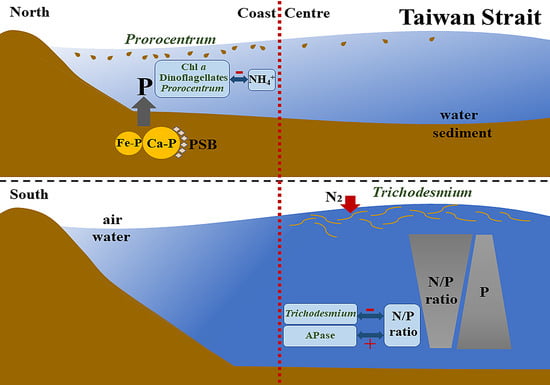
Coupling between Benthic Nutrient Cycling and Pelagic Phytoplankton Community in Taiwan Strait in Spring 2018
Abstract
1. Introduction
2. Materials and Methods
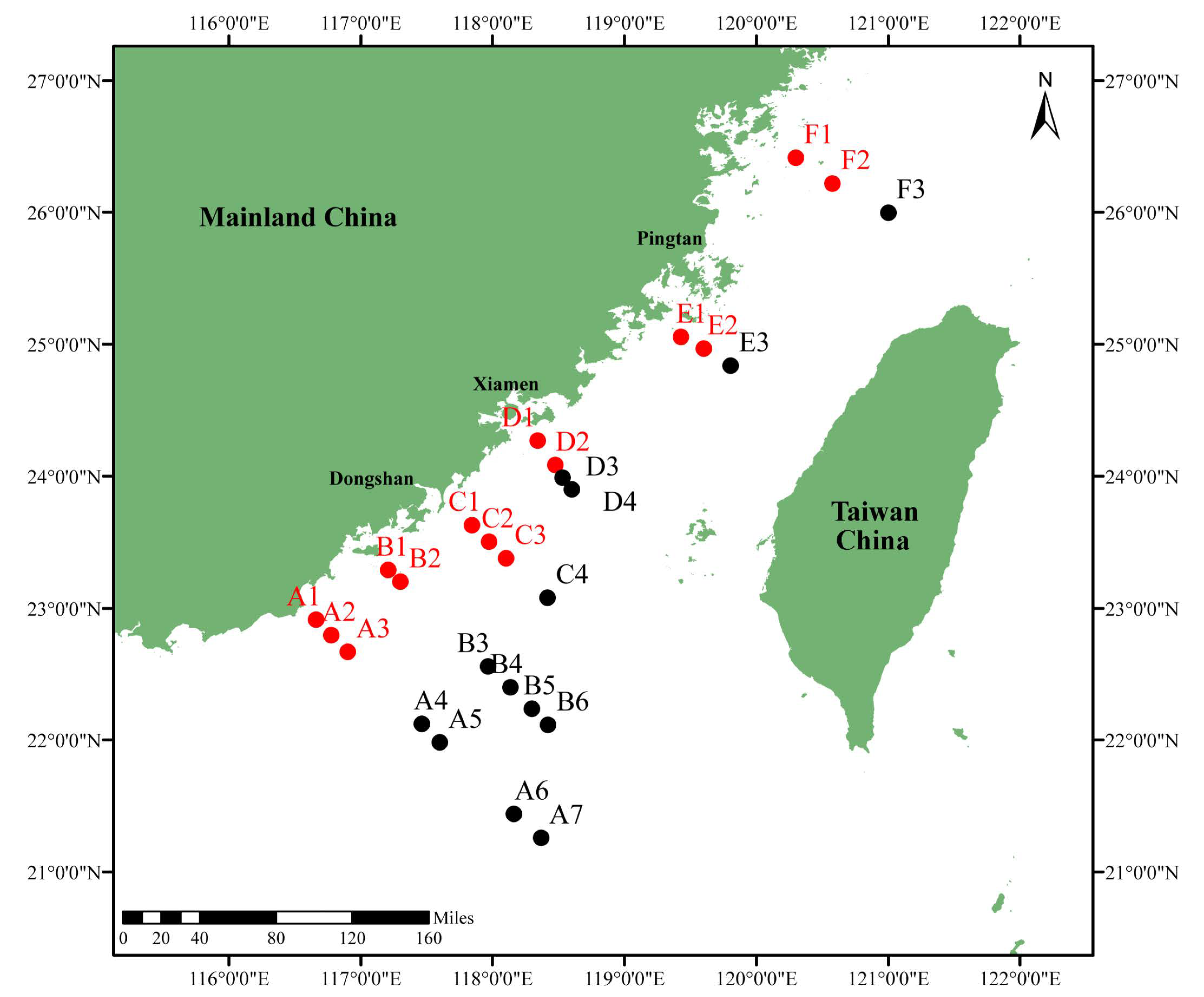
2.1. Study Area and Sampling Collection
2.2. Phytoplankton Analysis
2.3. Enzyme Labelled Fluorescence Measurements
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results
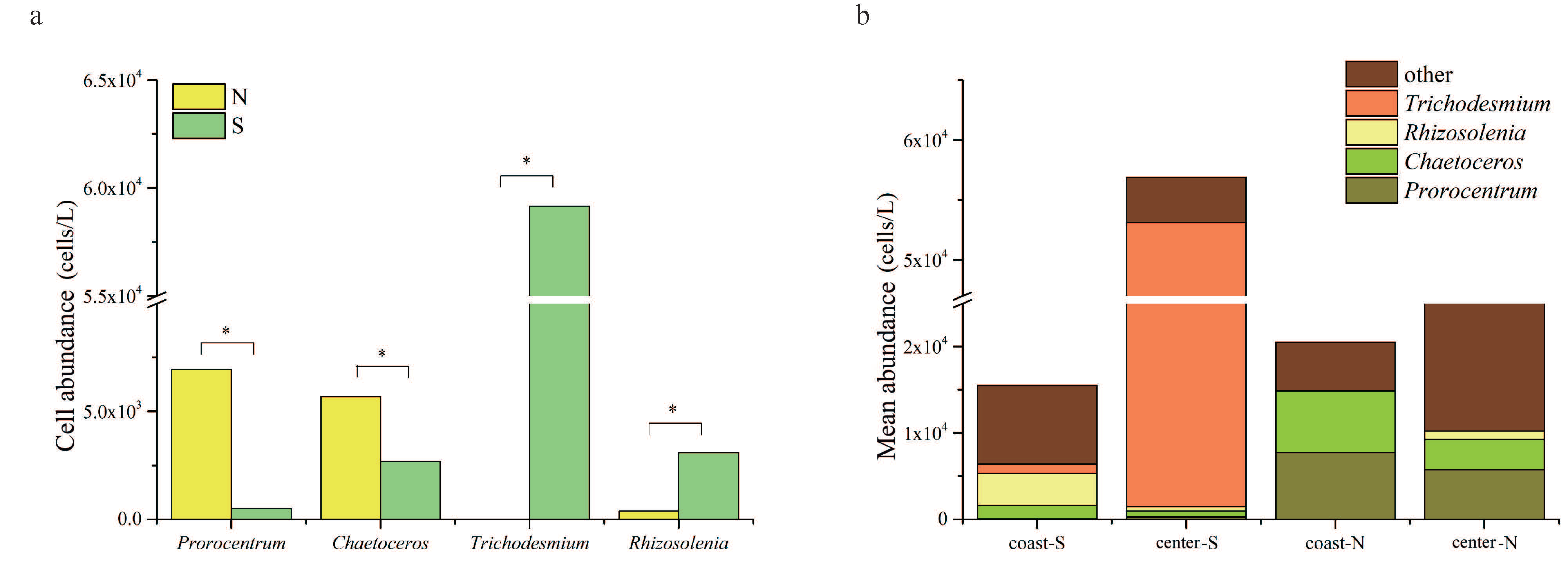
3.1. Abundance and Distribution of Phytoplankton
3.2. Nutrient Distribution and Its Relationship with Phytoplankton Community
3.3. Phytoplankton Extracellular Alkaline Phosphatase
3.4. Nutrient Status in Surface and Bottom Water
4. Discussion
4.1. Spatial Heterogeneity of Phytoplankton Community in the Taiwan Strait
4.2. Spatial Heterogeneity of Phytoplankton Community in Response to the Imbalanced Distribution of N and P
4.3. The Relationship between Bottom Nutrient Status and Spatial Heterogeneity of Phytoplankton Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schrope, M. Oceanography: Red Tide rising. Nature 2008, 452, 24–26. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Mang, J.H.; Ye, Y.Y.; Lin, G.M.; Yang, Q.L.; Lin, M. Phytoplankton community and environmental correlates in a coastal upwelling zone along western Taiwan Strait. J. Mar. Syst. 2016, 154, 252–263. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef]
- Hamilton, D.P.; Douglas, G.B.; Adeney, J.A.; Radke, L.C. Seasonal changes in major ions, nutrients and chlorophyll a at two sites in the Swan River estuary, Western Australia. Mar. Freshw. Res. 2006, 57, 803–815. [Google Scholar] [CrossRef]
- Weston, K.; Fernand, L.; Mills, D.K.; Delahunty, R.; Brown, J. Primary production in the deep chlorophyll maximum of the centre North Sea. J. Plankton. Res. 2005, 27, 909–922. [Google Scholar] [CrossRef]
- Liu, D.Y.; Shen, X.H.; Di, B.P.; Shi, Y.J.; Keesing, J.K.; Wang, Y.J.; Wang, Y.Q. Palaeoecological analysis of phytoplankton regime shifts in response to coastal eutrophication. Mar. Ecol. Prog. Ser. 2013, 475, 1–14. [Google Scholar] [CrossRef]
- Wu, G.F.; Zhou, X.P. Characterization of phosphorus-releasing bacteria in a small eutrophic shallow, Eastern Lake. Water Res. 2005, 39, 4623–4632. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Van Mooy, B.A.S.; Rocap, G.; Fredricks, H.F.; Evans, C.T.; Devol, A.H. Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligotrophic marine environments. Proc. Natl. Acad. Sci. USA 2006, 103, 8607–8612. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Dyhrman, S.T.; Lomas, M.W.; Poulton, N.J.; Van Mooy, B.A.S. Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proc. Natl. Acad. Sci. USA 2014, 111, 8089–8094. [Google Scholar] [CrossRef]
- Zhang, S.F.; Yuan, C.J.; Chen, Y.; Lin, L.; Wang, D.Z. Transcriptomic response to changing ambient phosphorus in the marine dinoflagellate Prorocentrum donghaiense. Sci. Total Environ. 2019, 692, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.J.; Huang, B.Q.; Lin, L.; Hong, H.; Zhang, F.; Chen, Z. Phosphorus stress of phytoplankton in the Taiwan Strait determined by bulk and single-cell alkaline phosphatase activity assays. Mar. Ecol. Prog. Ser. 2006, 327, 95–106. [Google Scholar] [CrossRef][Green Version]
- Marine Environment Status Bulletin in China 2016. China Oceanic Information Network. Available online: http://www.nmdis.org.cn/hygb/zghyhjzlgb/2016nzghyhjzkgb/ (accessed on 13 April 2017).
- Hu, J.; Liu, X.; Zhang, F.; Huang, B.Q.; Hong, H.S. Study on nutrient limitation of phytoplankton in southern Taiwan Strait during the summer. J. Oceanogr. Taiwan Strait 2008, 27, 452–458. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Palenik, B. Phosphate stress in cultures and field populations of the dinoflagellate Prorucentrum minimum detected by a single-cell alkaline phosphatase assay. Appl. Environ. Microb. 1999, 65, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Guo, H. Illustrations of Planktons Responsible for the Blooms in Chinese Coastal Water, 1st ed.; Science Press: Beijing, China, 2004. [Google Scholar]
- González-Gil, S.; Keafer, B.A.; Jovine, R.V.M.; Aguilera, A.; Lu, S.H.; Anderson, D.M. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Mar. Ecol. Prog. Ser. 1998, 164, 21–35. [Google Scholar] [CrossRef]
- Štrojsová, A.; Vrba, J.; Nedoma, J.; Komárková, J.; Znachor, P. Seasonal study on expression of extracellular phosphatase in the phytoplankton of a eutrophic reservoir. Eur. J. Phycol. 2003, 38, 295–306. [Google Scholar] [CrossRef]
- Beattie, D.M.; Golterman, H.L.; Vijverberg, J. Introduction to limnology of Friesian lakes. Hydrobiology 1978, 58, 49–64. [Google Scholar] [CrossRef]
- Greenberd, A.E.; Clesceri, L.S.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Golterman, H.L. Fractionation of sediment phosphate with chelating compounds: A simplification, and comparison with other methods. Hydrobiologia 1996, 335, 87–95. [Google Scholar] [CrossRef]
- Istvanovics, V. Fractional composition, adsorption and release of sediment phosphorus in the Kis-Balaton reservoir. Water Res. 1994, 28, 717–726. [Google Scholar] [CrossRef]
- Sakadevan, K.; Bavor, H.J. Phosphate adsorption characteristics of soils, slags and zeolite to be used as substrates in constructed wetland systems. Water Res. 1998, 32, 393–399. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. Fems. Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Cen, J.Y.; Ou, L.J.; Li, S.; Zhang, H.; Wang, J.Y.; Lu, S.H. The species composition of dinoflagellate and its role of indication to Kuroshio and adjacent waters in spring 2014. Sci. Rep. 2017, 48, 1022–1029. [Google Scholar] [CrossRef]
- Wen, Z.Z.; Lin, W.F.; Shen, R.; Hong, H.Z.; Kao, S.J.; Shi, D.L. Nitrogen fixation in two coastal upwelling regions of the Taiwan Strait. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Adachi, R. Red tide biology and red tide ecology. Aquat. Archit. 1973, 9, 31–36. [Google Scholar]
- Proenca, L.A.O.; Tamanaha, M.S.; Fonseca, R.S. Screening the Toxicity and Toxin Content of Blooms of the Cyanobacterium Trichodesmium Erythraeum (Ehrenberg) In Northeast Brazil. J. Venom. Anim Toxins 2009, 15, 204–215. [Google Scholar] [CrossRef]
- Lourenco, S.O.; Barbarino, E.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Basis for the calculation of specific nitrogen-to-protein conversion factors. J. Phycol. 1998, 34, 798–811. [Google Scholar] [CrossRef]
- Lomas, M.W.; Rumbley, C.J.; Glibert, P.M. Ammonium release by nitrogen sufficient diatoms in response to rapid increases in irradiance. J. Plankton. Res. 2000, 22, 2351–2366. [Google Scholar] [CrossRef]
- Huang, L.D.; Wu, Y.D. Effects of dissolved inorganic nitrogen in water bodies on intracellular reactive nitrogen compounds of Chaetoceros. J. Taiwan Strait 1991, 2, 74–81. [Google Scholar]
- Girault, M.; Arakawa, H.; Barani, A.; Ceccaldi, H.J.; Hashihama, F.; Kinouchi, S.; Gregori, G. Distribution of ultraphytoplankton in the western part of the North Pacific subtropical gyre during a strong La Nina condition: Relationship with the hydrological conditions. Biogeosciences 2013, 10, 5947–5965. [Google Scholar] [CrossRef]
- Orcutt, K.M.; Gundersen, K.; Ammerman, J.W. Intense ectoenzyme activities associated with Trichodesmium colonies in the Sargasso Sea. Mar. Ecol. Prog. Ser. 2013, 478, 101–113. [Google Scholar] [CrossRef]
- Sohm, J.A.; Capone, D.G. Phosphorus dynamics of the tropical and subtropical North Atlantic: Trichodesmium spp. versus bulk plankton. Mar. Ecol. Prog. Ser. 2006, 317, 21–28. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhao, J.G.; Zhang, Y.J.; Cao, Y. Phytoplankton community structure and environmental parameters in aquaculture areas of Daya Bay, South China Sea. J. Environ. Sci China 2009, 21, 1268–1275. [Google Scholar] [CrossRef]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with Iron-Oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Li, M.Z.; Li, L.; Shi, X.G.; Lin, L.X.; Lin, S.J. Effects of phosphorus deficiency and adenosine 5′-triphosphate (ATP) on growth and cell cycle of the dinoflagellate Prorocentrum donghaiense. Harmful Algae 2015, 47, 35–41. [Google Scholar] [CrossRef]
- Peters, F.; Arin, L.; Marrase, C.; Berdalet, E.; Sala, M.M. Effects of small-scale turbulence on the growth of two diatoms of different size in a phosphorus-limited medium. J. Marine Syst. 2006, 61, 134–148. [Google Scholar] [CrossRef]
- Ivancic, I.; Godrijan, J.; Pfannkuchen, M.; Maric, D.; Gasparovic, B.; Djakovac, T.; Najdek, M. Survival mechanisms of phytoplankton in conditions of stratification-induced deprivation of orthophosphate: Northern Adriatic case study. Limnol. Oceanogr. 2012, 57, 1721–1731. [Google Scholar] [CrossRef]
- Connelly, T.L.; Deibel, D.; Parrish, C.C. Biogeochemistry of coast-bottom suspended particulate matter of the Beaufort Sea shelf (Arctic Ocean): C, N, P, delta C-13 and fatty acids. Cont. Shelf Res. 2012, 43, 120–132. [Google Scholar] [CrossRef]
- Ingall, E.; Jahnke, R. Evidence for enhanced phosphorus regeneration from marine-sediments overlain by oxygen depleted waters. Geochim. Cosmochim. Ac. 1994, 58, 2571–2575. [Google Scholar] [CrossRef]
- Kraal, P.; Dijkstra, N.; Behrends, T.; Slomp, C.P. Phosphorus burial in sediments of the sulfidic deep Black Sea: Key roles for adsorption by calcium carbonate and apatite authigenesis. Geochim. Cosmochim. Ac. 2017, 204, 140–158. [Google Scholar] [CrossRef]
- Zhu, F.L.; Qu, L.Y.; Hong, X.G.; Sun, X.Q. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid Based Compl. Alt. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Ding, X.D.; Zhang, L.; Zhang, S.R.; Feng, G. Phytate utilization of maize mediated by different nitrogen forms in a plant-arbuscular mycorrhizal fungus-phosphate-solubilizing bacterium system. J. Plant. Interact. 2014, 9, 514–520. [Google Scholar] [CrossRef]
- Mackey, K.R.M.; Mioni, C.E.; Ryan, J.P.; Paytan, A. Phosphorus cycling in the red tide incubator region of Monterey Bay in response to upwelling. Front. Microbiol. 2012, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.P.; Holl, C.M.; Zehr, J.P.; Hansen, A.; Villareal, T.A.; Capone, D.G. High rates of N-2 fixation by unicellular diazotrophs in the oligotrophic. Nature 2004, 430, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.; Graneli, E.; Segatto, A.Z. Cycling of biologically available nitrogen in riverine humic substances between marine bacteria, a heterotrophic nanoflagellate and a photosynthetic dinoflagellate. Aquat. Microb. Ecol. 1999, 18, 23–36. [Google Scholar] [CrossRef]
- Bonnet, S.; Berthelot, H.; Turk-Kubo, K.; Fawcett, S.; Rahav, E.; L’Helguen, S.; Berman-Frank, I. Dynamics of N2 fixation and fate of diazotroph-derived nitrogen in a low-nutrient, low-chlorophyll ecosystem: Results from the VAHINE mesocosm experiment (New Caledonia). Biogeosciences 2016, 13, 2653–2673. [Google Scholar] [CrossRef]
- Yang, D.Z.; Yin, B.S.; Liu, Z.L.; Bai, T.; Qi, J.F.; Chen, H.Y. Numerical study on the pattern and origins of Kuroshio branches in the bottom water of southern East China Sea in summer. J. Geophys Res. Oceans 2012, 117, C02014. [Google Scholar] [CrossRef]
- Yu, S.J.; Gong, F.; He, X.Q.; Bai, Y.; Zhu, Q.K.; Wang, D.F.; Chen, P. Satellite views of the massive algal bloom in the Persian Gulf and the Gulf of Oman during 2008–2009. In Remote Sensing of the Ocean, Sea Ice, Coastal Waters, and Large Water Regions 2016; Charles, R.B., Xavier, N., Caroline, N., Oscar, A., Eds.; SPIE: Bellingham, WA, USA, 2016; Volume 9999, pp. 99990Z-1–99990Z-10. [Google Scholar] [CrossRef]
- Walsh, J.B. A theoretical analysis of sliding of rough surfaces. J. Geophys. Res.-Sol. Earth 2003, 108. [Google Scholar] [CrossRef]
- Rahav, E.; Bar-Zeev, E. Sewage outburst triggers Trichodesmium bloom and enhance N2 fixation rates. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Walsby, A.F. The properties and buoyancy-providing role of gas vacuoles in Trichodesmium Ehrenberg. Br. Phycol. J. 1978, 13, 103–116. [Google Scholar] [CrossRef]
- Böttger-Schnack, R.; Schnack, D. Vertical distribution and population structure of Macrosetella gracilis (Copepoda, Harpacticoida) in the Red Sea in relation to the occurrence of Oscillatoria (Trichodesmium) spp. (Cyanobacteria). Mar. Ecol. Prog. Ser. 1989, 52, 17–31. [Google Scholar] [CrossRef]
- Karl, D.M.; Letelier, R.; Hebel, D.V.; Bird, D.F.; Winn, C.D. Trichodesmium Blooms and new nitrogen in the North Pacific Gyre. In Marine Pelagic Cyanobacteria: Trichodesmium and other Diazotrophs, NATO ASI Series C: Mathematical and Physical Sciences; Carpenter, E.J., Capone, D.G., Reuter, J.G., Eds.; Springer: Dordrecht, The Netherlands, 1992; Volume 362, pp. 219–237. [Google Scholar]
- Villareal, T.A.; Carpenter, E.J. Buoyancy regulation and the potential for vertical migration in the oceanic cyanobacterium Trichodesmium. Microb. Ecol. 2003, 45, 1–10. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhou, L.B.; Li, J.J.; Lin, Y.Y.; Ke, Z.X.; Zhao, C.Y.; Liu, H.J.; Jiang, X.; He, Y.H.; Tan, Y.H. Effect of mesoscale eddies on diazotroph community structure and nitrogen fixation rates in the South China Sea. Reg. Stud. Mar. Sci. 2020, 35. [Google Scholar] [CrossRef]
- Shiozaki, T.; Chen, Y.L.L.; Lin, Y.H.; Taniuchi, Y.; Sheu, D.S.; Furuya, K.; Chen, H.Y. Seasonal variations of unicellular diazotroph groups A and B, and Trichodesmium in the northern South China Sea and neighboring upstream Kuroshio Current. Cont. Shelf Res. 2014, 80, 20–31. [Google Scholar] [CrossRef]





| Types | Regions | SRP | TP | NO3− | NO2− | NH4+ | DIN | TN | TN/TP | DIN/SRP | NH4+/SRP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface | S | 0.005 ± 0.003 | 0.021 ± 0.014 | 0.474 ± 0.049 | 0.000 ± 0.001** | 0.110 ± 0.018 ** | 0.584 ± 0.050 | 3.250 ± 0.340 | 187.033 ± 63.279 | 245.934 ± 233.053 | 45.579 ± 44.957 * |
| N | 0.007 ± 0.006 | 0.027 ± 0.016 | 0.481 ± 0.120 | 0.056 ± 0.022 | 0.031 ± 0.012 | 0.568 ± 0.129 | 3.415 ± 0.134 | 158.590 ± 66.362 | 144.464 ± 122.623 | 8.903 ± 7.531 | |
| Bottom | S | 0.023 ± 0.027 | 0.035 ± 0.024 | 0.563 ± 0.179 | 0.003 ± 0.005 | 0.082 ± 0.014 | 0.648 ± 0.176 | 3.093 ± 0.377 | 124.750 ± 57.570 | 60.738 ± 36.709 | 9.143 ± 6.290 |
| N | - | - | - | - | - | - | - | - | - | - | |
| Interstitial | S | - | - | - | - | - | - | - | - | - | - |
| N | 0.075 ± 0.014 | 0.976 ± 0.448 | 0.009 ± 0.005 | 1.538 ± 0.500 | 2.523 ± 0.456 | 34.610 ± 7.032 | 20.543 ± 5.081 |
| Regions | Types | Shore | SRP | TP | NO3− | NO2− | NH4+ | DIN | TN | TN/TP | DIN/SRP | NH4+/SRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Surface | coast | 0.006 ± 0.004 | 0.026 ± 0.020 | 0.453 ± 0.067 | 0.0003 ± 0.001 | 0.108 ± 0.013 | 0.561 ± 0.055 | 3.135 ± 0.325 | 164.55 ± 55.580 | 162.98 ± 106.410 | 29.804 ± 17.694 |
| center | 0.004 ± 0.003 | 0.018 ± 0.006 | 0.488 ± 0.022 | 0.0005 ± 0.001 | 0.111 ± 0.020 | 0.599 ± 0.039 | 3.326 ± 0.327 | 202.02 ± 63.661 | 301.24 ± 274.459 | 56.096 ± 53.696 | ||
| Bottom | coast | 0.007 ± 0.002 * | 0.017 ± 0.003 * | 0.444 ± 0.040 * | 0.000 ± 0.001 | 0.084 ± 0.011 | 0.528 ± 0.049 * | 2.836 ± 0.293 * | 167.418 ± 35.900 * | 83.009 ± 24.161 * | 13.147 ± 3.854 * | |
| center | 0.034 ± 0.030 | 0.046 ± 0.025 | 0.643 ± 0.190 | 0.005 ± 0.006 | 0.081 ± 0.015 | 0.728 ± 0.184 | 3.264 ± 0.326 | 96.304 ± 51.398 | 45.891 ± 36.133 | 6.473 ± 6.183 | ||
| Interstitial | coast | - | - | - | - | - | - | - | - | - | - | |
| center | - | - | - | - | - | - | - | - | - | - | ||
| N | Surface | coast | 0.009 ± 0.007 | 0.026 ± 0.007 | 0.548 ± 0.086 * | 0.064 ± 0.010 | 0.030 ± 0.013 | 0.642 ± 0.091 * | 3.343 ± 0.083 * | 136.554 ± 30.631 | 102.27 ± 52.869 | 5.639 ± 4.175 |
| center | 0.004 ± 0.003 | 0.029 ± 0.023 | 0.381 ± 0.089 | 0.045 ± 0.030 | 0.032 ± 0.009 | 0.458 ± 0.094 | 3.524 ± 0.123 | 191.64 ± 88.213 | 207.76 ± 163.466 | 13.800 ± 8.700 | ||
| Bottom | coast | - | - | - | - | - | - | - | - | - | - | |
| center | - | - | - | - | - | - | - | - | - | - | ||
| Interstitial | coast | 0.070 ± 0.009 | 1.028 ± 0.474 | 0.008 ± 0.004 | 1.418 ± 0.462 | 2.454 ± 0.470 | 35.729 ± 7.199 | 20.324 ± 5.540 | ||||
| center | 0.099 ± 0.000 | 0.715 ± 0.000 | 0.014 ± 0.000 | 2.138 ± 0.000 | 2.868 ± 0.000 | 29.013 ± 0.000 | 21.646 ± 0.000 |
| Station | A1 | A2 | A3 | A6 | A7 | B1 | B3 | B5 | B6 | C1 | C4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ELF (%) | 65.49 | 40.31 | 63.38 | 25.59 | 24.81 | 28.72 | 26.79 | 11.53 | 1.48 | 63.64 | 66.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chai, X.; Zheng, L.; Deng, Q.; Chen, X.; Zhang, Q.; Wan, L.; Song, C.; Song, L.; Zhou, Y.; et al. Coupling between Benthic Nutrient Cycling and Pelagic Phytoplankton Community in Taiwan Strait in Spring 2018. J. Mar. Sci. Eng. 2020, 8, 807. https://doi.org/10.3390/jmse8100807
Li X, Chai X, Zheng L, Deng Q, Chen X, Zhang Q, Wan L, Song C, Song L, Zhou Y, et al. Coupling between Benthic Nutrient Cycling and Pelagic Phytoplankton Community in Taiwan Strait in Spring 2018. Journal of Marine Science and Engineering. 2020; 8(10):807. https://doi.org/10.3390/jmse8100807
Chicago/Turabian StyleLi, Xiaowen, Xiaojie Chai, Lingling Zheng, Qinghui Deng, Xiaoyan Chen, Qi Zhang, Lingling Wan, Chunlei Song, Lirong Song, Yiyong Zhou, and et al. 2020. "Coupling between Benthic Nutrient Cycling and Pelagic Phytoplankton Community in Taiwan Strait in Spring 2018" Journal of Marine Science and Engineering 8, no. 10: 807. https://doi.org/10.3390/jmse8100807
APA StyleLi, X., Chai, X., Zheng, L., Deng, Q., Chen, X., Zhang, Q., Wan, L., Song, C., Song, L., Zhou, Y., & Cao, X. (2020). Coupling between Benthic Nutrient Cycling and Pelagic Phytoplankton Community in Taiwan Strait in Spring 2018. Journal of Marine Science and Engineering, 8(10), 807. https://doi.org/10.3390/jmse8100807