1. Introduction
Yellow squat lobster (
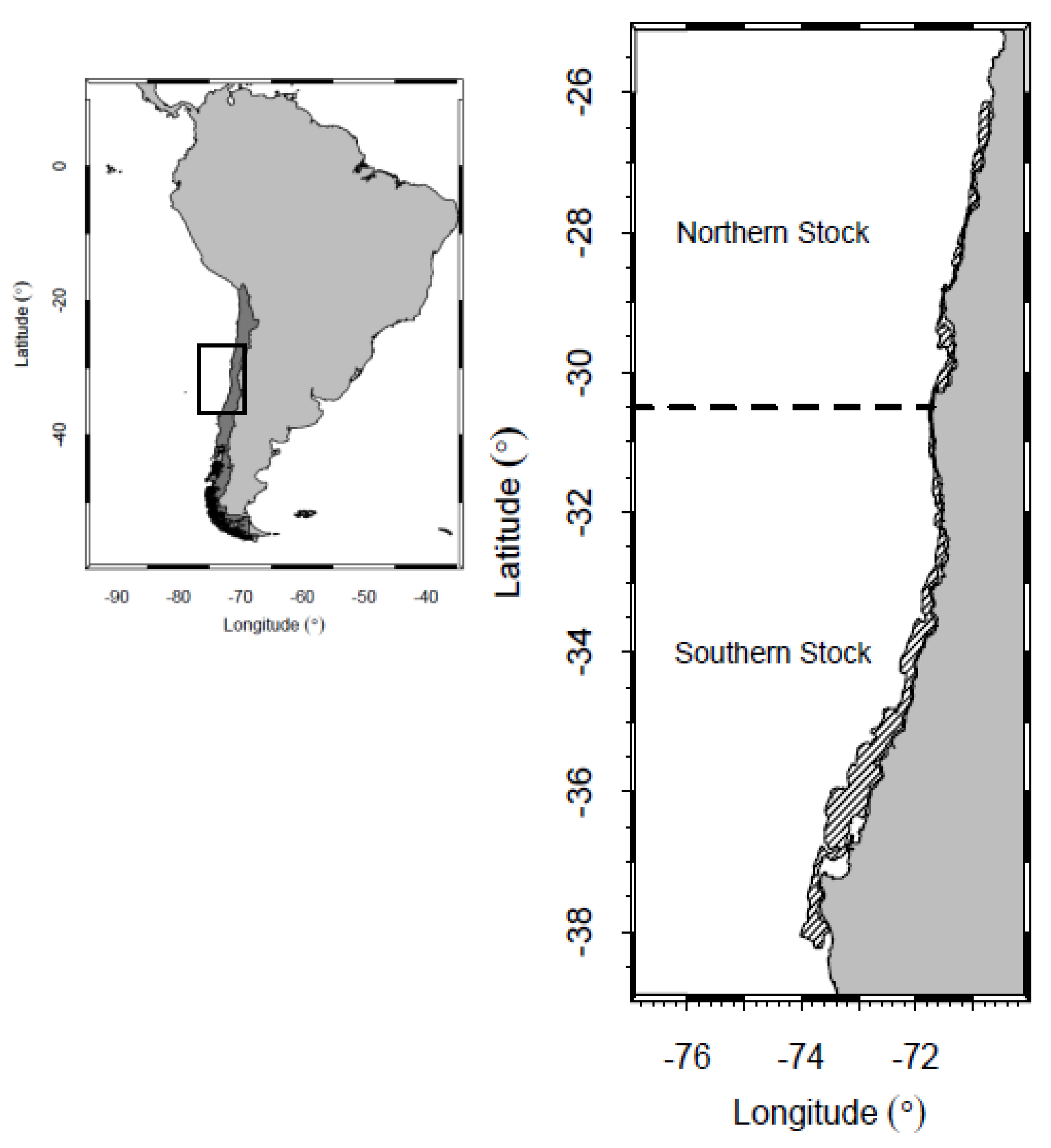
Cervimunida johni) is one of the most important crustaceans harvested in Chile in terms of landings and economical relevance. This species is a demersal crustacean inhabiting the Southeast Pacific coast between Taltal (29
19
S) and Mocha Island (38
20
S). Distribution is characterised by a narrow band on the continental shelf and upper part of the continental slope in depths of 100 to 500 m. The crustacean fishery in Chile started in the 1950s and features industrial and small-scale bottom trawlers, with a maximum landing of around 62,000 tons reached in 1976. Nowadays, landings of yellow squat lobster in Chile are around 5000 tons. Since 1996 this population has been separated into Northern Stock (26
03
S–30
30
S) and Southern Stock (30
30
S–38
48
S) (
Figure 1) for management purposes. Management of yellow squat lobster in Chile is based on total allowed catch (TAC) and reproductive closures.
Since 1991, fisheries management in Chile has been regulated by the “general law of fishing and aquaculture” including a system for quota allocation based on individual transferable quotas (ITQs) from 2001 to 2012. The Chilean fishing law was amended in 2013 to incorporate, among other changes, the maximum sustainable yield (MSY) as target reference point for managing fishery resources. This mandate triggered the estimation of MSY-based reference points (MSY-RPs) in each fishery subject to catch limits in Chile [
1].
In the case of the yellow squat lobster, MSY and depletion of spawning biomass are derived from an integrated age-structured stock assessment model, which combines data from different sources to estimate relevant population parameters. Direct assignation of age is difficult for crustaceans and debates still continue regarding the utility of age- or length-based stock assessment models [
2]. However, even when age cannot be assigned directly, age-structured models are useful for estimating recruitment cohorts, making population dynamics more tractable [
2].
For yellow squat lobster, the stock assessment is based on ages but fits observations of length structures; therefore, the conditional probability of the length-at-age (age-at-length key) is needed to convert observed lengths into ages. Age-at-length comes from individual growth parameters that are usually fixed within the stock assessment model. The assumption that growth parameters are known (for instance, updating) may add more uncertainty and complexity to the stock assessment of crustaceans, because small variations in growth parameters can have profound effects on the stock assessment and management variables and exploitation status [
3]. Steepness (
h) is a common measurement of stock resilience and is defined as the recruitment produced by a spawning biomass at 20% of unfished spawning biomass, relative to the recruitment produced by unfished spawning biomass [
4]. Incorrect assumptions on the steepness parameter will directly affect the assessment variables and the specific level at which each stock can be harvested.
Despite the importance of both growth and recruitment processes for the assessment and management variables of yellow squat lobster in Chile, the impact of the assumed parameters has not yet been studied. Thus, the aim of this paper is to explore how different assumptions about the parameters used in the growth and the stock recruitment relationship (SRR) affect the assessment and management variables (e.g., spawning biomass) of the yellow squat lobster fishery off Chile. We develop a sensitivity analysis to investigate the impact of the variation of growth and recruitment parameters on the different assessment and management variables. Using the yellow squat lobster stock assessment model as an operating model, we implement an extensive simulation analysis to assess the reliability in the estimation of those parameters that caused the higher variation in management quantities (e.g., ratio between the spawning biomass in the last year and the unfished spawning biomass, . We conclude that the parameters that describe individual growth have the largest impact in the assessment and management variables of the yellow squat lobster stock and we believe that our findings are extensive to all Chilean fisheries using the same assessment framework.
4. Discussion
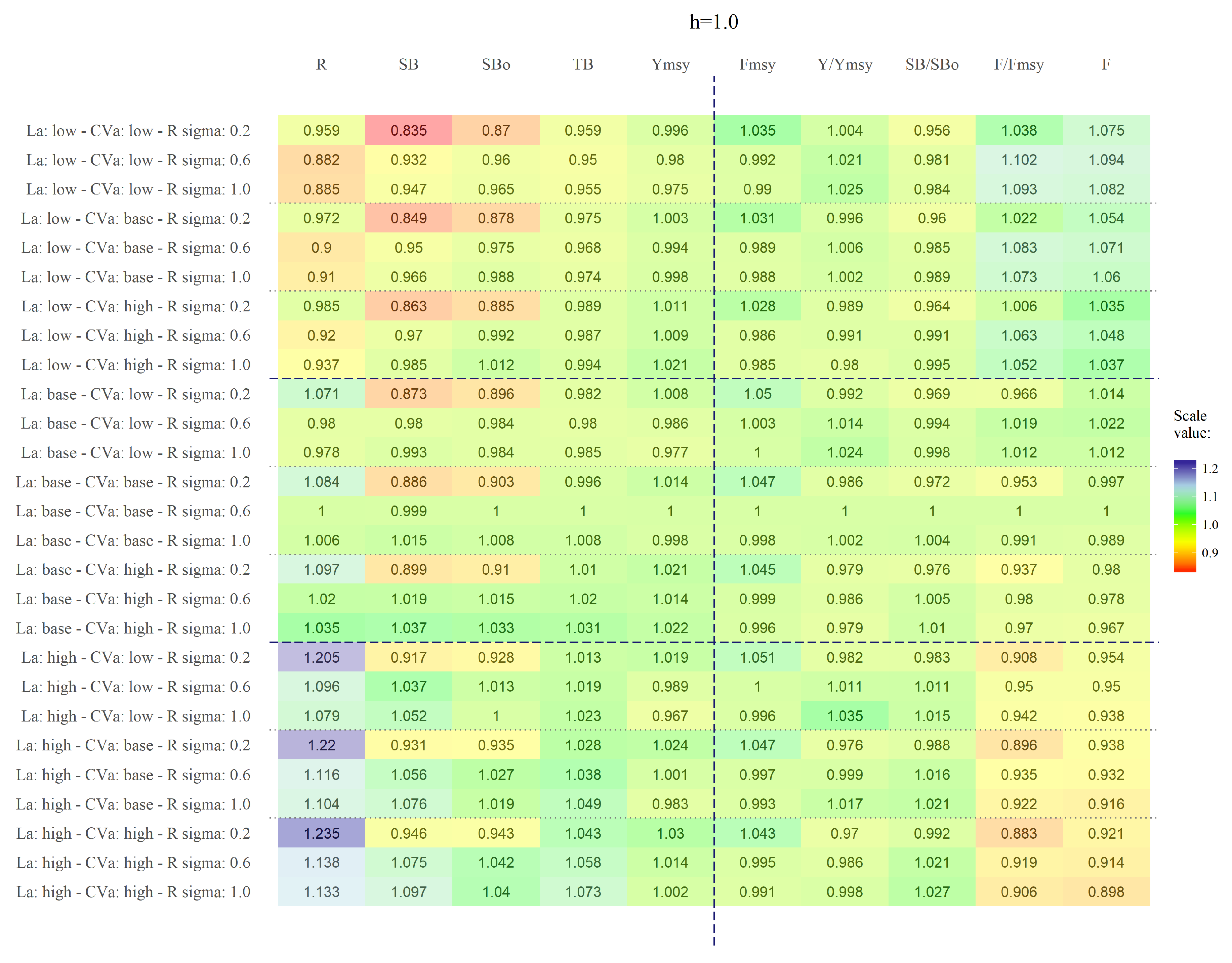
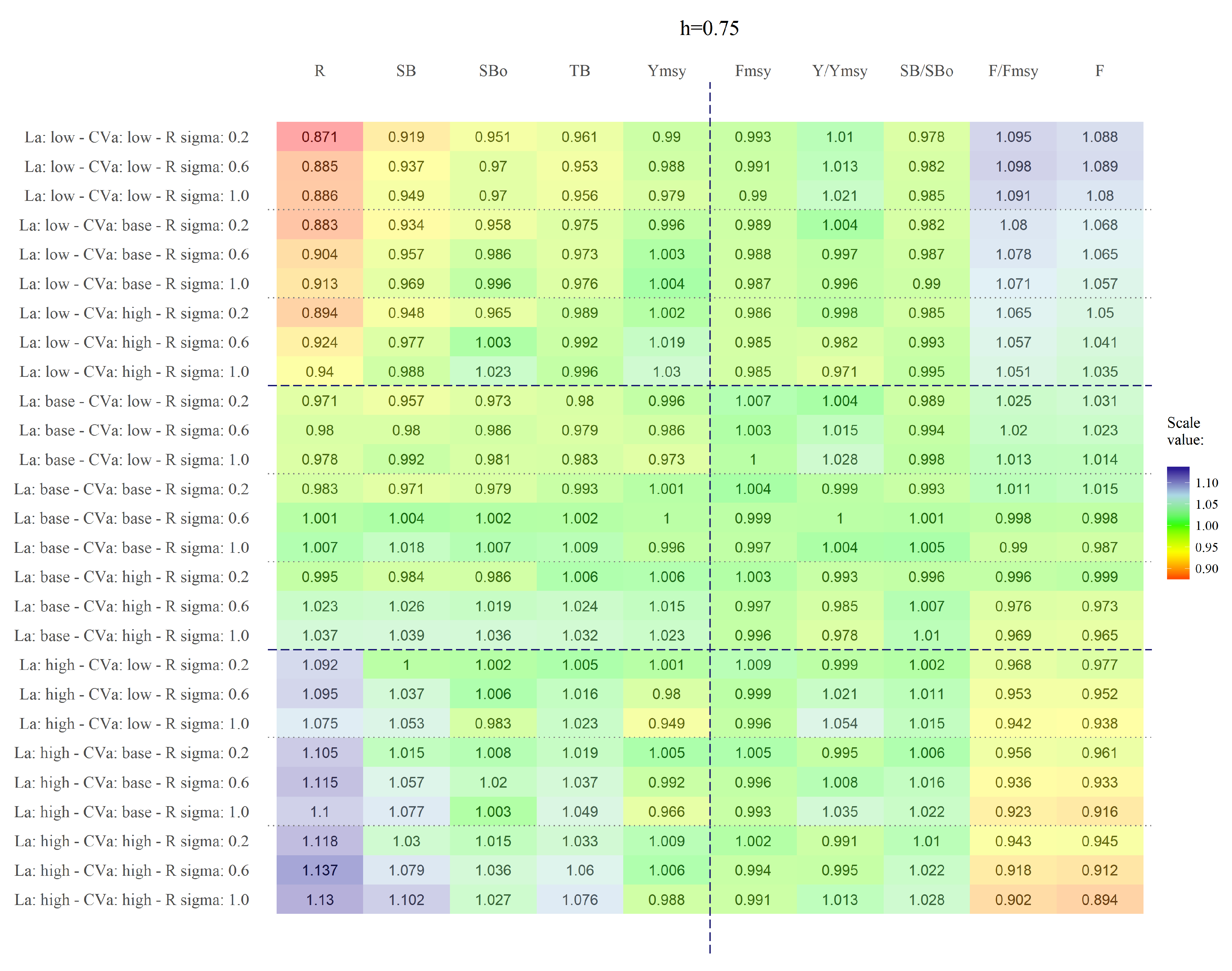
We have shown that the length at first capture, , has the highest impact on yellow squat lobster assessment and management variables. The variation of this parameter mainly affects recruitment (R) and secondarily, spawning biomass. Although, the other status variables were also affected, their variation was negligible. Contrary to our expectations, changes in the h assumption of the SRR and the variability of the recruitments () had a negligible impact on the studied variables. Changes in led to changes in individual mean weight, thus affecting directly the estimation of biomass and therefore the recruitments. Because the effects of the changes in h and were negligible, we concluded that the shape and variability of the SRR do not have a major impact on the assessment and management variables. Therefore, observed variations in the amount of recruitment were mainly caused by changing in the spawning biomass triggered by different values of .
Fishing mortality (F) and the ratio were the most affected management variables in all scenarios. However, when we analysed the effect of isolated; negligible variation arose across different scenarios for , , h and . Thus, variations in are down to changes in F across scenarios. Variability in F was a consequence of changes in across scenarios that triggered changes in vulnerable biomass. This imply that could significantly affect the exploitation status perceived in yellow squat lobster. Variation in steepness (h) became relevant only when . Under such condition, low variation of recruitment estimates around the theoretical curve is allowed, and thus steepness becomes more relevant in determining the population renewal therefore triggering variations in population abundance.
was the most biased parameter estimated in the simulation analysis. The bias on
increased when
remain fixed on the simulations in the estimation model, suggesting that the high correlation between these two parameters is important to maintain at least consistency on the estimation.
is assumed constant across time, but observed length structures show high variations. Therefore, processes such as selectivity, growth parameters and recruitment need to vary across time to cope with observed variations on length structures. Indeed, when trying to estimate growth and recruitment parameters simultaneously, a poor optimisation process was reported (e.g., non-invertible Hessian matrix). This agrees with previous investigations suggesting that a confounding effect is found when estimating at the same time life history and recruitment parameters on integrated stock assessment models [
11].
We propose that variations in
have a major impact on the assessment and management variables of Chilean yellow squat lobster. This agrees with other works stating that misspecification of growth can result in deep uncertainties in parameter and management quantity estimates when the stock assessment models are fit to length structure [
3]. The assessment framework of the yellow squat lobster depends on known growth parameter
k and
, leading to fixed values of
and
. This issue calls for caution because it may give a false sense of certainty about the exploitation status of this species. As with many other crustacean species, direct observation of ages in yellow squat lobster is difficult. In Chile, age estimations used in stock assessments come exclusively from age distribution mixtures using length structures [
12]. This method involves high levels of uncertainty because the numbers of age groups are essentially a subjective decision [
7]. Yellow squat lobster showed wide variability on reported growth parameters across different studies [
6,
13,
14,
15,
16]. Number of age groups assigned on the observed length structures varies from 5 [
13,
15] to 11 [
6]. These variations in age groups assigned are then propagated into the estimated growth parameters. Values of
k varied from 0.154 to 0.196 [year
] in females, and 0.118 to 0.221 [year
] in males. Additionally,
takes values in the range of 45.6 to 54.6 [cm] in females and 52.8 to 62.1 [cm] in males. For current stock assessment in Chilean yellow squat lobster, selected beforehand, a set of fixed growth parameters and no further or formal sensitivity analysis is performed. Given the importance of the growth parameters on
and
and therefore for the assessment and management variables of yellow squat lobster, we recommend to developing workable protocols that deal with these shortcomings. Alternatively, growth parameters could be estimate within the stock assessment model, thus including growth parameters and associate uncertainty as additional information to the integrated model [
3]. Further work should includes extensive sensitivity analysis on how growth parameters are affected by selectivity, and how intermediate and old ages are affected by changes
. This also should be extended to the perception of status, since
F was the variable most impacted by changes in
. In addition, others simulation scenarios could include a wider interval of values
h since the value used here and in the stock assessment (
h = 1) is at the boundary of the parameters space for
h and this sometime could influencing the Hessian estimation.
During 2014, the Instituto de Fomento Pesquero (IFOP) conducted a project on biological reference points in Chilean fisheries subjected to annual quotas [
9]. The project included extensive work, to classify stocks in tiers (groups) according to quantity and quality of the data available (poor, moderate, and rich data) and the reliability of the assessment estimates. Later, specific methods were proposed to estimate MSY-based RPs in each tier. Demersal crustacean fisheries, including yellow squat lobster, were classified in such tier in which proxy variables are used to estimate MSY- based RPs. This means that yellow squat lobster contains enough information to conduct an age-structured stock assessment, but
h cannot reliably be estimated within the stock assessment model. Thus, simulation results presented here should be seen as complementary to the simulations analysis developed for MSY-based RPs [
9]. Additionally, the simulation findings here suggest that in the process of parameterising the Chilean yellow squat lobster, the stock assessment model serves best to avoid the combined estimation of the parameters that describe the growth process and the stock-recruitment relationship. If the combined estimation is avoided, the bias in the estimation of the stock assessment and management variables will decrease.
We conclude that the parameters related to individual growth are the most important in determining the exploitation status of this stock. In an age-structured model where the fitted observed length structures show high inter-annual variability, an unbiased estimation of is very important. Thus, we recommend the use of workable simulation protocols for growth when using an age-structured model fitting length observations such as yellow squat lobster in Chile. The analysis was focused on the Northern stock of the Chilean yellow squat lobster, however our findings extend to all Chilean fisheries using age-structured models fitting length observations, because they shared the same assessment framework.