Knowledge on the Biological and Fisheries Aspects of the Japanese Sardine, Sardinops melanostictus (Schlegel, 1846)
Abstract
1. Introduction
2. Distribution, Migration, and Recruitment
2.1. Distribution and Migration
Factors Affecting the Distribution and Migration of Japanese Sardine
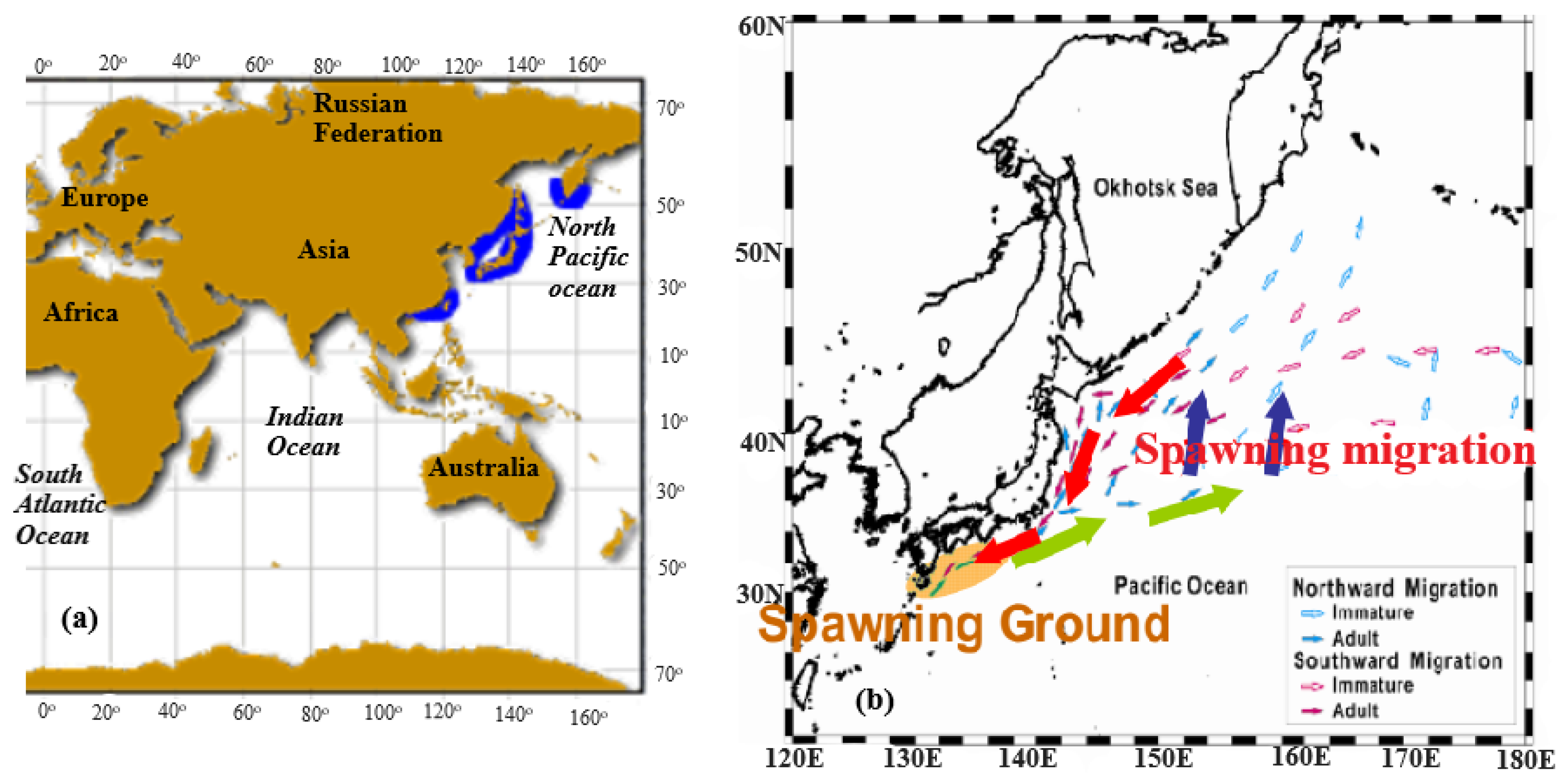
2.2. Mechanisms of Recruitment Variability
| Factors Affecting Their Distribution and Migration | Areas | Range Degree Celsius (°C) | References |
|---|---|---|---|
| Spawning grounds temperature | northern Pacific coast of Japan. | 14–17 °C | [14] |
| southern Pacific coast of Japan. | 17–19 °C | ||
| GSI maturity | Shikanoshima Island, | 11 °C | [38] |
| Spawning grounds temperature | Pacific coast of Japan. Kyushu and Shikoku in Japan, Sanriku coast | 11–22 °C | [17,19,20,39] |
| SST andGSI maturity | |||
| Spawning and feeding grounds temperature | |||
| Temperature | all reas | [41,42] | |
| Temperature/depth | northern parts of the Pacific | 8–20 °C/15–30 m | [43] |
| Spawning grounds, temperature, salinity, and depth | - | - | [44] |
| Optimal temperatures for growth | northwest Pacific | 16.2 °C | [45] |
| Spawning grounds temperature and salinity | offshore waters | 10–25 °C | [45] |
| spawning temperature index | western north Pacific | 16–17 °C | [46] |
| Temperature concentration of chlorophyll-a (Chl-a) | western north pacific. | - | [47] |
| Temperature growth and food density | Kuroshio region to the subarctic Oyashio region | 16–17 °C | [45,46,48,63,64] |
| Habitat temperature | - | 0.5–23.8 °C | [27] |
| GSI maturity | - | 11 °C | [49] |
2.2.1. Growth-Dependent Recruitment
2.2.2. Recruitment of Japanese Sardine and Climate Change
3. Trophic Position, Competition and Predation
3.1. Diet Composition
3.2. Trophic Position (TP)
3.3. Competition and Predation
4. Age, Growth and Ageing Methods
4.1. Ageing Methods
4.2. Age and Growth
5. Reproductive Aspects
5.1. Reproductive Biology
5.2. Maturity
5.3. Sex Ratio
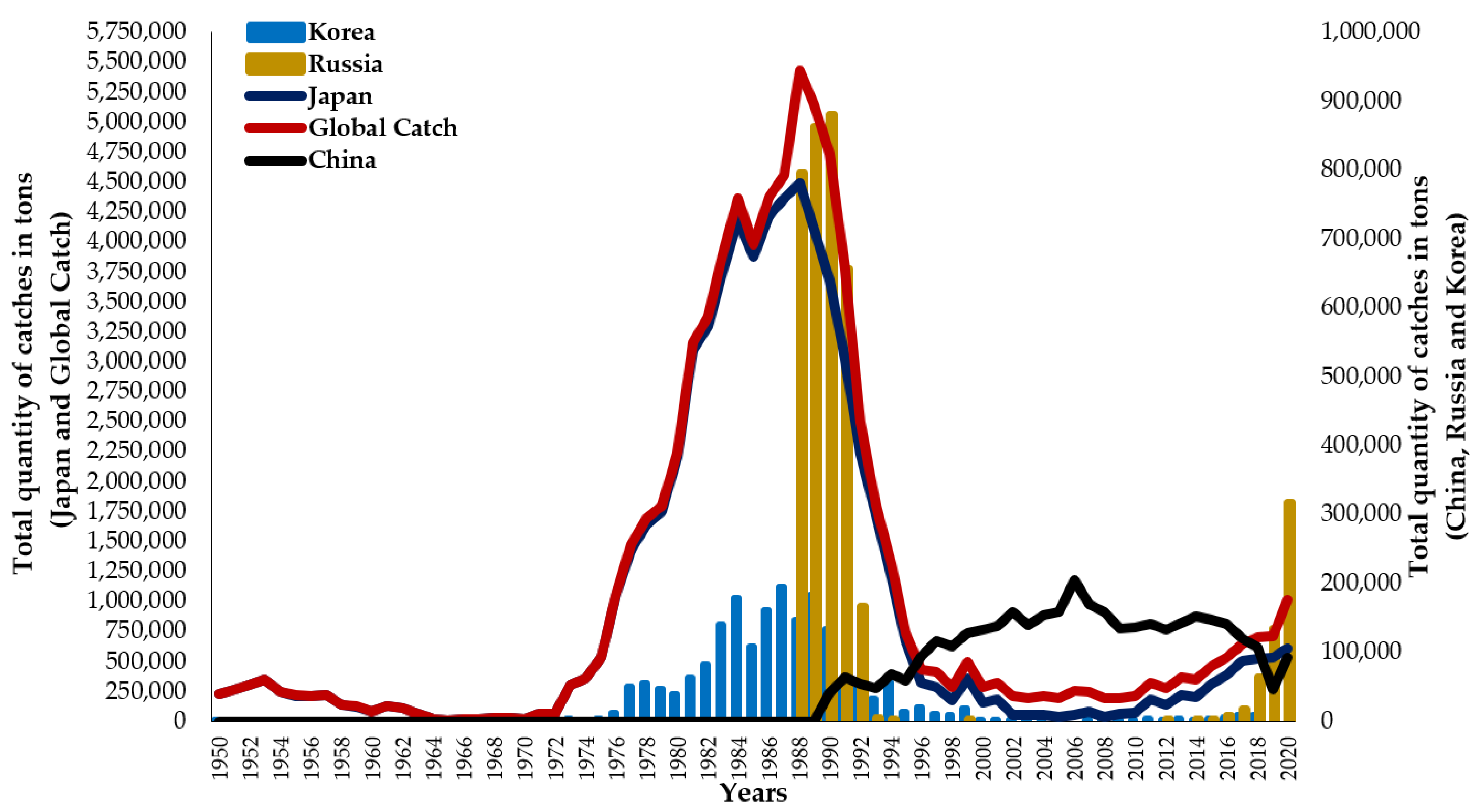
6. Fisheries
7. Stock Fluctuation and Assessment
8. Management Measures
9. Conclusions, Future Research Direction and Recommendations
- Research on the biology, population dynamics and stock assessment in China, Korea, and Russia is significant, necessary and essential for proper management;
- It is essential to carry out a global stock assessment of Japanese sardines using the available data to understand the current state of Japanese sardine and identify effective measures for its sustainable management;
- To reflect further on the natural conditions that hinder the recruitment of the Japanese sardine stocks and to find solutions;
- As a recommendation, the control of the fishing effort and the establishment of TAC in all catch areas is primordial. This measure will help minimize the pressure on the resource;
- It is also imperative to identify and control all areas of reproduction and the periods and put in place strong management measures to protect juveniles, which is the most sensitive period to ensure the recruitment of the stock.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takasuka, A. Biological Mechanisms Underlying Climate Impacts on Population Dynamics of Small Pelagic Fish. In Population Dynamics, Monitoring, and Management: Sustainable Fisheries in the Eternal Ocean; Yamakawa, A.I., Takasuka, A., Eds.; Springer Nature: Tokyo, Japan, 2018; pp. 19–50. [Google Scholar]
- COSEWIC, 2002. Évaluation et Rapport de Situation du COSEPAC sur la Sardine du Pacifique (Sardinops sagax) au Canada—Mise à Jour. Comité sur la Situation des Espèces en Péril au Canada; COSEWIC: Ottawa, ON, Canada, 2002; Volume 7, p. 22. [Google Scholar]
- Bakun, A. Patterns in the ocean: Ocean processes and marine population dynamics. Oceanogr. Lit. Rev. 1997, 44, 530. [Google Scholar]
- Gremillet, D.; Lewis, S.; Drapeau, L.; van Der Lingen, C.D.; Hugget, J.A.; Coetzee, J.C.; Verheye, H.M. Spatial match—Mismatch in the Benguela upwelling zone: Should we expect chlorophyll and sea-surface temperature to predict marine predator distributions? J. Appl. Ecol. 2008, 45, 610–621. [Google Scholar] [CrossRef]
- Nakai, Z. Studies relevant to mechanisms underlying the fluctuation in the catch of the Japanese sardine, Sardinops melanosticta. Jpn. J. Ichthyol. 1962, 9, 1–115. [Google Scholar] [CrossRef]
- Kondo, K.; Hori, Y.; Hiramoto, K.; Kondo, K.; Hori, Y.; Hiramoto, K. (1976): Life Pattern of the Japanese sardine, Sardinops melanosticta (Temminck and Schlegel), and Its Practical Procedure in Marine Resources Researches on the Stock, 2nd ed.; Fish. Res. Ser. No. 30; Jap. Fish. Resour. Conserv. Ass.: Tokyo, Japan, 1976; p. 67. (In Japanese) [Google Scholar]
- Sugimoto, T.; Kuroda, K.; Tsuboi, M.; Kuwae, M. Ocean environments and biological production estimated from old documents and sediment cores. Kaiyo Mon. 2005, 422, 563–567. [Google Scholar]
- FAO. Global Production Statistics 1950–2018. 2018. Available online: http://www.fao.org/figis/servlet/TabSelector (accessed on 17 August 2021).
- NPFC. 2021-AR-Annual Summary Footprint (Catch and Efforts) of Japanese Sardine Fisheries in the Convention Area in 1995–2020. Available online: https://www.npfc.int/summary-footprint-japanese-sardine-fisheries (accessed on 11 November 2021).
- Yatsu, A. Review of population dynamics and management of small pelagic fishes around the Japanese archipelago. Fish. Sci. 2019, 85, 611–639. [Google Scholar] [CrossRef]
- Ward, T.N.; Smart, J.; Ivey, A. Stock Assessment of Australian Sardine (Sardinops sagax) off South Australia 2017; Report Fisheries and Aquaculture, SARDI Publication No. F2007/65-6. SARDI Research Report Series No. 971; South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2017; 107p.
- Hill, K.T.; Crone, P.R.; Zwolinski, J.P. Assessment of the Pacific Sardine Resource in 2019 for US Management in 2019–20; NOAA Technical Memorandum NMFS; NOAA: Silver Spring, MA, USA, 2019.
- Taranetz, A.Y. A Handbook for identification of fishes of Soviet Far East and adjacent waters. Bull. Pacif. Sci. Inst. Fish. Oceanogr. 1937, 11, 1–200. [Google Scholar]
- Ito, S. Fishery biology of the sardine, Sardinops melanosticta (T. & S.), in the waters around Japan. Bull. Japan Sea Reg. Fish. Res. Lab. 1961, 9, 1–227, (In Japanese with English abstract). [Google Scholar]
- Fukushima, S.; Ogawa, Y. Changes in catch composition of coastal pelagic fishes in relation to long-term fluctuations in oceanographic condition of the northwestern Pacific Ocean. Bull. Tohoku Reg. Fish. Res. Lab. 1988, 50, 67–95, (In Japanese with English abstract). [Google Scholar]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Chapman & Hall: London, UK, 1990. [Google Scholar]
- Kuroda, K. Studies on the recruitment process focusing on the early life history of the Japanese sardine (Sardinops melanostictus) (Schlegel). Bull Natl. Res. Inst. Fish. Sci. 1991, 3, 25–278, (In Japanese with English abstract). [Google Scholar]
- Ivanov, A.N. Raspregelenie Iotzenka Chislennoisti Seboletkov Sarkinui v 1989g. Saury, Mackerel, Sardine, Squid and Pollack; Japan Fisheries Agency: Tokyo, Japan, 1992; pp. 399–408, (In Russian with Japanese translation).
- Tameishi, H.; Naramura, Y.; Shinomiya, H. Characteristics of the fishing ground of Japanese sardine off Sanriku during northward migration. Nippon. Suisan Gakkaishi 1994, 60, 39–44. [Google Scholar] [CrossRef]
- Tameishi, H.; Shinomiya, H.; Aoki, I.; Sugimoto, T. Understanding Japanese sardine migrations using acoustic and other aids. ICES J. Mar. Sci. 1996, 53, 167–171. [Google Scholar] [CrossRef]
- Sugisaki, H. Distribution of Larval and Juvenile Japanese sardine (Sardinops melanostictus) in the Western North Pacific and Its Relevance to Predation on These Stages. In Survival Strategies in Early Life Stages of Marine Resources; Watanabe, Y., Yamashita, Y., Oozeki, Y., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1996; pp. 261–270. [Google Scholar]
- Hiyama, Y. Migration Range and Growth Rate in the Tsushima Current Area. In Stock Fluctuations and Ecological Changes of the Japanese Sardine; Watanabe, Y., Wada, T., Eds.; Koseisha-Koseikaku: Tokyo, Japan, 1998; pp. 35–44. (In Japanese) [Google Scholar]
- Velikanov, A.Y. Migrations of Far Eastern Sardinops Melanostictus (Clupeidae) to the Sakhalin Coasts: Retrospective Insight Through the Century, Conf. “Sustainable Use of Biological Resources of the Russian Seas: Problems and Prospects”; Abstracts of Papers; Tezisy dokladov: Sochi, Russia, 2012; pp. 59–60. (In Russian) [Google Scholar]
- Sakuramoto, K. A recruitment-forecasting model for the Pacific stock of the Japanese sardine (Sardinops melanostictus) that does not assume density-dependent effects. J. Agric. Sci. 2013, 4. [Google Scholar] [CrossRef]
- Orlova, A.M.; Tokranovf, A. Checklist of deep-sea fishes of the Russian northwestern Pacific Ocean found at depths below 1000 m. Prog. Oceanogr. 2014. (In Russian) [Google Scholar] [CrossRef]
- Sakamoto, T. Temperature dependence of δ18O in otolith of juvenile Japanese sardine: Laboratory rearing experiment with micro-scale analysis. Fish. Res. 2017, 194, 55–59. [Google Scholar] [CrossRef]
- Muko, S.; Ohshimo, S.; Kurota, H.; Yasuda, T.; Fukuwaka, M.A. Long-term distribution changes in distribution of Japanese sardine in the Sea of Japan during the stock fluctuations. Mar. Ecol. Prog. Ser. 2018, 593, 141–154. [Google Scholar] [CrossRef]
- Furuichi, S.; Niino, Y.; Kamimura, Y.; Yukami, R. Time-varying relationships between early growth rate and recruitment in Japanese sardine. Fish. Res. 2020, 232, 105723. [Google Scholar] [CrossRef]
- Tomosada, A. Long-term variations in sea surface temperature and Japanese sardine catch. Bull. Tokai Reg. Fish. Res. Lab. 1988, 126, 1–9. (In Japanese) [Google Scholar]
- Kawasaki, T. Effects of Global Climatic Change on Marine Ecosystems and Fisheries. In Climatic Change: Science, Impacts and Policy; Jäger, J., Ferguson, H.L., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 291–299. [Google Scholar]
- Kodama, J.; Nagashima, H.; Izumi, Y. Long-term variations in the “mangoku herring”, Clupea pallasi Valenciennes, resources in relation to the ocean environments in the waters off Sanriku and Joban. Bull. Miyagi Pref. Fish. Res. Dev. Cent. 1995, 14, 17–36. (In Japanese) [Google Scholar]
- Kasai, A.; Sugimoto, T.; Nakata, H. The dependence of yearly recruitment of Japanese sardine Sardinops melanostictus on survival in the Kuroshio–Oyashio transition region. Fish. Sci. 1997, 63, 372–377. [Google Scholar] [CrossRef]
- Yasuda, I.; Sugisaki, H.; Watanabe, Y.; Minobe, S.; Oozeki, Y. Inter-decadal variations in Japanese sardine and ocean/climate. Fish. Oceanogr. 1999, 8, 18–24. [Google Scholar] [CrossRef]
- Gunther, A. Catalogue of the Fishes in the British Museum; London: British Museum (Natural History), Department of Zoology 1859–1868; Wheldon & Wesley: London, UK, 1868; Volume 7, p. 512. [Google Scholar]
- Kishinouye, K. Notes on the natural history of the sardine (Clupea melanosticta Schlegel). J. Fish. Bur. 1908, 14, 71–105. [Google Scholar]
- Watanabe, Y.; Zenitani, H.; Kimura, R. Offshore expansion of spawning of the Japanese sardine, Sardinops melanostictus, and its implication for egg and larval survival. Can. J. Fish. Aquat. Sci. 1995, 53, 55–61. [Google Scholar] [CrossRef]
- Sakamoto, T.; Komatsu, K.; Shirai, K.; Higuchi, T.; Ishimura, T.; Setou, T.; Kamimura, Y.; Watanabe, C.; Kawabata, A. Combining microvolume isotope analysis and numerical simulation to reproduce fish migration history. Methods Ecol. Evol. 2019, 10, 59–69. [Google Scholar] [CrossRef]
- Matsuyama, M.; Adachi, S.; Nagahama, Y.; Kitajima, C.; Matsuura, M.S. Annual reproductive cycle of the captive female Japanese sardine (Sardinops melanostictus): Relationship to ovarian development and serum levels of gonadal steroid hormones. J. Mar. Biol. 1991, 108, 21–29. [Google Scholar] [CrossRef]
- Murayama, T.; Shiraishi, M.; Aoki, I. Changes in ovarian development and plasma levels of sex steroid hormones in the wild female Japanese sardine (Sardinops melanostictus) during the spawning period. J. Fish Biol. 1994, 45, 235–245. [Google Scholar] [CrossRef]
- Fréon, P.; Misund, O.A. Dynamics of Pelagic Fish Distribution and Behaviour: Effects on Fisheries and Stock Assessment; Fishing News Books: Oxford, UK, 1999. [Google Scholar]
- Jacobson, L.D.; de Oliveira, J.A.A.; Barange, M.; Cisneros-Mata, M.; Felic-Uraga, R.; Hunter, J.R.; Kim, Y.J.; Mastuura, Y.; Ñiquen, M.; Porterior, C.; et al. Surplus production, variability, and climate change in the great sardine and anchovy fisheries. Can. J. Fish. Aquat. Sci. 2001, 58, 1891–1903. [Google Scholar] [CrossRef]
- Barange, M.; Manuel Barange, M.B.; Cubillos, L.A.; Daskalov, G.M.; de Moor, C.L.; de Oliveira, J.A.A.; Dickey-Collas, M.; Gaughan, D.J.; Hill, K.; Jacobson, L.D.; et al. Current Trends in the Assessment and Management of Small Pelagic Fish Stocks; Cambridge University Press: Cambridge, UK, 2009; pp. 191–255. [Google Scholar]
- Dudarev, V.A. Oceanological Principles of Distribution, Migration, and Dynamics of Population of Far Eastern Sardine. In Gidrometeorologiya i Gidrokhimiya Morei, Tom 8. Yaponskoe More; Hydrometeorology and Hydrochemistry of the Seas, Vol. 8: Sea of Japan; Gidrometeoizdat: St. Petersburg, Russia, 2004; Volume 8, pp. 229–234. [Google Scholar]
- Van der Lingen, C.D.; Castro, L.; Drapeau, L.; Checkley, D., Jr. (Eds.) Report of a GLOBEC-SPACC Workshop on Characterizing and Comparing the Spawning Habitats of Small Pelagic Fish; GLOBEC Report 21; GLOBEC: Swindon, UK, 2005; Volume 7, p. 33. [Google Scholar]
- Takasuka, A.; Oozeki, Y.; Aoki, I. Optimal growth temperature hypothesis: Why do anchovy flourish and sardine collapse or vice versa under the same ocean regime? Can. J. Fish. Aquat. Sci. 2007, 64, 768–776. [Google Scholar] [CrossRef]
- Takasuka, A.; Oozeki, Y.; Kubota, H.; Lluch-Cota, S.E. Contrasting spawning temperature optima: Why are anchovy and sardine regime shifts synchronous across the North Pacific? Prog. Oceanogr. 2008, 77, 225–232. [Google Scholar] [CrossRef]
- Okunishi, T.; Ito, S.-I.; Ambe, D.; Takasuka, A.; Kameda, T.; Tadokoro, K.; Setou, T.; Komatsu, K.; Kawabata, A.; Kubota, H.; et al. A modeling approach to evaluate growth and movement for recruitment success of Japanese sardine (Sardinops melanostictus) in the western Pacific. Fish. Oceanogr. 2011, 21, 44–57. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yasuda, I.; Komatsu, K.; Sasaki, H.; Sasai, Y.; Setou, T.; Shimizu, M. Winter mixed layer depth and spring bloom along the Kuroshio front: Implications for the Japanese sardine stock. Mar. Ecol. Prog. Ser. 2013, 487, 217–229. [Google Scholar] [CrossRef]
- Nyuji, M.; Hongo, Y.; Yoneda, M.; Nakamura, M. Transcriptome characterization of BPG axis and expression profiles of ovarian steroidogenesis-related genes in the Japanese sardine. BMC Genom. 2020, 21, 1–18. [Google Scholar] [CrossRef]
- Okunishi, T.; Yamanaka, Y.; Ito, S. A Migration Model of Japanese Sardine Using Artificial Neural Network PICES 2007. Available online: https://meetings.pices.int/publications/presentations/PICES_16/S3_Okunishi.pdf (accessed on 11 November 2021).
- Gavaris, S. An adaptive framework for the estimation of population size. Can. Atl. Fish. Sci. Adv. Comm. Res. Doc. 1988, 88, 1–12. [Google Scholar]
- Jacobson, L.D. ADEPT: Software for VPA Analysis Using Gavaris’s Procedure; Admin. Rep. No. LJ-93-02; National Marine Fisheries Service, Southwest Fisheries Science Center: La Jolla, CA, USA, 1993.
- Wada, T.; Jacobson, L.D. Regimes and stock-recruitment relationships in Japanese sardine (Sardinops melanostictus), 1951–1995. Can. J. Fish. Aquat. Sci. 1998, 55, 2455–2463. [Google Scholar] [CrossRef]
- Noto, M.; Yasuda, I. Population decline of the Japanese sardine, Sardinops melanostictus, in relation to sea surface temperature in the Kuroshio Extension. Can. J. Fish. Aquat. Sci. 1999, 56, 973–983. [Google Scholar] [CrossRef]
- Kodama, T.; Wagawa, T.; Ohshimo, S.; Morimoto, H.; Iguchi, N.; Fukudome, K.I.; Goto, T.; Takahashi, M.; Yasuda, T. Improvement in recruitment of Japanese sardine with delays of the spring phytoplankton bloom in the Sea of Japan. Fish. Oceanogr. 2018, 27, 289–301. [Google Scholar] [CrossRef]
- Yasuda, T.; Kuroda, T.; Hayashi, A.; Yoda, M.; Suzuki, K.; Takahashi, M. Stockassessment and Evaluation for the Tsushima Current Stock of Japanese Sardine (Fiscal Year 2017/2018). In Marine Fisheries Stock Assessment and Evaluation for Japanese Waters (Fiscal Year 2017/2018); Fisheries Agency of Japan and Fisheries Research and Education Agency of Japan: Japan, Tokyo, 2018; pp. 53–92. (In Japanese) [Google Scholar]
- Aoki, I.; Yamakawa, T.; Takasuka, A. (Eds.) Fish Population Dynamics, Monitoring, and Management Sustainable Fisheries in the Eternal Ocean; Springer Nature: Tokyo, Japan, 2018. [Google Scholar]
- Takasuka, A.; Yoneda, M.; Oozeki, Y. Disentangling density-dependent effects on egg production and survival from egg to recruitment in fish. Fish Fish. 2020, 20, 870–887. [Google Scholar] [CrossRef]
- Yatsu, A. Decadal-scale variability in ocean productivity and fishery management of small pelagic fishes. Nippon. Suisan Gakkaishi 2005, 71, 854–858. (In Japanese) [Google Scholar]
- Sakuramoto, K. Does the Ricker or Beverton and Holt type of stock-recruitment relationship truly exist? Fish. Sci. 2005, 71, 577–592. [Google Scholar] [CrossRef]
- Shimoyama, S.; Sakuramoto, K.; Suzuki, N. Proposal for stock-recruitment relationship for Japanese sardine (Sardinops melanostictus) in Northwestern Pacific. Fish. Sci. 2007, 73, 1035–1041. [Google Scholar] [CrossRef]
- Houde, E.D. Emerging from Hjort’s shadow. J. Northwest Atl. Fish. Sci. 2008, 41, 53–70. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishida, H.; Yatsu, A.; Watanabe, Y. Year-class strength and growth rates after metamorphosis of Japanese sardine (Sardinops melanostictus) in the western North Pacific Ocean during 1996–2003. Can. J. Fish. Aquat. Sci. 2008, 65, 1425–1434. [Google Scholar] [CrossRef]
- Takahashi, M.; Watanabe, Y.; Yatsu, A.; Nishida, H. Contrasting responses in larval and juvenile growth to a climate-ocean regime shift between anchovy and sardine. Can. J. Fish. Aquat. Sci. 2009, 66, 972–982. [Google Scholar] [CrossRef]
- Sakuramoto, K.; Suzuki, N. Effects of process and/or observation errors on the stock-recruitment curve and the validity of the proportional model as a stock-recruitment relationship. Fish. Sci. 2012, 78, 41–54. [Google Scholar] [CrossRef]
- Watanabe, Y.; Saito, H. Feeding and growth of early juvenile Japanese sardines in the Pacific waters off central Japan. J. Fish. Biol. 1998, 52, 519–530. [Google Scholar] [CrossRef]
- Wada, T.; Kashiwai, M. Changes in Growth and Feeding Ground of Japanese Sardine with Fluctuation in Stock Abundance. In Long-Term Variability of Pelagic Fish Populations and Their Environment; Kawasaki, T., Tanaka, S., Toba, Y., Taniguchi, A., Eds.; Pergamon Press: Oxford, UK, 1989; pp. 18–190. [Google Scholar]
- Hiyama, Y.; Nishida, H.; Goto, T. Interannual fluctuations in recruitment and growth of the sardine, (Sardinops melanostictus), in the Sea of Japan and adjacent waters. Res. Popul. Ecol. 1995, 37, 177–183. [Google Scholar] [CrossRef]
- Watanabe, S. A widely applicable bayesian information criterion. J. Mach. Learn. Res. 2013, 14, 867–897. [Google Scholar]
- Takasuka, A.; Yoneda, M.; Oozeki, Y. Density dependence in total egg production per spawner for marine fish. Fish Fish. 2019, 20, 125–137. [Google Scholar] [CrossRef]
- Campana, S.E. Year-class strength and growth rate in young Atlantic cod Gadus morhua. Mar. Ecol. Prog. Ser. 1996, 135, 21–26. [Google Scholar] [CrossRef]
- Ottersen, G.; Loeng, H. Covariability in early growth and year-class strength of Barents Sea cod, haddock, and herring: The environmental link. ICES J. Mar. Sci. 2000, 57, 339–348. [Google Scholar] [CrossRef]
- Jenkins, G.P.; King, D. Variation in larval growth can predict the recruitment of a temperate, seagrass-associated fish. Oecologia 2006, 147, 641–649. [Google Scholar] [CrossRef]
- Kamimura, Y.; Takahashi, M.; Yamashita, N.; Watanabe, C.; Kawabata, A. Larval and juvenile growth of chub mackerel Scomber japonicus in relation to recruitment in the western North Pacific. Fish. Sci. 2015, 81, 505–513. [Google Scholar] [CrossRef]
- Van Der Veer, H.W.; Berghahn, R.; Rijnsdorp, A.D. Impact of juvenile growth on recruitment in flatfish. Neth. J. Sea Res. 1994, 32, 153–173. [Google Scholar] [CrossRef]
- Bailey, K.M.; Brown, A.L.; Yoklavich, M.M.; Mier, K.L. Interannual variability in growth of larval and juvenile walleye Pollock Theragra chalcogramma in the western Gulf of Alaska, 1983–1991. Fish. Oceanogr. 1996, 5, 137–147. [Google Scholar] [CrossRef]
- Chavez, F.P.; Ryan, J.; Lluch-Cota, S.E.; Niquen, M. From anchovies to sardines and back: Multi decadal change in the Pacific Ocean. Science 2003, 299, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Ohshimo, S.; Tanaka, H.; Hiyama, Y. Long-term stock assessment and growth changes of the Japanese sardine (Sardinops melanostictus) in the Sea of Japan and East China Sea from 1953 to 2006. Fish. Oceanogr. 2009, 18, 346–358. [Google Scholar] [CrossRef]
- Okunishi, T.; Ito, S.-I.; Hashioka, T.; Sakamoto, T.T.; Yoshie, N.; Sumata, H.; Yara, Y.; Okada, N.; Yamanaka, Y. Impacts of climate change on growth, migration and recruitment success of Japanese sardine (Sardinops melanostictus) in the western North Pacific. Clim. Chang. 2012, 115, 485–503. [Google Scholar] [CrossRef]
- Nakayama, S.; Takasuka, A.; Ichinokawa, M.; Okamura, H. Climate change and interspecific interactions drive species alternations between anchovy and sardine in the western North Pacific: Detection of causality by convergent cross mapping. Fish. Oceanogr. 2018, 27, 312–322. [Google Scholar] [CrossRef]
- Nishikawa, H. Relationship between recruitment of Japanese sardine (Sardinops melanostictus) and environment of larval habitat in the low-stock period (1995–2010). Fish. Oceanogr. 2019, 28, 131–142. [Google Scholar] [CrossRef]
- Van der Lingen, C.D.; Hutchings, L.; Field, J.G. Comparative trophodynamics of Anchovy engraulis encrasicolusand Sardine sardinops sagax in the southern Benguela: Are species alternations between small pelagic fish trophodynamically mediated? Afr. J. Mar. Sci. 2006, 28, 465–477. [Google Scholar] [CrossRef]
- Van der Lingen, C.D.; Bertrand, A.; Bode, A.; Brodeur, R.; Cubillos, L.A.; Espinoza, P.; Temming, A. Trophic Dynamics. In Climate Change and Small Pelagic Fish; Checkley, D., Alheit, J., Oozeki, Y., Roy, C., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 112–157. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.; Strickland, J.D.H. Fluorometric determination of chlorophyll. J. Du Cons. Int. Pour L’exploration De La Mer 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Hirai, J.; Hidaka, K.; Nagai, S.; Ichikawa, T. Molecular-based diet analysis of the early post larvae of Japanese sardine (Sardinops melanostictus) and Pacific round herring (Etrumeus teres). Mar. Ecol. Prog. Ser. 2017, 564, 99–113. [Google Scholar] [CrossRef]
- Yoo, S.; An, Y.R.; Bae, S.; Choi, S.; Ishizaka, J.; Kang, Y.S.; Kim, Z.G.; Lee, C.; Lee, J.B.; Li, R.; et al. Status and Trends in the Yellow Sea and East China Sea Region. In Marine Ecosystems of the North Pacific Ocean, 2003–2008; McKinnell, S.M., Dagg, M.L., Eds.; PICES: Sydney, Australia, 2010; pp. 360–393. [Google Scholar]
- Yamamoto, M.; Katayama, S. Interspecific comparisons of feeding habit between Japanese anchovy Engraulis japonicus and Japanese sardine (Sardinops melanostictus) in eastern Hiuchi-nada, central Seto Inland Sea, Japan, in 1995. Bull. Jpn. Soc. Fish. Oceanogr. 2012, 76, 66–76. [Google Scholar]
- Kawasaki, T.; Kumagai, A. Food habits of the Far Eastern sardine and their implications in the fluctuation pattern of the sardine stock. Bull. Jpn. Soc. Sci. Fish 1984, 50, 1657–1663. [Google Scholar] [CrossRef]
- Yankovskaya, A.I. Zooplankton and iwashi feeding in northwest part of Japan Sea. Bull. Eastern Branch Acad. Sci. USSR 1937, 27, 63–84. [Google Scholar]
- Nakai, Z. On the relation between the structure of gillrakers and food of Sardinia melanosticta (T. & S.) with specical reference to the structure of the gill-rakers of other fishes. Ibid 1938, 33, 1–15. [Google Scholar]
- Yoshida, Y. Relation between the sardine and the food plankton-I on the feeding mechanism of Sardinops melanosticta. Bull. Japan Soc. Sci. Fish. 1955, 21, 467–470. [Google Scholar] [CrossRef]
- Yoshida, H. Distribution and food habits of the sardine, Sardinops melanostictus, off south-eastern Hokkaido and northeast-ern Honshu in spring and summer. Bull. Jpn. Soc. Fish. Oceanogr. 1987, 51, 324–329. [Google Scholar]
- Yamashita, H. The feeding habit of sardine, Sardinia melanosticta, in the waters adjacent to Kyushu, with reference to its growth. Bull. Jpn. Soc. Sci. Fish. 1955, 21, 471–475. [Google Scholar] [CrossRef]
- Honjo, K.T.; Kudoh, K.; Kudoh, S. Feeding habit of larval sardine. Rept. Coast. Mar. Res. Investig. 1956, 25–32. [Google Scholar]
- Nakata, K. Feeding conditions of Japanese sardine larvae in and near the Kuroshio examined from their gut contents. Bull. Natl. Res. Inst. Fish. Sci. 1995, 7, 265–275. [Google Scholar]
- Fukami, K.; Watanabe, A.; Fujita, S.; Yamaoka, K.; Nishijima, T. Predation on naked protozoan microzooplankton by fish larvae. Mar. Ecol. Prog. Ser. 1999, 185, 285–291. [Google Scholar] [CrossRef]
- Chiba, S.; Aita, M.; Tadokoro, K.; Saino, T.; Sugisaki, H.; Nakata, K. From climate regime shifts to lower-trophic level phe-nology: Synthesis of recent progress in retrospective studies of the western North Pacific. Prog. Oceanogr. 2008, 77, 112–126. [Google Scholar] [CrossRef]
- Takai, N.; Hirose, N.; Osawa, T.; Hagiwara, K.; Kojima, T.; Okazaki, Y.; Taniuchi, K.; Yoshihara, K. Carbon source and trophic position of pelagic fish in coastal waters of south-eastern Izu Peninsula, Japan, identified by stable isotope analysis. Fish. Sci. 2007, 73, 593–608. [Google Scholar] [CrossRef]
- Ohshimo, S.; Tanaka, H.; Nishiuchi, K.; Yasuda, T. Trophic positions and predator-prey mass ratio in the East China Sea and Sea of Japan. Mar. Freshw. Res. 2016, 67, 1692–1699. [Google Scholar] [CrossRef]
- Baba, T.; Morimoto, H.; Goto, T.; Nanjo, N.; Oda, M.; Ueno, Y. Comparison of stomach contents between Japanese sardine (Sardinops melanostictus) and anchovy (Engraulis japonicas) using commercial fisheries together with the two species in the Japan Sea during spring. Nippon. Suisan Gakkaishi 2018, 84, 288–290. [Google Scholar] [CrossRef]
- Takasuka, A.; Ozeki, Y.; Kuroda, H.; Okunishi, T. Resource variation and biological characteristics of small pelagic fish in the Kuroshio Sea basin from the viewpoint of comparison between marine ecosystems. Fish. Ocean Stud. 2015, 79, 352–374. [Google Scholar]
- Bailey, K.M.; Houde, E.D. Predation on eggs and larvae of marine fish and the recruitment problem. Adv. Mar. Biol. 1989, 25, 1–83. [Google Scholar]
- Hotta, H.; Ogawa, T. On the stomach contents of the skipjack, Katsuwonus pelamis. Bull. Tohoku Reg. Fish. Res. Lab. 1995, 43, 62–82. (In Japanese) [Google Scholar]
- Kohno, N. Distribution and Food of Epipelagic Fishes in the Waters around the Subarctic Boundaryin the North Pacific during Summer. Master’s Thesis, Faculty of Fisheries, Hokkaido University, Hakodate, Japan, 1984, unpublished (In Japanese). [Google Scholar]
- Kasamatsu, F.; Tanaka, S. Annual changes in prey species of minke whales taken off Japan 1948–87. Nippon. Suisan Gakkaishi 1992, 54, 637–651. [Google Scholar] [CrossRef]
- Aydin, K.Y.; McFarlane, G.A.; King, J.R.; Megrey, B.A. PICES-GLOBEC international program on climate change and carry-ing capacity. The BASS/MODEL report on trophic models of the Subarctic Pacific basin ecosystems. PICES Sci. Rep. 2003, 25, 1–93. [Google Scholar]
- Watanabe, H.; Kawaguchi, K. Decadal change in the diets of the surface migratorymyctophid fish Myctophum nitidulum in the Kuroshio region of the western North Pacific: Predation on sardine larvae bymyctophids. Fish. Sci. 2003, 69, 716–721. [Google Scholar] [CrossRef]
- Yatsu, A.; Nagasawa, K.; Wada, T. Decadal changes in abundance of dominant pelagic fishes and squids in the Northwestern Pacific Ocean since the 1970s and implications to fisheries management. Trans. Am. Fish. Soc. 2003, 38, 675–684. [Google Scholar]
- Yatsu, A.; Aydin, K.Y.; King, J.R.; McFarlane, G.; Chiba, S.; Tadokoro, T.; Kaeriyama, M.; Watanabe, Y. Elucidating dynamic responses of North Pacific fish populations to climate forcing: Influence of life-history strategy. Prog. Oceanogr. 2008, 77, 252–268. [Google Scholar] [CrossRef]
- Watanabe, K. Variation in the vertebrae number of 0-age sardine in the Tsushima Current region of the Japanese Islands. Ann. Rep. Jpn. Sea Reg. Fish. Res. Lab. 1958, 4, 121–133. (In Japanese) [Google Scholar]
- Panella, G. Fish otoliths: Daily growth layers and periodical pat terns. Science 1971, 173, 1124–1127. [Google Scholar] [CrossRef]
- Ohshimo, S.; Nagatani, H.; Ichimaru, T. Growth of 0-age Japanese sardine (Sardinops melanostictus) in the waters off the western coast of Kyushu. Fish. Sci. 1997, 63, 659–663. [Google Scholar] [CrossRef]
- Morimoto, H. Age and growth of Japanese sardine (Sardinops melanostictus) in Tosa Bay, southwestern Japan during a pe-riod of declining stock size. Fish. Sci. 2003, 69, 745–754. [Google Scholar] [CrossRef]
- McBride, R.S.; Richardson, A.K.; Maki, K.L. Age, growth, and mortality of wahoo, Acanthocybium solandri, from the Atlantic coast of Florida and the Bahamas. Mar. Freshw. Res. 2008, 59, 799–807. [Google Scholar] [CrossRef]
- Kawabata, A.; Yamaguchit, H.; Kubota, S.; Nakagami, M. Growth and fatness of 1975-2002 year classes of Japanese sardine in the Pacific waters around northern Japan. Fish. Sci. 2011, 77, 291–299. [Google Scholar] [CrossRef]
- Hayashi, A.; Yamashita, Y.; Kawaguchi, K.; Ishii, T. Rearing method and daily otolith ring of Japanese sardine larvae. Nippon Suisan Gakkaishi 1989, 55, 997–1000. [Google Scholar] [CrossRef]
- Yatsu, A.; Kawabata, A. Reconsidering Trans-Pacific “synchrony” in population fluctuations of sardines. Bull. Jpn. Soc. Fish. Oceanogr. 2017, 81, 1–13. [Google Scholar]
- Suda, M.; Kishida, T. A spatial model of population dynamics of the early life stages of Japanese sardine, Sardinops melanostictus, off the Pacific coast of Japan. Fish. Oceanogr. 2003, 12, 85–99. [Google Scholar] [CrossRef]
- Michio, Y.; Tanaka, H.; Honda, S.; Nishida, H.; Nashida, K.; Hirota, Y.; Ishida, M.; Ohshimo, S.; Miyabe, S.; Ito, H.; et al. Sex-ual maturation, spawning period and batch fecundity of Japanese sardine (Sardinops melanostictus) in the coastal waters of western Japan in 2008–2010. Bull. Jpn. Soc. Fish. Oceanogr. 2013, 77, 59–67. [Google Scholar]
- Morimoto, H. Relationship between the batch fecundity and the egg size on Japanese sardine (Sardinops melanostictus) in Tosa Bay and off Kii Channel, southwestern Japan from 1990 to 1993. Fish. Sci. 1998, 64, 220–227. [Google Scholar] [CrossRef][Green Version]
- Han, K.H.; Yu, T.S.; Lee, J.; Lee, S.H. Seasonal variation in species composition of Ichthyoplankton in Northern Jinhae Bay, Korea. Korean J. Fish. Aquat. Sci. 2018, 51, 72–78. [Google Scholar]
- Levavi-Sivan, B.; Bogerd, J.; Mañanós, E.L.; Gómez, A.; Lareyre, J.J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.A.; Dufour, S.; Karlsen, Ø.; Norberg, B.; et al. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Z.; Usami, S.; Hattori, S.; Honjô, K.; Hayashi, S. Progress report of the cooperative iwashi resources investigation April 1949–December 1951. Tokai Reg. Fisher. Res. Lab. 1955, 116. [Google Scholar]
- Shiraishi, M.; Azuma, N.; Aoki, I. Large schools of Japanese sardines (Sardinops melanostictus), mate in single pair units at night. Environ. Biol. Fishes 1996, 45, 405–409. [Google Scholar] [CrossRef]
- Wada, T. Population dynamics on Japanese sardine (Sardinops melanostictus), caught by the domestic purse seine fishery in the waters of the coast of southeastern Hokkaido. Bull. Hokkaido Reg. Fish. Res. Lab. 1988, 52, 1–138. [Google Scholar]
- Baik, C.H.; Park, J.H.; Choi, K.H.; Hwang, S.D. Seasonal and annual variations of catch by large purse seine off Korea. Sea 2001, 6, 164–179. [Google Scholar]
- Lee, J.H.; Lee, J.B.; Zhang, C.I.; Kang, S.; Choi, Y.M.; Lee, D.W. A study on fluctuation of the fishing grounds of target fishes by the Korean large purse seine fishery. J. Kor. Soc. Fish. Tech. 2012, 48, 107–117. [Google Scholar] [CrossRef]
- Makino, M. Rebuilding and full utilization of alternating pelagic species around Japan: A social-ecological approach. Re-Build. Mar. Fish. Part 2018, 630, 61–73. [Google Scholar]
- Yukami, R.; Nishijima, S.; Isu, S.; Watanabe, C.; Kamimura, Y.; Hashimoto, M. Stock assessment and evaluation for the Pacific stock of chub mackerel (fiscal year 2017/2018). In Marine Fisheries Stock Assessment and Evaluation for Japanese Waters (Fiscal Year 2017/2018); Fisheries Agency of Japan and Fisheries Research and Education Agency of Japan: Tokyo, Japan, 2018; pp. 157–200. (In Japanese) [Google Scholar]
- Caddy, J.F.; Mohon, R. Reference Points for Fisheries Management; FAO Fisheries Technical Paper, No. 347; FAO: Rome, Italy, 2005; p. 83. [Google Scholar]
- Nishida, H.; Ishida, M.; Noto, M.; Katsukawa, K. Marine Fisheries Stock Assessments and Evaluations for Japanese waters (Fiscal Year 2006/2007). In Stock Assessment and Evaluation for Japanese Sardine Pacific Stock (Fiscal Year 2006); Fisheries Agency and Fisheries Research Agency of Japan: Tokyo, Japan, 2006; pp. 11–49. (In Japanese) [Google Scholar]
- Ishida, Y.; Satoshi, H.T.; Keizou, Y.; Hiroshi, N.; Chikako, W. Management of declining Japanese sardine, chub mackerel and walleye pollock fisheries in Japan. J. Fish. Res. 2009, 100, 68–77. [Google Scholar] [CrossRef]
- Ichinokawa, M.; Okamura, H.; Kurota, H. The status of Japanese fisheries relative to fisheries around the world. ICES J. Mar. Sci. 2017, 74, 1277–1287. [Google Scholar] [CrossRef]
- Furuichi, S.; Watanabe, C.; Yukami, R.; Kamimura, Y.; Isu, S.; Udagawa, M. Stock Assessment and Evaluation for the Pacific stock of Japanese Sardine (FISCAL year 2017/2018). In Marine Fisheries Stock Assessment and Evaluation for Japanese Waters (Fis-cal Year 2017/2018); Fisheries Agency of Japan and Fisheries Research and Education Agency of Japan: Tokyo, Japan, 2018; pp. 15–52. (In Japanese) [Google Scholar]
- Wang, Y.; Liang, C.; Wang, Y.; Xian, W.; Palomares, M.L. Stock status assessments for 12 exploited fishery species in the Tsushima warm current region, southwest Japan and east China, using the CMSY and BSM methods. Front. Mar. Sci. 2020, 7, 640. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Anderson, S.C.; Gutierrez, N.L.; Kleisner, K.M.; Longo, C.; Walsh, J.C. Blood from a stone: Performance of catch-only methods in estimating stock biomass status. Fish. Res. 2020, 223, 105452. [Google Scholar] [CrossRef]
- Japan Fisheries Information Service Center. What Is Total Allowable Catch (TAC)? Fisheries Agency of Japan. 2002. Available online: http://www.jfa.maff.go.jp/j/suisin/s_tac/pdf/tacpanfu201501.pdf (accessed on 25 June 2021). (In Japanese).
- Ohshimo, S.; Yamakawa, T. Harvest control rules. Fish. Sci. Ser. 2018, 183–206. [Google Scholar] [CrossRef]
- Garcia, S.M.; Zerbi, A.; Aliaume, C.; Do Chi, T.; Lasserre, G. The Ecosystem Approach to Fisheries. In Issues, Terminology, Principles, Institutional Foundations, Implementation and Outlook; FAO Fisheries Technical Paper. 443; FAO: Rome, Italy, 2003; p. 71. [Google Scholar]
- North Pacific Fisheries Commission. 5th Meeting Report. NPFC-2 COM05-Final Report. 2019; p. 331. Available online: www.npfc.int (accessed on 25 June 2021).

| Japanese Sardine | Main Preys | References |
|---|---|---|
| Adults stage | Diatoms, Flagellata, Foraminifera, Radiolaria, Tintinnopsidae and Copepoda (Microsetella, Calanus), Schizopoda, Amphipoda, Decapoda, eggs, larvae of fishes. | [35] |
| Zooplankton (Euphausia pacifica, Neocalanus spp. and Calanus sinicus). | [86] | |
| Plankton. | [87] | |
| Copepoda, amphipoda, anchovy egg, spherical fish egg diatoms. | [38] | |
| Larval and Juvenile stages | Diatoms and dinoflagellates. | [88] |
| Zooplankton (Calanus finmarchicus, Pseudocalanus, elongatus, and Paracalanus parvus.). | [89] | |
| Paracalanus parvus, Calanus plumchrus, C. finmarchicus, Centropages abdominalis, Schizopoda, Rhizosolenia setigera, Thalassiosira decipiens, T.Clevei and Lauderia annulata. | [90] | |
| Plankton species as Chaetoceros affinis, C. danicus, Rhizosolenia, alata, R. setigera, Skeletonema costatum and Thalassionema itzschioides. | [91,92] | |
| Oncaea sp., Microsetella sp., Lamellibranchia larva, Gastropoda larva, microplankton. | [93] | |
| Microcopepods, nauplius Copepods, diatoms | [88,94] | |
| diatoms and dinoflagellates, zooplankton, copepod nauplii, | ||
| naupliar copepods, Copepod eggs and nauplii. | [66,95] | |
| Calanoid copepods, Oithona spp., Oncaea spp. Sapphirina spp. Corycaeus spp., Microsetella spp., Unidentified copepods, Larvaceans, unidentified. | ||
| Protozoa | [96] | |
| Zooplanktons (copepod, nauplii). | [96,97] | |
| Eukaryotic, fragile protozoa and gelatinous zooplankton. | ||
| Zooplankton, Copepods, protozoa (Radiolaria and Dinophyta, dinoflagellates), gelatinous zooplankton, chaetognaths, appendicularians, and hydrozoans. | [85] |
| Regions | Longevity | Recruitment Stage | Age at First Maturation, L50 | Spawning/Feeding Season | Spawning Area | Major Predators | References |
|---|---|---|---|---|---|---|---|
| Pacific Ocean | About 7+ years | Generally juveniles, but also larvae | 1 year (LSP), 2–3 years (HSP) | November–June, Mainly February–April | Kuroshio coastal area, expands to south of Kyushu in HSP, KOTZ, Oyashio, beyond in the HSP. | Common squid, skipjack tuna, other predatory marine species | [56,117] |
| Tsushima Current | January–June | Coastal area from Noto Penisula and southern Korea to western Kyushu, expands to East China Sea and southern Kyushu in HSP Kuroshio Current | yellow tail, sea birds and marine mammals | [48,54,78,81,109] | |||
| Sea of Japan and East China Sea | About 6+ years | - | - | Sea of Japan and East China Sea | [78] | ||
| Tosa Bay, south-western Japan | About 7+ years | - | - | Tosa Bay, south-western Japan | [113] | ||
| Pacific coast of Japan northern Japan | 2+ years, L50 = 180 mm/Wt ≥ 180 g | February–April | Pacific coast of Japan, Oyashio waters | [118] | |||
| Pacific waters around northern Japan | 7–9 years | - | - | Pacific waters around northern Japan | [115] | ||
| Coastal waters off western Japan | About 7+ years | - | 1-year-old L50 = 148 mm (KP), 144 mm (NWK) and 141 mm (SD) | - | Kochi Prefecture (KP), northwestern Kyushu (NWK), and the San-in district (SD) | [119] | |
| Central Pacific and sea of Japan | About 7+ years | - | - | All seasons | North and south sea area around Japan | [19,66] | |
| Sea of Japan | About 7+ years | juveniles, also larvae | during summer | west of Kyushu western part of central and northern areas | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarr, O.; Kindong, R.; Tian, S. Knowledge on the Biological and Fisheries Aspects of the Japanese Sardine, Sardinops melanostictus (Schlegel, 1846). J. Mar. Sci. Eng. 2021, 9, 1403. https://doi.org/10.3390/jmse9121403
Sarr O, Kindong R, Tian S. Knowledge on the Biological and Fisheries Aspects of the Japanese Sardine, Sardinops melanostictus (Schlegel, 1846). Journal of Marine Science and Engineering. 2021; 9(12):1403. https://doi.org/10.3390/jmse9121403
Chicago/Turabian StyleSarr, Ousmane, Richard Kindong, and Siquan Tian. 2021. "Knowledge on the Biological and Fisheries Aspects of the Japanese Sardine, Sardinops melanostictus (Schlegel, 1846)" Journal of Marine Science and Engineering 9, no. 12: 1403. https://doi.org/10.3390/jmse9121403
APA StyleSarr, O., Kindong, R., & Tian, S. (2021). Knowledge on the Biological and Fisheries Aspects of the Japanese Sardine, Sardinops melanostictus (Schlegel, 1846). Journal of Marine Science and Engineering, 9(12), 1403. https://doi.org/10.3390/jmse9121403







