Phytoplankton Distribution in Mar Menor Coastal Lagoon (SE Spain) during 2017
Abstract
:1. Introduction
2. Materials and Methods
3. Results
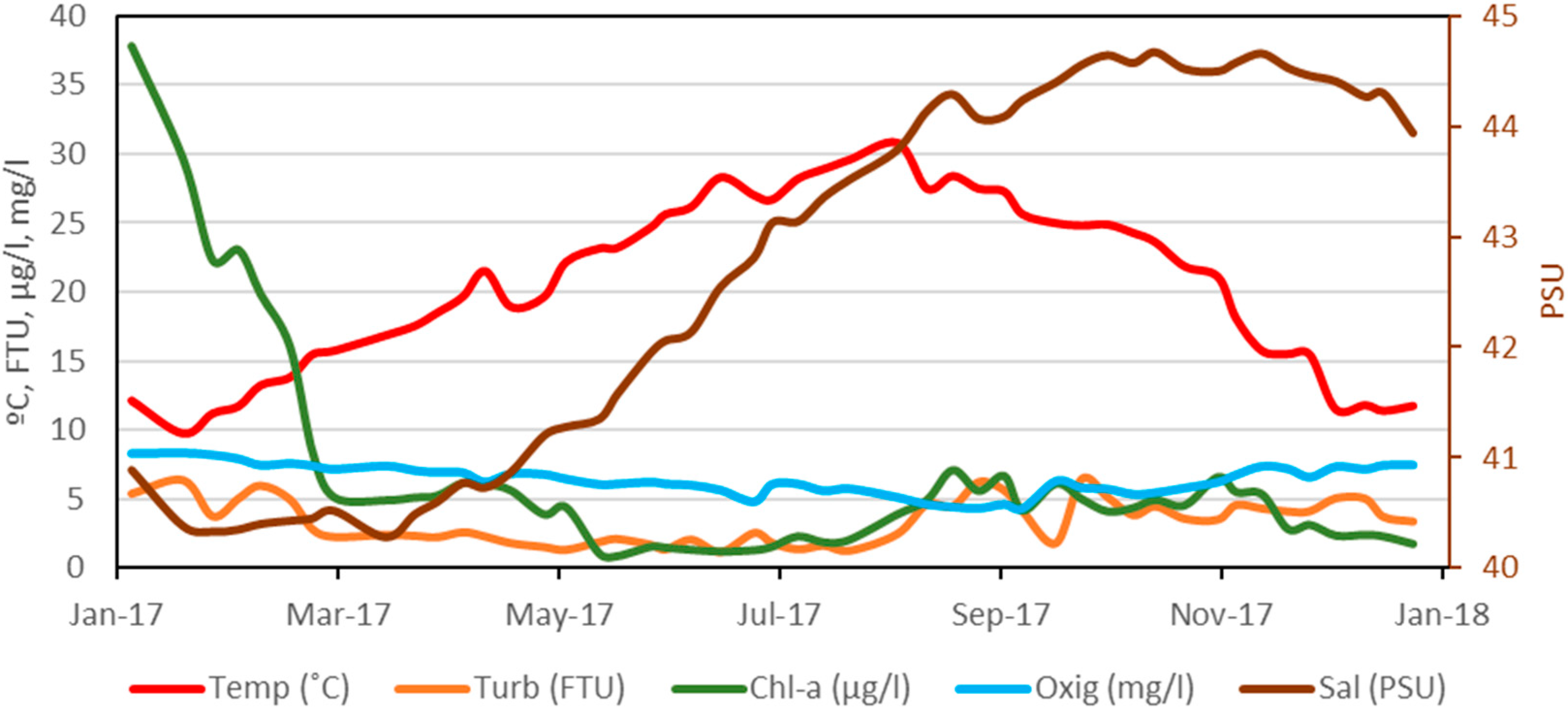
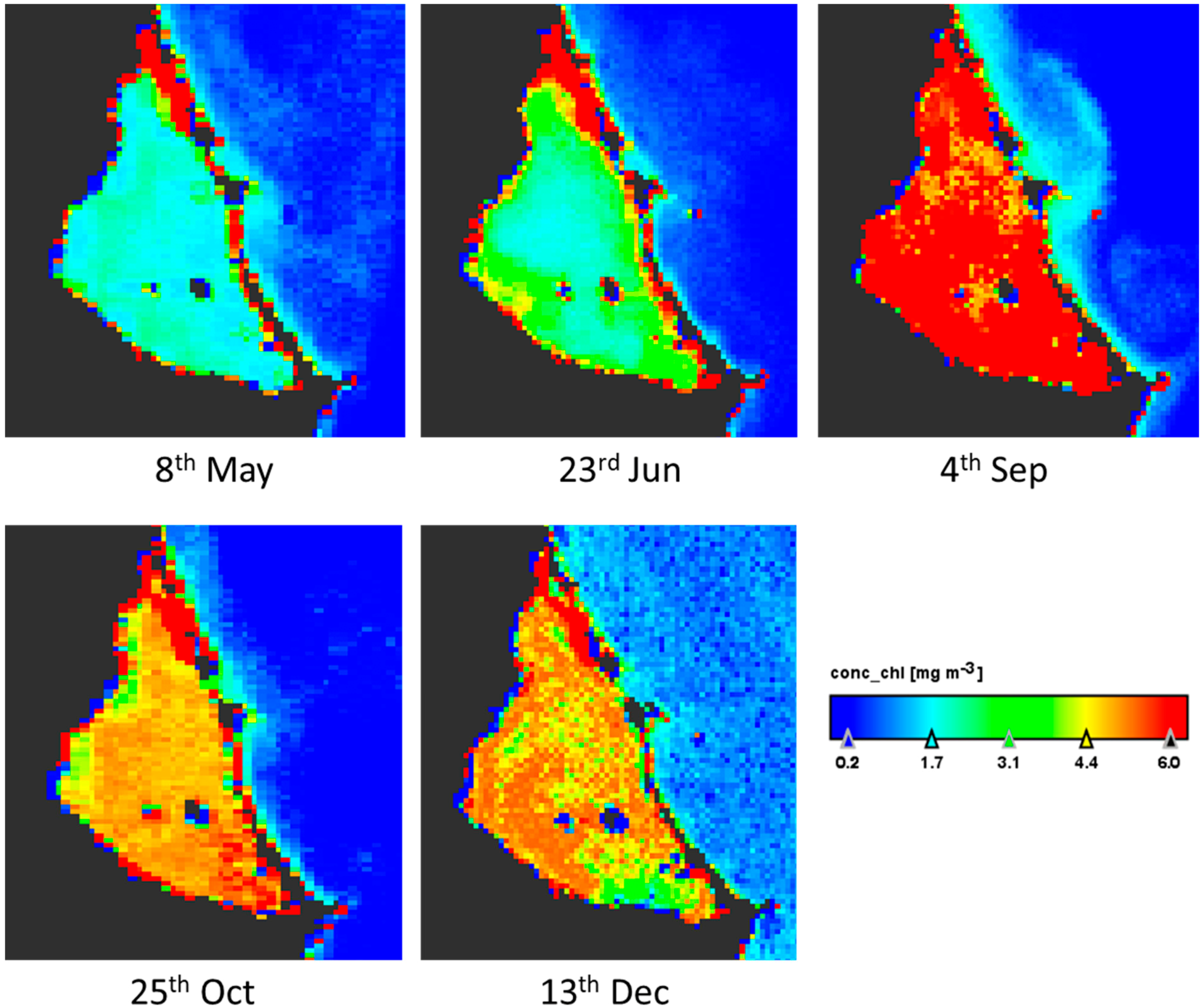
3.1. Changes in Environmental Variables
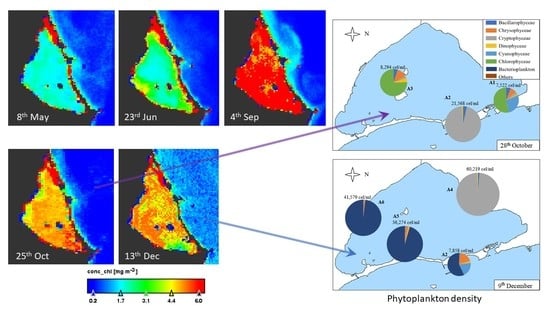
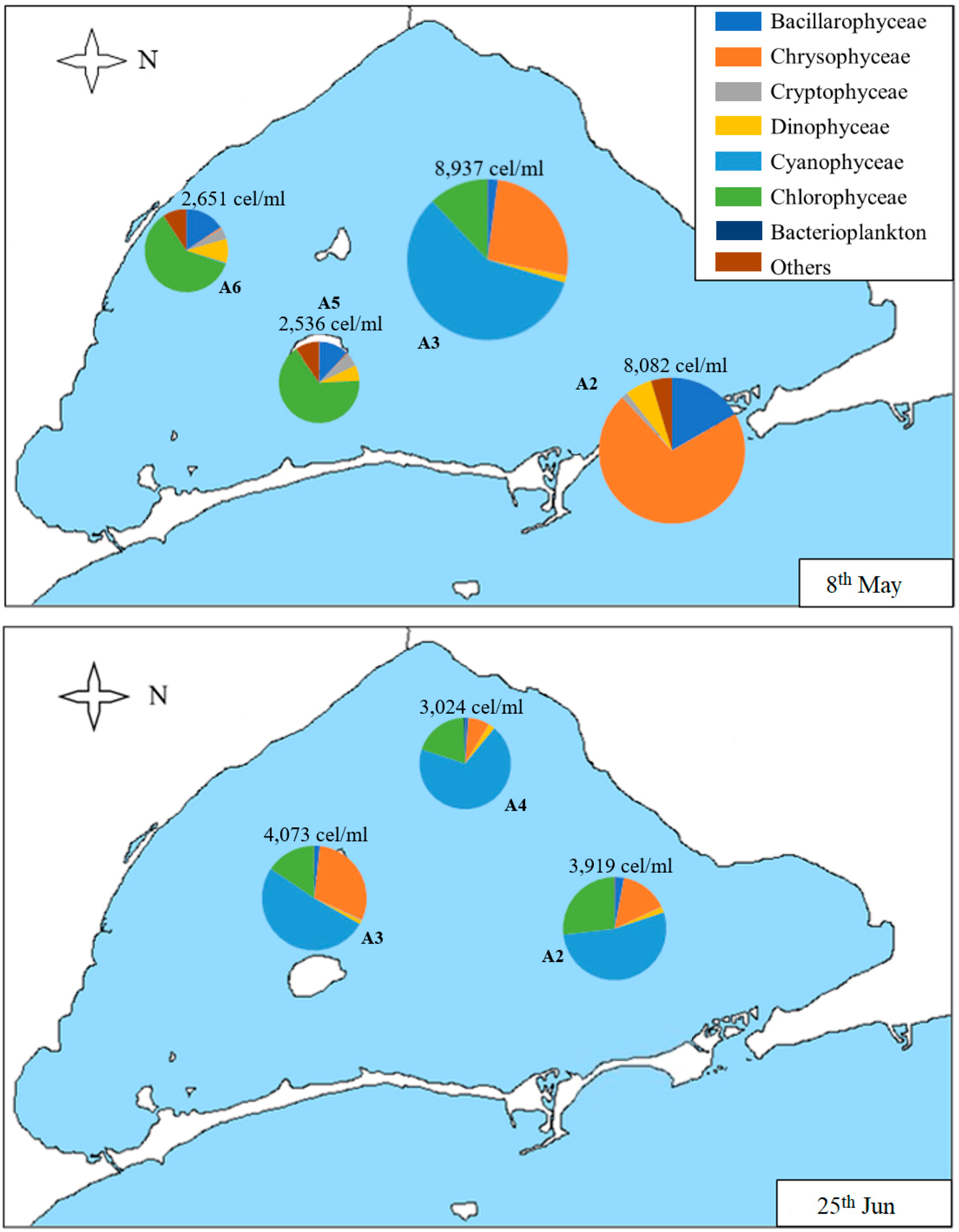
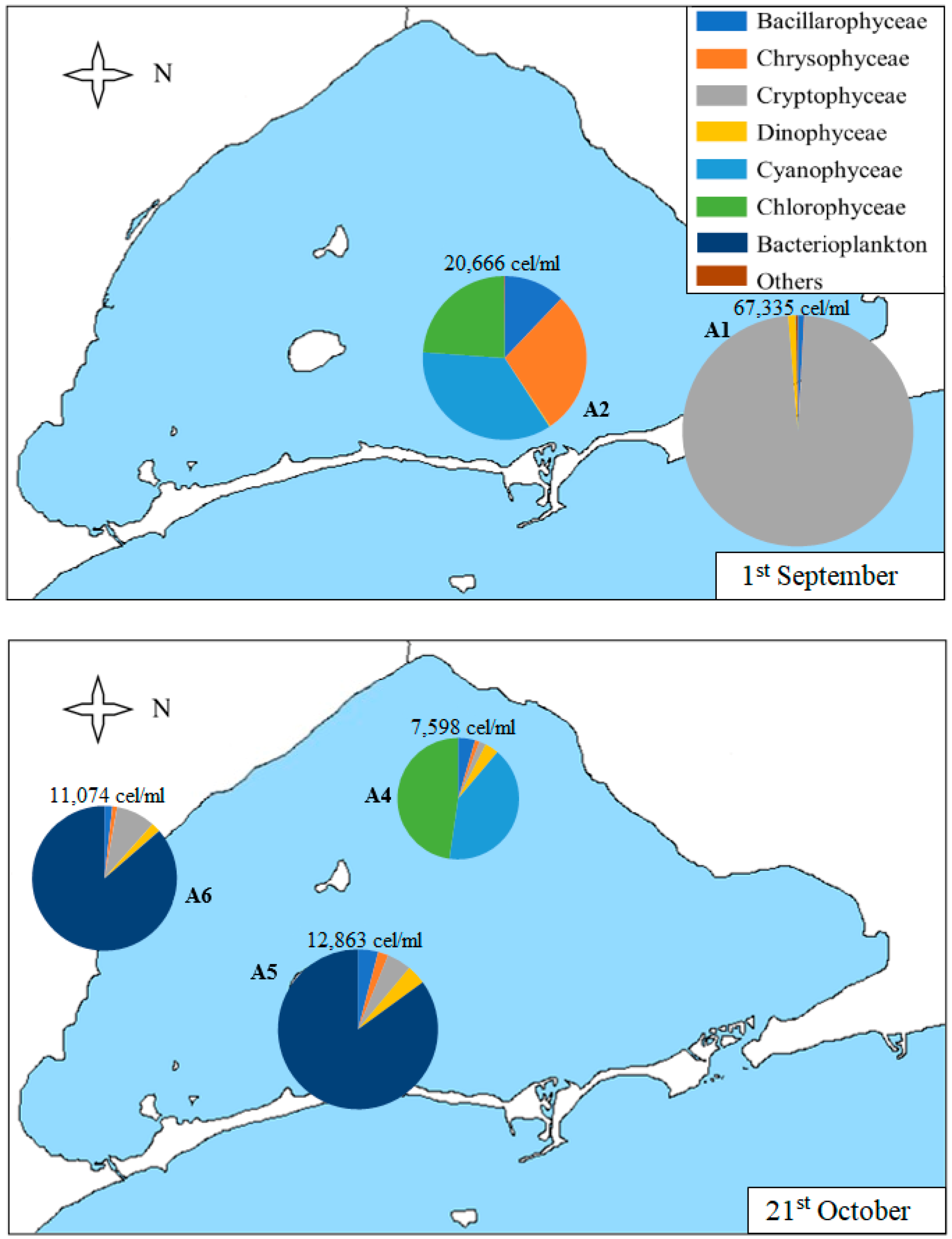
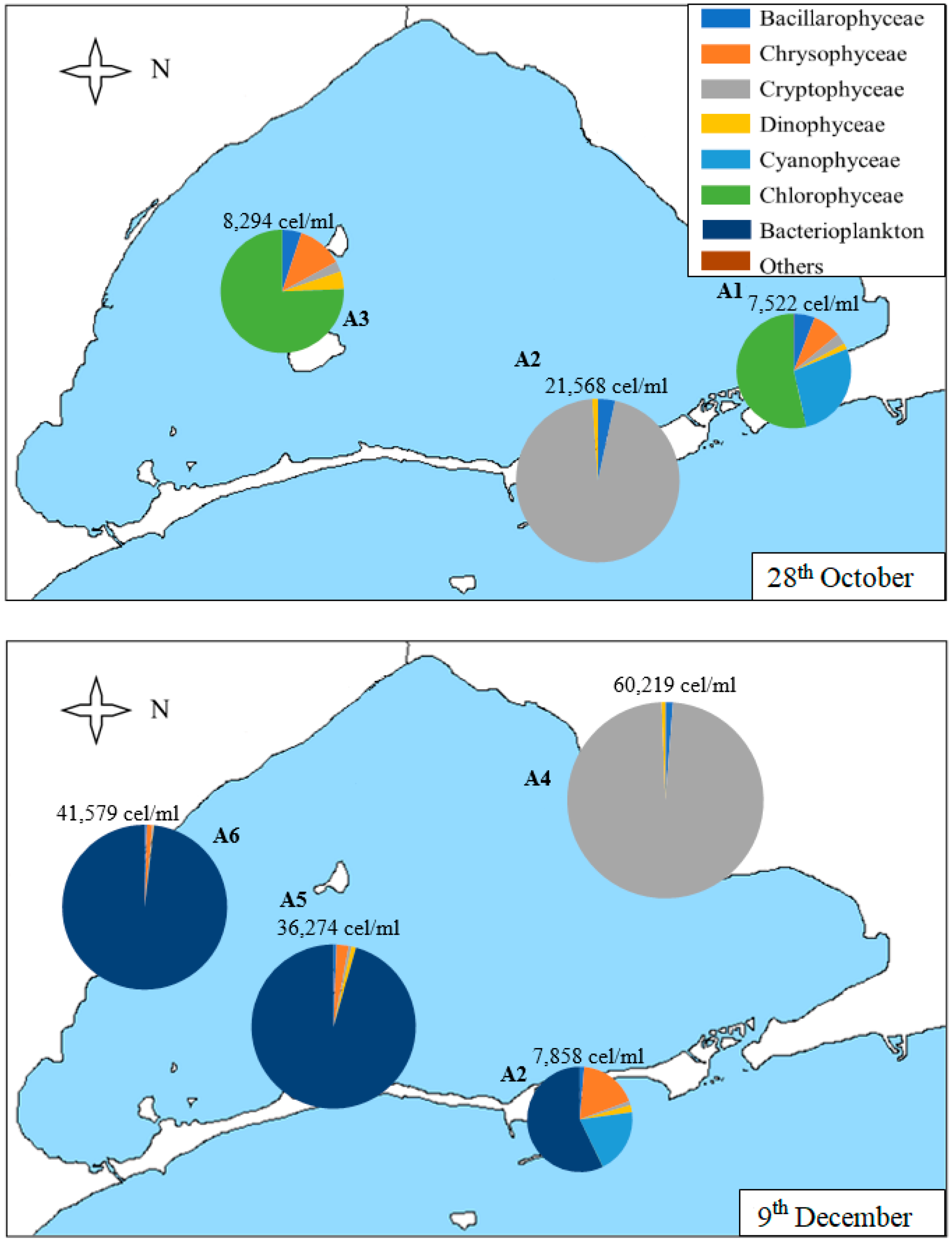
3.2. Geographical Distribution of Taxa
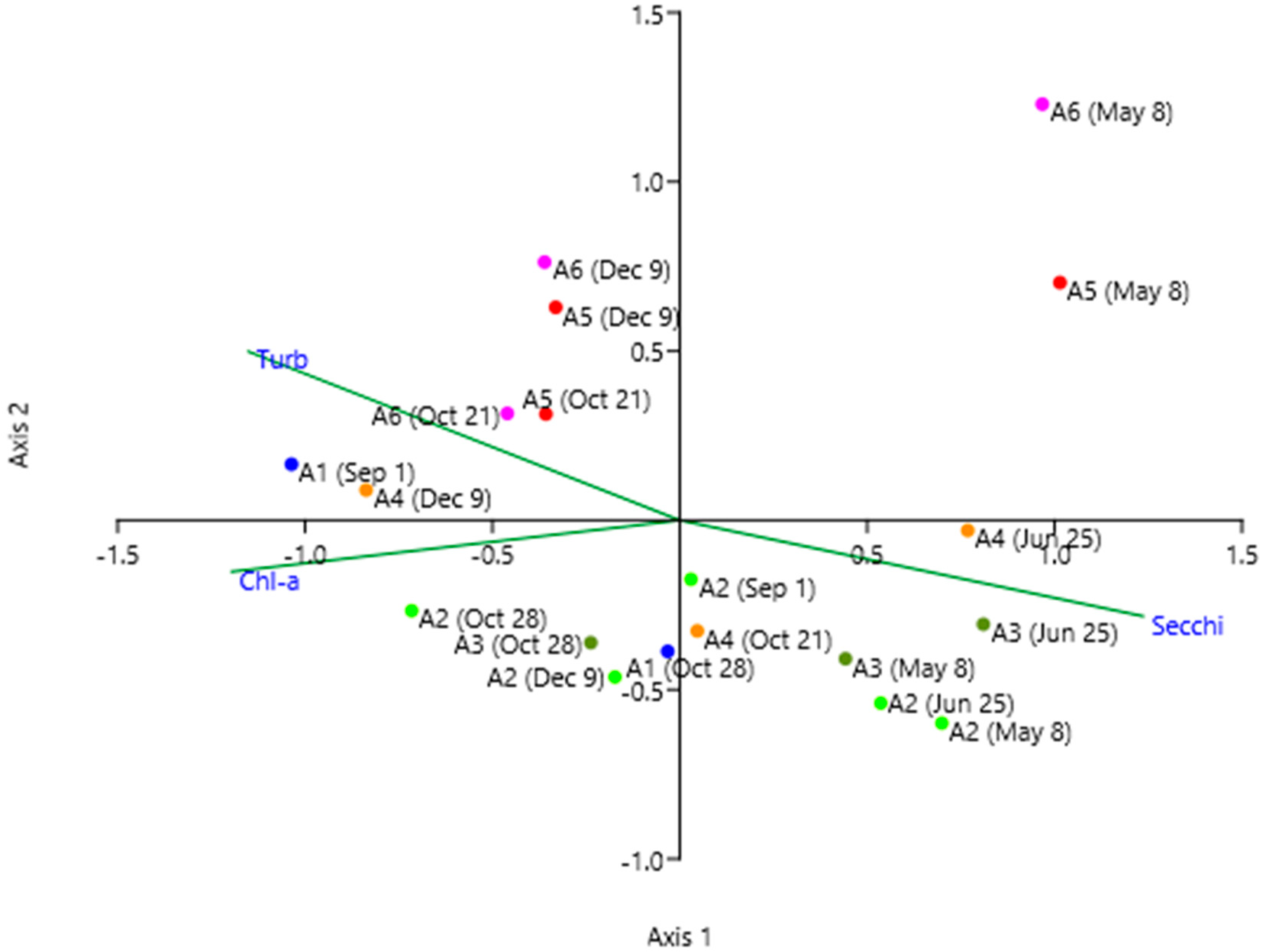
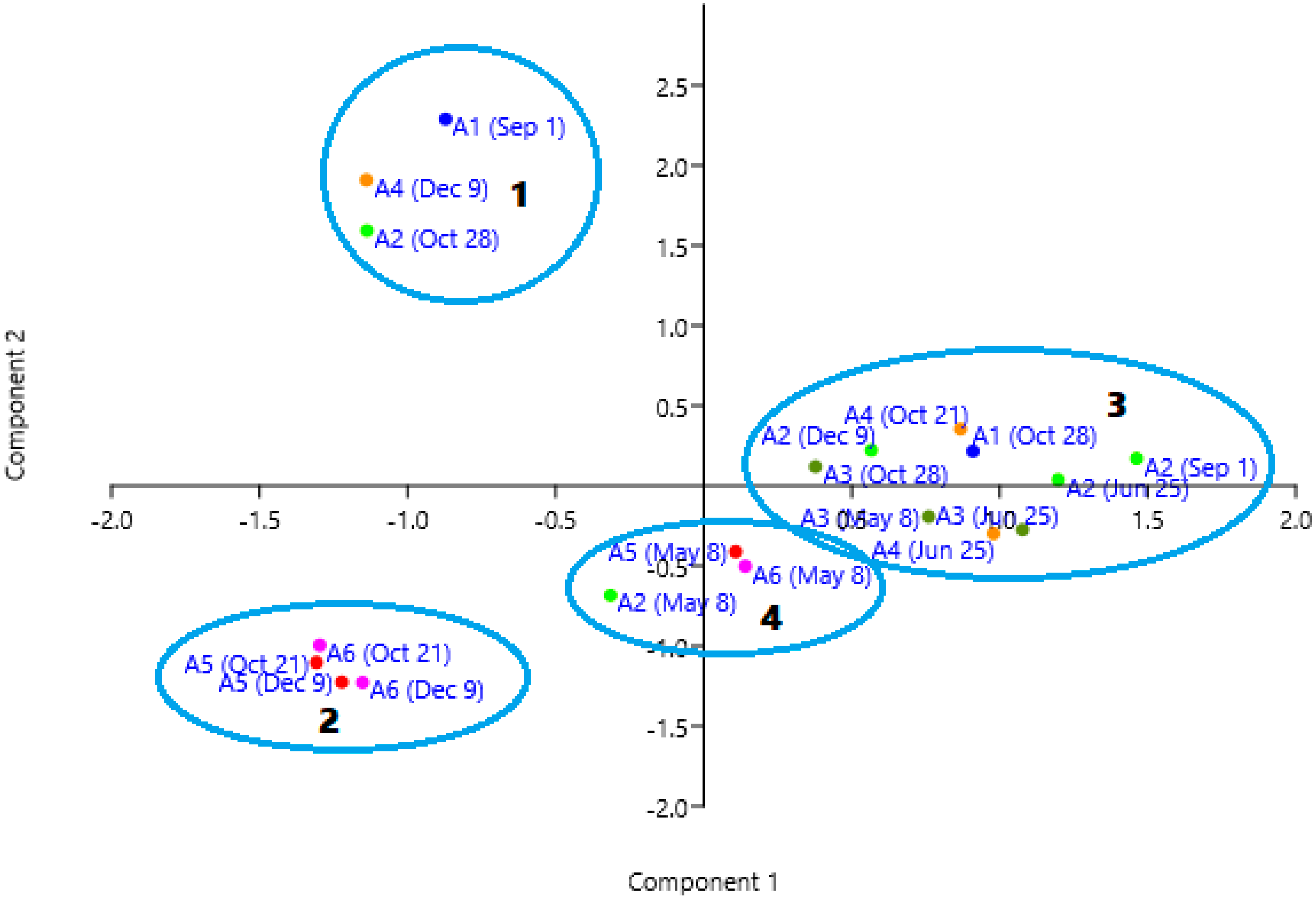
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ros, M.; Miracle, M.R. Variación estacional del fitoplancton del Mar Menor y su relación con las de un punto próximo del Mediterráneo. Limnetica 1984, 1, 32–42. [Google Scholar]
- Díaz del Río, V. Estudio Geoambiental del Mar. Menor; Instituto Español de Oceanografía: Madrid, Spain, 1993. [Google Scholar]
- Derruau, M. Geomorfología; Editorial Ariel: Barcelona, Spain, 1981. [Google Scholar]
- Pérez-Ruzafa, A.; Marcos, C.; Pérez-Ruzafa, I.M.; Domenec Ros, J. Evolución de las características ambientales y de los poblamientos del Mar Menor (Murcia, SE de España). An. Biol. 1987, 12, 53–65. [Google Scholar]
- García Aróstegui, J.L.; Marín Arnaldos, F.; Martínez Vicente, D. 1. Hidrogeología. In Informe Integral Sobre el Estado Ecológico del Mar. Menor; Comité de Asesoramiento Científico del Mar. Menor: Madrid, Spain, 2017; pp. 7–22. [Google Scholar]
- Bonilla, S.; Conde, D.; Aubriot, L.; Pérez, M.C. Influence of hydrology on phytoplankton species composition and life strategies in a subtropical coastal lagoon periodically connected with the Atlantic Ocean. Estuaries 2005, 28, 884–895. [Google Scholar] [CrossRef]
- Morote Seguido, A.F.; Rico Amorós, A.M. Perspectivas de funcionamiento del Trasvase Tajo-Segura (España): Efectos de las nuevas reglas de explotación e impulso de la desalinización como recurso sustitutivo. Bol. Asoc. Geogr. Esp. 2018, 79, 1–43. [Google Scholar] [CrossRef]
- Hanley, N.; Bell, D.; Alvarez-Farizo, B. Valuing the benefits of coastal water quality improvements using contingent and real behaviour. Environ. Res. Econ. 2003, 24, 273–285. [Google Scholar] [CrossRef]
- Álvarez Rogel, J.; García Alonso, C.; Gilavert Cervera, F.J.; Gómez Cerezo, R.; León León, V.M.; Marcos Diego, C.; Pérez-Ruzafa, A. 3. Oceanografía Física y Química. In Informe Integral Sobre el Estado Ecológico del Mar. Menor; Comité de Asesoramiento Científico del Mar. Menor: Madrid, Spain, 2017; pp. 71–86. [Google Scholar]
- Phlips, E.J.; Badylak, S. Spatial variability in phytoplankton standing crop and composition in a shallow inner-shelf lagoon, Florida Bay, Florida. Bull. Mar. Sci. 1996, 58, 203–216. [Google Scholar]
- Macedo, M.F.; Duarte, P.; Mendes, P. Annual variation of environmental variables, phytoplankton species composition and photosynthetic parameters in a coastal lagoon. J. Plankton Res. 2001, 23, 719–732. [Google Scholar] [CrossRef]
- Gimenez Casalduero, M.F.; Marcos Diego, C.; Oliva Paterna, F.J.; Pérez-Ruzafa, A.; Robledano Aymerich, F.; Torralva Forero, M.M. 2. Ecología Lagunar. In Informe Integral Sobre el Estado Ecológico del Mar. Menor; Comité de Asesoramiento Científico del Mar. Menor: Madrid, Spain, 2017; pp. 23–69. [Google Scholar]
- Jacquet, S.; Delesalle, B.; Torréton, J.P.; Blanchot, J. Response of phytoplankton communities to increased anthropogenic influences (southwestern lagoon, New Caledonia). Mar. Ecol. Prog. Ser. 2006, 320, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Bec, B.; Husseini-Ratrema, J.; Collos, Y.; Souchu, P.; Vaquer, A. Phytoplankton seasonal dynamics in a Mediterranean coastal lagoon: Emphasis on the picoeukaryote community. J. Plankton Res. 2005, 27, 881–894. [Google Scholar] [CrossRef] [Green Version]
- Sakka Hlaili, A.; Grami, B.; Hadj Mabrouk, H.; Gosselin, M.; Hamel, D. Phytoplankton growth and microzooplankton grazing rates in a restricted Mediterranean lagoon (Bizerte Lagoon, Tunisia). Mar. Biol. 2006, 151, 767–783. [Google Scholar] [CrossRef]
- Carrasco Palma, D.; Floraciones Algales Nocivas (FAN), un Fenómeno Natural Presente en Nuestras Costas. Labtox: Facultad de Medicina, Universidad de Chile. 2015. Available online: http://labtox.cl/?p=984 (accessed on 22 May 2019).
- European Union. Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. 1991. Available online: http://data.europa.eu/eli/dir/1991/271/oj (accessed on 22 May 2019).
- European Union. Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources. Available online: http://data.europa.eu/eli/dir/1991/676/oj (accessed on 22 May 2019).
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: http://data.europa.eu/eli/dir/2000/60/oj (accessed on 22 May 2019).
- De la Lanza Espino, G. Criterios generales para la elección de bioindicadores. In Organismos Indicadores de la Calidad del Agua y de la Contaminación (Bioindicadores); De la Lanza Espino, G., Hernández Pulido, S., Carbajal Pérez, J.L., Eds.; Plaza y Valdés, S.A.: Mexico City, Mexico, 2000; pp. 17–41. [Google Scholar]
- Loureiro, S.; Newton, A.; Icely, J. Boundary conditions for the European Water Framework Directive in the Ria Formosa lagoon, Portugal (physico-chemical and phytoplankton quality elements). Estuar. Coast. Shelf Sci. 2006, 67, 382–398. [Google Scholar] [CrossRef]
- Velasco, J.; Lloret, J.; Millán, A.; Marin, A.; Barahona, J.; Abellán, P.; Sánchez-Fernández, D. Nutrient and particulate inputs into the Mar Menor lagoon (SE Spain) from an intensive agricultural watershed. Water Air Soil Pollut. 2006, 176, 37–56. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Nienhuis, H.; Guerrero Caballero, R. A quantitative analysis of the annual phytoplankton cycle of the Magdalena lagoon complex (Mexico). J. Plankton Res. 1985, 7, 427–441. [Google Scholar] [CrossRef]
- Webster, I.T.; Ford, P.W.; Hodgson, B. Microphytobenthos contribution to nutrient-phytoplankton dynamics in a shallow coastal lagoon. Estuaries 2002, 25, 540–551. [Google Scholar] [CrossRef]
- Sarno, D.; Zingone, A.; Saggiomo, V.; Carrada, G.C. Phytoplankton biomass and species composition in a Mediterranean coastal lagoon. Hydrobiologia 1993, 271, 27–40. [Google Scholar] [CrossRef]
- Ros, M.; Miracle, M.R.; Serra, M. Análisis estadístico de las comunidades del fitoplancton de Mar Menor y su relación con el de la zona costera marina. Limnetica 1987, 3, 35–46. [Google Scholar]
- European Committee for Standardization. European Standard. EN 15204 2007. Water Quality—Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermöhl Technique); European Committee for Standardization: Brussels, Belgium, 2007; pp. 1–46. [Google Scholar]
- Vicente, E.; De Hoyos, C.; Sánchez, P.; Cambra, J. Protocolos de muestreo y análisis para fitoplancton. In Confederación Hidrográfica del Ebro. Metodología Para el Establecimiento del Estado Ecológico Según la Directiva del Marco del Agua; Ministerio de Medio Ambiente: Madrid, Spain, 2015. [Google Scholar]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Bourrelly, P. Les Algues D’eau Douce. 3. Algues Bleues et Rouges; Éditions N. Boubée & Cie: París, France, 1970; p. 512. [Google Scholar]
- Bourrelly, P. Les Algues D’eau Douce. 1. Algues Vertes; Éditions N. Boubée & Cie: París, France, 1972; p. 572. [Google Scholar]
- Delgado, M.; Fortuño, J.M. Atlas de fitoplancton del Mar Mediterráneo. Sci. Mar. 1991, 55, 1–133. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasserflora von Mitteleuropa. Bacillariophyceae. 1. Teil: Naviculaceae; VEB Gustav Fischer Verlag: Jena, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasserflora von Mitteleuropa. Bacillariophyceae. 2 Teil: Bacillariaceae, Epithemiaceae, Surirellaceae; VEB Gustav Fischer Verlag: Jena, Germany, 1986; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasserflora von Mitteleuropa H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae; VEB Gustav Fischer Verlag: Jena, Germany, 1986; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasserflora von Mitteleuropa. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema; VEB Gustav Fischer Verlag: Jena, Germany, 1986; p. 436. [Google Scholar]
- Schiller, J. Rabenhorst’s L. Kryptogamen-Flora von Deutschelad, Österreich un der Schweiz: Dinoflagellatae (Peridineaceae). 1. Teil; Akademische Verlagsgesellchaft M.B.M.: Leipzig, Germany, 1933. [Google Scholar]
- Schiller, J. Rabenhorst’s L. Kryptogamen-Flora von Deutschelad, Österreich un der Schweiz: Dinoflagellatae (Peridineaceae). 2. Teil; Akademische Verlagsgesellchaft M.B.M.: Leipzig, Germany, 1937. [Google Scholar]
- Streble, H.; Krauter, D. Atlas de los Microorganismos de Agua Dulce; Ediciones Omega S.A.: Barcelona, Spain, 1987. [Google Scholar]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Costas I; A.R.G. Ganter Verlag Kommanditgesellschaft: Munich, Germany, 2000. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 22 May 2019).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Peet, R.K. The Measurement of species diversity. Annu. Rev. Ecol. Syst. 2003, 5, 285–307. [Google Scholar] [CrossRef]
- Reyes, P.R.; Torres-Florez, J.P. Diversidad, distribución, riqueza y abundancia de condrictios de aguas profundas a través del archipiélago patagónico austral, Cabo de Hornos, Islas Diego Ramírez y el sector norte del paso Drake. Rev. Biol. Mar. Oceanogr. 2009, 44, 243–251. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell: Hoboken, NJ, USA, 2004. [Google Scholar]
- Dzierzbicka-Głowacka, L. Modelling the seasonal dynamics of marine plankton in the southern Baltic Sea. Part 1. A coupled ecosystem model. Oceanologia 2005, 47, 591–619. [Google Scholar]
- Bąk, M.; Witkowski, A.; Lange-Bertalot, H.; Dadał, A. Ecology of the Szczecin Lagoon diatom flora with reference to the utility of diatom indices in assessing water quality. Diatom 2004, 20, 23–31. [Google Scholar]
- Witak, M. A review of the diatom research in the Gulf of Gdańsk and Vistula Lagoon (southern Baltic Sea). Oceanol. Hydrobiol. Stud. 2013, 44, 336–346. [Google Scholar] [CrossRef]
- Pilkaitytë, R.; Razinkovas, A. Seasonal changes in phytoplankton composition and nutrient limitation in a shallow Baltic lagoon. Boreal Environ. Res. 2007, 12, 551–559. [Google Scholar]
- Pilkaitytë, R. Spring-Summer transition in the Curonian lagoon (SE Baltic Sea) phytoplankton community. Transit. Waters Bull. 2007, 1, 39–47. [Google Scholar] [CrossRef]
- Siegel, H.; Gerth, M. Remote-Sensing studies of the exceptional summer of 1997 in the Baltic Sea: The warmest August of the century, the Oder flood, and phytoplankton blooms. Elsevier Oceanogr. Ser. 2000, 63, 239–255. [Google Scholar] [CrossRef]
- Alves-de-Souza, C.; Iriarte, J.L.; Mardones, J.I. Interannual variability of Dinophysis acuminata and Protoceratium reticulatum in a Chilean Fjord: Insights from the realized niche analysis. Toxins 2019, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Martínez Fernández, J.; Esteve Selma, M.A. Gestión integrada de cuencas costeras: Dinámica de los nutrientes en la cuenca del Mar Menor (sudeste de España). Rev. Din. Sist. 2007, 3, 2–23. [Google Scholar]
- Stramska, M.; Aniskiewicz, P. Recent large scale environmental changes in the Mediterranean sea and their potential impacts on Posidonia oceanica. Remote Sens. 2019, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Thamm, R.; Schernewski, G.; Wasmund, N.; Neumann, T. Spatial phytoplankton pattern in the Baltic Sea. In Coastline Reports. Baltic Sea Typology; Schernewski, G., Wielgat, M., Eds.; EUCC: Leiden, The Netherlands, 2004; pp. 85–109. [Google Scholar]
- Bustillos–Guzmán, J.J.; Band–Schmidt, C.J.; López–Cortés, D.J.; Gárate–Lizárraga, I.; Núñez–Vázquez, E.J.; Hernández–Sandoval, F.E. Variaciones en el crecimiento y toxicidad en Gymnodinium catenatum Graham del golfo de California bajo diferentes proporciones de nitrógeno y fósforo. Cienc. Mar. 2012, 38, 101–117. [Google Scholar]
- Paldaviciene, A.; Mazur-Marzec, H.; Razinkovas, A. Toxic cyanobacteria blooms in the Lithuanian part of the Curonian Lagoon. Oceanologia 2009, 51, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, H.; Signoret, M. Fitoplancton de la Laguna del Mar Muerto en el sur del Pacífico de México. Anales del Centro de Ciencias del Mar. y Limnología. 1978. Available online: http://biblioweb.tic.unam.mx/cienciasdelmar/centro/1979-2/articulo72.html (accessed on 18 June 2019).
- Vadrucci, M.R.; Fiocca, A.; Vignes, F.; Fabbrocini, A.; Roselli, L.; D’Adamo, R.; Basset, A. Dynamics of phytoplankton guilds under dystrophic pressures in Lesina lagoon. Trans. Waters Bull. 2010, 3, 33–46. [Google Scholar] [CrossRef]
- Delpy, F.; Serranito, B.; Jamet, J.; Grégori, G.J.; LePoupon, C.; Jamet, D. Pico- and Nanophytoplankton Dynamics in Two Coupled but Contrasting Coastal Bays in the NW Mediterranean Sea (France). Estuaries Coasts 2012, 41, 2039–2055. [Google Scholar] [CrossRef]
- García-Pintado, J.; Martínez-Mena, M.; González-Barberá, G.; Albaladejo, J.; Castillo, V.M. Anthropogenic nutrient sources and loads from a Mediterranean catchment into a coastal lagoon: Mar Menor, Spain. Sci. Total Environ. 2007, 373, 220–239. [Google Scholar] [CrossRef] [PubMed]
- Pilkaitytë, R.; Razinkovas, A. Factors controlling phytoplankton blooms in a temperate estuary: Nutrient limitation and physical forcing. In Marine Biodiversity. Developments in Hydrobiology; Martens, K., Queiroga, H., Cunha, M.R., Cunha, A., Moreira, M.H., Quintino, V., Rodrigues, A.M., Seroôdio, J., Warwick, R.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 41–48. [Google Scholar]
- Faz Cano, A.; Lobera Lössel, J.B.; Mora Navarro, J.; Simón Andreu, P. 5. Depuración y descontaminación de aguas. In Informe Integral Sobre el Estado Ecológico del Mar Menor; Comité de Asesoramiento Científico del Mar Menor: Madrid, Spain, 2017; pp. 113–125. [Google Scholar]
- Dimitrieva, O.A.; Semenova, A.S. Seasonal dynamics and trophic interactions of phytoplankton and zooplankton in the Vistula Lagoon of the Baltic Sea. Oceanology 2012, 52, 785–789. [Google Scholar] [CrossRef]
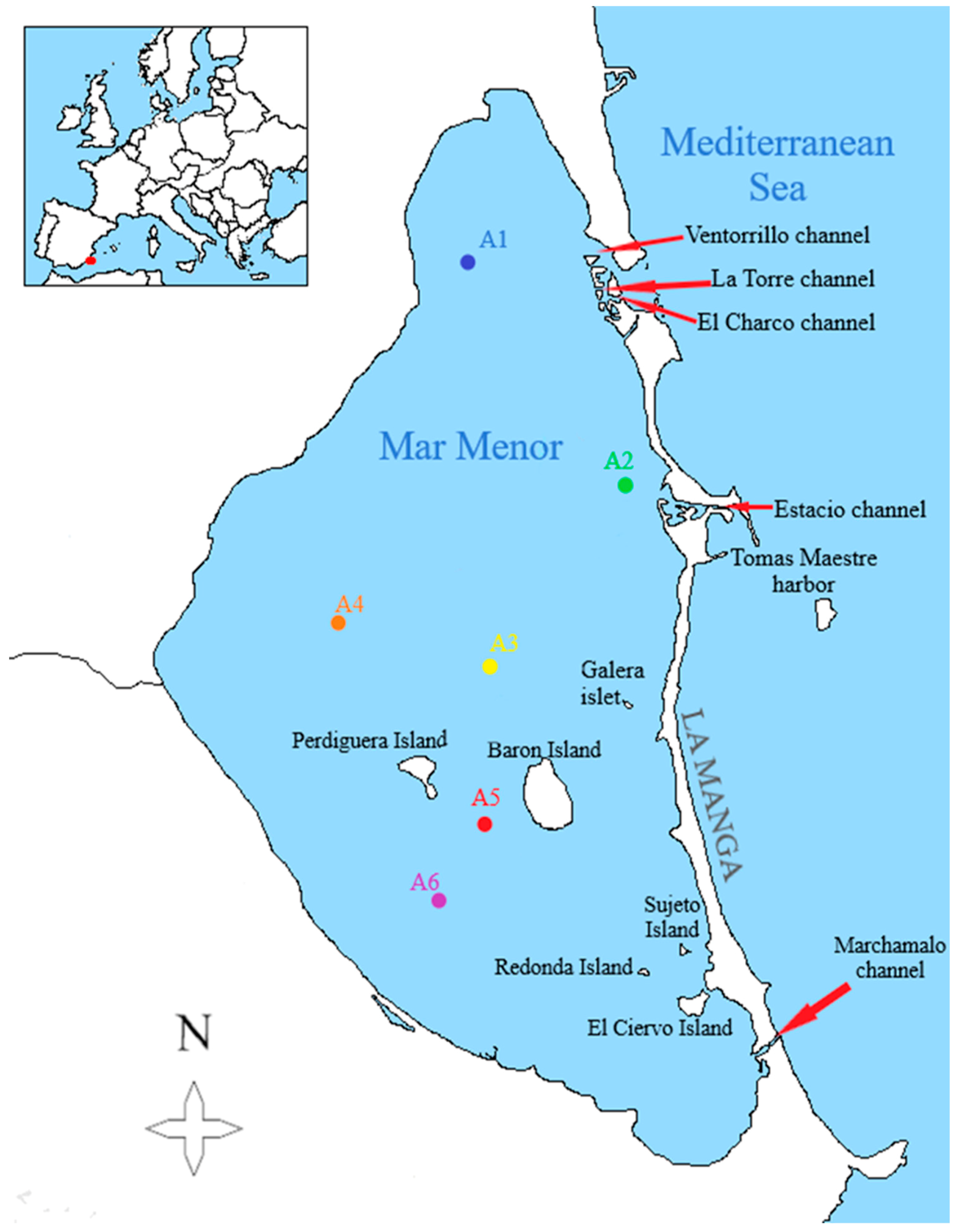

| Sample | Date | Chl-a (µg/L) | Secchi Disk Depth (m) | Density (cells/mL) | S | H’ | J |
|---|---|---|---|---|---|---|---|
| A1 | 1st Sep | 13.0 | 1.3 | 67,335 | 39 | 0.19 | 0.05 |
| 28th Oct | 7.0 | 2.3 | 7522 | 26 | 1.39 | 0.43 | |
| A2 | 8th May | 4.5 | 5.3 | 8082 | 31 | 1.21 | 0.35 |
| 25th Jun | 2.4 | 3.6 | 3919 | 24 | 1.40 | 0.44 | |
| 1st Sep | 9.0 | 1.3 | 20,666 | 17 | 1.61 | 0.57 | |
| 28th Oct | 7.9 | 2.1 | 21,568 | 20 | 0.52 | 0.17 | |
| 9th Dec | 6.2 | 1.9 | 7857 | 23 | 1.23 | 0.39 | |
| A3 | 8th May | 2.0 | 4.4 | 8937 | 37 | 1.12 | 0.31 |
| 25th Jun | 0.3 | 4.5 | 4073 | 19 | 1.29 | 0.44 | |
| 28th Oct | 10.7 | 1.6 | 8294 | 31 | 1.03 | 0.30 | |
| A4 | 25th Jun | 0.1 | 4.7 | 3024 | 26 | 1.08 | 0.33 |
| 21st Oct | 7.9 | 1.9 | 7598 | 22 | 1.22 | 0.39 | |
| 9th Dec | 6.1 | 1.9 | 60,219 | 14 | 0.23 | 0.09 | |
| A5 | 8th May | 1.2 | 3.7 | 2536 | 28 | 1.49 | 0.45 |
| 21st Oct | 7.7 | 1.5 | 12,863 | 18 | 0.74 | 0.25 | |
| 9th Dec | 4.3 | 1.7 | 36,274 | 17 | 0.26 | 0.09 | |
| A6 | 8th May | 2.2 | 3.1 | 2651 | 28 | 1.87 | 0.56 |
| 21st Oct | 8.1 | 1.8 | 11,074 | 25 | 0.65 | 0.20 | |
| 9th Dec | 5.2 | 1.8 | 41,579 | 15 | 0.12 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soria, J.; Caniego, G.; Hernández-Sáez, N.; Dominguez-Gomez, J.A.; Erena, M. Phytoplankton Distribution in Mar Menor Coastal Lagoon (SE Spain) during 2017. J. Mar. Sci. Eng. 2020, 8, 600. https://doi.org/10.3390/jmse8080600
Soria J, Caniego G, Hernández-Sáez N, Dominguez-Gomez JA, Erena M. Phytoplankton Distribution in Mar Menor Coastal Lagoon (SE Spain) during 2017. Journal of Marine Science and Engineering. 2020; 8(8):600. https://doi.org/10.3390/jmse8080600
Chicago/Turabian StyleSoria, Juan, Gema Caniego, Nuria Hernández-Sáez, Jose Antonio Dominguez-Gomez, and Manuel Erena. 2020. "Phytoplankton Distribution in Mar Menor Coastal Lagoon (SE Spain) during 2017" Journal of Marine Science and Engineering 8, no. 8: 600. https://doi.org/10.3390/jmse8080600