Valorization Use of Amphipod Meal, Gammarus pulex, as a Fishmeal Substitute on Growth Performance, Feed Utilization, Histological and Histometric Indices of the Gut, and Economic Revenue of Grey Mullet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Culture Technique
2.2. Preparation and Characterization of Amphipod Meal (APM)
2.3. Experimental Design
2.3.1. Growth Indices
2.3.2. Whole-Body Composition and Proximate Composition
2.3.3. Histological Examination
2.3.4. Economic Evaluation
2.4. Statistical Analyses
3. Results
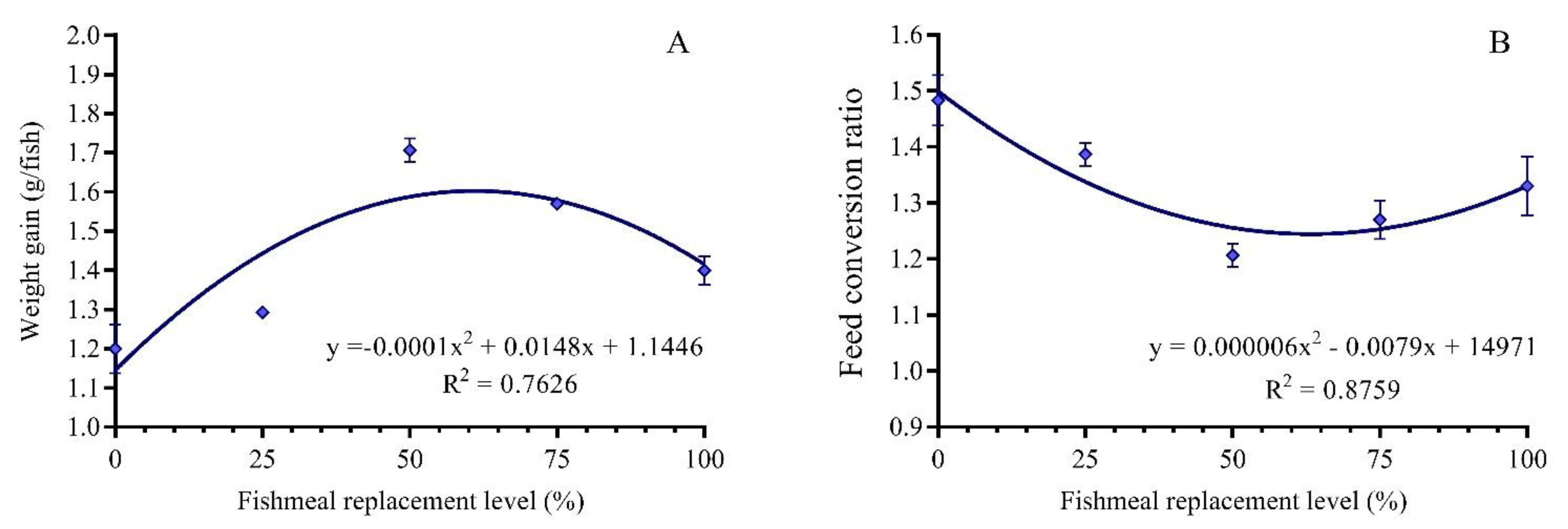
3.1. Growth Performance
3.2. Feed Utilization
3.3. Carcass Composition
3.4. Intestinal Histological Morphology Indices
3.5. Histological Observation of Liver
3.6. Economic Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals. Rome. Licenc. CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018; pp. 1–277. [Google Scholar]
- Granada, L.; Sousa, N.; Lopes, S.; Lemos, M.F.L. Is Integrated Multitrophic Aquaculture the Solution to the Sectors’ Major Challenges?–A Review. Rev. Aquac. 2016, 8, 283–300. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Trends in Global Aquaculture and Aquafeed Production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- GAFRD General Authority for Fish Resources Development. Fish Statistics Year Book; GAFRD: Cairo, Eqypt, 2012. [Google Scholar]
- Welcomme, R. Review of the State of the World Fishery Resources: Inland Fisheries. FAO Fish. Aquac. Circ. 2011, C942, I. [Google Scholar]
- Mabrouk, M.M.; Ashour, M.; Labena, A.; Zaki, M.A.A.; Abdelhamid, A.F.; Gewaily, M.S.; Dawood, M.A.O.; Abualnaja, K.M.; Ayoub, H.F. Nanoparticles of Arthrospira Platensis Improves Growth, Antioxidative and Immunological Responses of Nile Tilapia (Oreochromis Niloticus) and Its Resistance to Aeromonas Hydrophila. Aquac. Res. 2021. [Google Scholar] [CrossRef]
- Ashour, M.; Alprol, A.E.; Heneash, A.M.M.; Saleh, H.; Abualnaja, K.M.; Alhashmialameer, D.; Mansour, A.T. Ammonia Bioremediation from Aquaculture Wastewater Effluents Using Arthrospira Platensis NIOF17/003: Impact of Biodiesel Residue and Potential of Ammonia-Loaded Biomass as Rotifer Feed. Materials 2021, 14, 5460. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Mabrouk, M.M.; Abo-Taleb, H.A.; Sharawy, Z.Z.; Ayoub, H.F.; van Doan, H.; Davies, S.J.; El-Haroun, E.; Goda, A.M.-A. A Liquid Seaweed Extract (TAM®) Improves Aqueous Rearing Environment, Diversity of Zooplankton Community, Whilst Enhancing Growth and Immune Response of Nile Tilapia, Oreochromis Niloticus, Challenged by Aeromonas Hydrophila. Aquaculture 2021, 543, 736915. [Google Scholar] [CrossRef]
- Goda, A.A.; Srour, T.M.; Omar, E.; Mansour, A.T.; Baromh, M.Z.; Mohamed, S.A.; El-Haroun, E.; Davies, S.J. Appraisal of a High Protein Distiller’s Dried Grain (DDG) in Diets for European Sea Bass, Dicentrarchus Labrax Fingerlings on Growth Performance, Haematological Status and Related Gut Histology. Aquac. Nutr. 2019, 25, 808–816. [Google Scholar] [CrossRef]
- Allam, B.W.; Khalil, H.S.; Mansour, A.T.; Srour, T.M.; Omar, E.A.; Nour, A.A.M. Impact of Substitution of Fish Meal by High Protein Distillers Dried Grains on Growth Performance, Plasma Protein and Economic Benefit of Striped Catfish (Pangasianodon Hypophthalmus). Aquaculture 2020, 517, 734792. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Hoang, L.; Van Khanh, T.; Duy Hai, P.; Hung, L.T. Utilization of Fermented Soybean Meal for Fishmeal Substitution in Diets of Pacific White Shrimp (Litopenaeus Vannamei). Aquac. Nutr. 2018, 24, 1092–1100. [Google Scholar] [CrossRef]
- Amer, S.A.; Ahmed, S.A.A.; Ibrahim, R.E.; Al-Gabri, N.A.; Osman, A.; Sitohy, M. Impact of Partial Substitution of Fish Meal by Methylated Soy Protein Isolates on the Nutritional, Immunological, and Health Aspects of Nile Tilapia, Oreochromis niloticus, Fingerlings. Aquaculture 2020, 518, 734871. [Google Scholar] [CrossRef]
- Doughty, K.H.; Garner, S.R.; Bernards, M.A.; Heath, J.W.; Neff, B.D. Effects of Dietary Fishmeal Substitution with Corn Gluten Meal and Poultry Meal on Growth Rate and Flesh Characteristics of Chinook Salmon (Oncorhynchus Tshawytscha). Int. Aquat. Res. 2019, 11, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Rapatsa, M.M.; Moyo, N.A.G. Evaluation of Imbrasia Belina Meal as a Fishmeal Substitute in Oreochromis Mossambicus Diets: Growth Performance, Histological Analysis and Enzyme Activity. Aquac. Rep. 2017, 5, 18–26. [Google Scholar] [CrossRef]
- Mastoraki, M.; Ferrándiz, P.M.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. A Comparative Study on the Effect of Fish Meal Substitution with Three Different Insect Meals on Growth, Body Composition and Metabolism of European Sea Bass (Dicentrarchus Labrax L.). Aquaculture 2020, 528, 735511. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abbass, F.E. Dry Whey Meal as a Protein Source in Practical Diets for Nile Tilapia, Oreochromis Niloticus, Fingerlings. J. Appl. Aquac. 2016, 28, 276–284. [Google Scholar] [CrossRef]
- Fall, S.K.L.; Fall, J.; Loum, A.; Sagne, M.; Jatta, S.; Ndong, D.; Diouf, M.; Sheen, S.S. Effects of Partial Substitution of Fishmeal by Crustacean (Callianassa) Meal on the Growth Performance, Feed Efficiency and Survival Rate of Nile Tilapia (Oreochromis Niloticus). J. Biol. Life Sci. 2020, 11, 207–217. [Google Scholar] [CrossRef]
- Abdel-Rahim, M.M.; Mansour, A.T.; Mona, M.H.; El-Gamal, M.M.; El Atafy, M.M. To What Extent Can Maternal Inherited Immunity Acquired from a Crustacean-enhanced Diet Improve the Performance and Vitality of the Offspring and Enhance Profitability of European Sea Bass (Dicentrarchus Labrax)? J. World Aquac. Soc. 2019, 50, 550–574. [Google Scholar] [CrossRef]
- El-shabrawy, G.M.; Shadrin, N.V. Copepoda in the shallow hypersaline Bardawil coastal lake (Egypt): Are There Long-Term Changes in Composition and Abundance? Oceanol. Hydrobiol. Stud. 2018, 47, 219–229. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A.; Zeina, A.F.; Ashour, M.; Mabrouk, M.M.; Sallam, A.E.; El-Feky, M.M. Isolation and Cultivation of the Freshwater Amphipod Gammarus Pulex (Linnaeus, 1758), with an Evaluation of Its Chemical and Nutritional Content. Egypt. J. Aquat. Biol. Fish. 2020, 24, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Alprol, A.E.; Heneash, A.M.M.; Soliman, A.M.; Ashour, M.; Alsanie, W.F.; Gaber, A.; Mansour, A.T. Assessment of Water Quality, Eutrophication, and Zooplankton Community in Lake Burullus, Egypt. Diversity 2021, 13, 268. [Google Scholar] [CrossRef]
- Opstad, I.; Suontama, J.; Langmyhr, E.; Olsen, R.E. Growth, Survival, and Development of Atlantic Cod (Gadus Morhua L.) Weaned onto Diets Containing Various Sources of Marine Protein. ICES J. Mar. Sci. 2006, 63, 320–325. [Google Scholar] [CrossRef]
- Virtue, P.; Johannes, R.E.; Nichols, P.D.; Young, J.W. Biochemical Composition of Nyctiphanes Australis and Its Possible Use as an Aquaculture Feed Source: Lipids, Pigments and Fluoride Content. Mar. Biol. 1995, 122, 121–128. [Google Scholar] [CrossRef]
- Magouz, F.I.; Essa, M.A.; Matter, M.; Mansour, A.T.; Alkafafy, M.; Ashour, M. Population Dynamics, Fecundity and Fatty Acid Composition of Oithona Nana (Cyclopoida, Copepoda), Fed on Different Diets. Animals 2021, 11, 1188. [Google Scholar] [CrossRef] [PubMed]
- Lolas, A.; Karapanagiotidis, I.T.; Neofitou, N.; Panagiotaki, P. Use of Caprellid Amphipods as Alternative Protein and Lipid Source in Farmed Fish Nutrition. In Proceedings of the 3rd International Congress on Applied Ichthyology and Aquatic Environment, Volos, Greece, 8–11 November 2018. [Google Scholar]
- Wiebe, P.H.; Harris, R.; Gislason, A.; Margonski, P.; Skjoldal, H.R.; Benfield, M.; Hay, S.; O’Brien, T.; Valdés, L. The ICES Working Group on Zooplankton Ecology: Accomplishments of the First 25 Years. Prog. Oceanogr. 2016, 141, 179–201. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Gaber, A.; Ammar, G.A.G.A. Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Abbas, E.M.; Ali, F.S.; Desouky, M.G.; Ashour, M.; El-Shafei, A.; Maaty, M.M.; Sharawy, Z.Z. Novel Comprehensive Molecular and Ecological Study Introducing Coastal Mud Shrimp (Solenocera Crassicornis) Recorded at the Gulf of Suez, Egypt. J. Mar. Sci. Eng. 2021, 9, 9. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.F.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The Potential of a New Commercial Seaweed Extract in Stimulating Morpho-Agronomic and Bioactive Properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A.; Ashour, M.; Elokaby, M.A.; Mabrouk, M.M.; El-feky, M.M.M.; Abdelzaher, O.F.; Gaber, A.; Alsanie, W.F.; Mansour, A.T. Effect of a New Feed Daphnia Magna (Straus, 1820), as a Fish Meal Substitute on Growth, Feed Utilization, Histological Status, and Economic Revenue of Grey Mullet, Mugil Cephalus (Linnaeus 1758). Sustainability 2021, 13, 7093. [Google Scholar] [CrossRef]
- Sidorov, D.; Hou, Z.; Sket, B. Three New Remarkable Amphipod Species (Crustacea: Gammaridae) from Springs and Subterranean Waters of Central Asia. Zootaxa 2018, 4444, 437–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Prada, P.; Hachero-Cruzado, I.; Giráldez, I.; Fernández-Diaz, C.; Vilas, C.; Cañavate, J.P.; Guerra-García, J.M. Crustacean Amphipods from Marsh Ponds: A Nutritious Feed Resource with Potential for Application in Integrated Multi-Trophic Aquaculture. PeerJ 2018, 6, e4194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harlıoğlu, M.M.; Farhadi, A. Importance of Gammarus in Aquaculture. Aquac. Int. 2018, 26, 1327–1338. [Google Scholar] [CrossRef]
- Jiménez-Prada, P.; Hachero-Cruzado, I.; Guerra-García, J.M. Aquaculture Waste as Food for Amphipods: The Case of Gammarus Insensibilis in Marsh Ponds from Southern Spain. Aquac. Int. 2021, 29, 139–153. [Google Scholar] [CrossRef]
- Luzzana, U.; Valfrè, F.; Mangiarotti, M.; Domeneghini, C.; Radaelli, G.; Moretti, V.M.; Scolari, M. Evaluation of Different Protein Sources in Fingerling Grey Mullet, Mugil Cephalus, Practical Diets. Aquac. Int. 2005, 13, 291–303. [Google Scholar] [CrossRef]
- Sicuro, B. Reasons and possibilities of fish meal replacement in the Siberian sturgeon. In The Siberian Sturgeon (Acipenser baerii, Brandt, 1869) Volume 2-Farming; Springer: Cham, Switzerland, 2018; pp. 85–95. [Google Scholar]
- Sallam, A.E.; El-feky, M.M.M.; Ahmed, M.S.; Mansour, A.T. The Potential Use of Whey Protein as a Partial Substitute of Fishmeal on Growth Performance, Non-Specific Immunity, and Gut Histological Status of Juvenile European Seabass, Dicentrarchus Labrax. Aquac. Res. 2021, 1–10, in press. [Google Scholar] [CrossRef]
- Köprücü, K.; Özdemir, Y. Apparent Digestibility of Selected Feed Ingredients for Nile Tilapia (Oreochromis Niloticus). Aquaculture 2005, 250, 308–316. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemistry, Official Methods of Analysis; AOAC: Arlington, TX, USA, 2000. [Google Scholar]
- Al-Motabagani, M.A. Histological and Histochemical Studies on the Effects of Methotrexate on the Liver of Adult Male Albino Rat. Int. J. Morphol. 2006, 24, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Hamidian, G.; Zirak, K.; Sheikhzadeh, N.; Khani Oushani, A.; Shabanzadeh, S.; Divband, B. Intestinal Histology and Stereology in Rainbow Trout (Oncorhynchus Mykiss) Administrated with Nanochitosan/Zeolite and Chitosan/Zeolite Composites. Aquac. Res. 2018, 49, 1803–1815. [Google Scholar] [CrossRef]
- Goda, A.M.; Omar, E.A.; Srour, T.M.; Kotiet, A.M.; El-Haroun, E.; Davies, S.J. Effect of Diets Supplemented with Feed Additives on Growth, Feed Utilization, Survival, Body Composition and Intestinal Bacterial Load of Early Weaning European Seabass, Dicentrarchus Labrax Post-Larvae. Aquac. Int. 2018, 26, 169–183. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A.; El-feky, M.M.M.; Azab, A.M.; Mabrouk, M.M.; Elokaby, M.A.; Ashour, M.; Mansour, A.T.; Abdelzaher, O.F.; Abualnaja, K.M.; Sallam, A.E. Growth Performance, Feed Utilization, Gut Integrity, and Economic Revenue of Grey Mullet, Mugil Cephalus, Fed an Increasing Level of Dried Zooplankton Biomass Meal as Fishmeal Substitutions. Fishes 2021, 6, 38. [Google Scholar] [CrossRef]
- Waite, R.; Beveridge, M.; Brummett, R.; Castine, S.; Chaiyawannakarn, N.; Kaushik, S.; Mungkung, R.; Nawapakpilai, S.; Phillips, M. Improving Productivity and Environmental Performance of Aquaculture; WorldFish: Penang, Malaysia, 2014. [Google Scholar]
- Tacon, A.G.J.; Metian, M. Global Overview on the Use of Fish Meal and Fish Oil in Industrially Compounded Aquafeeds: Trends and Future Prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Jackson, A. Fish in–Fish out Ratios Explained. Aquac. Eur. 2009, 34, 5–10. [Google Scholar]
- Nair, K.K.C.; Anger, K. Seasonal Variation in Population Structure and Biochemical Composition of Jassa Falcata (Crustacea, Amphipoda) off the Island of Helgoland (North Sea). Estuar. Coast. Mar. Sci. 1980, 11, 505–513. [Google Scholar] [CrossRef]
- Percy, J.A. Seasonal Changes in Organic Composition and Caloric Content of an Arctic Marine Amphipod, Onisimus (= Boeckosimus) Affinis HJ Hansen. J. Exp. Mar. Biol. Ecol. 1979, 40, 183–192. [Google Scholar] [CrossRef]
- Massimo Perrone, F.; Della Croce, N.; Dell’anno, A. Biochemical Composition and Trophic Strategies of the Amphipod Eurythenes Gryllus at Hadal Depths (Atacama Trench, South Pacific). Chem. Ecol. 2003, 19, 441–449. [Google Scholar] [CrossRef]
- Baeza-Rojano, E.; Hachero-Cruzado, I.; Guerra-García, J.M. Nutritional Analysis of Freshwater and Marine Amphipods from the Strait of Gibraltar and Potential Aquaculture Applications. J. Sea Res. 2014, 85, 29–36. [Google Scholar] [CrossRef]
- Köprücü, K.; Harlioglu, M.M.; Konar, V. Use of Gammarus kischineffensis (Schellenberg 1937) as a protein, amino acid and pigment source in rainbow trout (Oncorhynchus mykiss Walbaum, 1792) rations. J. Fish. Aquat. Sci. 1998, 15, 199–210. [Google Scholar]
- Kolanowski, W.; Stolyhwo, A.; Grabowski, M. Fatty Acid Composition of Selected Fresh Water Gammarids (Amphipoda, Crustacea): A Potentially Innovative Source of Omega-3 LC PUFA. J. Am. Oil Chem. Soc. 2007, 84, 827–833. [Google Scholar] [CrossRef]
- Hyne, R.V.; Sánchez-Bayo, F.; Bryan, A.D.; Johnston, E.L.; Mann, R.M. Fatty Acid Composition of the Estuarine Amphipod, Melita Plumulosa (Zeidler): Link between Diet and Fecundity. Environ. Toxicol. Chem. Int. J. 2009, 28, 123–132. [Google Scholar] [CrossRef]
- Babin, A.; Motreuil, S.; Teixeira, M.; Bauer, A.; Rigaud, T.; Moreau, J.; Moret, Y. Origin of the Natural Variation in the Storage of Dietary Carotenoids in Freshwater Amphipod Crustaceans. PLoS ONE 2020, 15, e0231247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moren, M.; Suontama, J.; Hemre, G.-I.; Karlsen, Ø.; Olsen, R.E.; Mundheim, H.; Julshamn, K. Element Concentrations in Meals from Krill and Amphipods,—Possible Alternative Protein Sources in Complete Diets for Farmed Fish. Aquaculture 2006, 261, 174–181. [Google Scholar] [CrossRef]
- Mathias, J.A.; Martin, J.; Yurkowski, M.; Lark, J.G.I.; Papst, M.; Tabachek, J.L. Harvest and Nutritional Quality of Gammarus Lacustris for Trout Culture. Trans. Am. Fish. Soc. 1982, 111, 83–89. [Google Scholar] [CrossRef]
- Suontama, J.; Kiessling, A.; Melle, W.; Waagbø, R.; Olsen, R.E. Protein from Northern Krill (Thysanoessa Inermis), Antarctic Krill (Euphausia Superba) and the Arctic Amphipod (Themisto Libellula) Can Partially Replace Fish Meal in Diets to Atlantic Salmon (Salmo Salar) without Affecting Product Quality. Aquac. Nutr. 2007, 13, 50–58. [Google Scholar] [CrossRef]
- Azimi, A.; Hosseini, S.A.; Sudagar, M.; Aslanparviz, H. Effect of Replacement of Caspian Sea Gammarus Meal by Partial Kilka Fish Meal on Growth Performance, Feed Conversion Ratio and Survival of Juveniles of Rainbow Trout (Oncorhynchus Mykiss). Iran. Sci. Fish. J. 2011, 20, 63–74. [Google Scholar]
- Dehaghani, P.G.; Baboli, M.J.; Moghadam, A.T.; Ziaei-Nejad, S.; Pourfarhadi, M. Effect of Synbiotic Dietary Supplementation on Survival, Growth Performance, and Digestive Enzyme Activities of Common Carp (Cyprinus Carpio) Fingerlings. Czech J. Anim. Sci. 2015, 60, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Darjani, M.; Mohammadizadeh, K.M.; Shamsaee, M.M.; Abdollah, T.Y. Investigation on the Effects of Live and Commercial Food on Growth and Survival Rate of Caspian Salmon (Salmo Trutta Caspius). Breed. Aquac. Sci. Q. 2014, 2, 23–34. [Google Scholar]
- Sharahi, R.; Falahatkar, A.B.; Efatpanah, I. Effect of Fish Meal Replacement with Gammarus Meal on Growth and Body Composition of Juvenile Siberian Sturgeon, Acipenser Baerii (Brandt, 1869). J. Aquat. Ecol. 2016, 6, 102–113. [Google Scholar]
- Adhami, B.; Yeganeh, S.; Sara Jafari Kenari, S. Influence of Caspian Gammarus on Growth Parameters, Survival and Serum Biochemical Factors in Jewel Fish (Hemichromis Bimaculatus). Exp. Anim. Biol. 2017, 5, 31–37. [Google Scholar]
- Wang, L.; Li, J.; Jin, J.N.; Zhu, F.; Roffeis, M.; Zhang, X.Z. A Comprehensive Evaluation of Replacing Fishmeal with Housefly (Musca Domestica) Maggot Meal in the Diet of Nile Tilapia (Oreochromis Niloticus): Growth Performance, Flesh Quality, Innate Immunity and Water Environment. Aquac. Nutr. 2017, 23, 983–993. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Z.; Ren, X.; Li, P.; Wang, Y. Growth Performance, Feed Cost and Environmental Impact of Largemouth Bass Micropterus Salmoides Fed Low Fish Meal Diets. Aquac. Rep. 2021, 20, 100757. [Google Scholar] [CrossRef]
- Alberts-Hubatsch, H.; Jiménez-Prada, P.; Beermann, J.; Slater, M.J. Amphipod Meal in Formulated Diets for Juvenile Turbot Psetta Maxima. In Proceedings of the Conference of Aquaculture Europe, Berlin, Germany, 7–11 October 2019. [Google Scholar]
- Promthale, P.; Withyachumnarnkul, B.; Bossier, P.; Wongprasert, K. Nutritional Value of the Amphipod Bemlos Quadrimanus Sp. Grown in Shrimp Biofloc Ponds as Influenced by Different Carbon Sources. Aquaculture 2021, 533, 736128. [Google Scholar] [CrossRef]
- Aleya, H.; Taizo, S.; Daiichi, K. Microflora in the Alimentary Tract of Gray Mullet. IV. Estimation of Enzymic Activities of the Intestinal Bacteria. Nippon Suisan Gakkaishi 1979, 45, 99–106. [Google Scholar]
- Moutinho, S.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jover-Cerdá, M.; Oliva-Teles, A.; Peres, H. Meat and Bone Meal as Partial Replacement for Fish Meal in Diets for Gilthead Seabream (Sparus Aurata) Juveniles: Growth, Feed Efficiency, Amino Acid Utilization, and Economic Efficiency. Aquaculture 2017, 468, 271–277. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of Intestinal Morphology and Immunity in Nile Tilapia (Oreochromis Niloticus) by Lactobacillus Rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Pirarat, N.; Boonananthanasarn, S.; Krongpong, L.; Katagiri, T.; Maita, M. Effect of Activated Charcoal-Supplemented Diet on Growth Performance and Intestinal Morphology of Nile Tilapia (Oreochromis Niloticus). Thai J. Vet. Med. 2015, 45, 113. [Google Scholar]
- Hershberg, R.; Blumberg, R.S. The lymphocyte-epithelial-bacterial interface. In Inflammatory Bowel Disease: From Bench to Bedside; Springer: Berlin/Heidelberg, Germany, 2003; pp. 121–146. [Google Scholar]

| Ingredients | DM (%) | CP (%) | EE (%) | CF (%) | Ash (%) | NFE (%) | GE (Kcal kg−1) |
|---|---|---|---|---|---|---|---|
| Fishmeal (FM) | 92.03 | 60.17 | 12.50 | 0.62 | 15.40 | 11.50 | 3930.14 |
| Amphipod meal (APM) | 93.15 | 40.02 | 14.21 | 1.50 | 19.82 | 24.51 | 3364.05 |
| Soybean meal | 90.23 | 45.32 | 1.10 | 7.31 | 6.34 | 40.32 | 4451.10 |
| Yellow corn | 88.16 | 10.10 | 3.62 | 2.32 | 1.30 | 82.80 | 3985.01 |
| Rice bran | 89.28 | 14.01 | 6.43 | 9.90 | 5.31 | 64.43 | 3792.04 |
| Amino Acid Profile (% of Dry Weight) 1 | ||
|---|---|---|
| Fishmeal [37] | Gammarid Meal [38] | |
| Methionine | 1.99 | 1.1 |
| Lysine | 5.04 | 3.0 |
| Cystine | 0.86 | 0.5 |
| Arginine | 3.77 | 3.2 |
| Glycine | 4.4 | 2.3 |
| Histidine | 1.61 | 1.3 |
| Isoleucine | 3.1 | 1.9 |
| Leucine | 3.99 | 3.4 |
| Phenylalanine | 2.78 | 2.3 |
| Tyrosine | 2.52 | 1.9 |
| Serine | 3.29 | 1.9 |
| Threonine | 2.76 | 2.1 |
| Tryptophan | 0.75 | - |
| Valine | 3.5 | 2.3 |
| Diets * | D0 | D25 | D50 | D75 | D100 |
|---|---|---|---|---|---|
| Formulation (% DM) | |||||
| Fishmeal (FM) 1 | 20.00 | 15.00 | 10.00 | 5.00 | 0.00 |
| Cost | 509.55 | 382.17 | 254.78 | 127.39 | 0.00 |
| Amphipod meal (APM) | 0.00 | 8.5.00 | 17.00 | 25.50 | 34.00 |
| Cost | 0.00 | 108.28 | 216.56 | 324.84 | 433.12 |
| Soybean meal 2 | 42.5.00 | 42.5.00 | 42.00 | 42.00 | 42.00 |
| Cost | 189.17 | 189.17 | 187.26 | 187.26 | 187.26 |
| Yellow corn 2 | 17.00 | 15.00 | 13.00 | 11.5.00 | 10.00 |
| Cost | 39.49 | 34.39 | 29.94 | 26.18 | 22.93 |
| Rice bran 2 | 16.50 | 15.00 | 14.00 | 12.00 | 10.00 |
| Cost | 37.58 | 34.39 | 31.85 | 27.39 | 22.93 |
| Vitamin and mineral premix 3 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Cost | 127.39 | 127.39 | 127.39 | 127.39 | 127.39 |
| Fish Oil 4 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Cost | 25.48 | 25.48 | 25.48 | 25.48 | 25.48 |
| Total Cost (U.S.A. $/ton diet) | 928.66 | 901.27 | 873.25 | 845.92 | 819.11 |
| Proximate composition (%) | |||||
| Crude protein | 35.14 | 35.13 | 34.96 | 34.96 | 34.9 |
| Ether extract | 9.5 | 10.1 | 10.9 | 10.8 | 11.2 |
| Ash | 10.2 | 9.8 | 10.1 | 10.4 | 10.2 |
| Crude fiber | 2.4 | 2.6 | 2.5 | 2.7 | 2.5 |
| Nitrogen free extract | 37.9 | 37.5 | 36.5 | 36.1 | 36.1 |
| Dry matter | 93 | 95 | 94 | 93 | 95 |
| Gross energy (Kcal/kg) 5 | 4495 | 4543 | 4565 | 4548 | 4574 |
| Diets * | D0 | D25 | D50 | D75 | D100 |
|---|---|---|---|---|---|
| Initial weight (g/fish) | 0.096 ± 0.00 | 0.096 ± 0.00 | 0.098 ± 0.00 | 0.097 ± 0.00 | 0.097 ± 0.001 |
| Final weight (g/fish) | 1.29 ± 0.03 d | 1.38 ± 0.01 d | 1.80 ± 0.02 a | 1.68 ± 0.01 b | 1.49 ± 0.03 c |
| Weight gain (g/fish) | 1.20 ± 0.03 d | 1.29 ± 0.01 d | 1.70 ± 0.02 a | 1.57 ± 0.01 b | 1.40 ± 0.03 c |
| Initial length (cm/fish) | 1.33 ± 0.03 b | 1.37 ± 0.03 ab | 1.40 ± 0.00 a | 1.30 ± 0.00 b | 1.40 ± 0.00 a |
| Final length (cm/fish) | 3.83 ± 0.03 c | 3.93 ± 0.03 c | 4.47 ± 0.03 a | 4.03 ± 0.06 ab | 4.13 ± 0.03 b |
| Length gain (cm/fish) | 2.50 ± 0.06 c | 2.57 ± 0.03 bc | 3.06 ± 0.03 a | 3.00 ± 0.06 a | 2.73 ± 0.03 b |
| Survival (%) | 73.33 ± 3.3 d | 73.33 ± 3.3 d | 86.67 ± 3.3 a | 83.33 ± 3.3 b | 76.33 ± 3.3 c |
| Condition factor | 2.30 ± 0.05 | 2.27 ± 0.04 | 2.02 ± 0.06 | 2.11 ± 0.08 | 2.12 ± 0.07 |
| Diets * | D0 | D25 | D50 | D75 | D100 |
|---|---|---|---|---|---|
| Feed intake (g/fish) | 1.77 ± 0.03 b | 1.79 ± 0.01 b | 2.06 ± 0.02 a | 2.01 ± 0.05 a | 1.86 ± 0.01 b |
| Feed conversion ratio (Feed: gain) | 1.48 ± 0.03 a | 1.39 ± 0.01 ab | 1.21 ± 0.01 d | 1.27 ± 0.02 cd | 1.33 ± 0.03 bc |
| Protein intake (g/fish) | 0.71 ± 0.01 b | 0.71 ± 0.01 b | 0.82 ± 0.01 a | 0.80 ± 0.02 a | 0.74 ± 0.02 b |
| Protein efficiency ratio | 0.68 ± 0.01 d | 0.72 ± 0.03 cd | 0.83 ± 0.05 a | 0.79 ± 0.01 ab | 0.75 ± 0.02 bc |
| Protein productive value (%) | 23.51 ± 0.48 d | 25.18 ± 0.51 cd | 30.65 ± 0.54 a | 28.35 ± 0.83 b | 26.50 ± 0.46 c |
| Diets * | D0 | D25 | D50 | D75 | D100 |
|---|---|---|---|---|---|
| Moisture content | 77.9 ± 0.88 | 78.07 ± 1.53 | 77.97 ± 1.49 | 77.9 ± 1.13 | 77.93 ± 0.71 |
| Crude protein | 13.94 ± 0.08 c | 14.01 ± 0.05 bc | 14.81 ± 0.09 a | 14.39 ± 0.11 b | 14.11 ± 0.07 bc |
| Ether extract | 11.08 ± 0.08 | 11.07 ± 11.07 | 11.56 ± 0.04 | 11.22 ± 0.20 | 11.16 ± 0.06 |
| Ash | 3.40 ± 0.05 a | 3.26 ± 3.26 ab | 2.68 ± 0.06 c | 3.04 ± 0.03 b | 3.25 ± 0.04 ab |
| Gross energy (Kcal kg−1) | 5223 ± 10.0 c | 5245 ± 14.0 c | 5424 ± 19.0 a | 5308 ± 3.00 b | 5262 ± 8.00 bc |
| Diets * | D0 | D25 | D50 | D75 | D100 |
|---|---|---|---|---|---|
| Villus length (μm) | 413.7 ± 3.10 d | 416.5 ± 1.10 d | 475.8 ± 5.70 a | 457.10 ± 3.60 b | 425.70 ± 0.80 c |
| Villus width (μm) | 90.70 ± 1.30 e | 99.70 ± 1.50 d | 131.80 ± 2.20 a | 128.60 ± 1.10 b | 108.60 ± 1.40 c |
| Crypts depth (μm) | 44.00 ± 0.60 e | 48.50 ± 1.10 d | 77.5 ± 2.10 a | 68.20 ± 1.10 b | 55.20 ± 1.40 c |
| Muscle thickness (μm) | 20.80 ± 0.40 e | 22.00 ± 0.60 d | 29.20 ± 0.70 a | 27.30 ± 0.66 b | 24.70 ± 0.33 c |
| Diets * | Fish Price (US$ 1000 fish−1) | Feed Cost (US$ 1000 fish−1) | Value of Fish (US$) | Incidence Cost (US$) | Change in Incidence Cost (%) | Profit Index (LE) | Change in Profit Index (%) | Economic Conversion Rate (US$) | Change in Economic Conversion Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| D0 | 101.91 | 1532.10 | 74.71 | 1606.82 | 100 | 0.049 | 100 | 20.11 | 100 |
| D25 | 101.91 | 1500.38 | 78.79 | 1579.17 | 98.06 | 0.053 | 107.68 | 18.29 | 90.95 |
| D50 | 101.91 | 1668.98 | 88.34 | 1757.32 | 108.38 | 0.053 | 108.50 | 15.39 | 76.52 |
| D75 | 101.91 | 1573.44 | 84.90 | 1658.34 | 102.53 | 0.054 | 110.65 | 15.61 | 77.60 |
| D100 | 101.91 | 1405.10 | 77.77 | 1482.87 | 92.22 | 0.055 | 113.50 | 15.77 | 78.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, M.; Abo-Taleb, H.A.; Hassan, A.-K.M.; Abdelzaher, O.F.; Mabrouk, M.M.; Elokaby, M.A.; Alzahrani, O.M.; Mahmoud, S.F.; El-feky, M.M.M.; Shaban, W.M.; et al. Valorization Use of Amphipod Meal, Gammarus pulex, as a Fishmeal Substitute on Growth Performance, Feed Utilization, Histological and Histometric Indices of the Gut, and Economic Revenue of Grey Mullet. J. Mar. Sci. Eng. 2021, 9, 1336. https://doi.org/10.3390/jmse9121336
Ashour M, Abo-Taleb HA, Hassan A-KM, Abdelzaher OF, Mabrouk MM, Elokaby MA, Alzahrani OM, Mahmoud SF, El-feky MMM, Shaban WM, et al. Valorization Use of Amphipod Meal, Gammarus pulex, as a Fishmeal Substitute on Growth Performance, Feed Utilization, Histological and Histometric Indices of the Gut, and Economic Revenue of Grey Mullet. Journal of Marine Science and Engineering. 2021; 9(12):1336. https://doi.org/10.3390/jmse9121336
Chicago/Turabian StyleAshour, Mohamed, Hamdy A. Abo-Taleb, Abdel-Kader M. Hassan, Othman F. Abdelzaher, Mohamed M. Mabrouk, Mohamed A. Elokaby, Othman M. Alzahrani, Samy F. Mahmoud, Mohamed M. M. El-feky, Walaa M. Shaban, and et al. 2021. "Valorization Use of Amphipod Meal, Gammarus pulex, as a Fishmeal Substitute on Growth Performance, Feed Utilization, Histological and Histometric Indices of the Gut, and Economic Revenue of Grey Mullet" Journal of Marine Science and Engineering 9, no. 12: 1336. https://doi.org/10.3390/jmse9121336
APA StyleAshour, M., Abo-Taleb, H. A., Hassan, A.-K. M., Abdelzaher, O. F., Mabrouk, M. M., Elokaby, M. A., Alzahrani, O. M., Mahmoud, S. F., El-feky, M. M. M., Shaban, W. M., & Mansour, A. T. (2021). Valorization Use of Amphipod Meal, Gammarus pulex, as a Fishmeal Substitute on Growth Performance, Feed Utilization, Histological and Histometric Indices of the Gut, and Economic Revenue of Grey Mullet. Journal of Marine Science and Engineering, 9(12), 1336. https://doi.org/10.3390/jmse9121336









