Phytoplankton Biomass Dynamics in Tropical Coastal Waters of Jakarta Bay, Indonesia in the Period between 2001 and 2019
Abstract
1. Introduction
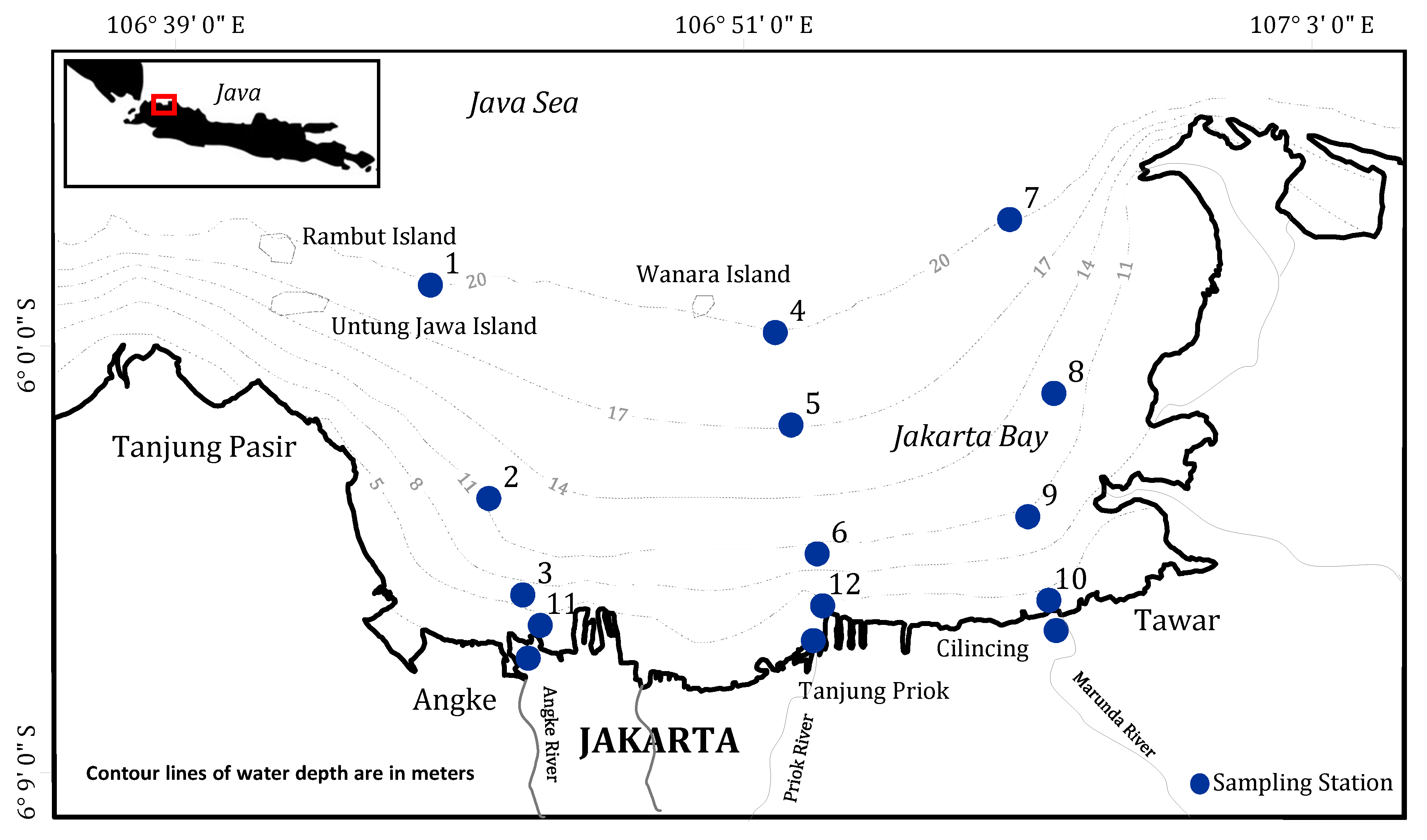
2. Materials and Methods
3. Results
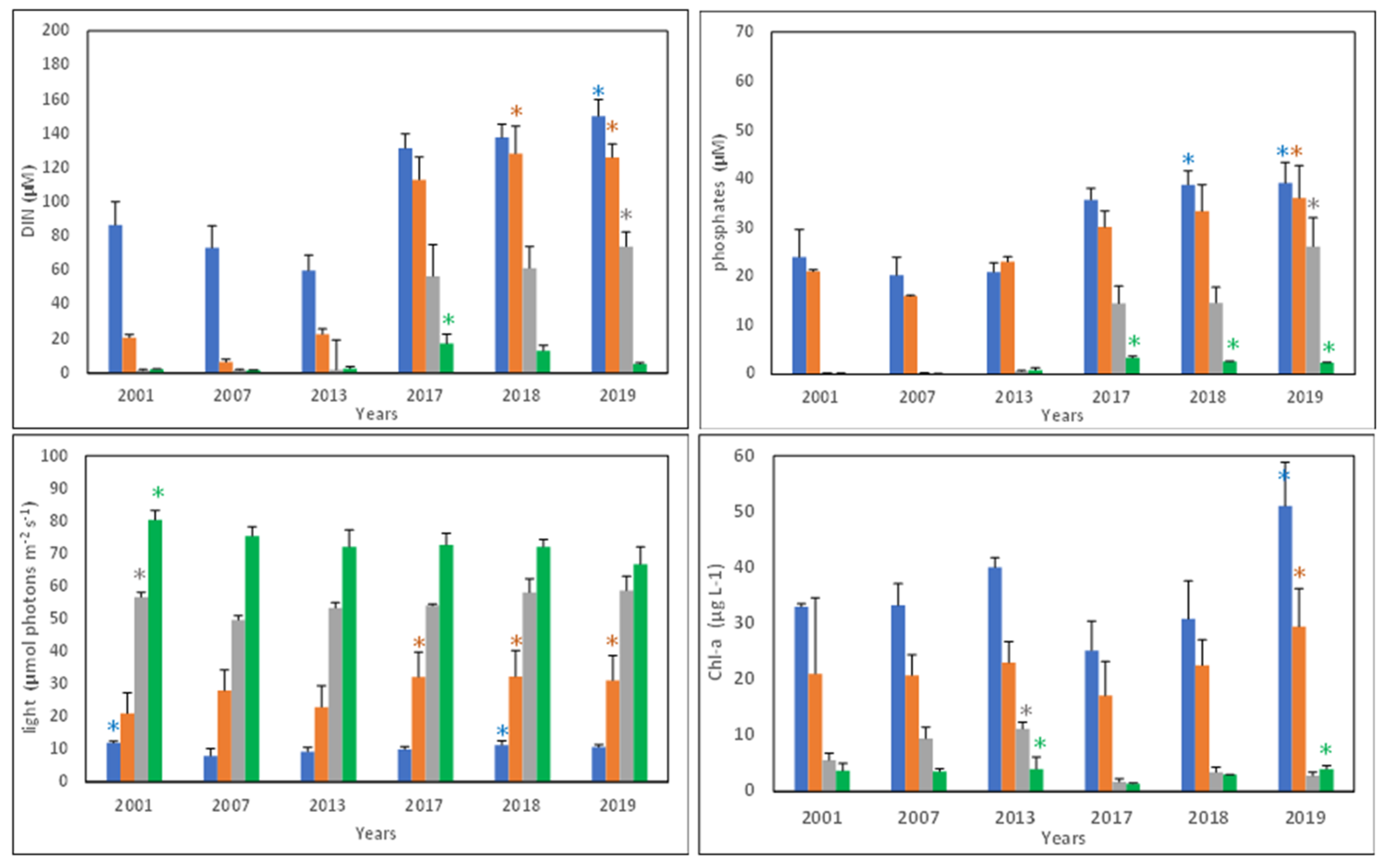
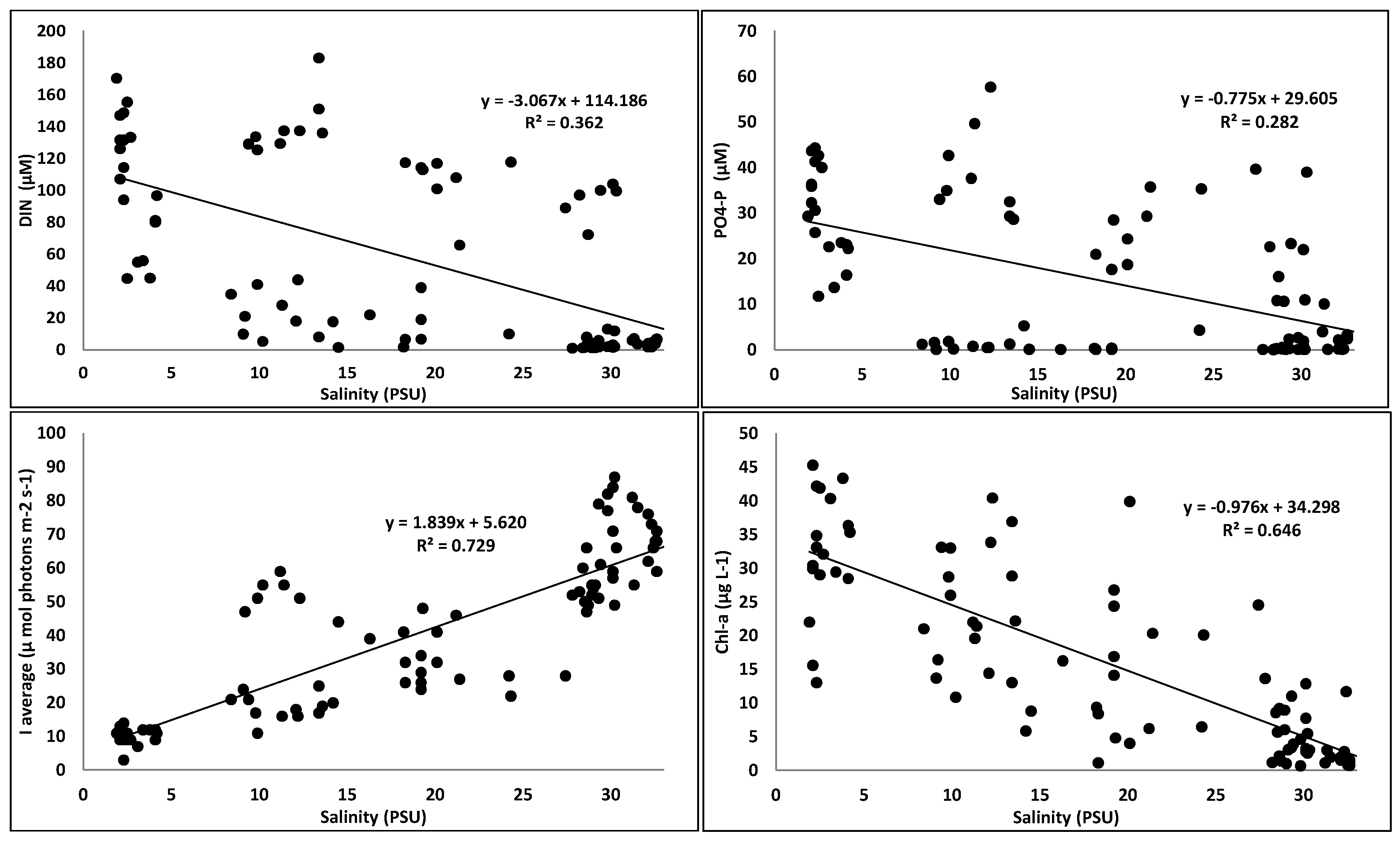
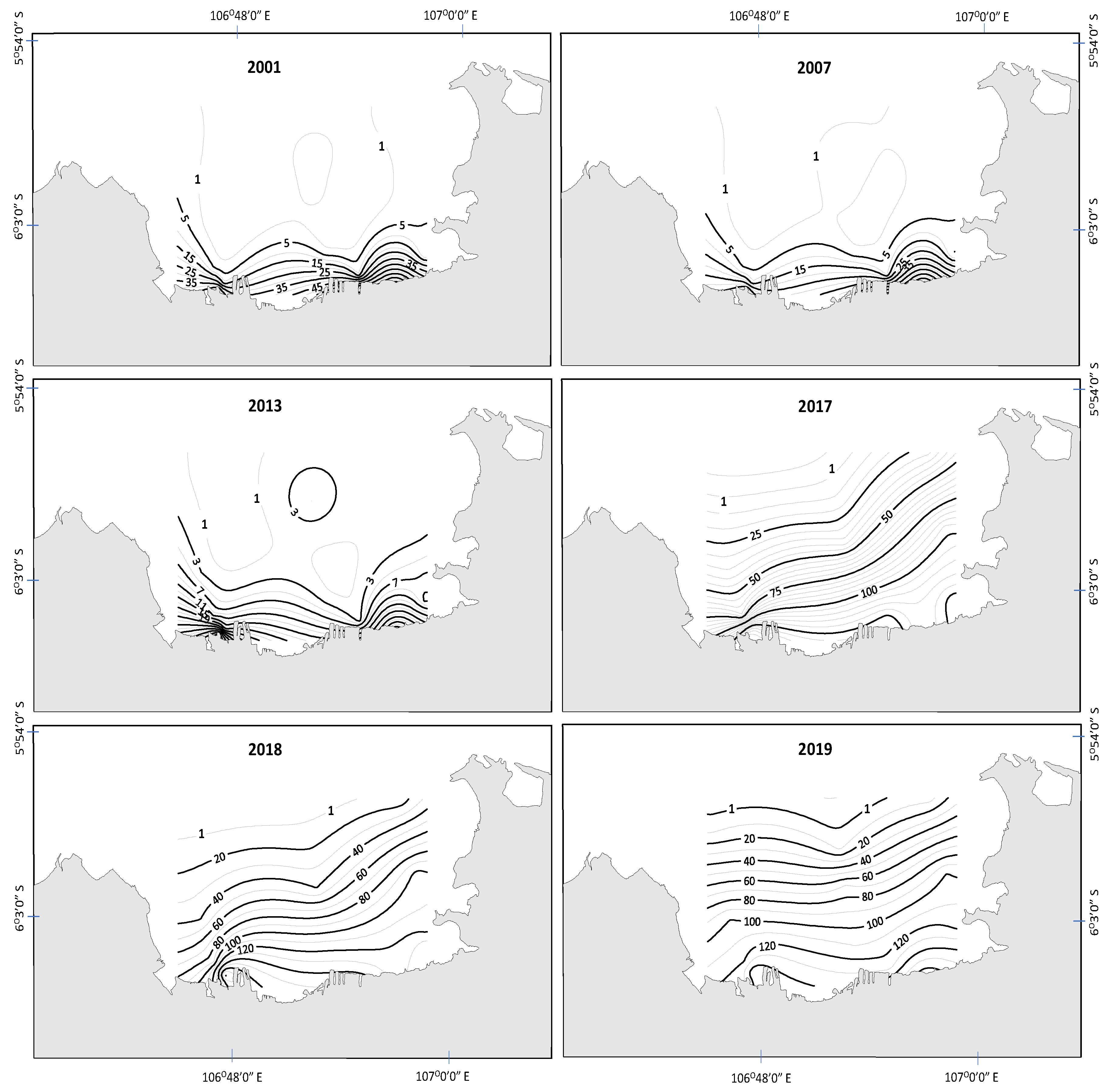
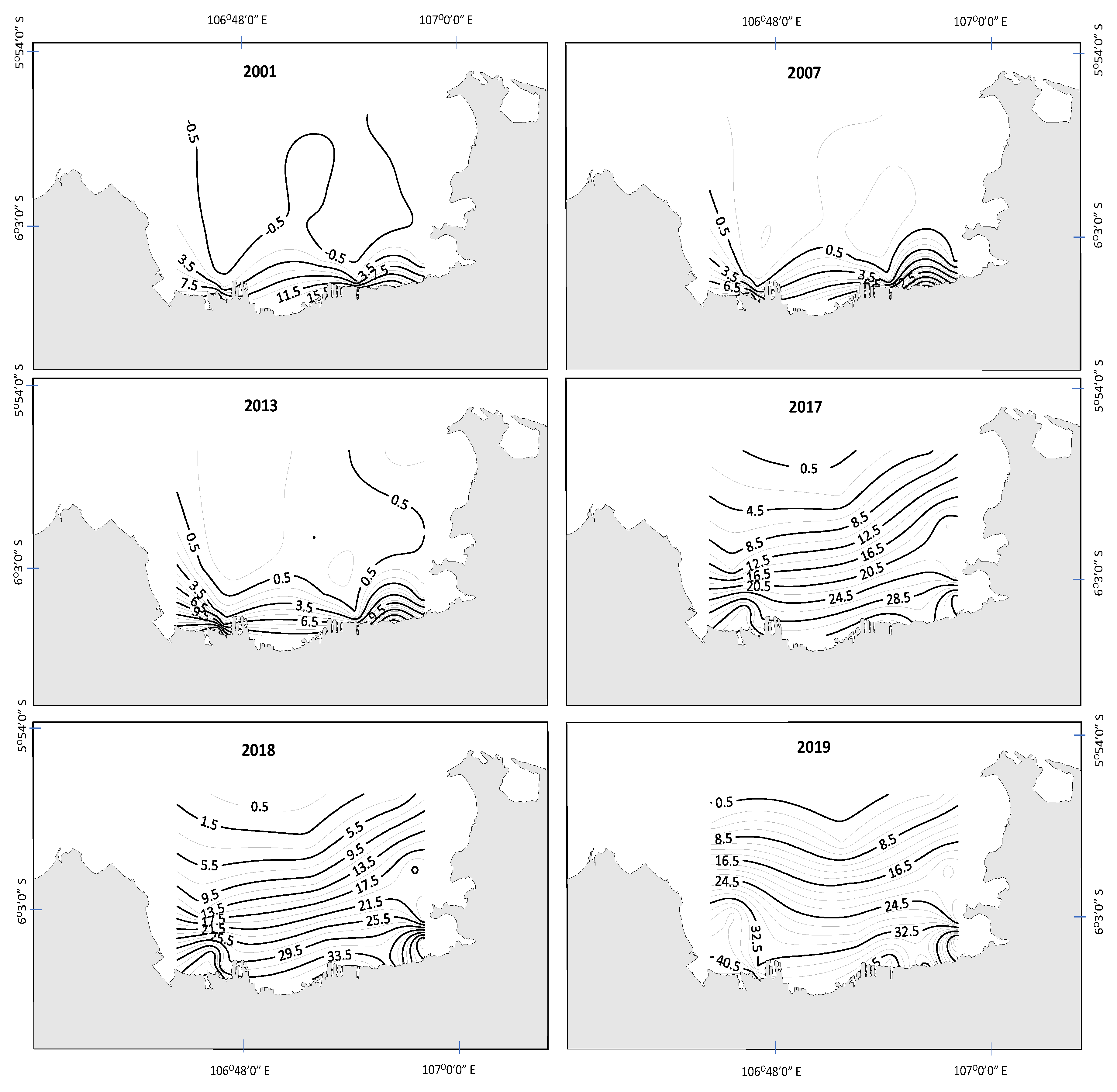
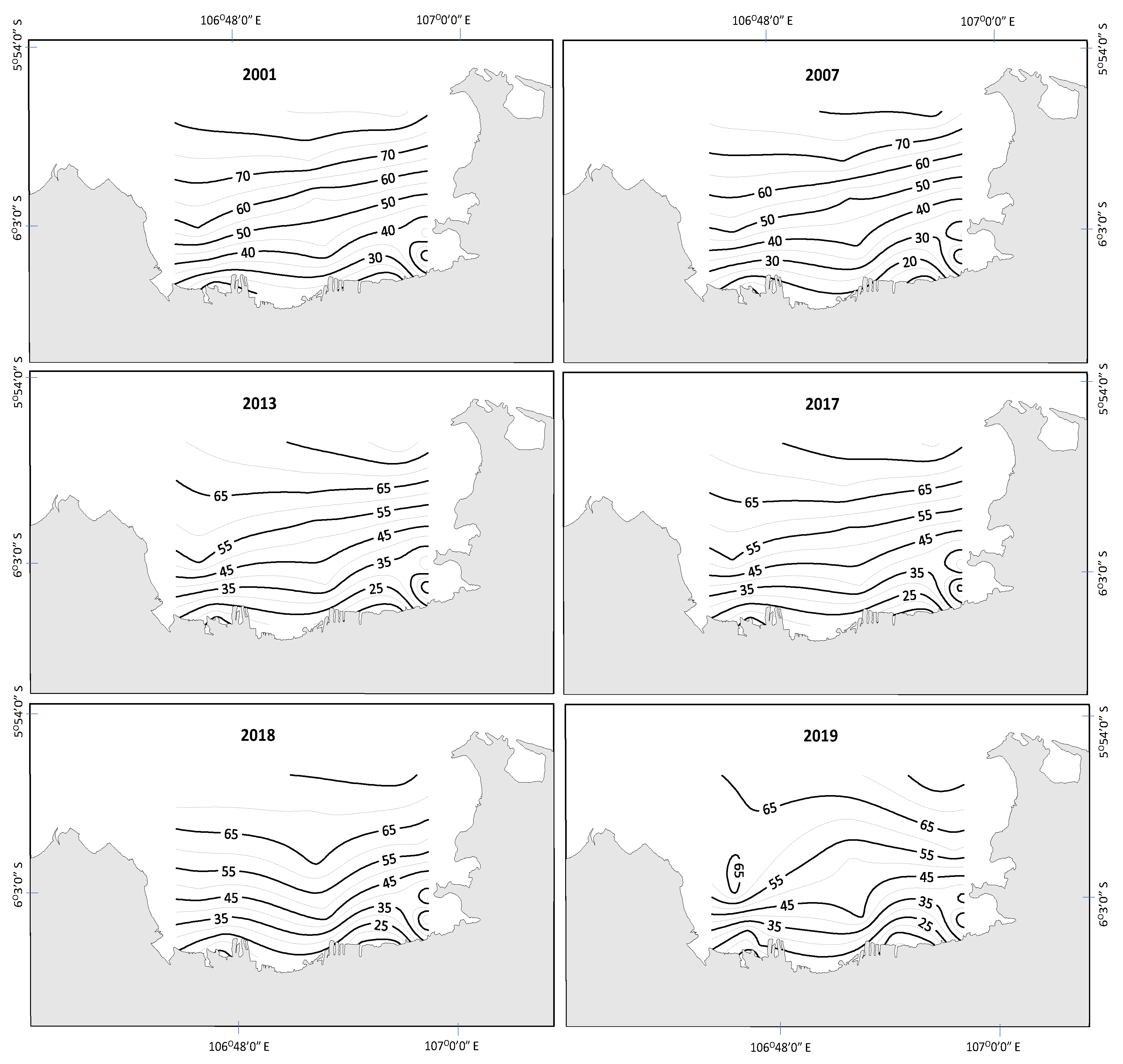
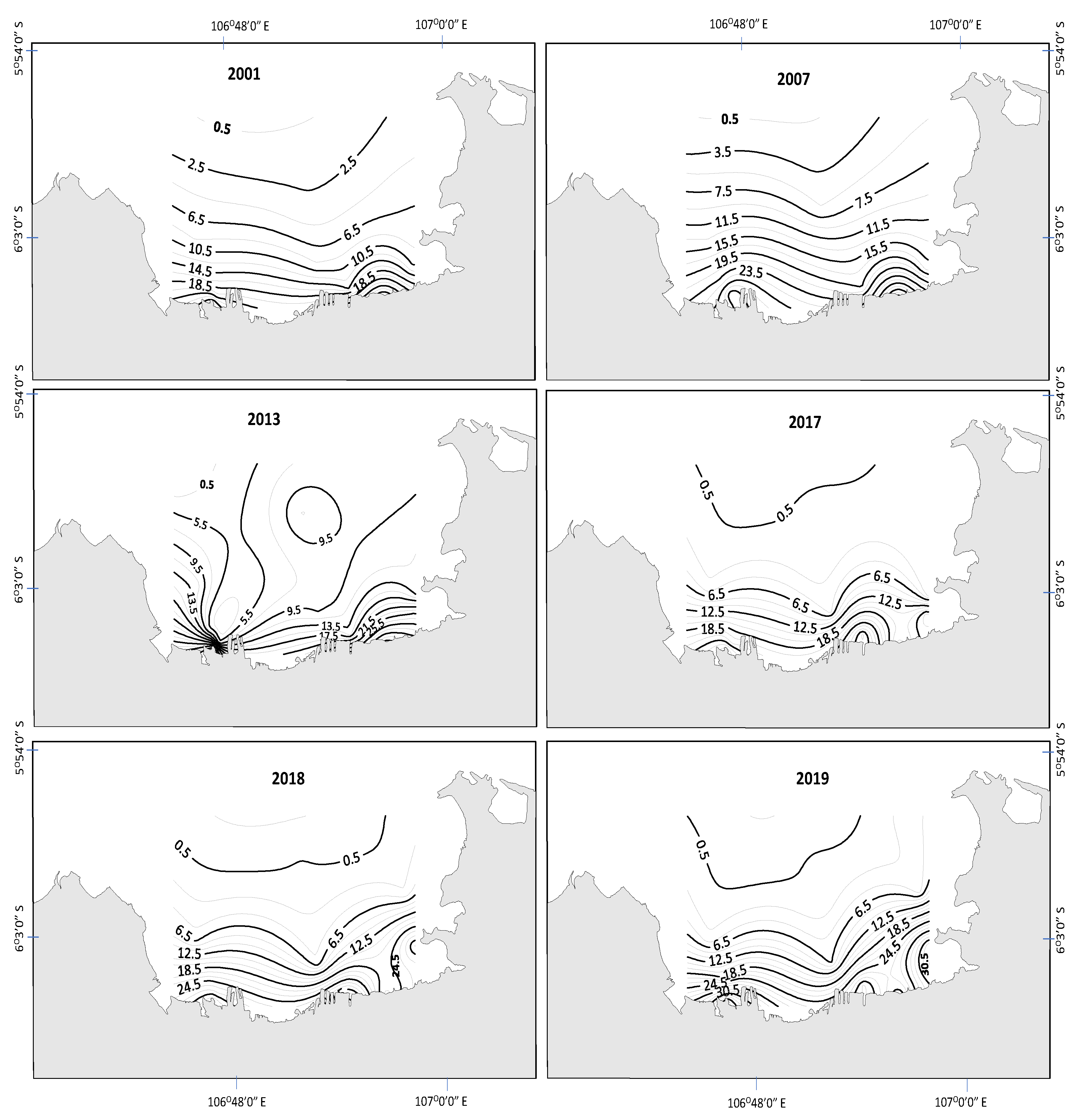
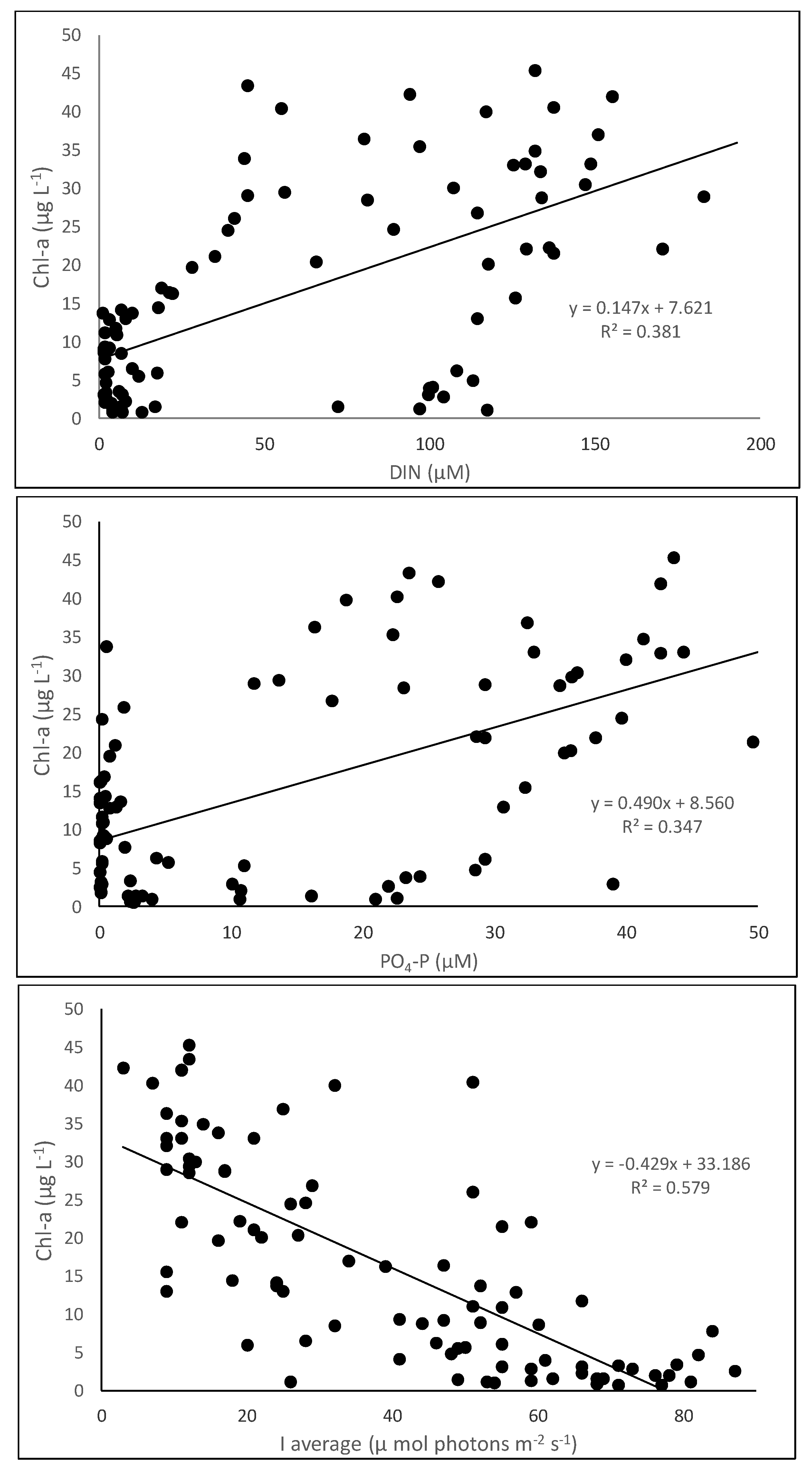
3.1. Spatial and Temporal Changes in Nutrients, Light, and Phytoplankton Biomass
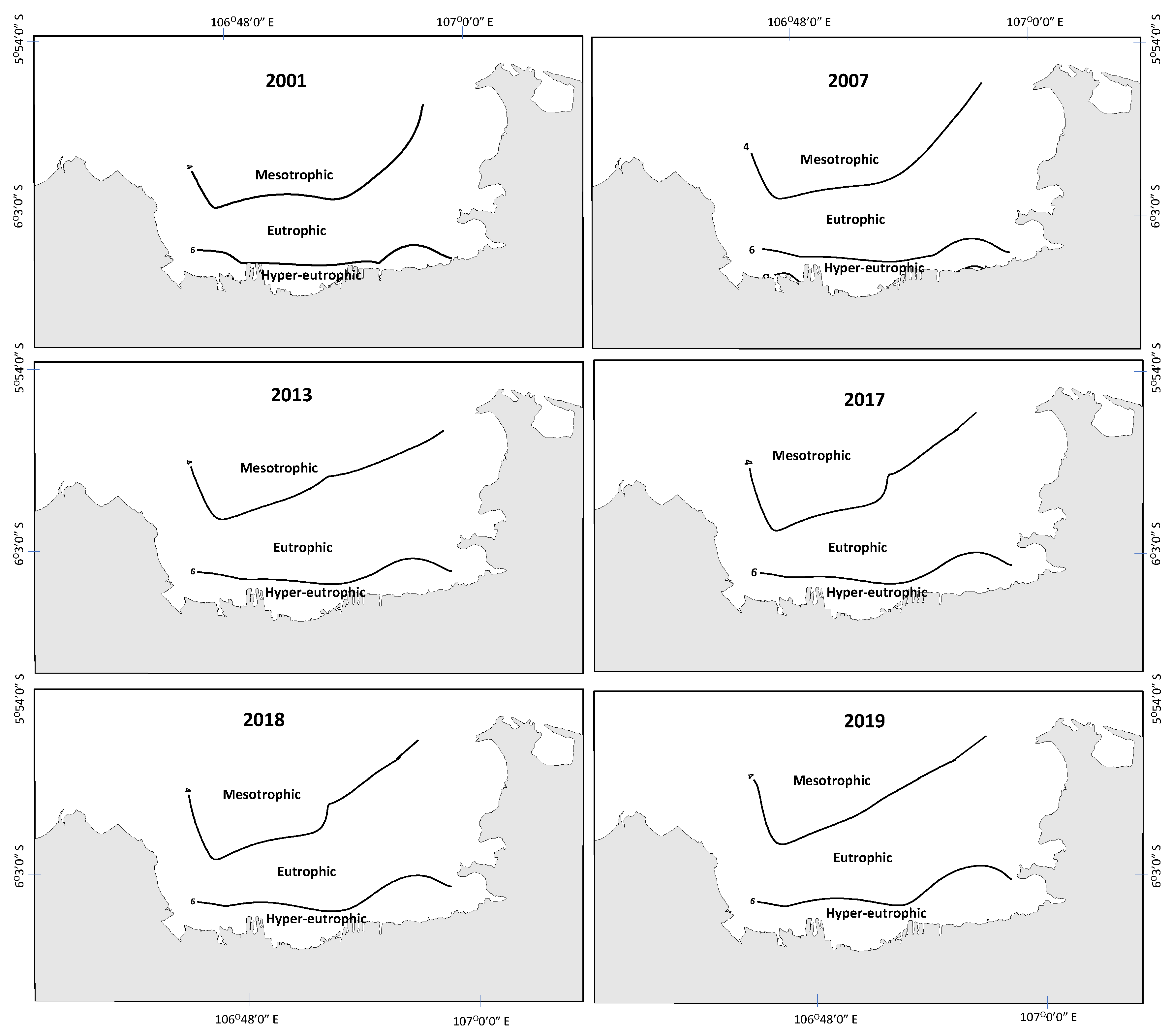
3.2. Eutrophication State
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Cloern, J.E.; Schraga, T.S.; Nejad, E.; Martin, C. Nutrient Status of San Francisco Bay and Its Management Implications. Estuar. Coast. 2020, 43, 1299–1317. [Google Scholar] [CrossRef]
- Golubkov, M.; Golubkov, S. Eutrophication in the Neva Estuary (Baltic Sea): Response to temperature and precipitation patterns. Mar. Freshw. Res. 2020, 71, 583–595. [Google Scholar] [CrossRef]
- Townhill, B.L.; Tinker, J.; Jones, M.; Pitois, S.; Creach, V.; Dye, S.D.S.; Bear, E.; Pinnegar, J.K. Harmful algal blooms and climate change: Exploring future distribution changes. ICES J. Mar. Sci. 2018, 75, 1882–1893. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, T.; Huang, D.; Liu, S.M.; Fang, J. Eutrophication and hypoxia and their impacts on the ecosystem of the Changjiang Estuary and adjacent coastal environment. J. Mar. Syst. 2016, 154, 1–4. [Google Scholar] [CrossRef]
- Cloern, J.E. The relative importance of light and nutrient limitation of phytoplankton growth: A simple index of coastal ecosystem sensitivity to nutrient enrichment. Aquat. Ecol. 1999, 33, 3–16. [Google Scholar] [CrossRef]
- Colijn, F.; Hesse, K.-J.; Ladwig, N.; Tillmann, U. Effects of the large-scale uncontrolled fertilisation process along the continental coastal North Sea. Hydrobiologia 2002, 484, 133–148. [Google Scholar] [CrossRef]
- Kocum, E.; Underwood, G.J.C.; Nedwell, D.B. Simultaneous measurement of phytoplanktonic primary production, nutrient, and light availability along a turbid, eutrophic UK east coast estuary (the Colne Estuary). Mar. Ecol. Prog. Ser. 2002, 231, 1–12. [Google Scholar] [CrossRef]
- Damar, A.; Colijn, F.; Hesse, K.-J. Effects of different nutrient loadings on planktonic primary production in embayments of Indonesia. J. Trop. Biol. Conserv. 2014, 11, 63–85. [Google Scholar]
- Damar, A.; Colijn, F.; Hesse, K.-J.; Wardiatno, Y. The eutrophication states of Jakarta, Lampung and Semangka Bays: Nutrient and phytoplankton dynamics in Indonesian tropical waters. J. Trop. Biol. Conserv. 2012, 9, 61–81. [Google Scholar]
- Ladwig, N.; Hesse, K.-J.; van der Wulp, S.A.; Damar, A.; Koch, D. Pressure on oxygen levels of Jakarta Bay. Mar. Pollut. Bull. 2016, 110, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Sidabutar, T.; Srimariana, E.S. The Connectivity of Nutrient Ratios on The Abundance of Phytoplankton Population In Jakarta Bay. E3S Web Conf. 2020, 147, 02012. [Google Scholar] [CrossRef]
- Sidabutar, T.; Srimariana, E.S.; Wouthuyzen, S. The potential role of eutrophication, tidal and climatic on the rise of algal bloom phenomenon in Jakarta Bay. IOP Conf. Ser. EES 2020, 429, 012021. [Google Scholar] [CrossRef]
- Yuliana. Implikasi perubahan ketersediaan nutrien terhadap perkembangan pesat (blooming) fitoplankton di Perairan Teluk Jakarta. Ph.D. Thesis, Bogor Agricultural University, Bogor, Indonesia, 2012. [Google Scholar]
- van der Wulp, S.A.; Damar, A.; Ladwig, N.; Hesse, K.-J. Numerical simulations of river discharges, nutrient flux and nutrient dispersal in Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2016, 110, 675–685. [Google Scholar] [CrossRef]
- BPS. Provinsi DKI Jakarta Dalam Angka 2018/DKI Jakarta Province in Figures 2018; Badan Pusat Statistik (BPS) Provinsi DKI Jakarta: Jakarta, Indonesia, 2018; p. 581, Nomor Katalog: 1102001.31, Nomor Publikasi: 31560.1803. (Unpublished Document). [Google Scholar]
- Damar, A.; Hesse, K.-J.; Colijn, F.; Yonvitner. The eutrophication states of the Indonesian sea large marine ecosystem: Jakarta Bay, 2001–2013. Deep Sea Res. Part II 2019, 163, 72–86. [Google Scholar] [CrossRef]
- Prismayanti, A.D.; Damar, A.; Pratiwi, N.T.M. Distributions of dissolved inorganic nitrogen to estimate trophic state in Jakarta Bay. IOP Conf. Series: EES 2019, 241, 012023. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Verlag Chemie: Weinheim/Deerfield Beach, FL, USA, 1983; p. 419. [Google Scholar]
- Lorenzen, C.J. Determination of Chlorophyll and Pheopigments: Spectrophotometric Equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Lalli, C.M.; Parsons, P.R. Biological Oceanography. An Introduction, 2nd ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Tillmann, U.; Hesse, K.-J.; Colijn, F. Planktonic primary production in the German Wadden Sea. J. Plankton Res. 2000, 22, 1253–1276. [Google Scholar] [CrossRef]
- Vollenweider, R.A.; Giovanardi, F.; Montanari, G.; Rinaldi, A. Characterisation of the trophic conditions of marine coastal waters with special reference to the NW Adriatic Sea: Proposal for a trophic scale, turbidity and generalised water quality index. Environmetrics 1998, 9, 329–357. [Google Scholar] [CrossRef]
- Caiaffa, E. European marine information system: EUMARIS. In Proceedings of the OCEANS 2000 MTS/IEEE Conference and Exhibition. Conference Proceedings (Cat. No.00CH37158), Providence, RI, USA, 11–14 September 2000; p. 44. [Google Scholar]
- Ærtebjerg, G.; Carstensen, J.; Dahl, K.; Hansen, J.; Nygard, K.; Rygg, B.; Sørensen, K.; Severinsen, G.; Casartelli, S.; Schrimpf, W.; et al. Eutrophication in Europe’s Coastal Waters; European Environmental Agency: Copenhagen, Denmark, 2001; p. 86. [Google Scholar]
- Pérez-Ruzafa, A.; Campillo, S.; Fernández-Palacios, J.M.; García-Lacunza, A.; García-Oliva, M.; Ibañez, H.; Navarro-Martínez, P.C.; Pérez-Marcos, M.; Pérez-Ruzafa, I.M.; Quispe-Becerra, J.I.; et al. Long-term dynamic in nutrients, chlorophyll a, and water quality parameters in a coastal lagoon during a process of eutrophication for decades, a sudden break and a relatively rapid recovery. Front. Mar. Sci. 2019, 6, 26. [Google Scholar] [CrossRef]
- Berthold, M.; Karsten, U.; Weber, M.v.; Bachor, A.; Schumann, R. Phytoplankton can bypass nutrient reductions in eutrophic coastal water bodies. Ambio 2018, 47 (Suppl. 1), 146–158. [Google Scholar] [CrossRef] [PubMed]
- Bužančić, M.; Gladan, Ž.N.; Marasović, I.; Kušpilić, G.; Grbec, B. Eutrophication influence on phytoplankton community composition in three bays on the eastern Adriatic coast. Oceanologia 2016, 58, 302–316. [Google Scholar] [CrossRef]
- Damar, A. Net Phytoplankton Community Structure and Its Biomass Dynamics in the Brantas River Estuary, Java, Indonesia. In Coastal Environments: Focus on Asian Regions; Subramanian, V., Ed.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Sidabutar, T.; Bengen, D.G.; Wouthuyzen, S.; Partono, T. The abundance of phytoplankton and its relationship to the N/P ratio in Jakarta Bay, Indonesia. Biodiversitas 2016, 17, 673–678. [Google Scholar] [CrossRef]
- Koropitan, A.F.; Ikeda, M.; Damar, A.; Yamanaka, Y. Influences of physical processes on the ecosystem of Jakarta Bay: A coupled physical-ecosystem model experiment. ICES J. Mar. Sci. 2009, 66, 336–348. [Google Scholar] [CrossRef][Green Version]
- Dring, M.J. The Biology of Marine Plants; Edward Arnold Limited: London, UK, 1982; p. 129. [Google Scholar]
- Margalef, R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Colijn, F.; Cadée, G.C. Is phytoplankton growth in the Wadden Sea light or nitrogen limited? J. Sea Res. 2003, 49, 83–93. [Google Scholar] [CrossRef]
- Colijn, F. Primary Production in the Ems-Dollard Estuary. Ph.D. Thesis, State University Groningen, Groningen, The Netherlands, 1983. [Google Scholar]
- Lohrenz, S.E.; Fahnenstiel, G.L.; Redalje, D.G.; Lang, G.A.; Dagg, M.J.; Whitledge, T.E.; Dortch, Q. Nutrients, irradiance, and mixing as factors regulating primary production in coastal waters impacted by the Mississippi River plume. Cont. Shelf Res. 1999, 19, 1113–1141. [Google Scholar] [CrossRef]
- Tang, S.; Rachman, A.; Fitria, N.; Thoha, H.; Chen, B. Phytoplankton changes during SE monsoonal period in the Lembeh Strait of North Sulawesi, Indonesia, from 2012 to 2015. Acta Oceanol. Sin. 2018, 37, 9–17. [Google Scholar] [CrossRef]
- Domingues, R.B.; Barbosa, A.; Galvão, H. Nutrients, light and phytoplankton succession in a temperate estuary (the Guadiana, south-western Iberia). Estuar. Coast. Shelf Sci. 2005, 64, 249–260. [Google Scholar] [CrossRef]
- Lemley, D.A.; Adams, J.B.; Strydom, N.A. Triggers of phytoplankton bloom dynamics in permanently eutrophic waters of a South African estuary. Afr. J. Aquat. Sci. 2018, 43, 229–240. [Google Scholar] [CrossRef]
- Huisman, J.; Sommeijer, B. Population dynamics of sinking phytoplankton in light-limited environments: Simulation techniques and critical parameters. J. Sea Res. 2002, 48, 83–96. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.K.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Lippemeier, S.; Hintze, R.; Vanselow, K.H.; Hartig, P.; Colijn, F. In-line recording of PAM fluorescence of phytoplankton cultures as a new tool for studying effects of fluctuating nutrient supply on photosynthesis. Eur. J. Phycol. 2001, 36, 89–100. [Google Scholar] [CrossRef]
- Damar, A.; Colijn, F.; Hesse, K.-J.; Kurniawan, F. Coastal Phytoplankton Pigments Composition in Three Tropical Estuaries of Indonesia. J. Mar. Sci. Eng. 2020, 8, 311. [Google Scholar] [CrossRef]
- Aryawati, R.; Bengen, D.G.; Prartono, T.; Zulkifli, H. Harmful Algal in Banyuasin Coastal Waters, South Sumatera. Biosaintifika 2016, 8, 231–239. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.T.N.; Némery, J.; Gratiot, N.; Strady, E.; Tran, V.Q.; Nguyen, A.T.; Aimé, J.; Peyne, A. Nutrient dynamics and eutrophication assessment in the tropical river system of Saigon-Dongnai (southern Vietnam). Sci. Total Environ. 2019, 653, 370–383. [Google Scholar] [CrossRef]
- Breckwoldt, A.; Dsikowitzky, L.; Baum, G.; Ferse, S.C.A.; Wulp, S.v.d.; Kusumanti, I.; Ramadhan, A.; Adrianto, L. A review of stressors, uses and management perspectives for the larger Jakarta Bay Area, Indonesia. Mar. Pollut. Bull. 2016, 110, 790–794. [Google Scholar] [CrossRef]
- Choirun, A.; Sari, S.H.J.; Iranawati, F. Phytoplankton Harmfull Algae Bloom (Hab) Identification during Tide Period in Brondong Coastal Waters, Lamongan, East Java. Torani J. Fish. Mar. Sci. 2015, 25, 58–66. [Google Scholar]
- Barokah, G.R.; Putri, A.K.; Gunawan. The Abundance of Phytoplankton Causing HAB (Harmful Algal Bloom) in Lampung Bay during West and East Monsoon. JPB Kelaut. dan Perikan. 2016, 11, 115–126. [Google Scholar]
- Buditama, G.; Damayanti, A.; Pin, T.G. Identifying Distribution of Chlorophyll-a Concentration Using Landsat 8 OLI on Marine Waters Area of Cirebon. IOP Conf. Ser. EES 2017, 98, 012040. [Google Scholar] [CrossRef]
- Arifin, Z. Trend of coastal pollution of Jakarta Bay Indonesia: Its implication for fishery and recreational activities. In Proceedings of the International Workshop on Coastal Resources Exploration and Conservation; Rachmawati, Aldrian, R.E., Hendiarti, N., Tejakusuma, I., Eds.; Badan Pengkajian dan Penerapan Teknologi Jakarta: Jakarta, Indonesia, 2004; pp. 16–21. [Google Scholar]
- Arifin, Z.; Fitriati, M. Green mussels cultured in highly polluted area of Jakarta Bay, in Indonesia. In International Conference on Hubs, Harbours and Deltas in Southeast Asia: Multidisciplinary and Intercultural Perspectives; Verhasselt, Y., Ed.; Royal Academy of Overseas Sciences Brussel: Brussels, Belgium, 2006; pp. 525–536. [Google Scholar]
- Dsikowitzky, L.; Sträter, M.; Dwiyitno, D.; Ariyani, F.; Irianto, H.E.; Schwarzbauer, J. First comprehensive screening of lipophilic organic contaminants in surface waters of the megacity Jakarta, Indonesia. Mar. Pollut. Bull. 2016, 110, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Dsikowitzky, L.; Van der Wulp, S.A.; Dwiyitno, D.; Ariyani, F.; Hesse, K.-J.; Damar, A.; Schwarzbauer, J. Transport of pollution from the megacity Jakarta into the ocean: Insights from organic pollutant mass fluxes along the Ciliwung River. Estuar. Coast. Shelf Sci. 2018, 215, 219–228. [Google Scholar] [CrossRef]
- Dwiyitno, D.; Dsikowitzky, L.; Nordhaus, I.; Andarwulan, N.; Irianto, H.E.; Lioe, H.N.; Ariyani, F.; Kleinertz, S.; Schwarzbauer, J. Accumulation patterns of lipophilic organic contaminants in surface sediments and in economic important mussel and fish species from Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2016, 110, 767–777. [Google Scholar] [CrossRef]
- Baum, G.; Kegler, P.; Scholz-Böttcher, B.M.; Alfiansah, Y.R.; Abrar, M.; Kunzmann, A. Metabolic performance of the coral reef fish Siganus guttatus exposed to combinations of water borne diesel, an anionic surfactant and elevated temperature in Indonesia. Mar. Pollut. Bull. 2016, 110, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Huhn, M.; Hattich, G.S.I.; Zamani, N.P.; Juterzenka, K.V.; Lenz, M. Tolerance to stress differs between Asian greenmussels Perna viridis from the impacted Jakarta Bay and from natural habitats along the coast of West Java. Mar. Pollut. Bull. 2016, 110, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.; Arifin, Z.; Baum, G. Pollution of Coastal Areas of Jakarta Bay: Water Quality And Biological Responses. Mar. Res. Indones. 2018, 43, 37–51. [Google Scholar] [CrossRef]
- Oetama, V.S.P.; Hennersdorf, P.; Abdul-Aziz, M.A.; Mrotzek, G.; Haryanti, H.; Saluz, H.P. Microbiome analysis and detection of pathogenic bacteria of Penaeus monodon from Jakarta Bay and Bali. Mar. Pollut. Bull. 2016, 110, 718–725. [Google Scholar] [CrossRef]
| The Surveys | 2001 | 2007 | 2013 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| 1st | 24th Apr. | 3rd Mar. | 4th Mar. | 14th Jul. | 30th Jun. | 10th Apr. |
| 2nd | 9th Jul. | 21st Apr. | 17th May | 3rd Aug. | 28th Jul. | 24th Apr. |
| 3rd | 20th Sep. | 27th Jul. | 21st Jul. | 11th Sep. | 1st Sep. | 2nd May |
| 4th | 20th Nov. | 1st Oct. | 2nd Oct. | 3rd Oct. | 2nd Oct. | 4th Jun. |
| Variable | Coefficients | SE | t Stat | p-Value | Variance Inflation Factor |
|---|---|---|---|---|---|
| DIN, phosphates, and light to Chl-a | |||||
| Intercept | 26.45 | 2.70 | 9.78 | 0.13 × 10−15 | |
| DIN | 0.00107 | 0.04 | 0.03 | 0.9784 | 6.597 |
| Phosphates | 0.23 | 0.13 | 1.81 | 0.0744 | 5.797 |
| Light | −0.35 | 0.04 | −7.82 | 0.12 × 10−11 | 1.511 |
| DIN and light to Chl-a | |||||
| Intercept | 26.09 | 2.73 | 9.55 | 3.33 × 10−15 | |
| DIN | 0.064 | 0.019 | 3.34 | 0.0012 | 1.492 |
| Light | −0.34 | 0.045 | −7.58 | 3.46 × 10−11 | 1.491 |
| Phosphates and light to Chl-a | |||||
| Intercept | 26.47803 | 2.4234 | 10.93 | 5.34 × 10−18 | |
| Phosphates | 0.236732 | 0.0612 | 3.866 | 0.00021 | 1.31243 |
| Light | −0.35059 | 0.0415 | −8.447 | 6.09 × 10−13 | 1.3135 |
| Eutrophication Status | 2001 | 2007 | 2013 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Hyper-eutrophic | 75.1 | 73.14 | 83.33 | 87.58 | 92.64 | 114 |
| Eutrophic | 186.21 | 188.76 | 214.66 | 235.39 | 207.08 | 211.73 |
| Mesotrophic | 210.69 | 210.1 | 174.01 | 149.03 | 172.28 | 146.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damar, A.; Colijn, F.; Hesse, K.-J.; Adrianto, L.; Yonvitner; Fahrudin, A.; Kurniawan, F.; Prismayanti, A.D.; Rahayu, S.M.; Rudianto, B.Y.; et al. Phytoplankton Biomass Dynamics in Tropical Coastal Waters of Jakarta Bay, Indonesia in the Period between 2001 and 2019. J. Mar. Sci. Eng. 2020, 8, 674. https://doi.org/10.3390/jmse8090674
Damar A, Colijn F, Hesse K-J, Adrianto L, Yonvitner, Fahrudin A, Kurniawan F, Prismayanti AD, Rahayu SM, Rudianto BY, et al. Phytoplankton Biomass Dynamics in Tropical Coastal Waters of Jakarta Bay, Indonesia in the Period between 2001 and 2019. Journal of Marine Science and Engineering. 2020; 8(9):674. https://doi.org/10.3390/jmse8090674
Chicago/Turabian StyleDamar, Ario, Franciscus Colijn, Karl-Juergen Hesse, Luky Adrianto, Yonvitner, Achmad Fahrudin, Fery Kurniawan, Ageng Dwi Prismayanti, Siti Mira Rahayu, Bambang Yudho Rudianto, and et al. 2020. "Phytoplankton Biomass Dynamics in Tropical Coastal Waters of Jakarta Bay, Indonesia in the Period between 2001 and 2019" Journal of Marine Science and Engineering 8, no. 9: 674. https://doi.org/10.3390/jmse8090674
APA StyleDamar, A., Colijn, F., Hesse, K.-J., Adrianto, L., Yonvitner, Fahrudin, A., Kurniawan, F., Prismayanti, A. D., Rahayu, S. M., Rudianto, B. Y., & Ramli, A. (2020). Phytoplankton Biomass Dynamics in Tropical Coastal Waters of Jakarta Bay, Indonesia in the Period between 2001 and 2019. Journal of Marine Science and Engineering, 8(9), 674. https://doi.org/10.3390/jmse8090674





