ± 4.8 | 44.1 ± 4.6 | 43.8 ± 4.3 | 38.6 ± 4.1 | 37.1 ± 4.7 |
| WAF-CLB | 46.2 ± 6.2 | 43.5 ± 6.1 | 44.4 ± 5.3 | 45.3 ± 5.3 | 36.4 ± 5.0 | 38.9 ± 5.4 |
| WAF-Diesel | 45.7 ± 6.3 | 38.4 ± 5.0 | 41.0 ± 4.6 | 40.6 ± 4.3 | 32.1 ± 4.1 | 26.2 ± 3.1 |
| Treatment | End of Exposure | Recovery Period | ||||
|---|---|---|---|---|---|---|
| 96 h | 24 h | 72 h | 168 h | 336 h | 504 h | |
| Series A | ||||||
| Control | 9.8 ± 1.2 | 8.7 ± 1.8 | 8.3 ± 1.2 | 7.8 ± 1.4 | 8.8 ± 1.5 | 9.8 ± 1.2 |
| WAF-CLB | 18.3 ± 3.5 * | 9.6 ± 2.6 | 9.1 ± 0.8 | 8.0 ± 1.6 | 8.2 ± 1.0 | 10.0 ± 1.6 |
| WAF-Diesel | 10.4 ± 1.0 | 9.1 ± 0.7 | 9.7 ± 1.3 | 10.0 ± 2.8 | 9.0 ± 0.9 | 9.6 ± 1.3 |
| Series B | ||||||
| Control | 7.2 ± 1.1 | 12.2 ± 1.2 | 11.8 ± 2.1 | 12.4 ± 1.6 | 5.8 ± 0.7 | 5.6 ± 1.2 |
| WAF-CLB | 21.6 ± 5.2 * | 19.6 ± 3.2 * | 14.2 ± 1.6 | 7.3 ± 0.9 | 8.7 ± 2.1 | 6.7 ± 1.4 |
| WAF-Diesel | 8.4 ± 1.4 | 8.7 ± 1.7 | 15.6 ± 3.1 | 8.2 ± 1.3 | 5.9 ± 0.9 | 5.4 ± 1.2 |
| Treatment | End of Exposure | Recovery Phase | ||||
|---|---|---|---|---|---|---|
| 96 h | 24 h | 72 h | 168 h | 336 h | 504 h | |
| Series A | ||||||
| Control | 12.8 ± 1.6 | 17.8 ± 0.7 | 17.6 ± 0.7 | 14.9 ± 1.0 | 13.1 ± 1.3 | 10.2 ± 1.8 |
| WAF-CLB | 46.0 ± 3.1 * | 40.7 ± 3.8 * | 39.3 ± 2.6 * | 26.7 ± 2.7 * | 16.9 ± 0.7 | 11.1 ± 1.5 |
| WAF-Diesel | 40.2 ± 2.6 * | 38.6 ± 2.1 * | 45.9 ± 1.8 * | 32.8 ± 4.0 * | 18.7 ± 1.8 | 11.8 ± 1.6 |
| Series B | ||||||
| Control | 18.4 ± 2.8 | 18.9 ± 1.2 | 19.6 ± 0.9 | 14.7 ± 2.2 | 11.8 ± 1.4 | 11.3 ± 1.7 |
| WAF-CLB | 44.9 ± 1.9 * | 32.2 ± 6.3 * | 25.3 ± 2.1 | 24.0 ± 1.7 | 11.8 ± 1.9 | 10.0 ± 1.9 |
| WAF-Diesel | 40.2 ± 3.5 * | 40.9 ± 4.9 * | 34.7 ± 4.2 * | 18.7 ± 2.0 | 13.0 ± 1.5 | 13.7 ± 0.6 |
| Treatment | End of Exposure | Recovery Phase | ||||
|---|---|---|---|---|---|---|
| 96 h | 24 h | 72 h | 168 h | 336 h | 504 h | |
| Series A | ||||||
| Control | 5.8 ± 0.4 | 5.6 ± 0.4 | 6.0 ± 0.8 | 5.0 ± 0.4 | 5.7 ± 0.5 | 5.6 ± 0.8 |
| WAF-CLB | 10.6 ± 0.5 * | 10.6 ± 0.8 * | 8.9 ± 0.7 | 6.7 ± 0.6 | 5.7 ± 0.3 | 4.8 ± 0.2 |
| WAF-Diesel | 11.5 ± 0.7 * | 10.8 ± 0.6 * | 9.1 ± 0.7 | 6.3 ± 0.9 | 6.0 ± 0.5 | 5.3 ± 0.2 |
| Series B | ||||||
| Control | 5.5 ± 0.4 | 5.9 ± 0.5 | 6.2 ± 0.7 | 6.1 ± 0.4 | 5.7 ± 0.4 | 6.1 ± 0.3 |
| WAF-CLB | 13.6 ± 2.1 * | 9.4 ± 0.6 | 8.3 ± 0.6 | 7.8 ± 0.3 | 7.0 ± 0.7 | 6.7 ± 0.4 |
| WAF-Diesel | 16.4 ± 1.2 * | 14.2 ± 1.4 * | 9.6 ± 0.6 | 7.4 ± 0.5 | 7.0 ± 0.5 | 5.8 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthod, C.; Bénard-Déraspe, M.-H.; Laplante, J.-F.; Lemaire, N.; Nadeau, M.; Toupoint, N.; Triffault-Bouchet, G.; Saint-Louis, R. Hemocyte Health Status Based on Four Biomarkers to Assess Recovery Capacity in American Lobster (Homarus americanus) after Exposure to Marine Diesel and Diluted Bitumen. J. Mar. Sci. Eng. 2021, 9, 370. https://doi.org/10.3390/jmse9040370
Berthod C, Bénard-Déraspe M-H, Laplante J-F, Lemaire N, Nadeau M, Toupoint N, Triffault-Bouchet G, Saint-Louis R. Hemocyte Health Status Based on Four Biomarkers to Assess Recovery Capacity in American Lobster (Homarus americanus) after Exposure to Marine Diesel and Diluted Bitumen. Journal of Marine Science and Engineering. 2021; 9(4):370. https://doi.org/10.3390/jmse9040370
Chicago/Turabian StyleBerthod, Camille, Marie-Hélène Bénard-Déraspe, Jean-François Laplante, Nicolas Lemaire, Madeleine Nadeau, Nicolas Toupoint, Gaëlle Triffault-Bouchet, and Richard Saint-Louis. 2021. "Hemocyte Health Status Based on Four Biomarkers to Assess Recovery Capacity in American Lobster (Homarus americanus) after Exposure to Marine Diesel and Diluted Bitumen" Journal of Marine Science and Engineering 9, no. 4: 370. https://doi.org/10.3390/jmse9040370
APA StyleBerthod, C., Bénard-Déraspe, M.-H., Laplante, J.-F., Lemaire, N., Nadeau, M., Toupoint, N., Triffault-Bouchet, G., & Saint-Louis, R. (2021). Hemocyte Health Status Based on Four Biomarkers to Assess Recovery Capacity in American Lobster (Homarus americanus) after Exposure to Marine Diesel and Diluted Bitumen. Journal of Marine Science and Engineering, 9(4), 370. https://doi.org/10.3390/jmse9040370







