The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in Laboratory Batch Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Purification
2.2. Experimental Conditions
2.3. Tested Parameters and Statistical Analysis
3. Results
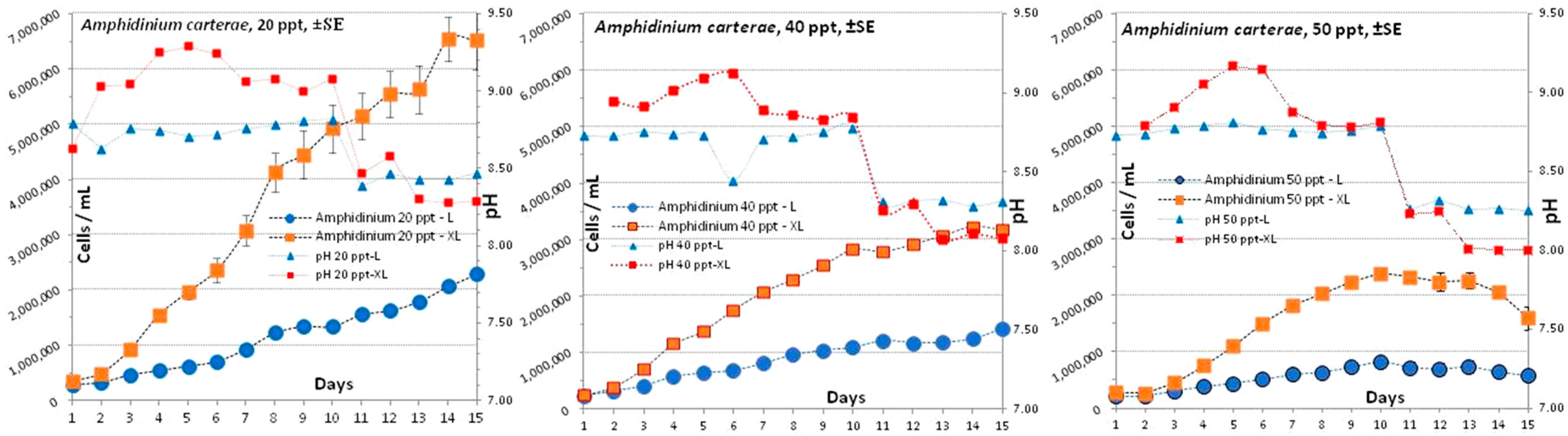
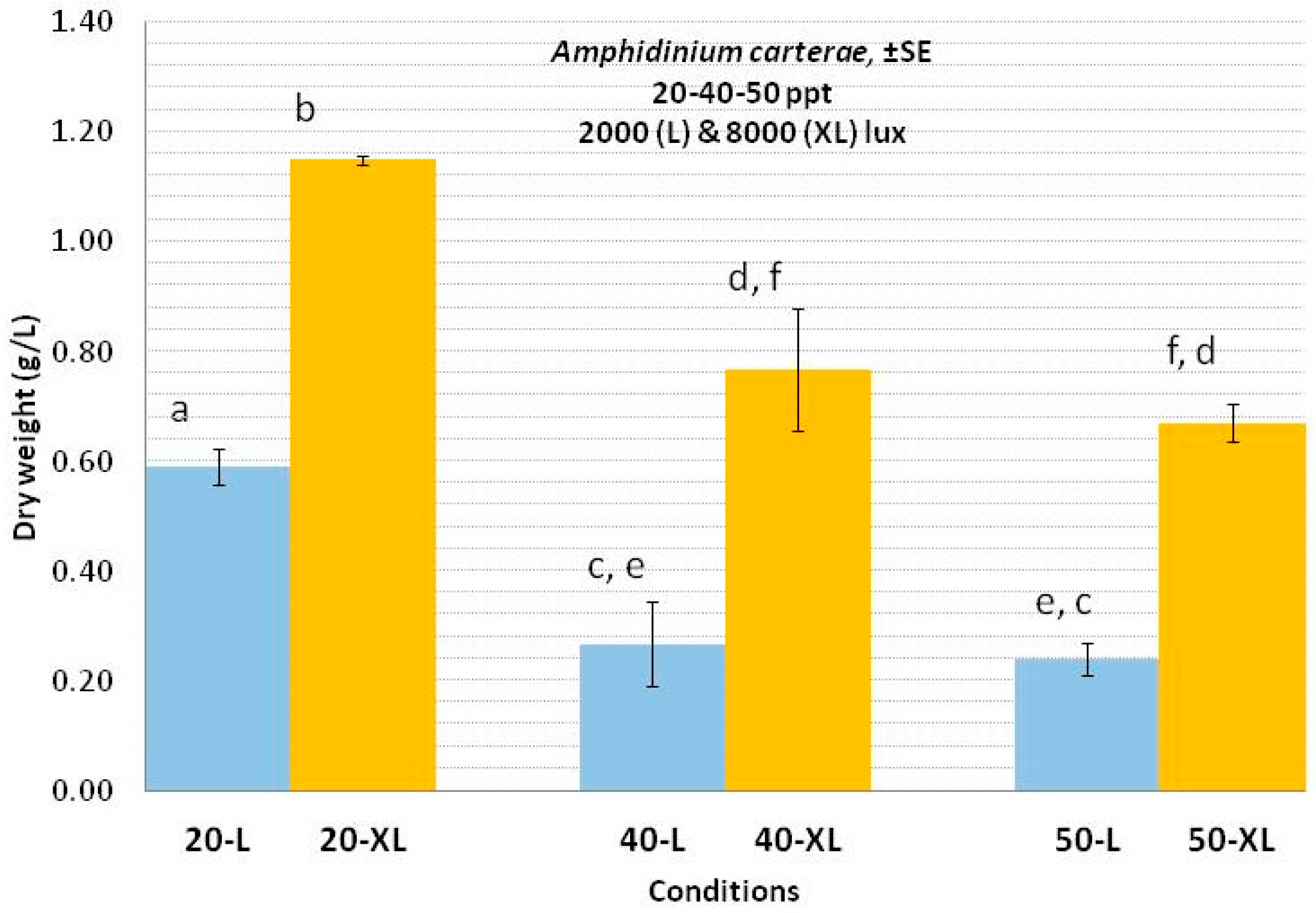
3.1. Amphidinium carterae
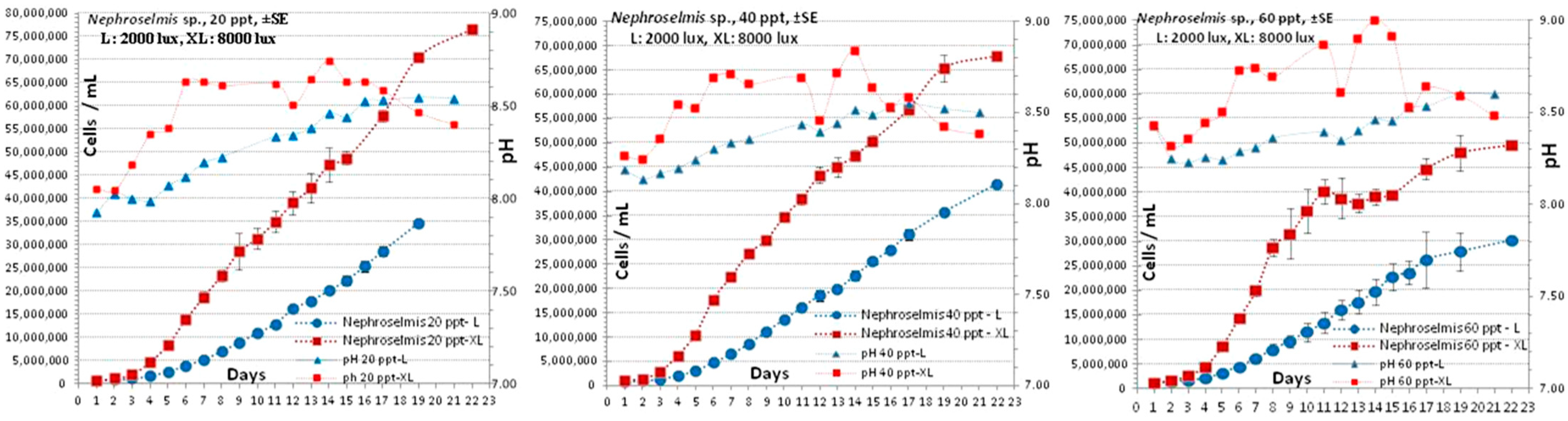
3.2. Nephroselmis sp.
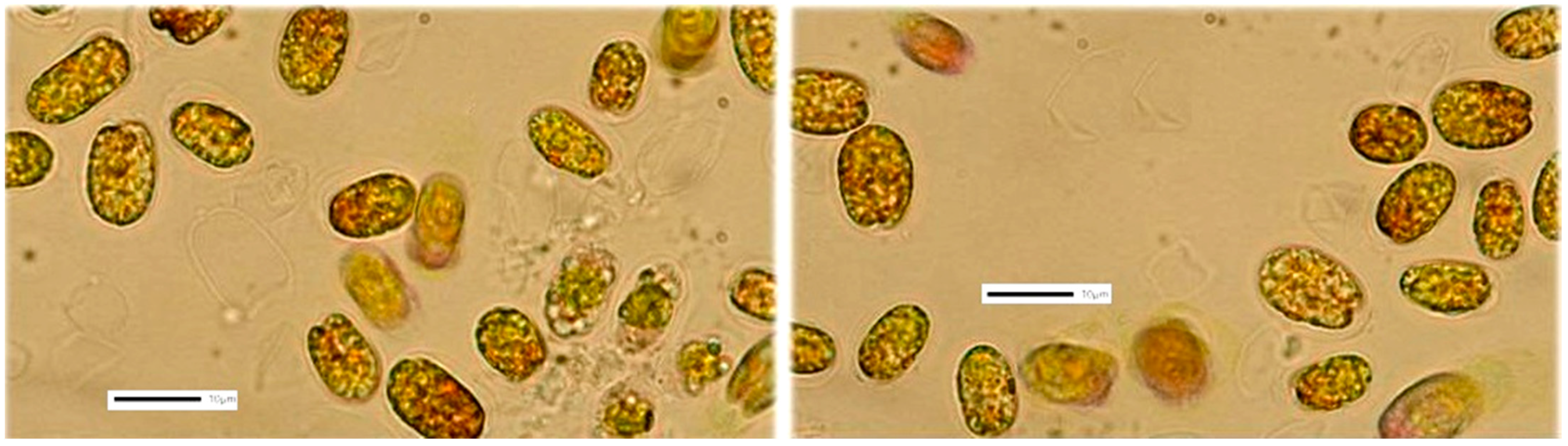
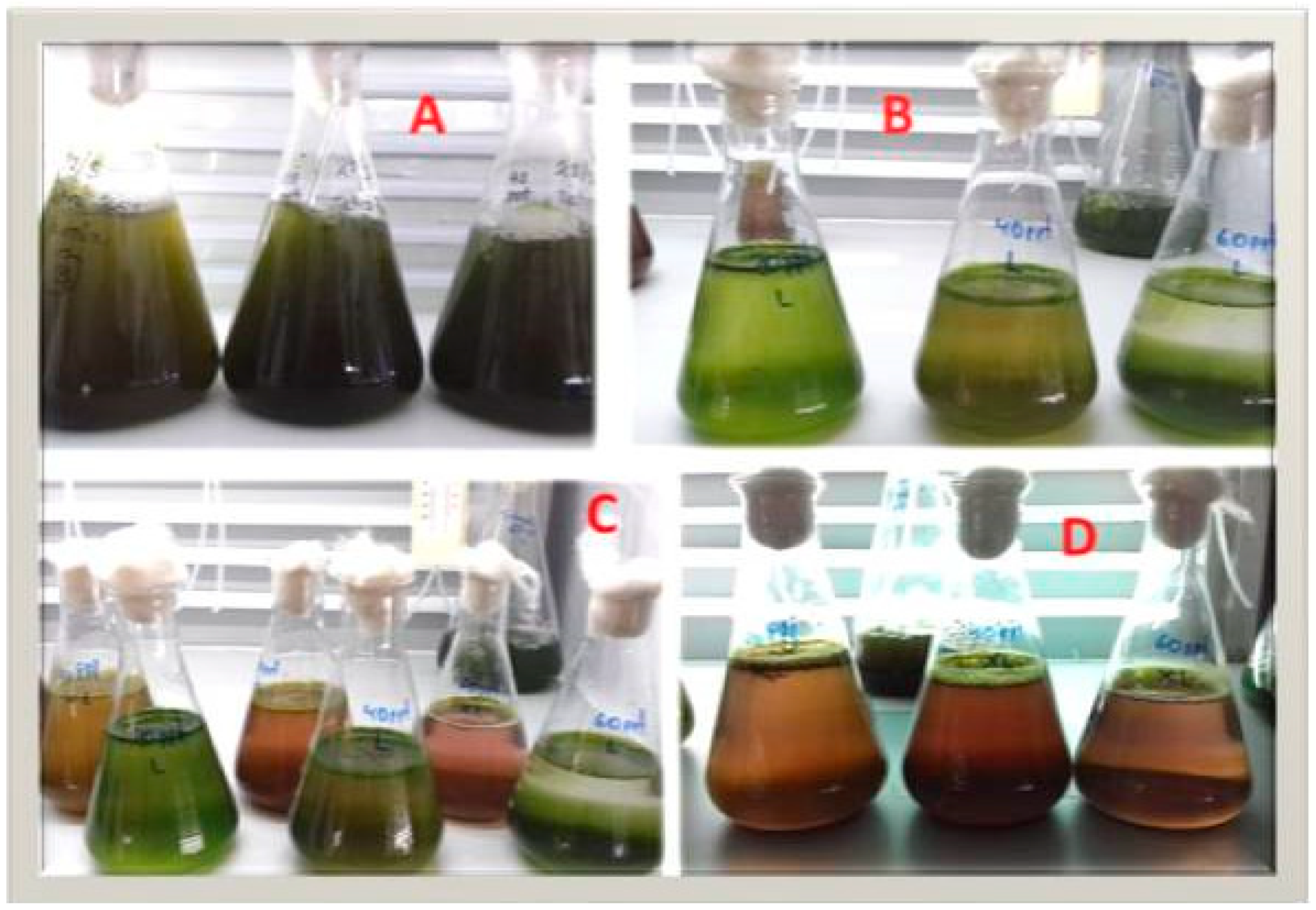
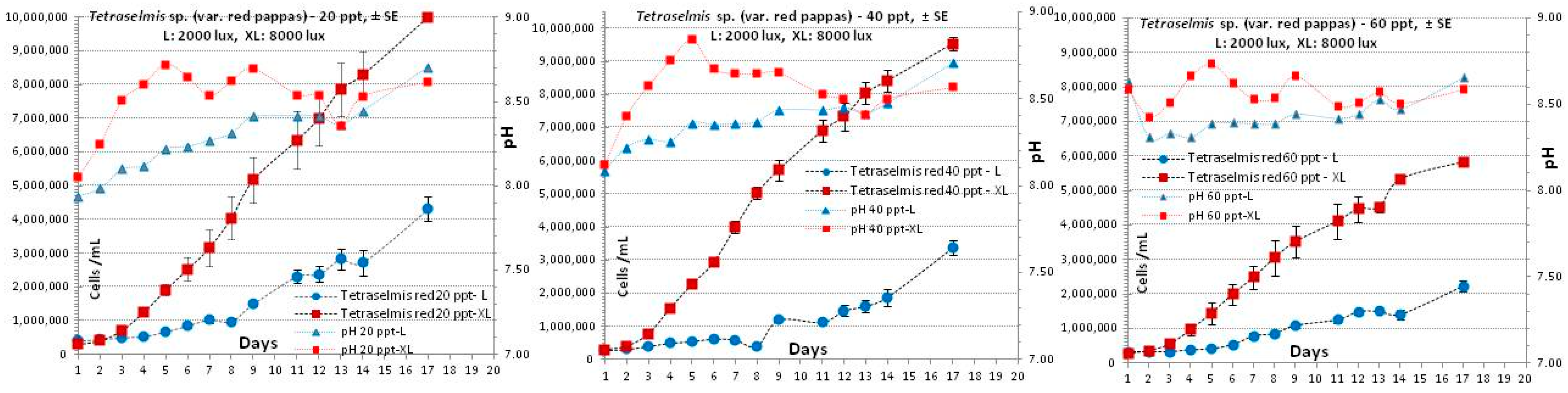
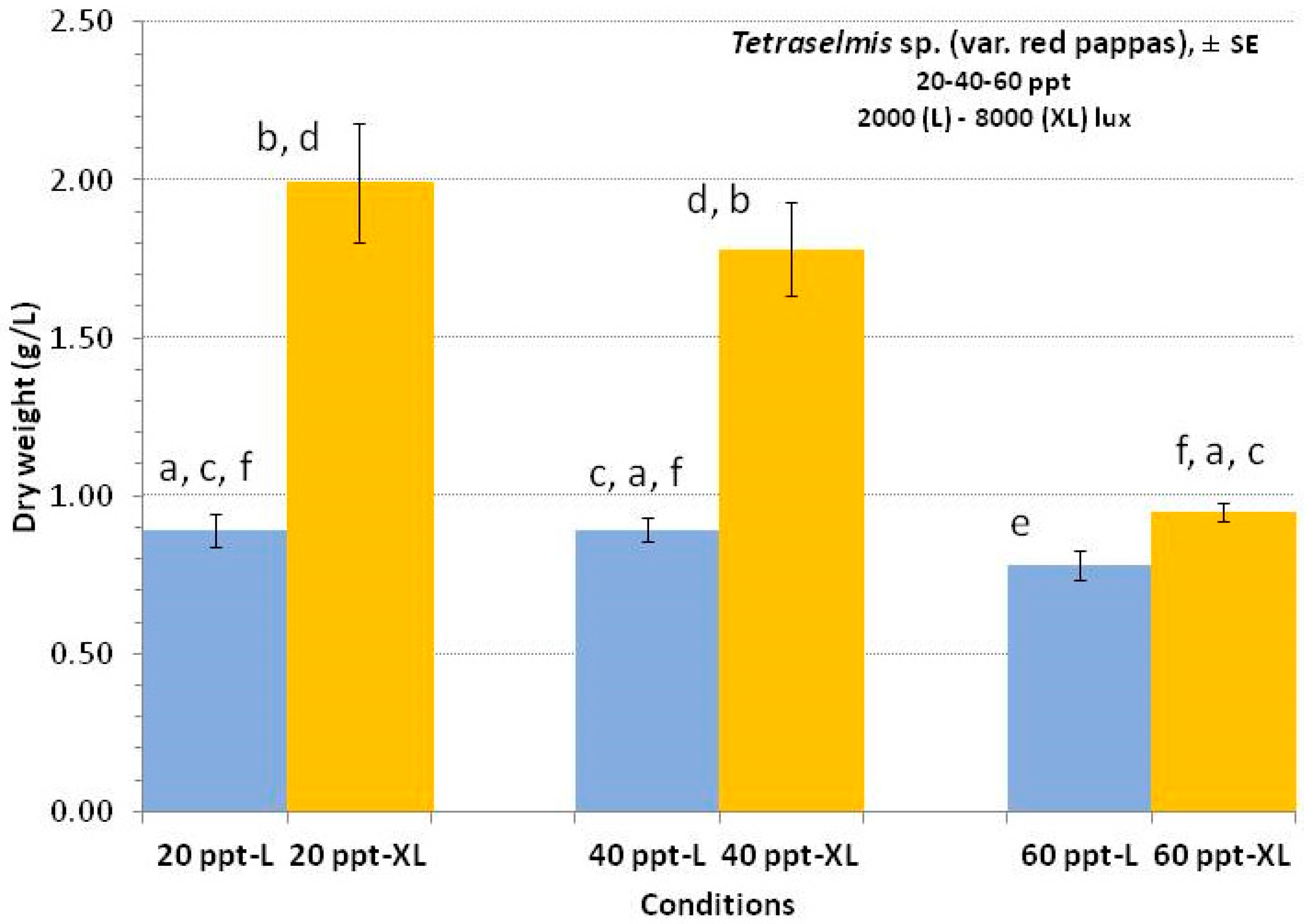
3.3. Tetraselmis sp. (var. red pappas)
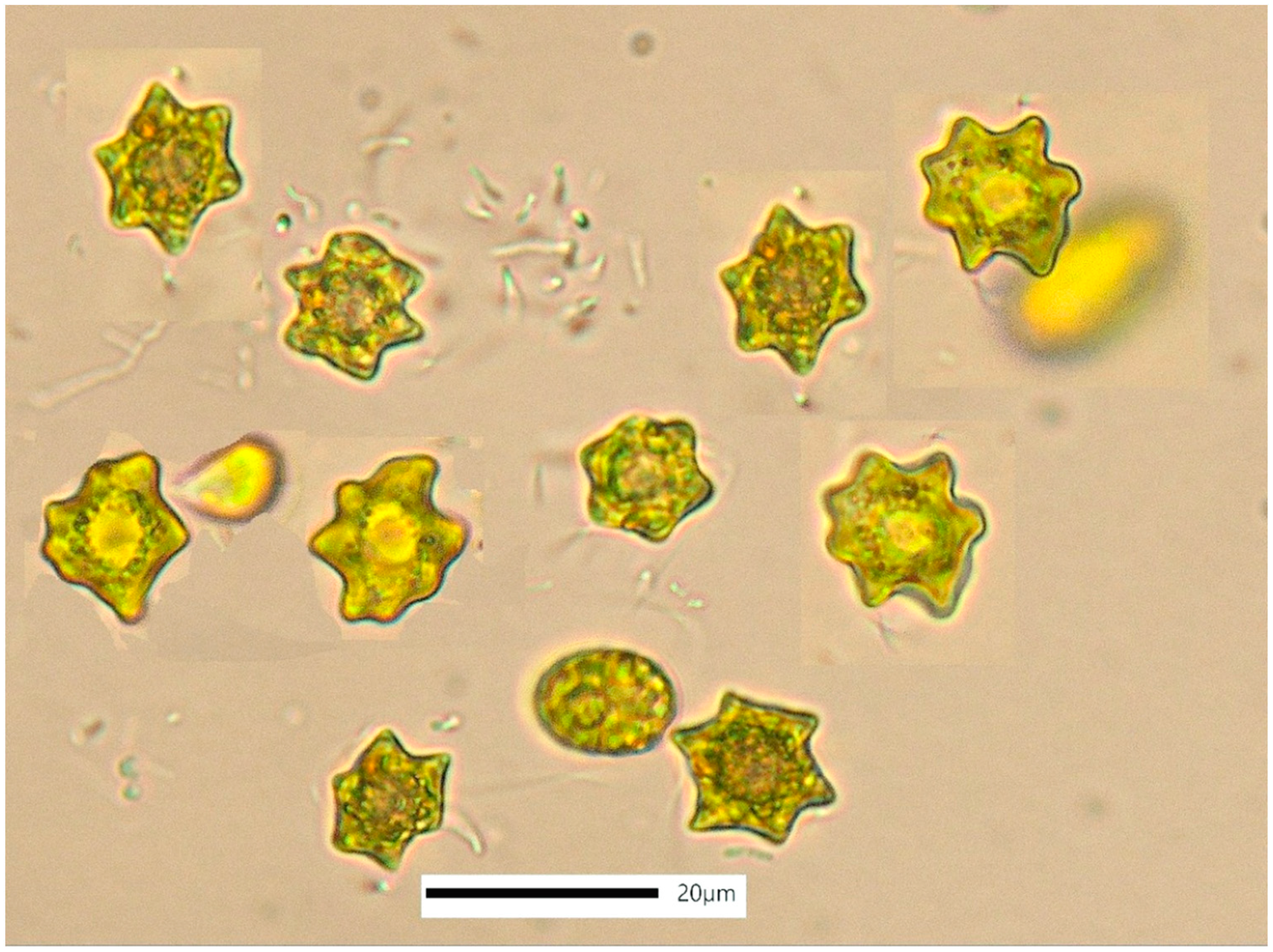
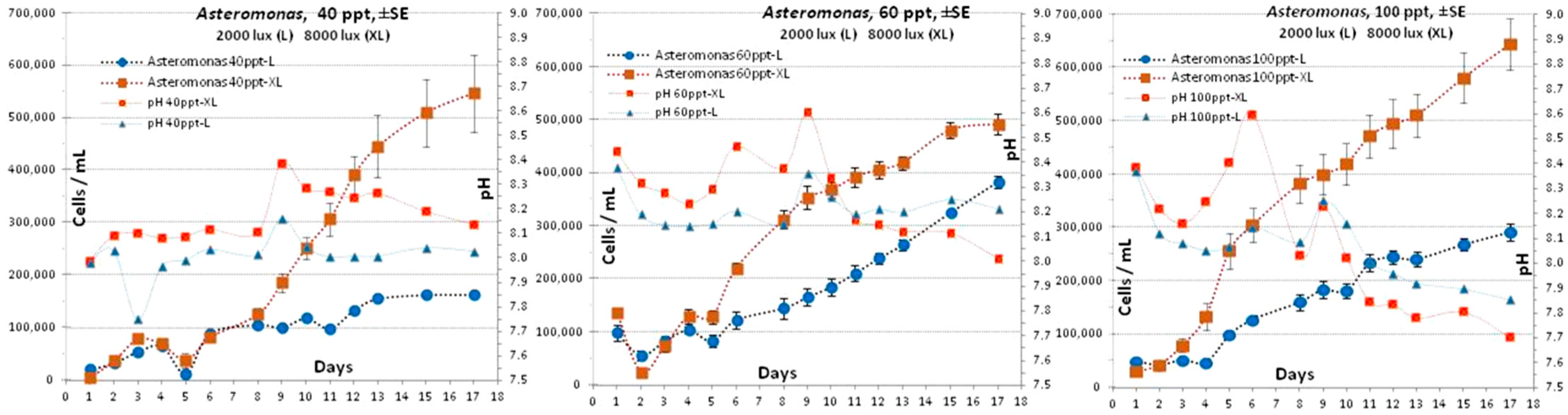
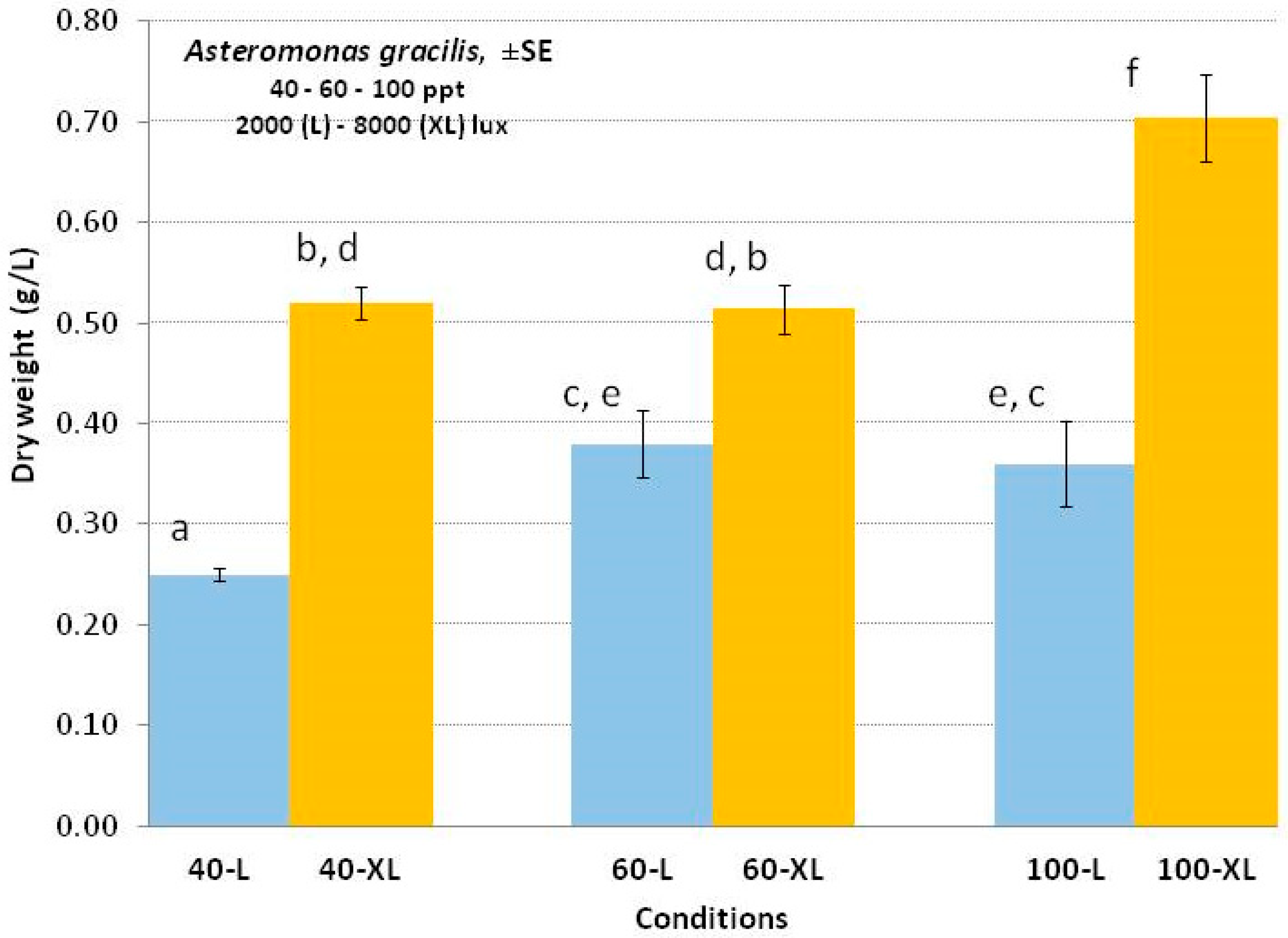
3.4. Asteromonas gracilis
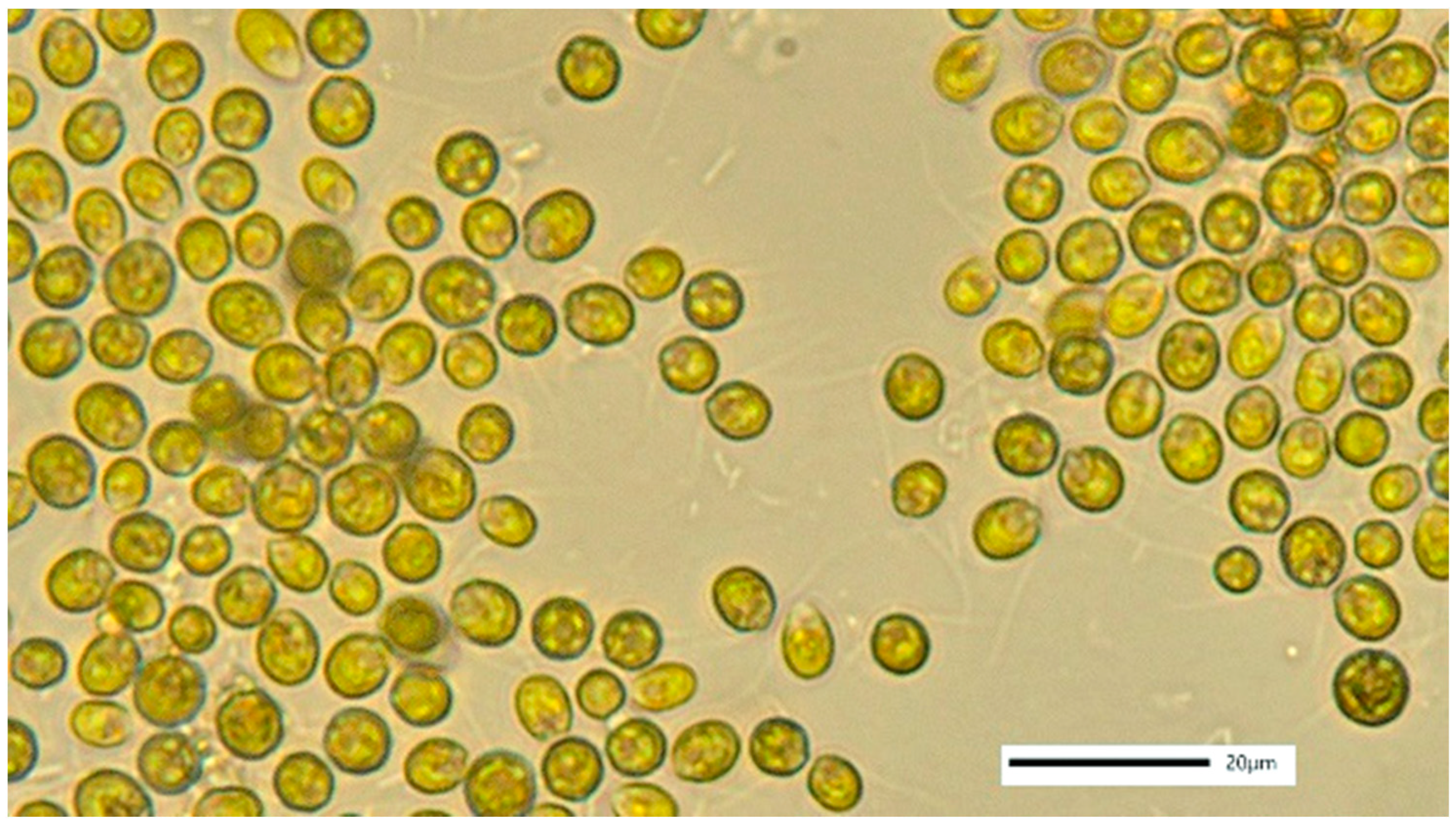
3.5. Dunaliella sp.
4. Discussion
4.1. Amphidinium carterae
4.2. Nephroselmis sp.
4.3. Tetraselmis sp. (var. red pappas)
4.4. Asteromonas gracilis
4.5. Dunaliella sp.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravindran, B.; Gupta, S.K.; Cho, W.-M.; Kim, J.K.; Lee, S.R.; Jeong, K.-H.; Lee, D.J.; Choi, H.-C. Microalgae Potential and Multiple Roles—Current Progress and Future Prospects—An Overview. Sustainability 2016, 8, 1215. [Google Scholar] [CrossRef]
- Muller-Feuga, A.; Cahu, R.R.; Robin, J.; Divanach, P. Uses of Microalgae in Aquaculture. In Live Feeds in Marine Aquaculture; Støttrup, J.G., McEvoy, L.A., Eds.; Blackwell Science Ltd.: Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Gatamaneni, B.L.; Orsat, V.; Lefsrud, M. Factors affecting growth of various microalgal species. Environ. Eng. Sci. 2018, 35, 1037–1048. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-value biomass from microalgae production platforms: Strategies and progress based on carbon metabolism and energy conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef]
- Hotos, G.N. Culture Growth of the Cyanobacterium Phormidium sp. in Various Salinity and Light Regimes and their Influence on its Phycocyanin and Other Pigments Content. J. Mar. Sci. Eng. 2021, 9, 798. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Lai, Y.T.; Tsang, P.M.; Chow, K.P.; Yau, Y.H.; Choi, M.C.; Ho, R.S.C. Effects of light intensity, illumination cycles on microalgae Haematococcus pluvialis for production of astaxanthin. J. Marine Biol. Aquacult. 2016, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Raqiba, H.; Sibi, G. Light emitting diode (LED) illumination for enhanced growth and cellular composition in three microalgae. Adv. Microb. Res. 2019, 3, 7. [Google Scholar] [CrossRef]
- Bilanovic, D.; Andargatchew, A.; Kroeger, T.; Shelef, G. Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations Response surface methodology analysis. Energy Convers. Manag. 2009, 50, 262–267. [Google Scholar] [CrossRef]
- Vonshak, A.; Torzillo, G. Environmental Stress Physiology. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Hoboken, NJ, USA, 2004; pp. 73–75. [Google Scholar]
- Fisher, N.S.; Schwarzenbach, R.P. Fatty acid dynamics in Thalassiosira pseudonana (Bacillariophyceae), Implications for physiological ecology. J. Phycol. 1978, 14, 143–150. [Google Scholar] [CrossRef]
- Tzovenis, I.; Fountoulaki, E.; Dolapsakis, N.; Kotzamanis, I.; Nengas, I.; Bitis, I.; Cladas, Y.; Economou-Amilli, A. Screening for marine nanoplanktic microalgae from Greek coastal lagoons (Ionian Sea) for use in mariculture. J. Appl. Phycol. 2009, 21, 457–469. [Google Scholar] [CrossRef]
- Walne, P.R. Experiments in the Large-Scale Culture of the Larvae of Ostrea edulis L.; HMSO: London, UK, 1966; Volume 25. [Google Scholar]
- Xu, Y.; Ibrahim, M.I.; Harvey, J.P. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016, 106, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G. A Short Review on the Halotolerant Green Microalga Asteromonas gracilis Artari with Emphasis on Its Uses. Asian J. Fish. Aquat. Res. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Hotos, G.; Avramidou, D. Growth study of the one-celled halophilous alga Asteromonas gracilis (Chlorophyta) in mass culture condition using different salinities, photoperiod and without addition of vitamins (in Greek with English summary). Geotech. Sci. Issues Geotech. Chamb. Greece 1995, 6, 37–45. [Google Scholar]
- Shah, M.M.R.; Samarakoon, W.K.; An, S.-J.; Jeon, Y.-J.; Lee, J.-B. Growth characteristics of three benthic dinoflagellates in mass culture and their antioxidant properties. J. Fish. Aquat. Sci. 2016, 11, 268–277. [Google Scholar] [CrossRef]
- Hotos, G. Selectivity of the rotifer Brachionus plicatilis fed mixtures of algal species with various cell volumes and cell densities. Aquacult. Res. 2002, 33, 949–957. [Google Scholar] [CrossRef]
- Hotos, G.N. Growth, filtration and ingestion rate of the rotifer Brachionus plicatilis fed with large (Asteromonas gracilis) and small (Chlorella sp.) celled algal species. Aquacult. Res. 2003, 34, 793–802. [Google Scholar] [CrossRef]
- Molina-Miras, A.; López-Rosales, L.; Sánchez-Mirón, A.; López-Rodríguez, M.; Cerón-García, M.C.; García-Camacho, F.; Molina-Grima, E. Influence of culture medium recycling on the growth of a marine dinoflagellate microalga and bioactives production in a raceway photobioreactor. Algal Res. 2020, 47, 101820. [Google Scholar] [CrossRef]
- López-Rosales, L.; García-Camacho, F.; Sánchez-Mirón, A.; Contreras-Gómez, A.; Molina-Grima, E. An optimization approach for culturing shear-sensitive dinoflagellate microalgae in bench-scale bubble column photobioreactors. Bioresour. Technol. 2015, 197, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; García-Camacho, F.; Cerón-García, M.C.; Belarbi, E.H.; Chisti, Y.; Molina-Grima, E. Causes of shear sensitivity of the toxic dinoflagellate Protoceratium reticulatum. Biotechnol. Prog. 2009, 25, 792–800. [Google Scholar] [CrossRef]
- García-Camacho, F.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Cerón-García, M.C.; Belarbi, E.H.; Molina Grima, E. Determination of shear stress thresholds in toxic dinoflagellates cultured in shaken flasks: Implications in bioprocess engineering. Proc. Biochem. 2007, 42, 1506–1515. [Google Scholar] [CrossRef]
- Morton, S.L.; Norris, D.R.; Bomber, J.W. Effect of temperature, salinity and light intensity on the growth and seasonality of toxic dinoflagellates associated with ciguatera. J. Exp. Mar. Biol. Ecol. 1992, 157, 79–90. [Google Scholar] [CrossRef]
- Dixon, G.K.; Syrett, P.J. The growth of dinoflagellates in laboratory cultures. New Phytol. 1988, 109, 297–302. [Google Scholar] [CrossRef]
- Aquino-Cruz, A.; Okolodkov, Y.B. Impact of increasing water temperature on growth, photosynthetic efficiency, nutrient consumption, and potential toxicity of Amphidinium cf. carterae and Coolia monotis (Dinoflagellata). Rev. Biol. Mar. Oceanogr. 2016, 51, 565–580. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Barthelemy, V.; Schreiber, N.; Brun, P.; Lebouvier, N.; Jauffrais, T. Effects of Nitrogen Availability on the Antioxidant Activity and Carotenoid Content of the Microalgae Nephroselmis sp. Mar. Drugs 2020, 18, 453. [Google Scholar] [CrossRef]
- Park, S.; Ahn, Y.; Pandi, K.; Ji, M.-K.; Yun, H.-S.; Choi, J.-Y. Microalgae Cultivation in Pilot Scale for Biomass Production Using Exhaust Gas from Thermal Power Plants. Energies 2019, 12, 3497. [Google Scholar] [CrossRef]
- Anderson, R.; Charvet, S.; Hansen, P.J. Mixotrophy in Chlorophytes and Haptophytes—Effect of Irradiance, Macronutrient, Micronutrient and Vitamin Limitation. Front. Microbiol. 2018, 9, 1704. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2018.01704 (accessed on 1 November 2021). [CrossRef]
- Thinh, L.-V.; Renaud, M.S.; Parry, L.D. Evaluation of recently isolated Australian tropical microalgae for the enrichment of the dietary value of brine shrimp, Artemia nauplii. Aquaculture 1999, 170, 161–173. [Google Scholar] [CrossRef]
- Fogg, G.E. The Ecological Significance of Extracellular Products of Phytoplankton Photosynthesis. Bot. Mar. 1983, 26, 3–14. [Google Scholar] [CrossRef]
- Hadj-Romdhane, F.; Zheng, X.; Jaouen, P.; Pruvost, J.; Grizeau, D.; Croué, P.J.; Bourseau, P. The culture of Chlorella vulgaris in a recycled supernatant: Effects on biomass production and medium quality. Bioresour. Technol. 2013, 132, 285–292. [Google Scholar] [CrossRef]
- Loftus, E.S.; Johnson, I.Z. Cross-study analysis of factors affecting algae cultivation in recycled medium for biofuel production. Algal Res. 2017, 24, 154–166. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Wu, Y.-H.; Espinosa, D.M.V.; Zhang, T.-Y.; Dao, G.-H.; Hu, H.-Y. Soluble Algal Products (SAPs) in large scale cultivation of microalgae for biomass/bioenergy production: A review. Renew. Sustain. Energy Rev. 2016, 59, 141–148. [Google Scholar] [CrossRef]
- Nguyen, M.-L.; Westerhoff, P.; Baker, L.; Hu, Q.; Esparza-Soto, M.; Sommerfeld, M. Characteristics and reactivity of algae-produced dissolved organic carbon. J. Environ. Eng. 2005, 131, 1574–1582. [Google Scholar] [CrossRef]
- Prakash, A.; Rashid, M.A.; Jensen, A.; Subha Rao, D.V. Influence of Humic Substances On The Growth of Marine Phytoplankton: Diatoms. Limnol. Oceanogr. 1973, 18, 15–30. [Google Scholar] [CrossRef]
- Depraetere, O.; Pierre, G.; Noppe, W.; Vandamme, D.; Foubert, I.; Michaud, P.; Muylaert, K. Influence of culture medium recycling on the performance of Arthrospira platensis cultures. Algal Res. 2015, 10, 48–54. [Google Scholar] [CrossRef]
- Henderson, K.R.; Baker, A.; Parsons, A.S.; Jefferson, B. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms. Water Res. 2008, 42, 3435–3445. [Google Scholar] [CrossRef]
- Montes-González, O.; González-Silvera, A.; Valenzuela-Espinoza, E.; Santamaría-Del-Ángel, E.; López-Calderón, J. Effect of light intensity and nutrient concentration on growth and pigments of the green microalga Tetraselmis suecica. Lat. Am. J. Aquat. Res. 2021, 49, 431–441. [Google Scholar] [CrossRef]
- Venckus, P.; Cicchi, B.; Chini Zittelli, G. Effects of medium salinity on growth and biochemical composition of the green microalga Tetraselmis suecica. J. Appl. Phycol. 2021, 1–9. [Google Scholar] [CrossRef]
- Trovão, M.; Pereira, H.; Silva, J.; Páramo, J.; Quelhas, P.; Santos, T.; Silva, T.J.; Machado, A.; Gouveia, L.; Barreira, L.; et al. Growth performance, biochemical composition and sedimentation velocity of Tetraselmis sp. CTP4 under different salinities using low-cost lab- and pilot-scale systems. Heliyon 2019, 5, e01553. [Google Scholar] [CrossRef]
- Pugkaew, W.; Meetam, M.; Yokthongwattana, K.; Leeratsuwan, N.; Pokethitiyook, P. Effects of salinity changes on growth, photosynthetic activity, biochemical composition, and lipid productivity of marine microalga Tetraselmis suecica. J. Appl. Phycol. 2019, 31, 969–979. [Google Scholar] [CrossRef]
- Dammak, M.; Hadrich, B.; Barkallah, M.; Hentati, F.; Ben Hlima, H.; Pichon, C.; Denis, M.; Fendri, I.; Michaud, P.; Abdelkafi, S. Modelling Tetraselmis sp. growth-kinetics and optimizing bioactive-compound production through environmental conditions. Bioresour. Technol. 2018, 249, 510–518. [Google Scholar] [CrossRef]
- Khatoon, H.; Abdu Rahman, N.; Banerjee, S.; Harun, N.; Suleiman, S.S.; Zakaria, H.N.; Lananan, F.; Abdul Hamid, H.S.; Endut, A. Effects of different salinities and pH on the growth and proximate composition of Nannochloropsis sp. and Tetraselmis sp. isolated from South China Sea cultured under control and natural conditions. Int. Biodeterior. Biodegrad. 2014, 95, 11–18. [Google Scholar] [CrossRef]
- Go, S.; Lee, S.-J.; Jeong, G.T.; Kim, S.-K. Factors affecting the growth and the oil accumulation of marine microalgae, Tetraselmis suecica. Bioproc. Biosyst. Eng. 2012, 35, 145–150. [Google Scholar] [CrossRef]
- Meseck, L.S.; Alix, H.J.; Wikfors, H.G. Photoperiod and light intensity effects on growth and utilization of nutrients by the aquaculture feed microalga, Tetraselmis chui (PLY429). Aquaculture 2005, 246, 393–404. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae for aquaculture: Opportunities and constraints. J. Appl. Phycol. 1997, 9, 393–401. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Z.; Wen, W.; Yan, J. Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J. Appl. Phycol. 2012, 25, 129–137. [Google Scholar] [CrossRef]
- Fon Sing, S.; Isdepsky, A.; Borowitzka, M.A.; Lewis, D.M. Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: A novel protocol for commercial microalgal biomass production. Bioresour. Technol. 2014, 161, 47–54. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. Accumulation of metabolites by halotolerant algae and its industrial potential. Annu. Rev. Microbiol. 1983, 37, 95–119. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Grunwald, T. Osmoregulation in the Halotolerant Alga Asteromonas gracilis. Plant Physiol. 1981, 67, 613–616. [Google Scholar] [CrossRef]
- Fawzy, M.A. Fatty acid characterization and biodiesel production by the marine Microalga Asteromonas gracilis: Statistical optimization of medium for biomass and lipid enhancement. Mar. Biotechnol. 2017, 19, 219–231. [Google Scholar] [CrossRef]
- Oren, A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005, 1, 1–14. [Google Scholar] [CrossRef]
- Ramos, A.A.; Polle, J.; Tran, D.; Cushman, C.J.; Jin, E.-S.; Varela, C.J. The Unicellular Green Alga Dunaliella Salina Teod. as a Model for Abiotic Stress Tolerance: Genetic Advances and Future Perspectives. Algae 2011, 26, 3–20. [Google Scholar] [CrossRef]
- Schlipalius, L. The extensive commercial cultivation of Dunaliella salina. Bioresour. Technol. 1991, 38, 241–243. [Google Scholar] [CrossRef]
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microb. 2009, 107, 14–35. [Google Scholar] [CrossRef]
- Hermawan, J.; Masithah, E.D.; Tjahjaningsih, W.; Abdillah, A.A. Increasing β-carotene content of phytoplankton Dunaliella salina using different salinity media. IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 012034. [Google Scholar] [CrossRef]
- Reshma, R.; Chitra Devi, K.; Dinesh Kumar, S.; Santhanam, P.; Perumal, P.; Krishnaveni, N.; Begum, A.; Pragnya, M.; Arthikha, R.; Dhanalakshmi, B.; et al. Enhancement of pigments production in the green microalga Dunaliella salina (PSBDU05) under optimized culture condition. Bioresour. Technol. Rep. 2021, 14, 100672. [Google Scholar] [CrossRef]
- May, Q.S. The Effect of Various Environmental Factors of a Red Pigmented Dunaliella Species from the Great Salt Lake. Master’s Thesis, Utah State University, Logan, UT, USA, 1978; p. 51. Available online: https://digitalcommons.usu.edu/etd/3381 (accessed on 1 November 2021).
- Nguyen, A.; Tran, D.; Ho, M.; Louime, C.; Tran, D. High light stress regimen on Dunaliella salina strains for carotenoids induction. Integr. Food Nutr. Metab. 2016, 3, 347–350. [Google Scholar] [CrossRef]
- Abu-Rezq, T.; Al-Hooti, S.; Dangly, A.J. Optimum culture conditions required for the locally isolated Dunaliella salina. J. Algal Biomass Util. 2010, 1, 12–19. [Google Scholar]
- Park, S.; Lee, Y.; Jin, E.-S. Comparison of the responses of two Dunaliella strains, Dunaliella salina CCAP 19/18 and Dunaliella bardawil to light intensity with special emphasis on carotenogenesis. Algae 2013, 28, 203–211. [Google Scholar] [CrossRef]
- Zerveas, S.; Mente, M.S.; Tsakiri, D.; Kotzabasis, K. Microalgal photosynthesis induces alkalization of aquatic environment as a result of H+ uptake independently from CO2 concentration—New perspectives for environmental applications. J. Environ. Manag. 2021, 289, 112546. [Google Scholar] [CrossRef]






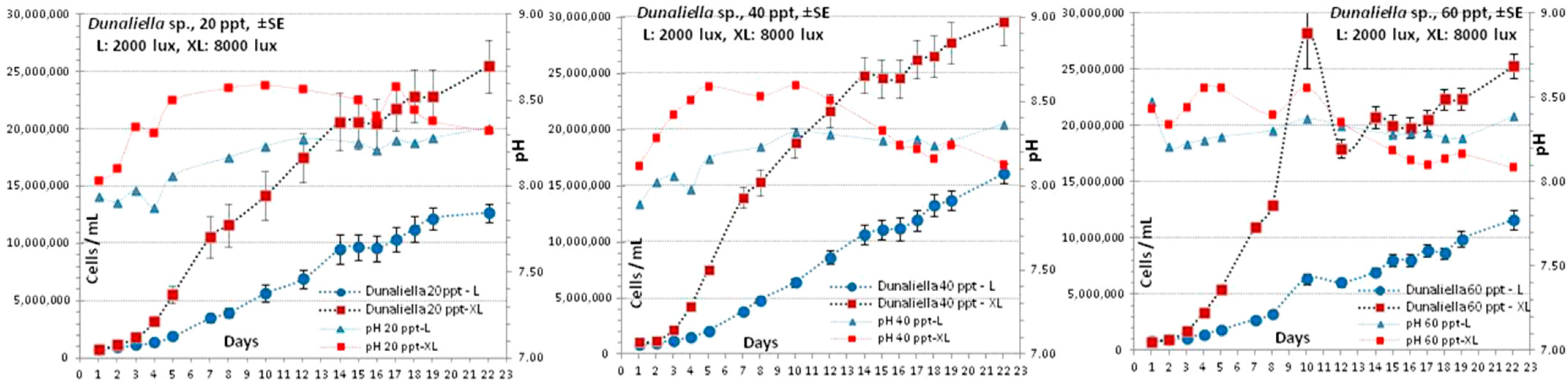
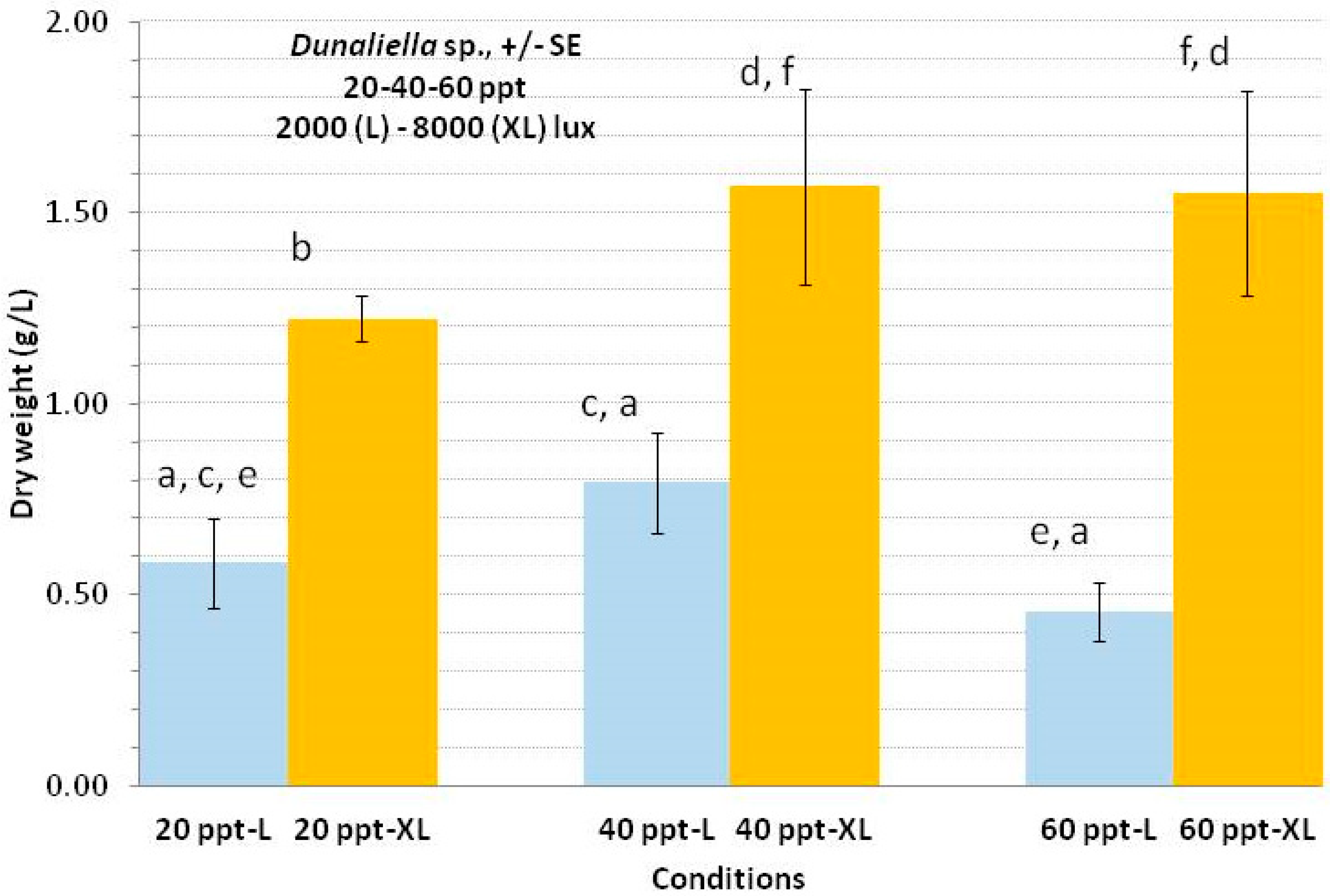
| Conditions | 20 ppt-L | 20 ppt-XL | 40 ppt-L | 40 ppt-XL | 50 ppt-L | 50 ppt-XL |
|---|---|---|---|---|---|---|
| SGR ± SE | 0.189 a ± 0.0077 | 0.295 b ± 0.0064 | 0.167 c ± 0.0025 | 0.232 d ± 0.0022 | 0.143 e ± 0.0074 | 0.302 f,b ± 0.0023 |
| tg (days) ± SE | 3.67 ± 0.185 | 2.35 ± 0.049 | 4.16 ± 0.062 | 2.98 ± 0.028 | 4.85 ± 0.323 | 2.29 ± 0.018 |
| Conditions | 20 ppt-L | 20 ppt-XL | 40 ppt-L | 40 ppt-XL | 60 ppt-L | 60 ppt-XL |
|---|---|---|---|---|---|---|
| SGR ± SE | 0.338 a ± 0.001 | 0.401 b ± 0.0021 | 0.341 c,a,e ± 0.0016 | 0.370 d ± 0.0006 | 0.336 e,a,c ± 0.0008 | 0.462 f ± 0.0023 |
| tg (days) ± SE | 2.051 ± 0.003 | 1.729 ± 0.009 | 2.032 ± 0.01 | 1.875 ± 0.003 | 2.063 ± 0.005 | 1.499 ± 0.011 |
| Conditions | 20 ppt-L | 20 ppt-XL | 40 ppt-L | 40 ppt-XL | 60 ppt-L | 60 ppt-XL |
|---|---|---|---|---|---|---|
| SGR ± SE | 0.181 a ± 0.0035 | 0.323 b ± 0.0069 | 0.180 c,a ± 0.0023 | 0.331 d,b ± 0.0037 | 0.203 e ± 0.0031 | 0.310 f ± 0.0019 |
| tg (days) ± SE | 3.860 ± 0.074 | 2.163 ± 0.046 | 3.870 ± 0.052 | 2.097 ± 0.023 | 3.429 ± 0.052 | 2.236 ± 0.013 |
| Conditions | 40 ppt-L | 40 ppt-XL | 60 ppt-L | 60 ppt-XL | 100 ppt-L | 100 ppt-XL |
|---|---|---|---|---|---|---|
| SGR ± SE | 0.106 a ± 0.007 | 0.143 b ± 0.01 | 0.190 c,e ± 0.005 | 0.273 d,f ± 0.014 | 0.205 e ± 0.009 | 0.280 f ± 0.01 |
| tg (days) ± SE | 8.034 ± 0.773 | 5.674 ± 0.426 | 3.711 ± 0.101 | 2.768 ± 0.154 | 3.607 ± 0.175 | 2.551 ± 0.07 |
| Conditions | 20 ppt-L | 20 ppt-XL | 40 ppt-L | 40 ppt-XL | 60 ppt-L | 60 ppt-XL |
|---|---|---|---|---|---|---|
| SGR | 0.239 a ± 0.01 | 0.350 b ± 0.015 | 0.279 c,a ± 0.004 | 0.387 d,b ± 0.0037 | 0.215 e ± 0.003 | 0.405 f,d ± 0.007 |
| tg (days) | 3.020 ± 0.111 | 2.09 ± 0.095 | 2.495 ± 0.036 | 1.815 ± 0.042 | 3.246 ± 0.052 | 1.774 ± 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotos, G.N.; Avramidou, D. The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in Laboratory Batch Cultures. J. Mar. Sci. Eng. 2021, 9, 1275. https://doi.org/10.3390/jmse9111275
Hotos GN, Avramidou D. The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in Laboratory Batch Cultures. Journal of Marine Science and Engineering. 2021; 9(11):1275. https://doi.org/10.3390/jmse9111275
Chicago/Turabian StyleHotos, George N., and Despoina Avramidou. 2021. "The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in Laboratory Batch Cultures" Journal of Marine Science and Engineering 9, no. 11: 1275. https://doi.org/10.3390/jmse9111275
APA StyleHotos, G. N., & Avramidou, D. (2021). The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in Laboratory Batch Cultures. Journal of Marine Science and Engineering, 9(11), 1275. https://doi.org/10.3390/jmse9111275







