Coastal Phytoplankton Pigments Composition in Three Tropical Estuaries of Indonesia
Abstract
1. Introduction
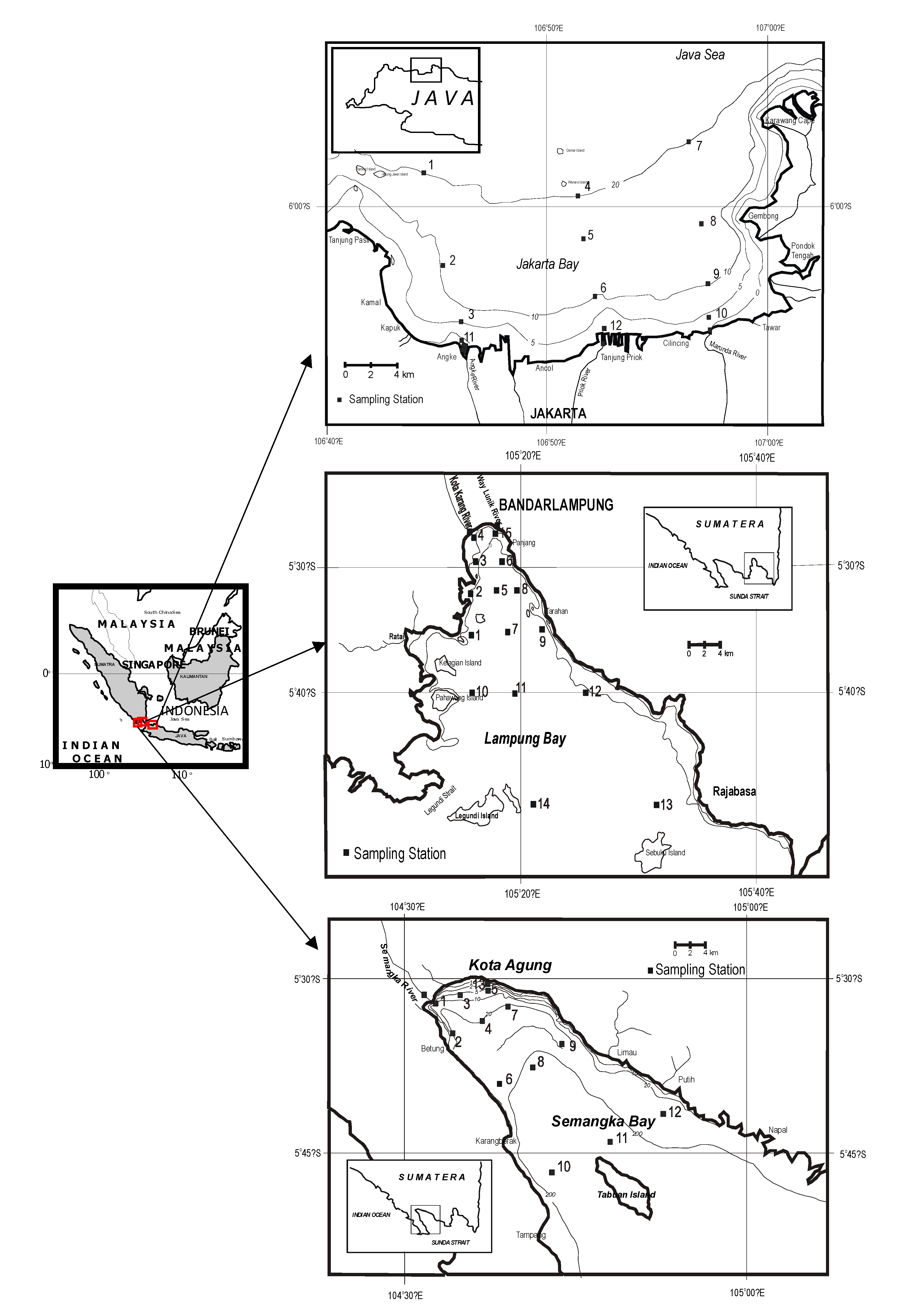
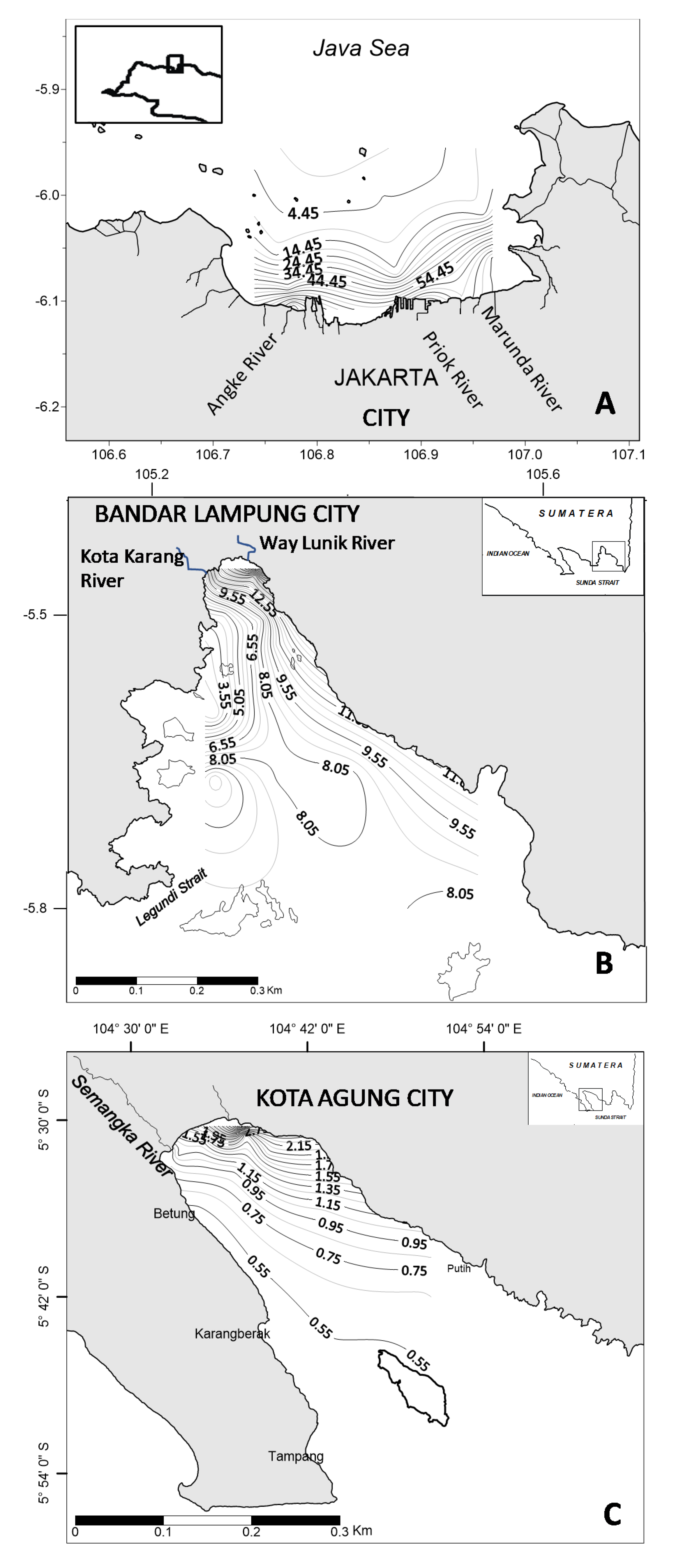
2. Context and Methodology
3. Results
3.1. Pigment Distribution
3.2. Pigments Correlation with Nutrients and Underwater Light
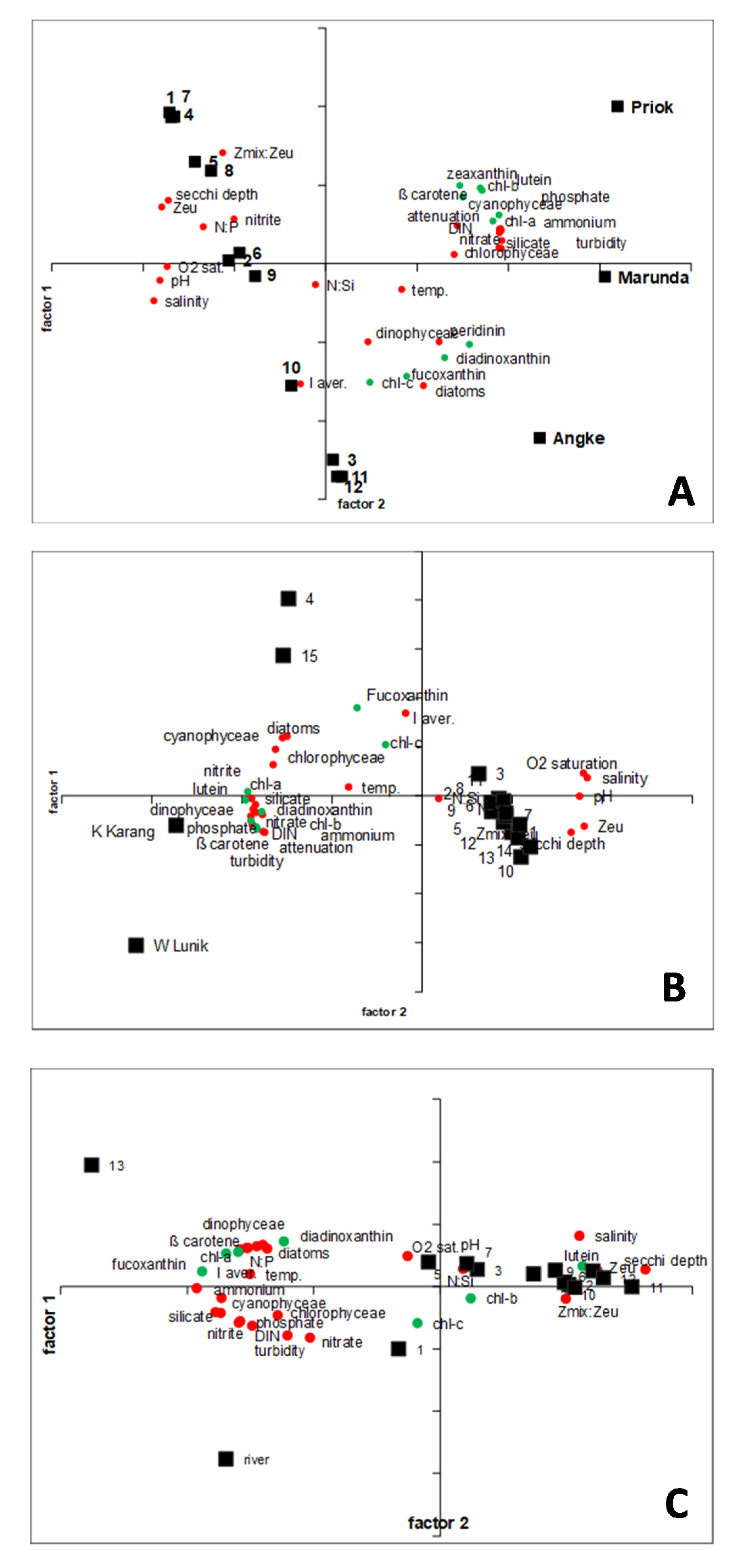
3.3. General Proxy for Phytoplankton Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrier-Pages, C.; Gattuso, J.-P.; Dallot, S.; Daubert, J. Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 2000, 19, 103–113. [Google Scholar] [CrossRef]
- Riemann, L.; Nielsen, T.G.; Kragh, T.; Richardson, K.; Parner, H.; Jakobsen, H.H.; Munk, P. Distribution and production of plankton communities in the subtropical convergence zone of the Sargasso Sea. I. Phytoplankton and bacterioplankton. Mar. Ecol. Prog. Ser. 2011, 426, 57–70. [Google Scholar] [CrossRef]
- Cloern, J.E. The relative importance of light and nutrient limitation of phytoplankton growth: A simple index of coastal ecosystem sensitivity to nutrient enrichment. Aquat. Ecol. 1999, 33, 3–16. [Google Scholar] [CrossRef]
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Colijn, F.; Hesse, K.-J.; Ladwig, N.; Tillmann, U. Effects of the large-scale uncontrolled fertilisation process along the continental coastal North Sea. Hydrobiologia 2002, 484, 133–148. [Google Scholar] [CrossRef]
- Kitsiou, D.; Karydis, M. Coastal marine eutrophication assessment: A review on data analysis. Environ. Int. 2011, 37, 778–801. [Google Scholar] [CrossRef]
- Raveh, O.; Angel, D.L.; Astrahan, P.; Belkin, N.; Bar-Zeev, E.; Rahava, E. Phytoplankton response to N-rich well amelioration brines: A mesocosm study from the southeastern Mediterranean Sea. Mar. Pollut. Bull. 2019, 146, 355–365. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef]
- Agirbas, E.; Koca, L.; Aytan, U. Spatio-temporal pattern of phytoplankton and pigment composition in surface waters of south-eastern Black Sea. Oceanologia 2017, 59, 283–299. [Google Scholar] [CrossRef]
- Mishra, R.K.; Senga, Y.; Nakata, K.; Mishra, S.; Sahu, B.K. Spatio-temporal variation of Prochlorococcus and phytoplankton community between Shimizu coast and Suruga bay, Northwest Pacific Ocean. Reg. Stud. Mar. Sci. 2020, 33, 100890. [Google Scholar] [CrossRef]
- Reuss, N. Sediment Pigments as Biomarkers of Environmental Change. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, March 2005. [Google Scholar]
- Barlow, R.; Lamont, T.; Kyewalyanga, M.; Sessions, H.; Morris, T. Phytoplankton production and physiological adaptation on the southeastern shelf of the Agulhas ecosystem. Cont. Shelf Res. 2010, 30, 1472–1486. [Google Scholar] [CrossRef]
- Lamont, T.; Barlow, R.G.; Kyewalyanga, M.S. Physical drivers of phytoplankton production in the southern Benguela upwelling system. Deep Sea Res. Part I 2014, 90, 1–16. [Google Scholar] [CrossRef]
- Hashihama, F.; Umeda, H.; Hamada, C.; Kudoh, S.; Hirawake, T.; Satoh, K.; Fukuchi, M.; Kashino, Y. Light acclimation states of phytoplankton in the Southern Ocean, determined using photosynthetic pigment distribution. Mar. Biol. 2010, 157, 2263–2278. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Mantoura, R.F.C.; Wright, S.W. Phytoplankton Pigments in Oceanography; UNESCO Publishing: Paris, France, 1997; p. 661. [Google Scholar]
- Jeffrey, S.W.; Wright, S.W.; Zapata, M. Recent advances in HPLC pigment analysis of phytoplankton. Mar. Freshw. Res. 1999, 50, 879–896. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffry, S.W. Fucoxanthin pigment markers of marine phytoplankton analyzed by HPLC and HPTLC. Mar. Ecol. Prog. Ser. 1987, 38, 259–266. [Google Scholar] [CrossRef]
- Aneeshkumar, N.; Sujatha, C.H. Biomarker pigment signatures in Cochin back water system—A tropical estuary south west coast of India. Estuar. Coast. Shelf Sci. 2012, 99, 182–190. [Google Scholar] [CrossRef]
- Lima, C.R.; Mendesa, C.R.B.; Tavanoa, V.M.; Detonia, A.M.S.; Secchi, E.R. Chemotaxonomy-based mapping of phytoplankton communities in the subtropical Southwestern Atlantic Ocean, with emphasis on the marine cyanobacterium Trichodesmium. Prog. Oceanogr. 2019, 172, 77–88. [Google Scholar] [CrossRef]
- Yang, S.; Cui, Z.; Zhang, Y.; Jiang, T.; Yang, Q.; Sun, Y. Photosynthetic pigments in surface sediments in the northwest of the Bohai Sea, China: Potential implications for sediment deposition of brown tides of Aureococcus anophagefferens in coastal waters. Ecol. Indic. 2019, 102, 145–153. [Google Scholar] [CrossRef]
- Foy, R.H. The phycocyanin to chlorophyll a ratio and other cell components as indicators of nutrient limitation in two planktonic cyanobacteria subjected to low-light exposures. J. Plankton Res. 1993, 15, 1263–1276. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Hodgson, D.A. Sedimentary Pigments. In Tracking Environmental Change Using Lake Sediments; Smol, J.P., Birks, H.J.B., Last, W.M., Bradley, R.S., Alverson, K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 295–325. [Google Scholar]
- Damar, A.; Colijn, F.; Hesse, K.-J.; Wardiatno, Y. The eutrophication states of Jakarta, Lampung and Semangka Bays: Nutrient and phytoplankton dynamics in Indonesian tropical waters. J. Trop. Biol. Conserv. 2012, 9, 61–81. [Google Scholar]
- Gieskes, W.W.C.; Kraay, G.W.; Nontji, A.; Setiapermana, D.; Sutomo. Monsoonal alternation of a mixed and a layered structure in the phytoplankton of the euphotic zone of the Banda Sea (Indonesia): A mathematical analysis of algal pigment fingerprints. Neth. J. Sea Res. 1988, 22, 123–137. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.H.; Zhao, J.G.; Xiao, L.; Jiang, T. Effects of inorganic nutrients on the phytoplankton community in the sea surface microlayer of Daya Bay, South China Sea. J. Sea Res. 2020, 156, 101830. [Google Scholar] [CrossRef]
- Chacko, N. Chlorophyll bloom in response to tropical cyclone Hudhud in the Bay of Bengal: Bio-Argo subsurface observations. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 124, 66–72. [Google Scholar] [CrossRef]
- Chakraborty, K.; Nimit, K.; Akhand, A.; Prakash, S.; Paul, A.; Ghosh, J.; Bhaskar, T.V.S.U.; Chanda, A. Modeling the enhancement of sea surface chlorophyll concentration during the cyclonic events in the Arabian Sea. J. Sea Res. 2018, 140, 22–31. [Google Scholar] [CrossRef]
- George, J.V.; Nuncio, M.; Chacko, R.; Anilkumar, N.; Noronha, S.B.; Patil, S.M.; Pavithran, S.; Alappattu, D.P.; Krishnan, K.P.; Achuthankutty, C.T. Role of physical processes in chlorophyll distribution in the western tropical Indian Ocean. J. Mar. Syst. 2013, 113–114, 1–12. [Google Scholar] [CrossRef]
- Hou, X.; Dong, Q.; Xue, C.; Wu, S. Seasonal and interannual variability of chlorophyll-a and associated physicalsynchronous variability in the western tropical Pacific. J. Mar. Syst. 2016, 158, 59–71. [Google Scholar] [CrossRef]
- Krishna, K.M. Variability of SeaWiFS-derived chlorophyll-a concentrations in waters off central east coast of India, 1998–2003. Int. J. Appl. Earth Obs. Geoinf. 2010, 12, 101–109. [Google Scholar] [CrossRef]
- Nukapothula, S.; Chen, C.; Yunus, A.P.; Wu, J. Satellite-based observations of intense chlorophyll-a bloom in response of cold core eddy formation: A study in the Arabian Sea, Southwest Coast of India. Reg. Stud. Mar. Sci. 2018, 24, 303–310. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, L.; Hou, Y.; Zhao, H.; Qi, Y.; Yi, X. Tropical storm-induced turbulent mixing and chlorophyll-a enhancement in the continental shelf southeast of Hainan Island. J. Mar. Syst. 2014, 129, 405–414. [Google Scholar] [CrossRef]
- Sañé, E.; Valente, A.; Fatela, F.; Cabral, M.C.; Beltrán, C.; Drago, T. Assessment of sedimentary pigments and phytoplankton determined by CHEMTAX analysis as biomarkers of unusual upwelling conditions in summer 2014 off the SE coast of Algarve. J. Sea Res. 2019, 146, 33–45. [Google Scholar] [CrossRef]
- Damar, A.; Colijn, F.; Hesse, K.-J. Effects of different nutrient loadings on planktonic primary production in embayments of Indonesia. J. Trop. Biol. Conserv. 2014, 11, 63–85. [Google Scholar]
- Damar, A. Effects of Enrichment on Nutrient Dynamics, Phytoplankton Dynamics and Primary Production in Indonesian Tropical Waters: A Comparison between Jakarta Bay, Lampung Bay and Semangka Bay. Ph.D. Thesis, University of Kiel, Kiel, Germany, 2003. [Google Scholar]
- Mantoura, R.F.C.; Llewellyn, C.A. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase-high-performance liquid chromatography. Anal. Chim. Acta 1983, 151, 297–314. [Google Scholar] [CrossRef]
- Lalli, C.M.; Parsons, P.R. Biological Oceanography. An Introduction, 2nd ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Tillmann, U.; Hesse, K.-J.; Colijn, F. Planktonic primary production in the German Wadden Sea. J. Plankton Res. 2000, 22, 1253–1276. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Verlag Chemie Weinheim: Deerfield Beach, FL, USA, 1983; p. 419. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Int. Ver. Theor. Angew. Limnol. Mitt. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Wright, S.W. Analysis of phytoplankton populations using pigment markers. In Proceedings of the Course Notes for a Workshop “Pigment Analysis of Antarctic Microorganisms”, Kuala Lumpur, Malaysia, 29 June–1 July 2005. [Google Scholar]
- Oseji, O.F.; Chigbu, P.; Oghenekaro, E.; Waguespack, Y.; Chen, N. Spatiotemporal patterns of phytoplankton composition and abundance in the Maryland Coastal Bays: The influence of freshwater discharge and anthropogenic activities. Estuar. Coast. Shelf Sci. 2018, 207, 119–131. [Google Scholar] [CrossRef]
- Sidabutar, T.; Bengen, D.G.; Wouthuyzen, S.; Partono, T. The abundance of phytoplankton and its relationship to the N/P ratio in Jakarta Bay, Indonesia. Biodiversitas 2016, 17, 673–678. [Google Scholar] [CrossRef]
- Dring, M.J. The Biology of Marine Plants; Edward Arnold (Publishers) Limited: London, UK, 1982; p. 129. [Google Scholar]
- Platt, T.; Gallegos, C.K.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Chai, C.; Jiang, T.; Cen, J.; Ge, W.; Lu, S. Phytoplankton pigments and functional community structure in relation to environmental factors in the Pearl River Estuary. Oceanologia 2016, 58, 201–211. [Google Scholar] [CrossRef]
- Lagaria, A.; Mandalakis, M.; Mara, P.; Frangoulis, C.; Karatsolis, B.-T.; Pitta, P.; Triantaphyllou, M.; Tsiola, A.; Psarra, S. Phytoplankton variability and community structure in relation to hydrographic features in the NE Aegean frontal area (NE Mediterranean Sea). Cont. Shelf Res. 2017, 149, 124–137. [Google Scholar] [CrossRef]
- Van de Vyver, E.; Van Wichelen, J.; Vanormelingen, P.; Van Nieuwenhuyze, W.; Daveloose, I.; De Jong, R.; De Blok, R.; Urrutia, R.; Tytgat, B.; Verleyen, E.; et al. Variation in phytoplankton pigment composition in relation to mixing conditions in temperate South-Central Chilean lakes. Limnologica 2019, 79, 125715. [Google Scholar] [CrossRef]
- Kocum, E.; Underwood, G.J.C.; Nedwell, D.B. Simultaneous measurement of phytoplanktonic primary production, nutrient, and light availability along a turbid, eutrophic UK east coast estuary (the Colne Estuary). Mar. Ecol. Prog. Ser. 2002, 231, 1–12. [Google Scholar] [CrossRef]
- Wilkerson, F.P.; Lassiter, A.M.; Dugdale, R.C.; Marchi, A.; Hogue, V.E. The phytoplankton bloom response to wind events and upwelled nutrients during the CoOP WEST study. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 3023–3048. [Google Scholar] [CrossRef]
- Guinder, V.A.; Molinero, J.C.; Popovich, C.A.; Marcovecchio, J.E.; Sommer, U. Dominance of the planktonic diatom Thalassiosira minima in recent summers in the Bahı´a Blanca Estuary, Argentina. J. Plankton Res. 2012, 34, 995–1000. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, L.; Laws, E.; Xie, Y.; Chen, J.; Liu, X.; Chen, B.; Huang, B. Realized niches explain spatial gradients in seasonal abundance of phytoplankton groups in the South China Sea. Prog. Oceanogr. 2018, 162, 223–239. [Google Scholar] [CrossRef]
- Irigoien, X.; Meyer, B.; Harris, R.; Harbour, D. Using HPLC pigment analysis to investigate phytoplankton taxonomy: The importance of knowing your species. Helgol. Mar. Res. 2004, 58, 77–82. [Google Scholar] [CrossRef]
- Thrane, J.-E.; Kyle, M.; Striebel, M.; Haande, S.; Grung, M.; Rohrlack, T.; Andersen, T. Spectrophotometric analysis of pigments: A critical assessment of a high-throughput method for analysis of algal pigment mixtures by spectral deconvolution. PLoS ONE 2015, 10, e0137645. [Google Scholar] [CrossRef] [PubMed]
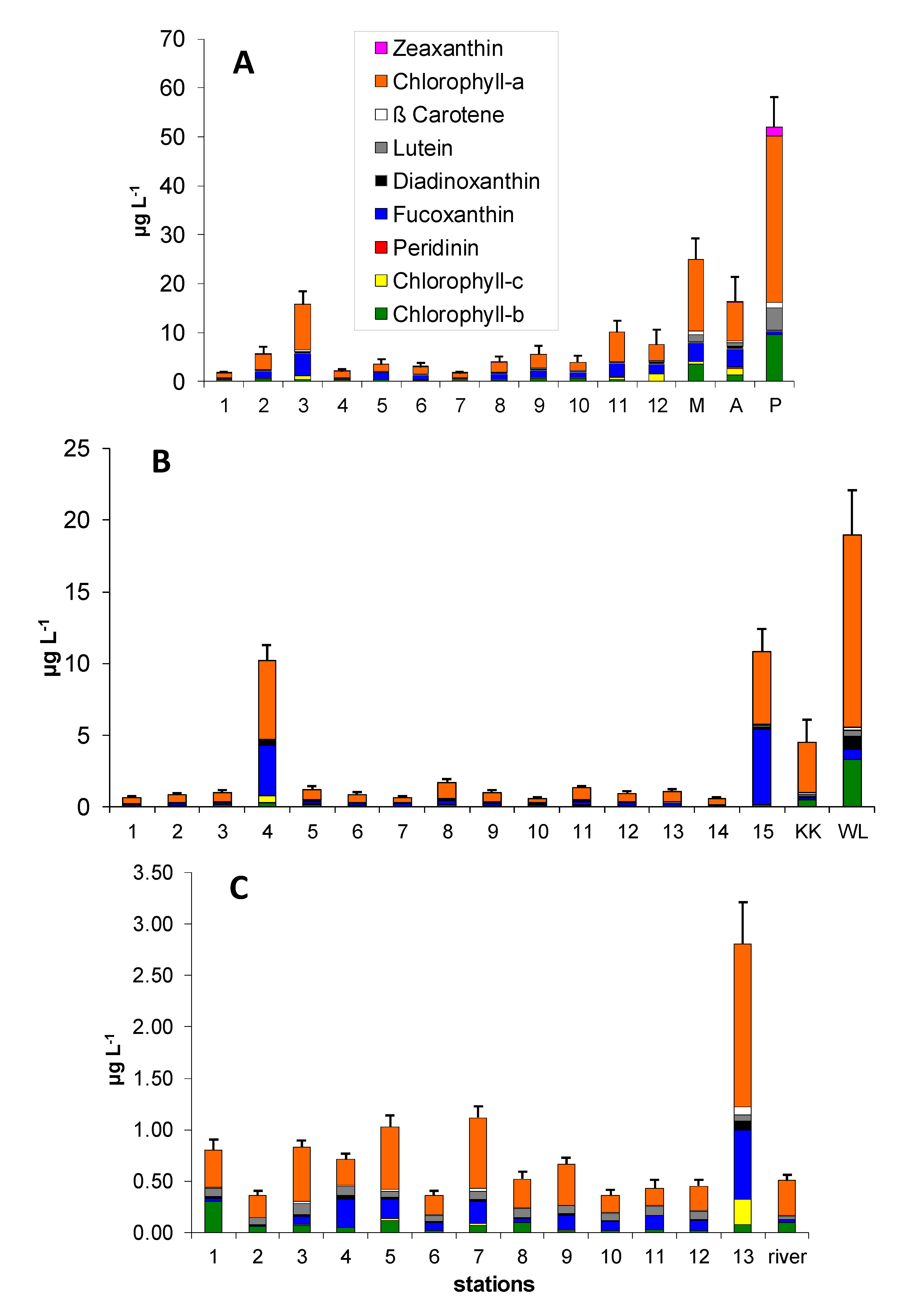


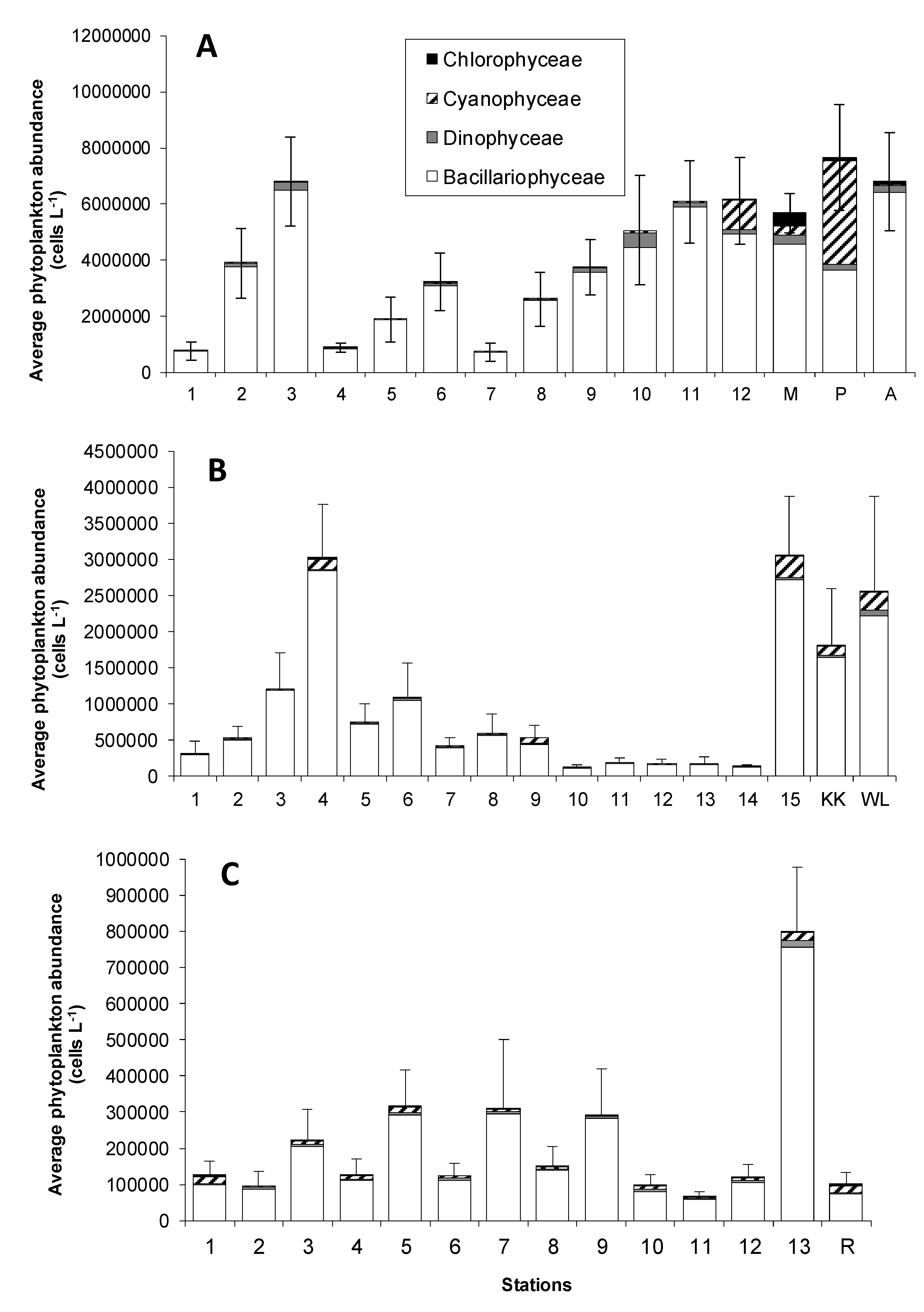
| Pigments | Linear Regression | R2 |
|---|---|---|
| Jakarta Bay | ||
| Chlorophyll-a | y = 1.4761x + 1.4235 | R2 = 0.99 |
| Chlorophyll-b | y = 4.9069x + 4.4668 | R2 = 0.91 |
| Chlorophyll-c | y = 1.55x + 9.7722 | R2 = 0.003 |
| Fucoxanthin | y = 2.674x + 5.8019 | R2 = 0.08 |
| Diadinoxanthin | y = 48.413x + 1.5975 | R2 = 0.28 |
| Lutein | y = 10.69x + 4.3464 | R2 = 0.89 |
| β-carotene | y = 42.669x + 0.9266 | R2 = 0.95 |
| Zeaxanthin | y = 22.781x + 7.0696 | R2 = 0.75 |
| Lampung Bay | ||
| Chlorophyll-a | y = 1.5079x + 0.2209 | R2 = 0.96 |
| Chlorophyll-b | y = 5.4355x + 1.6574 | R2 = 0.69 |
| Chlorophyll-c | y = 0.4482x − 0.6728 | R2 = 0.19 |
| Fucoxanthin | y = 2.116x + 1.906 | R2 = 0.35 |
| Diadinoxanthin | y = 21.071x + 1.4949 | R2 = 0.82 |
| Lutein | y = 52.313x − 1.7543 | R2 = 0.80 |
| β-carotene | y = 87.282x − 0.6708 | R2 = 0.82 |
| Semangka Bay | ||
| Chlorophyll-a | y = 1.6801x + 0.0621 | R2 = 0.98 |
| Chlorophyll-b | y = 1.4928x + 0.6721 | R2 = 0.03 |
| Chlorophyll-c | y = 9.2563x + 0.578 | R2 = 0.90 |
| Fucoxanthin | y = 3.4329x + 0.2712 | R2 = 0.84 |
| Diadinoxanthin | y = 25.055x + 0.3504 | R2 = 0.85 |
| Lutein | y = −5.9702x + 1.2246 | R2 = 0.03 |
| β-carotene | y = 33.127x + 0.2315 | R2 = 0.93 |
| Correlation | Jakarta Bay Linear Regression and R2 | p values | Lampung Bay Linear Regression and R2 | p values | Semangka Bay Linear Regression and R2 | p values |
|---|---|---|---|---|---|---|
| DIN | ||||||
| DIN vs. Total pigments | y = 0.30x + 3.10; R2 = 0.94; | 7.94 × 10−9 | y = 0.15x + 6.48; R2 = 0.27 | 0.034 | y = 0.33x − 0.11; R2 = 0.58 | 0.0002 |
| DIN vs. Chl-b | y = 0.06x + 0.02; R2 = 0.79 | 2.49 × 10−5 | y = 0.05x − 0.01; R2 = 0.69 | 6.53 × 10−5 | y = 0.01x + 0.10; R2 = 0.04 | 0.564 |
| DIN vs. Chl-c | y = 0.01x + 0.36; R2 = 0.01 | 0.852 | y = −0.0001x + 0.05; R2 = 0.01 | 0.937 | y = 0.03x − 0.06; R2 = 0.49 | 0.0003 |
| DIN vs. Fucoxanthin | y = 0.01x + 1.37; R2 = 0.10 | 0.299 | y = 0.01x + 0.53; R2 = 0.02 | 0.637 | y = 0.09x − 0.13; R2 = 0.52 | 7.95 × 10−5 |
| DIN vs. Diadinoxanthin | y = 0.01x + 0.14; R2 = 0.23 | 0.091 | y = 0.01x − 0.01; R2 = 0.59 | 0.0005 | y = 0.01x − 0.01; R2 = 0.38 | 0.005 |
| DIN vs. β-carotene | y = 0.01x + 0.05; R2 = 0.93 | 3.23 × 10−8 | y = 0.004x + 0.02; R2 = 0.88 | 8.09 × 10−8 | y = 0.01x − 0.01; R2 = 0.51 | 6.92 × 10−5 |
| DIN vs. Lutein | y = 0.03x − 0.03; R2 = 0.78 | 2.81 × 10−5 | y = 0.01x + 0.05; R2 = 0.76 | 1.12 × 10−5 | y = −0.004x + 0.08; R2 = 0.12 | 0.135 |
| PO4-P | ||||||
| PO4-P vs. Total pigments | y = 0.68x + 5.80; R2 = 0.68 | 0.0002 | y = 0.93x + 8.59; R2 = 0.26 | 0.039 | y = 1.98x + 0.31; R2 = 0.78 | 1.48 × 10−5 |
| PO4-P vs. Chl-b | y = 0.17x + 0.36; R2 = 0.77 | 4.02 × 10−5 | y = 0.21x − 0.05; R2 = 0.85 | 4.74 × 10−7 | y = 0.03x + 0.12; R2 = 0.01 | 0.778 |
| PO4-P vs. Chl-c | y = 0.002x + 0.37; R2 = 0.004 | 0.889 | y = 0.002x + 0.04; R2 = 0.005 | 4.75 × 10−7 | y = 0.21x − 0.03; R2 = 0.82 | 8.25 × 10−8 |
| PO4-P vs. Fucoxanthin | y = 0.003x + 1.62; R2 = 0.0008 | 0.988 | y = 0.15x + 0.35; R2 = 0.12 | 0.191 | y = 0.53x − 0.02; R2 = 0.73 | 6.89 × 10−6 |
| PO4-P vs. Diadinoxanthin | y = 0.005x + 0.15; R2 = 0.22 | 0.096 | y = 0.06x − 0.03; R2 = 0.86 | 2.76 × 10−7 | y = 0.07x + 0.001; R2 = 0.68 | 0.0001 |
| PO4-P vs. β-carotene | y = 0.02x + 0.12; R2 = 0.64 | 0.0006 | y = 0.01x + 0.02; R2 = 0.87 | 9.65 × 10−8 | y = 0.06x + 0.003; R2 = 0.76 | 1.05 × 10−6 |
| PO4-P vs. Lutein | y = 0.08x + 0.11; R2 = 0.84 | 4.86 × 10−6 | y = 0.03x + 0.05; R2 = 0.94 | 8.74 × 10−10 | y = −0.02x + 0.08; R2 = 0.10 | 0.249 |
| I | ||||||
| I vs. Total pigments | y = −0.30x + 22.35; R2 = 0.51 | 0.0046 | y = −0.12x + 13.19; R2 = 0.31 | 0.045 | y = −0.01x + 1.36; R2 = 0.03 | 0.599 |
| I vs. Chl-b | y = −0.02x + 1.67; R2 = 0.28 | 0.049 | y = −0.02x + 1.29; R2 = 0.23 | 0.063 | y = 0.002x + 0.01; R2 = 0.09 | 0.477 |
| I vs. Chl-c | y = −0.009x + 0.79; R2 = 0.27 | 0.076 | y = −0.002x + 0.15; R2 = 0.13 | 0.189 | y = −0.002x + 0.12; R2 = 0.08 | 0.387 |
| I vs. Fucoxanthin | y = −0.038x + 3.30; R2 = 0.47 | 0.011 | y = −0.04x + 2.57; R2 = 0.28 | 0.039 | y = −0.002x + 0.28; R2 = 0.03 | 0.631 |
| I vs. Diadinoxanthin | y = −0.004x + 0.38; R2 = 0.63 | 0.001 | y = −0.007x + 0.43; R2 = 0.36 | 0.013 | y = −0.01x + 0.04; R2 = 0.03 | 0.573 |
| I vs. β-carotene | y = −0.008x + 0.57; R2 = 0.46 | 0.008 | y = −0.002x + 0.12; R2 = 0.27 | 0.044 | y = −0.001x + 0.05; R2 = 0.09 | 0.364 |
| I vs. Lutein | y = −0.022x + 1.51; R2 = 0.23 | 0.082 | y = −0.003x + 0.22; R2 = 0.31 | 0.022 | y = 0.001x + 0.04; R2 = 0.13 | 0.233 |
| Location | Multiple Linear Regression Equation | R | R2 |
|---|---|---|---|
| Combined bays | Total pigment = 8.84 + 0.16 DIN + 0.25 PO4 − 0.09 Light | 0.92 | 0.85 |
| Jakarta Bay | Total pigment = 5.84 + 0.22 DIN + 0.19 PO4 − 0.05 Light | 0.98 | 0.96 |
| Lampung Bay | Total pigment = 7.78 + 0.14 DIN + 1.28 PO4 − 0.03 Light | 0.76 | 0.57 |
| Semangka Bay | Total pigment = 1.28 + 0.04 DIN + 1.87 PO4 − 0.05 Light | 0.89 | 0.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damar, A.; Colijn, F.; Hesse, K.-J.; Kurniawan, F. Coastal Phytoplankton Pigments Composition in Three Tropical Estuaries of Indonesia. J. Mar. Sci. Eng. 2020, 8, 311. https://doi.org/10.3390/jmse8050311
Damar A, Colijn F, Hesse K-J, Kurniawan F. Coastal Phytoplankton Pigments Composition in Three Tropical Estuaries of Indonesia. Journal of Marine Science and Engineering. 2020; 8(5):311. https://doi.org/10.3390/jmse8050311
Chicago/Turabian StyleDamar, Ario, Franciscus Colijn, Karl-Juergen Hesse, and Fery Kurniawan. 2020. "Coastal Phytoplankton Pigments Composition in Three Tropical Estuaries of Indonesia" Journal of Marine Science and Engineering 8, no. 5: 311. https://doi.org/10.3390/jmse8050311
APA StyleDamar, A., Colijn, F., Hesse, K.-J., & Kurniawan, F. (2020). Coastal Phytoplankton Pigments Composition in Three Tropical Estuaries of Indonesia. Journal of Marine Science and Engineering, 8(5), 311. https://doi.org/10.3390/jmse8050311




