In-Situ Variability of DOM in Relation with Biogeochemical and Physical Parameters in December 2017 in Laucala Bay (Fiji Islands) after a Strong Rain Event
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. In-Situ Sampling and Sea Measurements
2.3. Physical Parameters
2.4. Biogeochemical Parameters Sample Conditioning
2.5. Biogeochemical Parameters Laboratory Methodology
2.5.1. aCDOM(m−1)
2.5.2. DOC (µM)
2.5.3. Nutrients (µM L−1)
2.5.4. SPM-Dry Weight (mg L−1)
2.5.5. Chla and Pheophytin, Pheo (µg L−1)
2.5.6. FDOM (Fluorescent Dissolved Organic Matter)
2.6. Statistical Analysis, Standardization, and Visualization
3. Results and Discussion
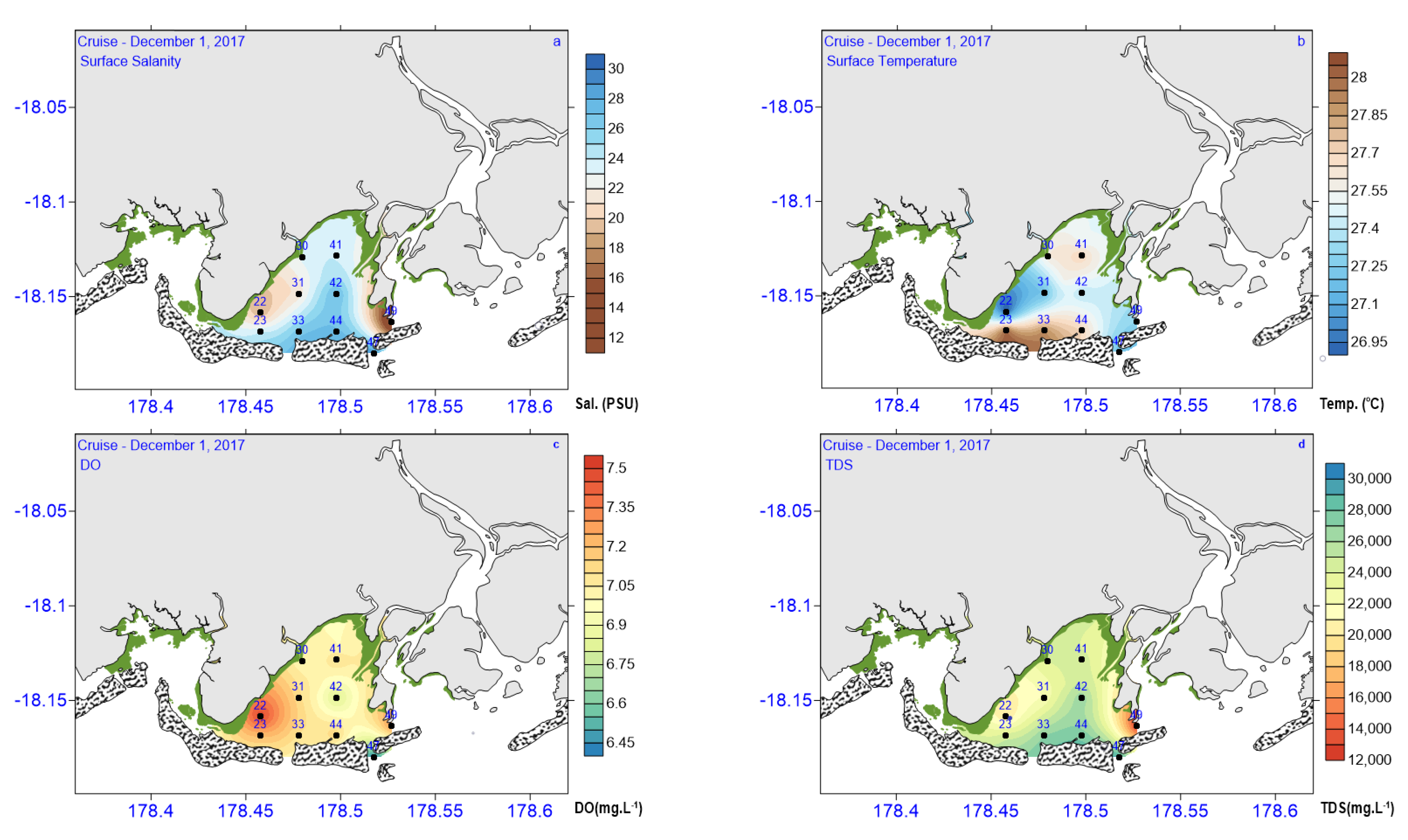
3.1. Physical Conditions in Laucala Bay in December 2017
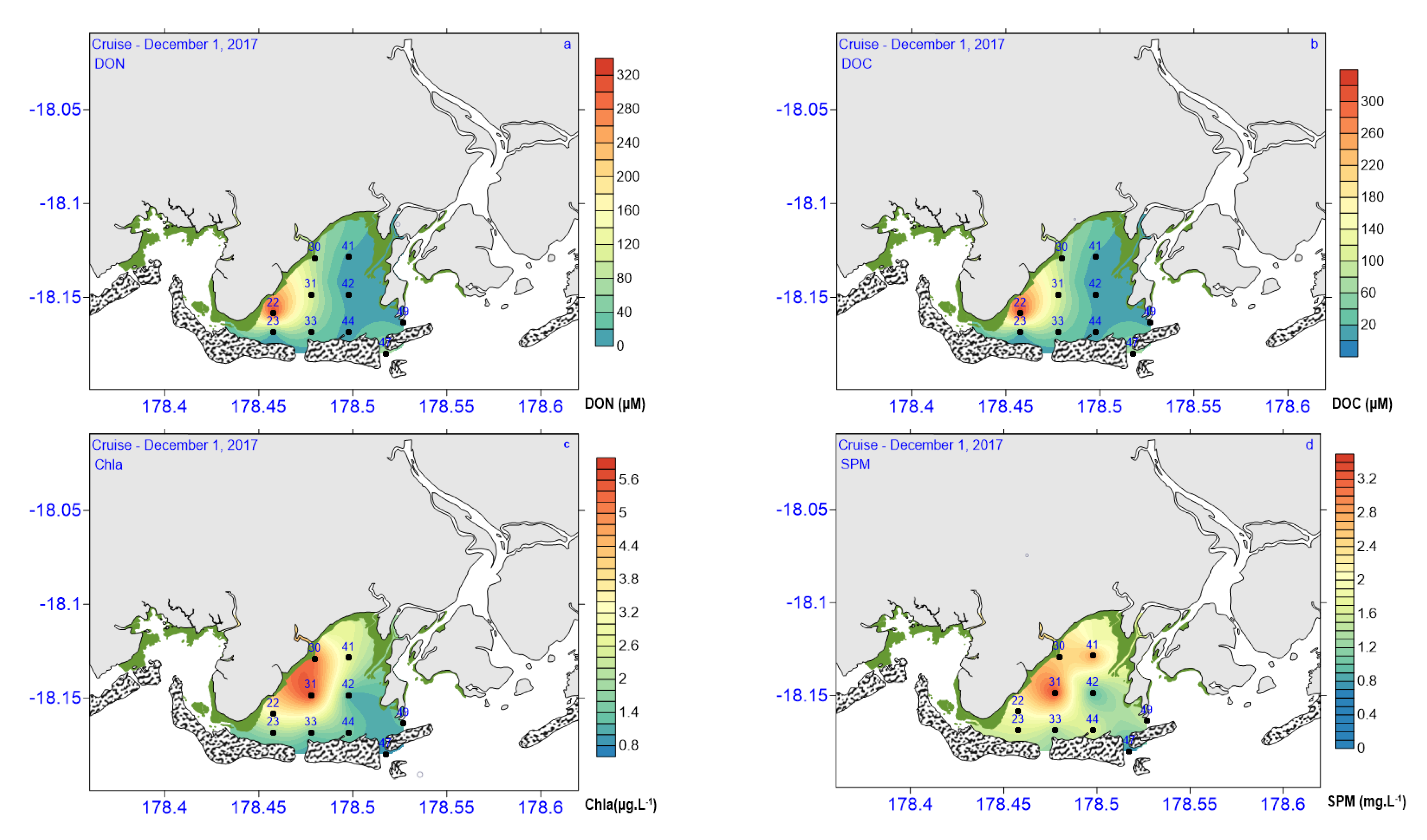
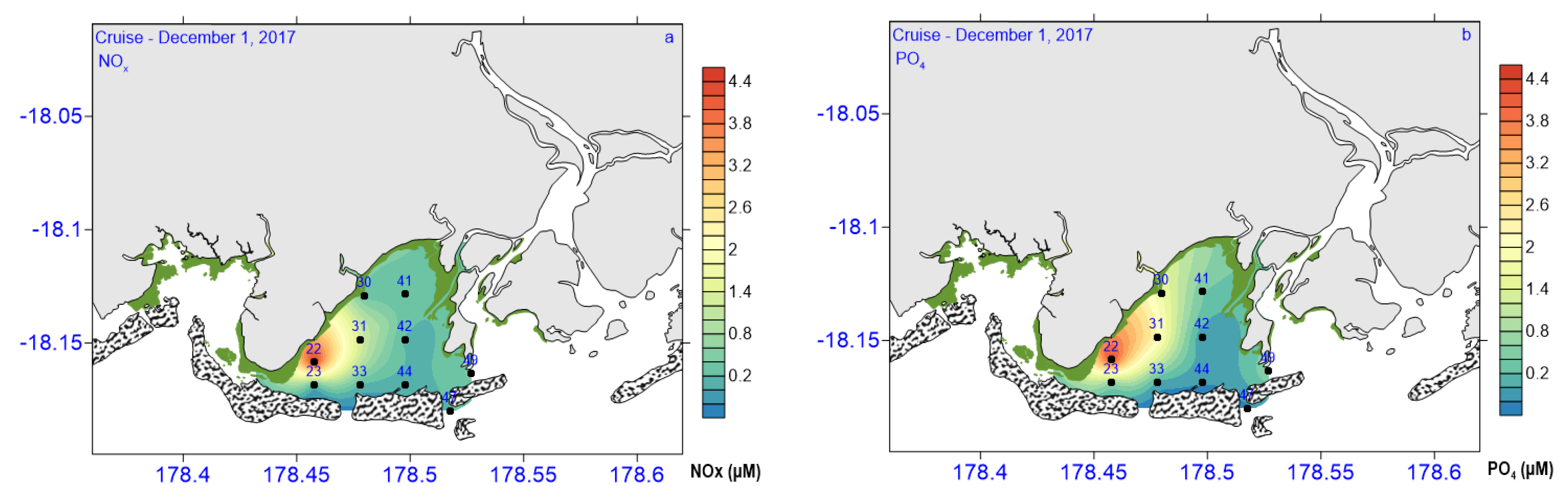
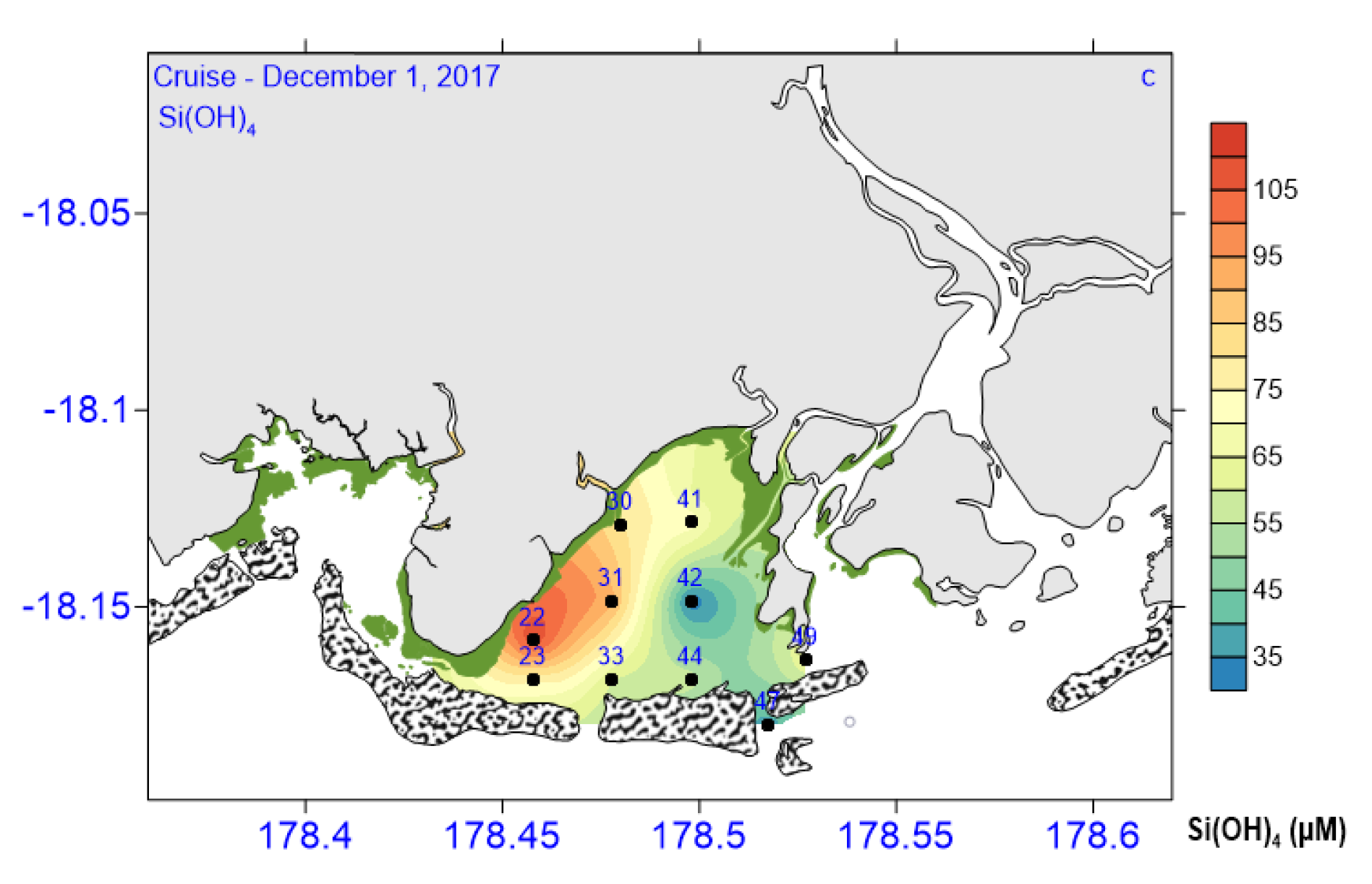
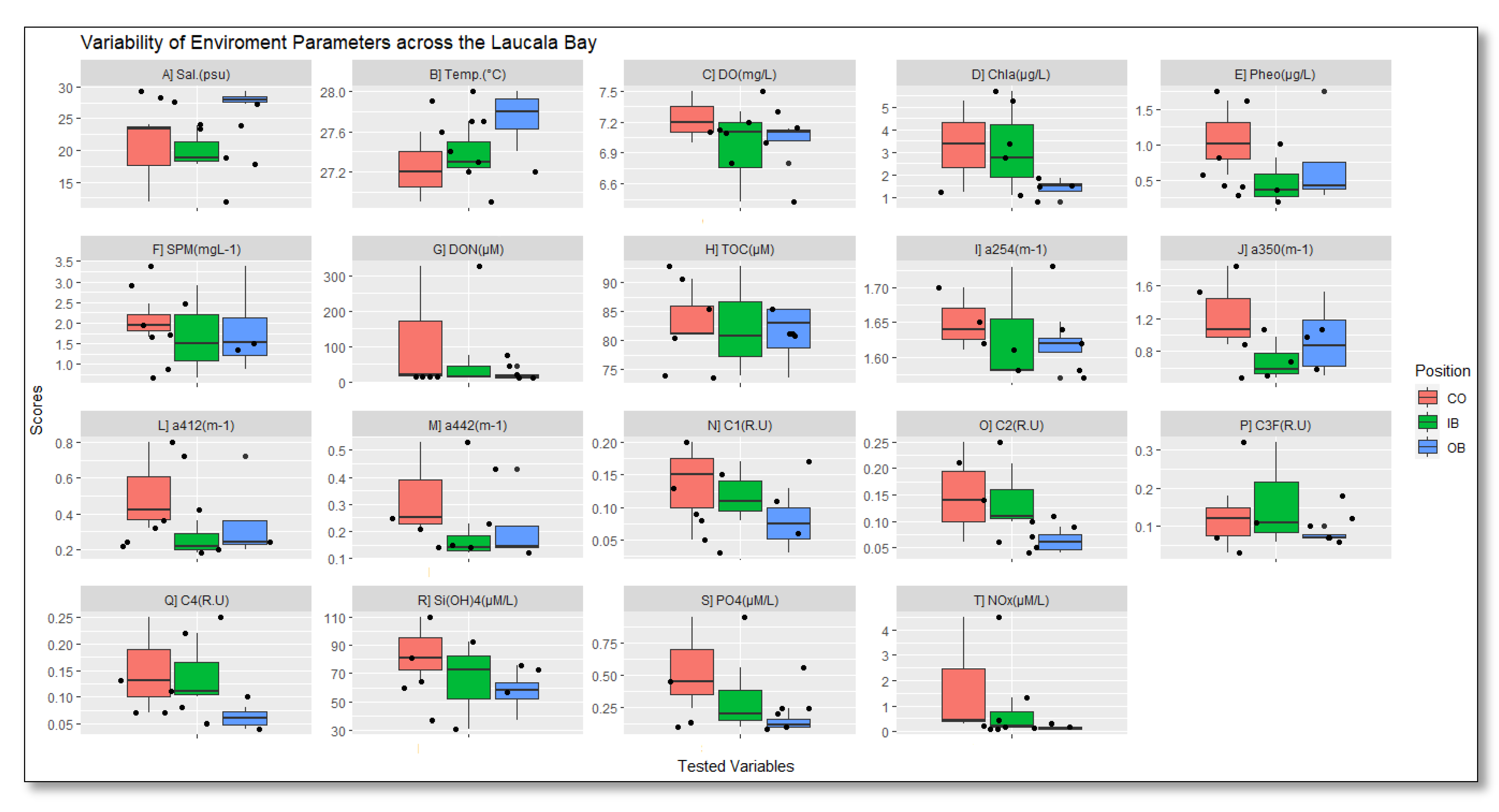
3.2. Biogeochemical and Nutrient Variables
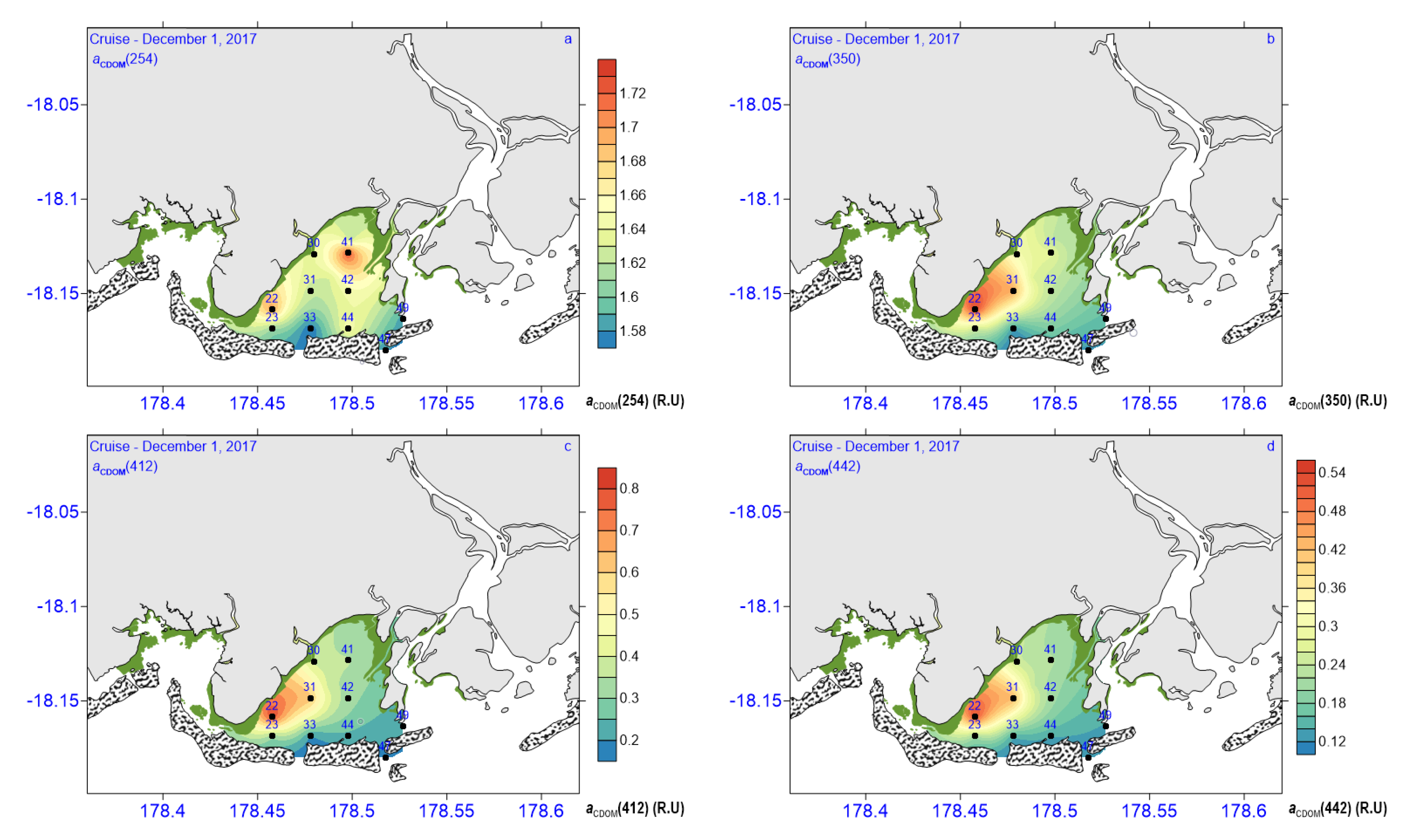
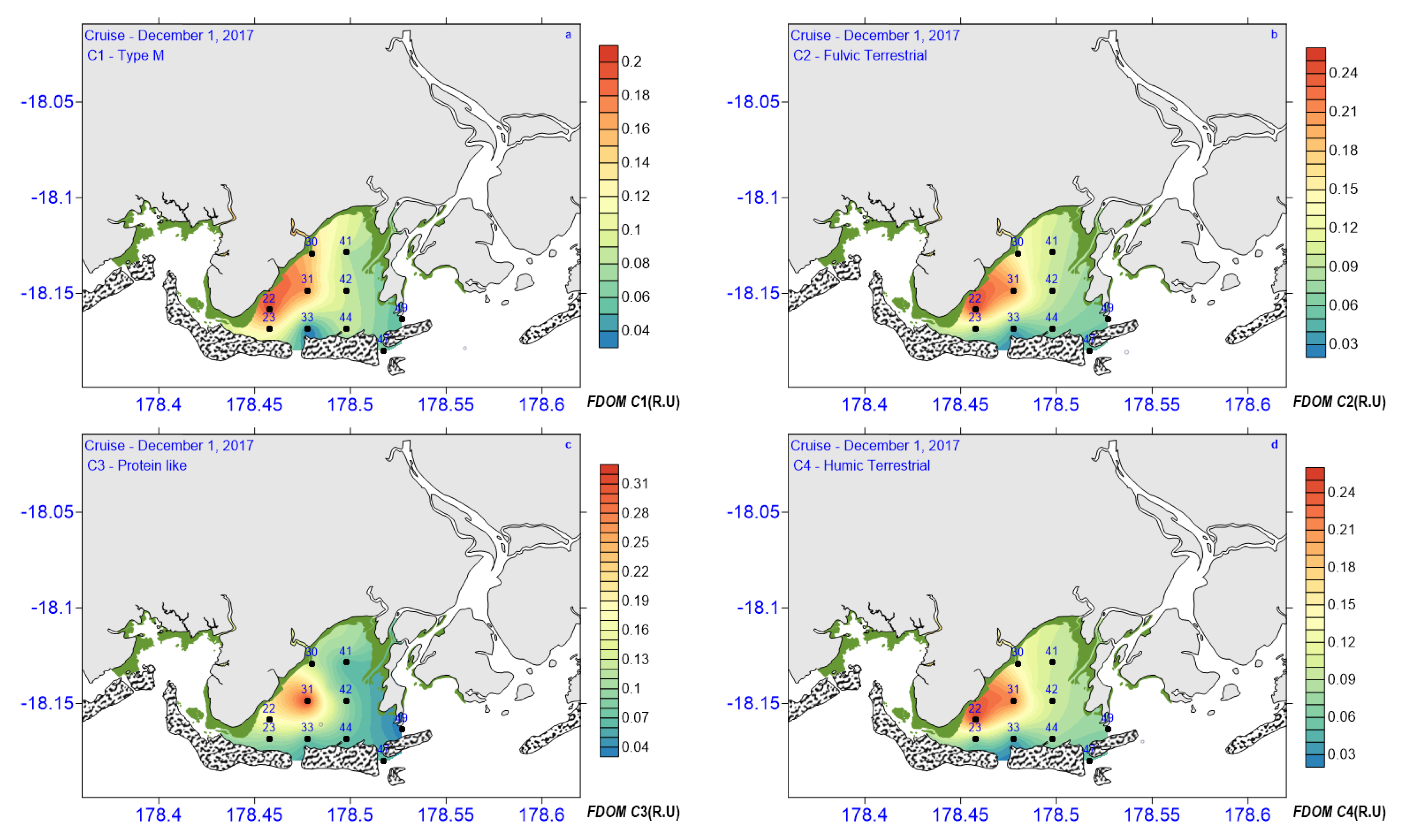
3.3. CDOM Absorption and Fluorescence
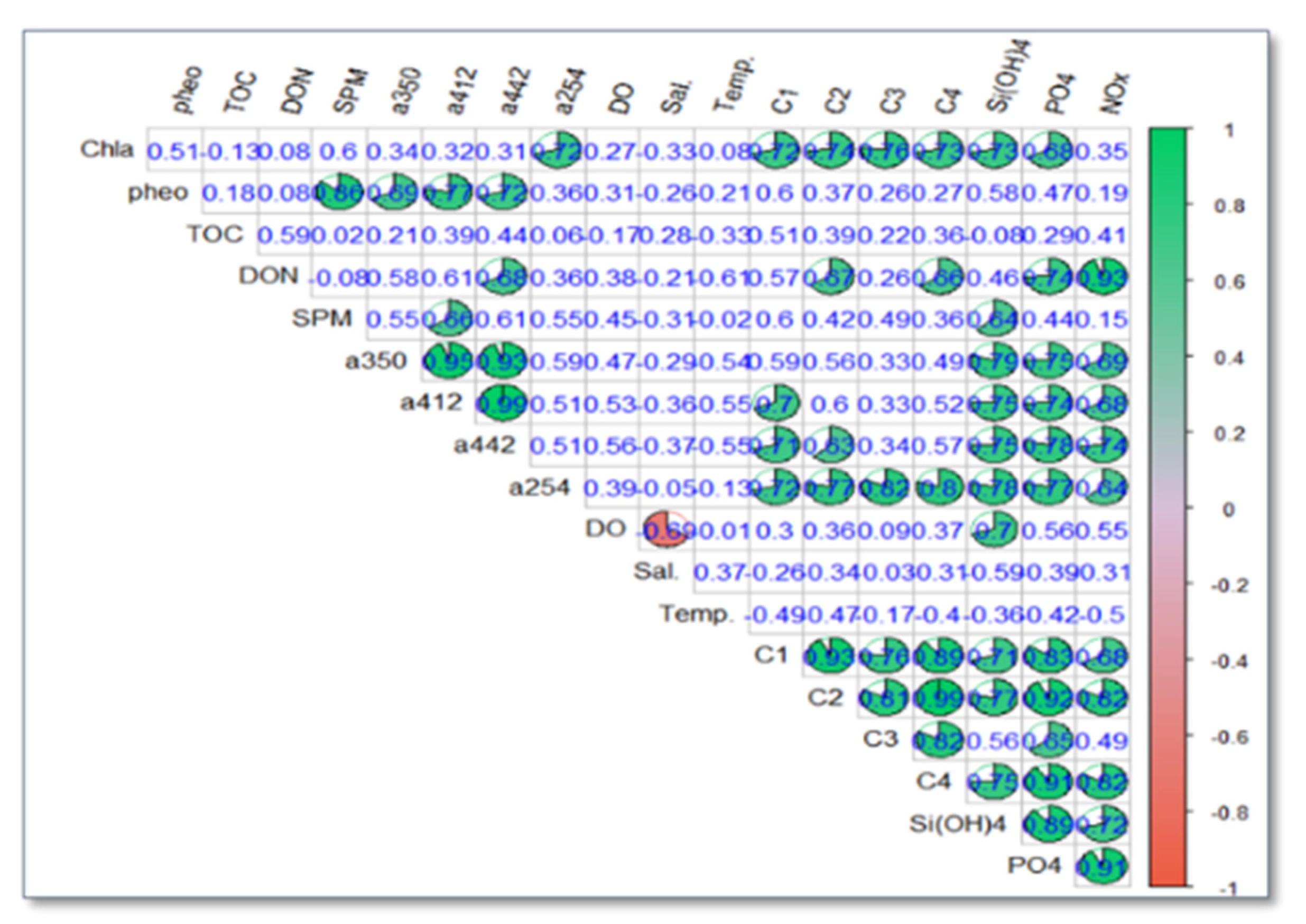
3.3.1. Association and Dependence of Environment Variables
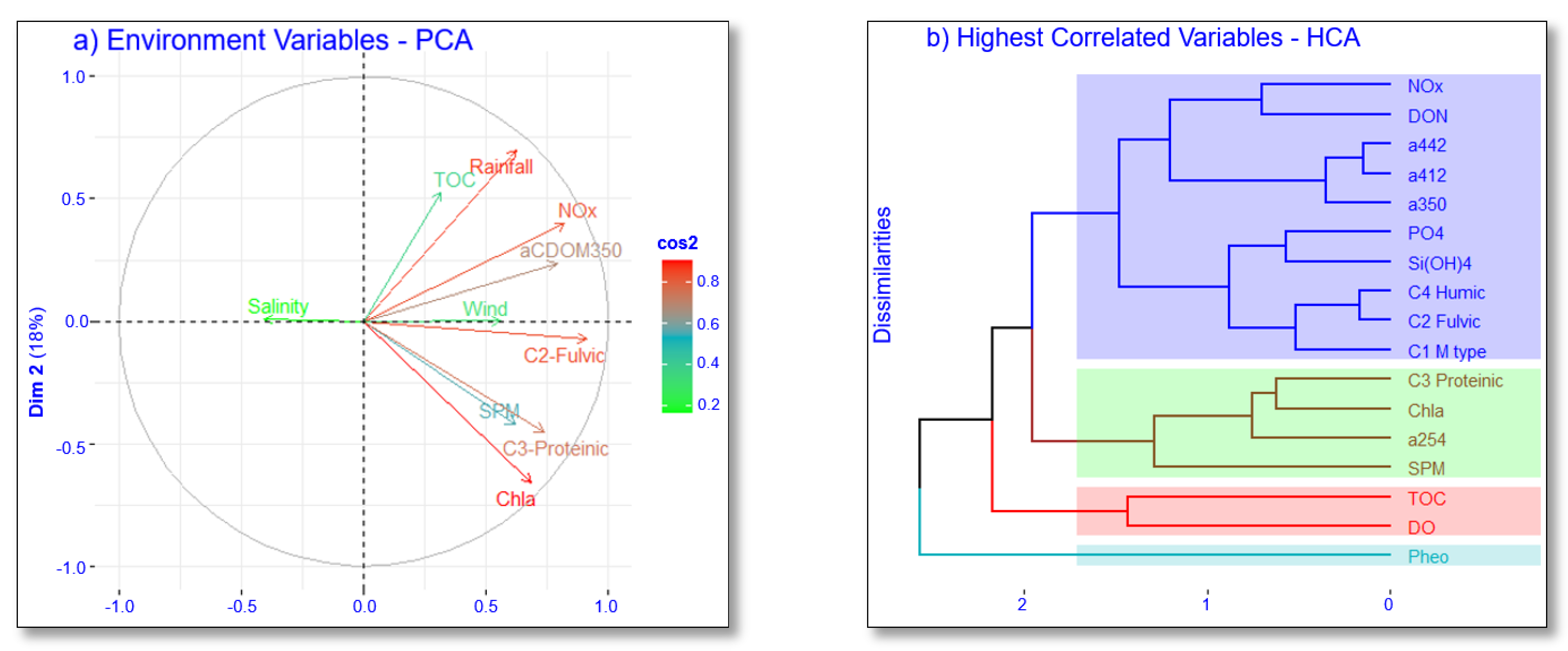
3.3.2. Association with the Climate Variables—PCA Results
3.3.3. Association with DOM Sources—HCA Results
3.4. CDOM Inputs Enhancement and Conservative Dilution by Heavy Rain
3.5. In-situ CDOM from Biogenic Sources
3.6. South-West Plume Formation during Heavy Rain via the LBW
3.7. Association of Terrigenous and Oceanic Inputs
3.8. DOM Responses as Energy and Nutrient Sources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koliyavu, T.; Martias, C.; Singh, A.; Dupouy, C. Characterisation of chlorophyll plumes in the Fiji waters using ocean color remote sensing. In Proceedings of the Pacific GIS & RS User Conference, Suva, Fiji, 27–30 November 2017. [Google Scholar]
- Jenkins, A.P. Freshwater’s fish survey methodology from streams & rivers in tropical oceanic islands: A Brief Guide Based on the survey work conducted as part of the Fiji Ecosystem-Based Management Project. Available online: https://pacific-data.sprep.org/dataset/freshwater-fish-survey-methodology-streams-rivers-tropical-oceanic-islands-brief-guide-based (accessed on 31 December 2020).
- Singh, A.; Aung, T. Salinity, Temperature and Turbidity Structure in the Suva Lagoon, Fiji. Am. J. Environ. Sci. 2008, 4, 266–275. [Google Scholar] [CrossRef]
- Singh, S.; Aalbersberg, W.G.L.; Morrison, R.J. Nutrient Pollution in Laucala Bay, Fiji Islands. Water Air Soil Pollut. 2009, 363–372. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Logan, M.; Weeks, S.J.; Brodie, J. The effects of river run-off on water clarity across the central Great Barrier Reef. Mar. Pollut. Bull. 2014, 84, 191–200. [Google Scholar] [CrossRef]
- Wymore, A.S.; Rodríguez-Cardona, B.; McDowell, W.H. Understanding dissolved organic matter biogeochemistry through In-Situ nutrients manipulations in stream ecosystems. J. Vis. Exp. 2016, 116, 54704. [Google Scholar] [CrossRef] [PubMed]
- Angly, F.E.; Candice, H.; Thomas, C.M.; Hemerson, T.; Virginia, R.; Britta, S.; Bourne, D.; Tyson, G.W. Marine microbial communities of the Great Barrier Reef lagoon are influenced by riverine floodwaters and seasonal weather events. PeerJ 2016, 4, 1511. [Google Scholar] [CrossRef]
- Shilla, D.J.; Makoto, T.; Daniel, A. Terrigenous nutrient and organic matter in a subtropical river estuary Okinawa Japan: Origin, distribution and pattern across the estuarine salinity gradient. Chem. Ecol. 2011, 27, 532–542. [Google Scholar] [CrossRef]
- Kowalczuk, P.; Zabłocka, M.; Sagan, S.; Kuliński, K. Fluorescence measured in situ as a proxy of CDOM absorption and DOC concentration in the Baltic Sea. Oceanologia 2010, 52, 431–471. [Google Scholar] [CrossRef]
- Dupouy, C.; Frouin, R.; Tedetti, M.; Maillard, M.; Rodier, M.; Fabien, L.; Guidi, L.; Picheral, M.; Duhamel, S.; Charriere, B.; et al. Diazotrophic Trichodesmium influence on ocean color and pigment composition in the South West tropical Pacific. Biogeosci. Discuss. 2018, 1–43. [Google Scholar] [CrossRef]
- Kim, S.; Kaplan, L.A.; Hatche, P.G. Biodegradable dissolved organic matter in a temperate and tropical stream determined from ultra-high resolution mass spectroscopy. Limnol. Oceanogr. 2006, 51, 1054–1063. [Google Scholar]
- Mostovaya, A.; Hawkes, J.A.; Dittmar, T.; Tranvik, J.L. Molecular Determinants of Dissolved Organic Matter Reactivity in Lake Water. Front. Earth Sci. 2017, 106. [Google Scholar] [CrossRef]
- Downing, B.D.; Pellerin, B.A.; Bergamaschi, B.A.; Sarceno, J.F.; Koans, T.S. The effects of particles dissolved and temperature on in situ measurement of DOM fluorescence in rivers and streams. Limnol. Oceanogr. Methods 2012, 10, 767–785. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Liu, C.-Q.; Yoshioka, T.; Vione, D.; Zhang, Y.; Sakugawa, H. Fluorescent dissolved organic matter in natural waters. In Photobiogeochemistry of Organic Matter; Mostofa, K.M.G., Yoshioka, T., Mottaleb, A., Vione, D., Eds.; Springer: Berlin, Germany, 2013; pp. 429–559. [Google Scholar]
- Dupouy, C.; Röttgers, R.; Tedetti, M.; Frouin, R.; Lantoine, F.; Rodier, M.; Martias, C.; Goutx, M. Impact of Contrasted Weather Conditions on CDOM Absorption/Fluorescence and Biogeochemistry in the Eastern Lagoon of New Caledonia. Front. Earth Sci. 2020, 8, 54. [Google Scholar] [CrossRef]
- Martias, C.; Tedetti, M.; Lantoine, F.; Jamet, L.; Dupouy, C. Characterization and sources of colored dissolved organic matter in a coral reef ecosystem subject to ultramafic erosion pressure (New Caledonia, Southwest Pacific). Sci. Total Environ. 2018, 616, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Guillemette, F.; McCallister, S.L.; del Giorgio, P.A. Differentiating the degradation dynamics of algal and terrestrial carbon within complex natural dissolved organic carbon in temperate lakes. J. Geophys. Res. Biogeosci. 2013, 118, 963–973. [Google Scholar] [CrossRef]
- Thurman, E.M. Classification of dissolved organic carbon. In Organic Geochemistry of Natural Waters; Springer: Dordrecht, The Netherlands; Amsterdam, The Netherlands, 1985; pp. 103–110. [Google Scholar]
- McKone, H.T. An Introduction to Marine Biogeochemistry (Libes, Susan M.). J. Chem. Educ. 1992, 69, 250–283. [Google Scholar] [CrossRef]
- Luculano, F.; Álverez-Salgado, X.A.; Otero, J.; Catalá, T.S.; Sobrino, C.; Duarte, C.M.; Agustí, S. Patterns and Drivers of UV Absorbing Chromophoric Dissolved Organic Matter in the Euphotic Layer of the Open Ocean. Front. Mar. Sci. 2019, 6, 320. [Google Scholar] [CrossRef]
- Vecchio, R.D.; Blough, N.V. Photobleaching of chromophoric dissolved organic matter in natural waters: Kinetics and modeling. Mar. Chem. 2002, 78, 231–253. [Google Scholar] [CrossRef]
- Follett, C.L.; Repeta, J.D.; Rothman, H.D.; Santineli, C. Hidden cycle of dissolved organic carbon in the deep ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 16706–16711. [Google Scholar] [CrossRef] [PubMed]
- Urban-Rich, J.; McCarty, J.T.; Fernandez, D.; Acun, J.L. Larvaceans and copepods excrete fluorescent dissolved organic matter (FDOM). J. Exp. Mar. Biol. Ecol. 2006, 332, 96–105. [Google Scholar] [CrossRef]
- Urban-Rich, J. Grazing impact of Chromophoric dissolved organic matter (CDOM) by the larvacean Oikopleura dioica. Mar. Ecol. 2006, 317, 101–110. [Google Scholar]
- Dupouy, C.; Neveux, J.; Ouillon, S.; Frouin, R.; Murakami, H.; Hochard, S.; Dirberg, G. Inherent optical properties and satellite retrieval of chlorophyll concentration in the lagoon and open ocean waters of New Caledonia. Mar. Pollut. Bull. 2010, 61, 503–518. [Google Scholar] [CrossRef]
- Dupouy, C.; Wattelez, G.; Fuchs, R.; Lefèvre, J.; Mangeas, M.; Murakami, H.; Frouin, R. The color of the Coral Sea. In Proceedings of the 12th International Coral Reef Symposium, 18E—The Future of the Coral Sea reefs and seamounts, Cairns, Australia, 9–13 July 2012; pp. 9–13. [Google Scholar]
- Dupouy, C.; Röttgers, R.; Tedetti, M.; Martias, C.; Murakami, H.; Doxaran, D.; Lantoine, F.; Rodier, M.; Favareto, L.; Kampel, M.; et al. Influence of CDOM and Particle Composition on Ocean Color of the Eastern New Caledonia Lagoon during the CALIOPE Cruises. Proc. SPIE 2014, 9261, 932–1102. [Google Scholar]
- Lee, E.-J.; Yoo, G.-Y.; Jeong, Y.; Kim, K.-U.; Park, J.-H.; Oh, N.-H. Comparison of UV–VIS and FDOM sensors for in situ monitoring of stream DOC concentrations. Biogeosciences 2015, 12, 3109–3118. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E. Riverine export of aged terrestrial organic matter to the North Atlantic Ocean. Nat. Cell Biol. 2001, 409, 497–500. [Google Scholar] [CrossRef]
- Di Toro, D.M.; Allen, H.E.; Bergman, H.L.; Meyer, J.S.; Paquin, P.R.; Santore, R.C. Biotic ligand model of the acute toxicity of metals, 1. Technical basis. Environ. Toxicol. Chem. 2001, 20, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Mounier, S.; Zhao, H.; Garnier, C.; Redon, R. Copper complexing properties of dissolved organic matter: PARAFAC treatment of fluorescence quenching. Biogeochemistry 2010, 106, 107–116. [Google Scholar] [CrossRef]
- Berggren, M.; Lapierre, J.F.; del Giorgio, P. Magnitude and regulation of bacterioplankton respiratory quotient across freshwater environmental gradients. ISME J. 2011, 6, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Coble, P.G. Marine optical biogeochemistry—The chemistry of ocean color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef]
- Campbell, J.W. The lognormal distribution as a model for bio-optical variability in the sea. J. Geophys. Res. Space Phys. 1995, 100, 13237–13254. [Google Scholar] [CrossRef]
- Carstea, M.E.; Bridgeman, J.; Baker, A.; Reynolds, M.D. Fluorescence spectroscopy for wastewater monitoring: Review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef]
- Tedetti, M.; Cuet, P.; Guigue, C.; Goutx, M. Characterization of dissolved organic matter in a coral reef ecosystem subjected to anthropogenic pressures (La Réunion Island, Indian Ocean) using multi-dimensional fluorescence spectroscopy. Sci. Total. Environ. 2011, 409, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Tedetti, M.; Longhitano, R.; Garcia, N.; Guigue, C.; Ferretto, N.; Goutx, M. Fluorescence properties of dissolved organic matter in coastal Mediterranean waters influenced by municipal sewage effluent (Bay of Marseilles, France). Environ. Chem. 2012, 9, 438–449. [Google Scholar] [CrossRef]
- Tedetti, M.; Bigot, L.; Turquet, J.; Guigue, C.; Ferretto, N.; Goutx, M.; Cuet, P. Influence of Freshwater discharge on biogeochemistry and benthic communities of a Coral Reef Ecosystem (La Reunion Island, Indian Ocean. Front. Mar. Sci. 2020, 1–18. [Google Scholar] [CrossRef]
- Newall, E.R.; Nguyen, T.M.H.; Le, T.P.Q.; Sengtaheuanghoung, O.; Ribolzi., O. A short review of fecal indicator bacteria in tropical aquatic ecosystems: Knowledge gaps and future directions. Front. Microbiol. 2015, 6, 308. [Google Scholar] [CrossRef]
- Naidu, S.D.; Morison, R.J. Contamination of Suva Habour, Fiji. Mar. Pollut. Bull. 1994, 29, 126–130. [Google Scholar] [CrossRef]
- Pratap, A.; Francis, S.M.; Prasad, S. Heavy metals contamination and risk assessment in sediments of Laucala Bay, Suva, Fiji. Mar. Pollut. Bull. 2020, 56, 1–10. [Google Scholar] [CrossRef]
- Morrison, R.J.; Narayan, S.P.; Gangaiya, P. Trace Element Studies in Laucala Bay, Suva, Fiji. Mar. Pollut. Bull. 2001, 42, 397–404. [Google Scholar] [CrossRef]
- Fichez, R.D.P.; Chevillon, C.; Torreton, J.P.; Aung, T.H.; Chifflet, S.; Fernandez, J.M.; Gangaiya, P.; Garimella, S.; Gerard, P.; Ouillon, S.; et al. The Suva Lagoon Environment: An overview of a joint IRD Camelia Research Unit and USP Study. In At the Cross Roads: Science and Management of the Suva Lagoon; Morrison, R.J., Aalbersberg, W., Eds.; Institute of the Applied Sciences Press: Suva, Fiji, 2006; Volume 1, pp. 93–105. [Google Scholar]
- Torréton, J.P.; Pringault, O.; Jacquet, S.; Chifflet, S.; Moreton, B.; Panché, J.-Y.; Rodier, M.; Gérard, P.; Blanchot, J. Rapport des missions BULA 3 (mars 2002) el BULA 4 (août 2003) dans le lagon de Suva (Fidji). 2004. Available online: https://agris.fao.org/agris-search/search.do?recordID=AV20120163637 (accessed on 31 December 2020).
- Fichez, R.; Chifflet, S.; Douillet, P.; Gérard, P.; Gutierrez, F.; Jouon, A.; Ouillon, S.; Grenz, C. Biogeochemical typology and temporal variability of lagoon waters in a coral reef ecosystem subject to terrigenous and anthropogenic inputs (New Caledonia). Mar. Poll. Bull. 2010, 61, 309–322. [Google Scholar] [CrossRef]
- Organelli, E.; Claustre, E. Small Phytoplankton Shapes Colored Dissolved Organic Matter Dynamics in the North Atlantic Subtropical Gyre. Geophys. Res. Lett. 2019, 46, 12183–12191. [Google Scholar] [CrossRef] [PubMed]
- Cartisano, C.M.; Del Vecchio, R.; Bianca, M.R.; Blough, N.V. Investigating the sources and structure of chromophoric dissolved organic matter (CDOM) in the North Pacific Ocean (NPO) utilizing optical spectroscopy combined with solid-phase extraction and borohydride reduction. Mar. Chem. 2018, 204, 20–35. [Google Scholar] [CrossRef]
- Raimbault, P.; Gentilhomme, V.; Slawyk, G. Short-term responses of 24-hour N-starved cultures of Phaeodactylum tricornutum to pulsed additions of nitrate at nanomolar levels. Mar. Ecol. Prog. Ser. 1990, 63, 47–52. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Fanning, K.A.; Pilson, M.E.Q. The diffusion of dissolved silica out of deep-sea sediments. J. Geophys. Res. Space Phys. 1974, 79, 1293–1297. [Google Scholar] [CrossRef]
- Robards, K.; McKelvie, D.I.; Benson, L.R.; Bundle, N.J.W.; Casey, H. Determination of carbon, phosphorus, nitrogen and silicon species in waters. Anal. Chim. Acta Elsevier Sci. 1994, 287, 147–190. [Google Scholar] [CrossRef]
- Dommenget, D.; Latif, M. A cautionary note on the interpretation of EOFs. J. Clim. 2002, 15, 216–225. [Google Scholar] [CrossRef]
- Andrew, A.A.; Del Vecchio, R.; Subramaniam, A.; Blough, N.V. Chromophoric dissolved organic matter (CDOM) in the equatorial Atlantic ocean: Optical properties and their relation to CDOM structure and source. Mar. Chem. 2013, 148, 33–43. [Google Scholar] [CrossRef]
- Brando, V.; Dekker, A.; Marks, A.; Qin, Y.; Oubelkheir, K. Chlorophyll and suspended sediment assessment in a macrotidal tropical estuary adjacent to the Great Barrier Reef: Spatial and temporal assessment using remote sensing. Cooperative Research Centre for Coastal Zone. Estuary Waterw. Manag. Technical Rep. 2006, 74, 70–92. [Google Scholar]

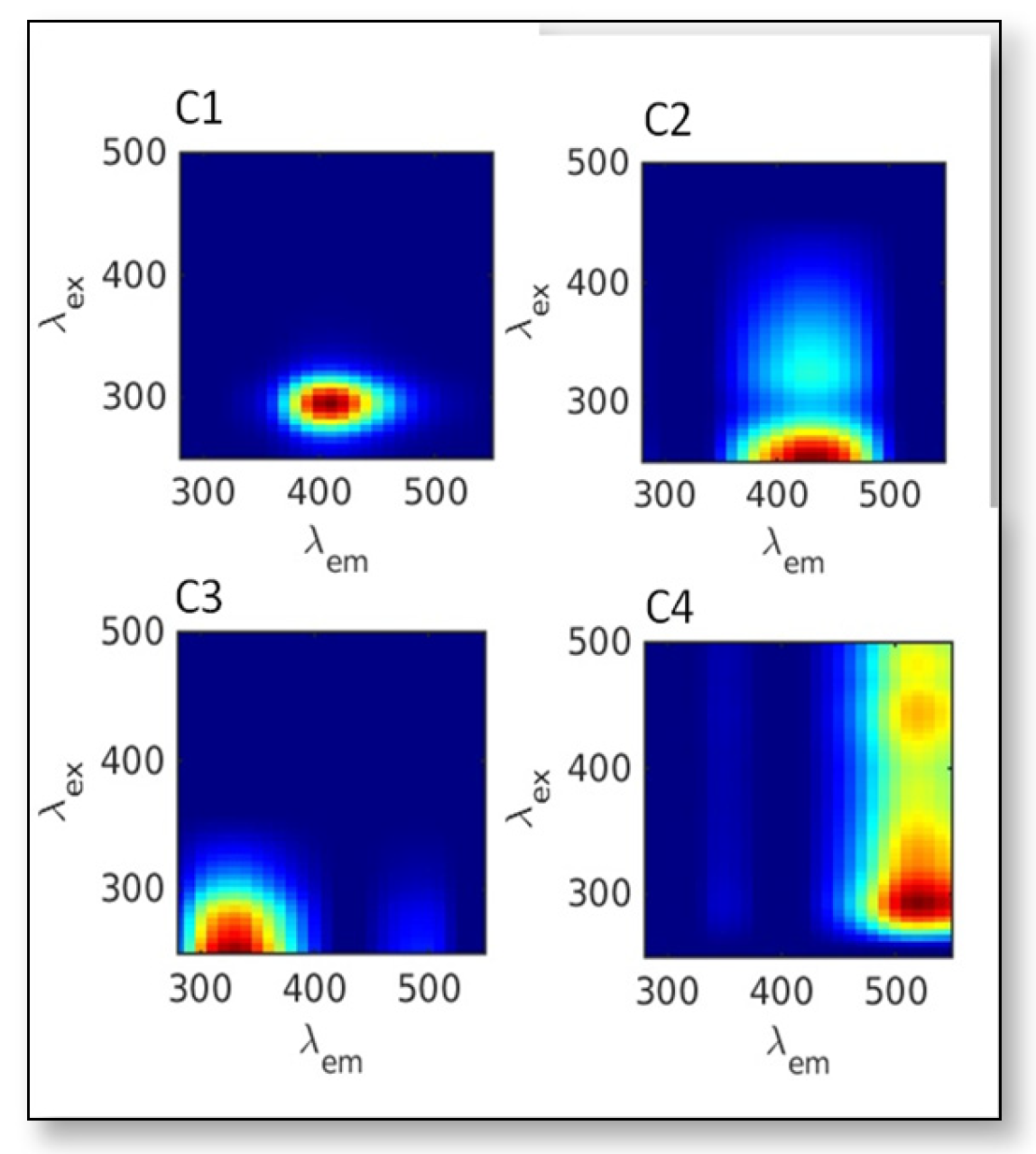
| Station | Longitude | Latitude | Transect | Zones | Depth(m) | Tide |
|---|---|---|---|---|---|---|
| 22 | 178.4578 | 18.1585 | T1 | CO | 5.93 | Ebb |
| 23 | 178.458 | 18.165 | T1 | OB | 20.35 | Decreasing |
| 30 | 178.4800 | 18.1291 | T2 | CO | 6.79 | Decreasing |
| 31 | 178.4778 | 18.1485 | T2 | IB | 5.87 | Decreasing |
| 33 | 178.4778 | 18.1685 | T2 | CO | 30.53 | Decreasing |
| 41 | 178.4978 | 18.1285 | T3 | IB | 15.78 | Low |
| 42 | 178.4978 | 18.1485 | T3 | IB | 15.53 | Flood |
| 44 | 178.4978 | 18.1685 | T3 | OB | 15.21 | Increasing |
| 47 | 178.5175 | 18.1798 | T4 | OB | 22.79 | Increasing |
| 49 | 178.5269 | 18.1635 | T4 | OB | 12.54 | Increasing |
| Physical Variables | CO (N = 3) | IB (N = 3) | OB (N = 4) |
|---|---|---|---|
| YSI DO (mg L−1) | 7.23 ± 0.25 | 6.94 ± 0.96 | 7.04 ± 0.18 |
| CTD Sal. | 17.96 ± 5.79 | 24.17 ± 3.0 | 26.8 ± 2.19 |
| YSI Temp. (°C) | 27.26 ± 0.35 | 27.43 ± 0.25 | 27.40 ± 0.36 |
| YSI TDS (mg L−1) | 18,778 ± 5726 | 24,429 ± 2308 | 27,146 ± 2462 |
| Variables | All Stations | Mean Values and STD | Mean All Stations | ||
|---|---|---|---|---|---|
| [Range] | CO (N = 3) | IB (N = 3) | OB (N = 4) | N = 10 | |
| Chl (µg L−1) | 0.79–5.60 | 3.29 ± 1.99 | 3.17 ± 2.33 | 1.42 ± 0.41 | 2.62 ± 1.05 |
| Pheo (µg L−1) | 0.46–1.5 | 1.06 ± 0.51 | 0.46 ± 0.32 | 0.72 ± 0.08 | 0.78 ± 0.33 |
| DOC (µM) | 73.56–92.73 | 84 ± 5.4 | 83 ± 9.5 | 81 ± 6 | 81.45 ± 3.95 |
| DON (µM) | 10–326 | 119 ± 7.0 | 33 ± 36 | 20 ± 19 | 72.8 ± 84.6 |
| SPM (mg L−1) | 0.67–3.37 | 2.03 ± 0.41 | 1.70 ± 1.13 | 1.83 ± 0.42 | 1.90 ± 0.52 |
| aCDOM(254) (m−1) | 1.57–1.73 | 1.65 ± 0.05 | 1.63 ± 0.09 | 1.62 ± 0.04 | 1.63 ± 0.036 |
| aCDOM(350) (m−1) | 0.48–1.83 | 1.26 ± 0.50 | 0.68 ± 0.26 | 0.94 ± 0.29 | 1.01 ± 0.33 |
| aCDOM(412) (m−1) | 0.18–0.8 | 0.51 ± 0.25 | 0.25 ± 0.09 | 0.35 ± 0.02 | 0.398 ± 0.14 |
| aCDOM(442) (m−1) | 0.12–0.53 | 0.33 ± 1.74 | 0.16 ± 0.05 | 0.22 ± 0.01 | 0.26 ± 0.09 |
| C1 (R.U) | 0.3–0.20 | 0.13 ± 0.08 | 0.12 ± 0.05 | 0.08 ± 0.03 | 0.11 ± 0.03 |
| C2 (R.U) | 0.04–0.25 | 0.15 ± 0.1 | 0.14 ± 0.06 | 0.06 ± 0.01 | 12.25 ± 0.05 |
| C3 (R.U) | 0.03–0.32 | 0.11 ± 0.01 | 0.16 ± 0.14 | 0.07 ± 0.01 | 0.123 ± 0.05 |
| C4 (R.U) | 0.04–0.25 | 0.15 ± 0.09 | 0.14 ± 0.01 | 0.06 ± 0.02 | 0.123 ± 0.05 |
| Si(OH)4 (µM L−1) | 31.07–109.4 | 84.83 ± 22.75 | 65.3 ± 31.16 | 57.36 ± 12.33 | 69.17 ± 14.14 |
| PO4 (µM L−1) | 0.08–0.94 | 0.55 ± 0.36 | 0.29 ± 0.24 | 0.14 ± 0.02 | 0.325 ± 0.24 |
| NOx (µM L−1) | 0.11–4.49 | 1.75 ± 2.38 | 0.56 ± 0.65 | 0.8 ± 0.40 | 0.813 ± 0.834 |
| Wind (ms−1) | 0.3–1.8 | ||||
| Rainfall (mm) | 0–98.10 | ||||
| SL(m) | 0.581–4.45 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koliyavu, T.; Martias, C.; Singh, A.; Mounier, S.; Gérard, P.; Dupouy, C. In-Situ Variability of DOM in Relation with Biogeochemical and Physical Parameters in December 2017 in Laucala Bay (Fiji Islands) after a Strong Rain Event. J. Mar. Sci. Eng. 2021, 9, 241. https://doi.org/10.3390/jmse9030241
Koliyavu T, Martias C, Singh A, Mounier S, Gérard P, Dupouy C. In-Situ Variability of DOM in Relation with Biogeochemical and Physical Parameters in December 2017 in Laucala Bay (Fiji Islands) after a Strong Rain Event. Journal of Marine Science and Engineering. 2021; 9(3):241. https://doi.org/10.3390/jmse9030241
Chicago/Turabian StyleKoliyavu, Timoci, Chloe Martias, Awnesh Singh, Stéphane Mounier, Philippe Gérard, and Cecile Dupouy. 2021. "In-Situ Variability of DOM in Relation with Biogeochemical and Physical Parameters in December 2017 in Laucala Bay (Fiji Islands) after a Strong Rain Event" Journal of Marine Science and Engineering 9, no. 3: 241. https://doi.org/10.3390/jmse9030241
APA StyleKoliyavu, T., Martias, C., Singh, A., Mounier, S., Gérard, P., & Dupouy, C. (2021). In-Situ Variability of DOM in Relation with Biogeochemical and Physical Parameters in December 2017 in Laucala Bay (Fiji Islands) after a Strong Rain Event. Journal of Marine Science and Engineering, 9(3), 241. https://doi.org/10.3390/jmse9030241








