Trends in the Recent Evolution of Coastal Lagoons and Lakes in Galicia (NW Iberian Peninsula)
Abstract
:1. Introduction
2. Material and Methods
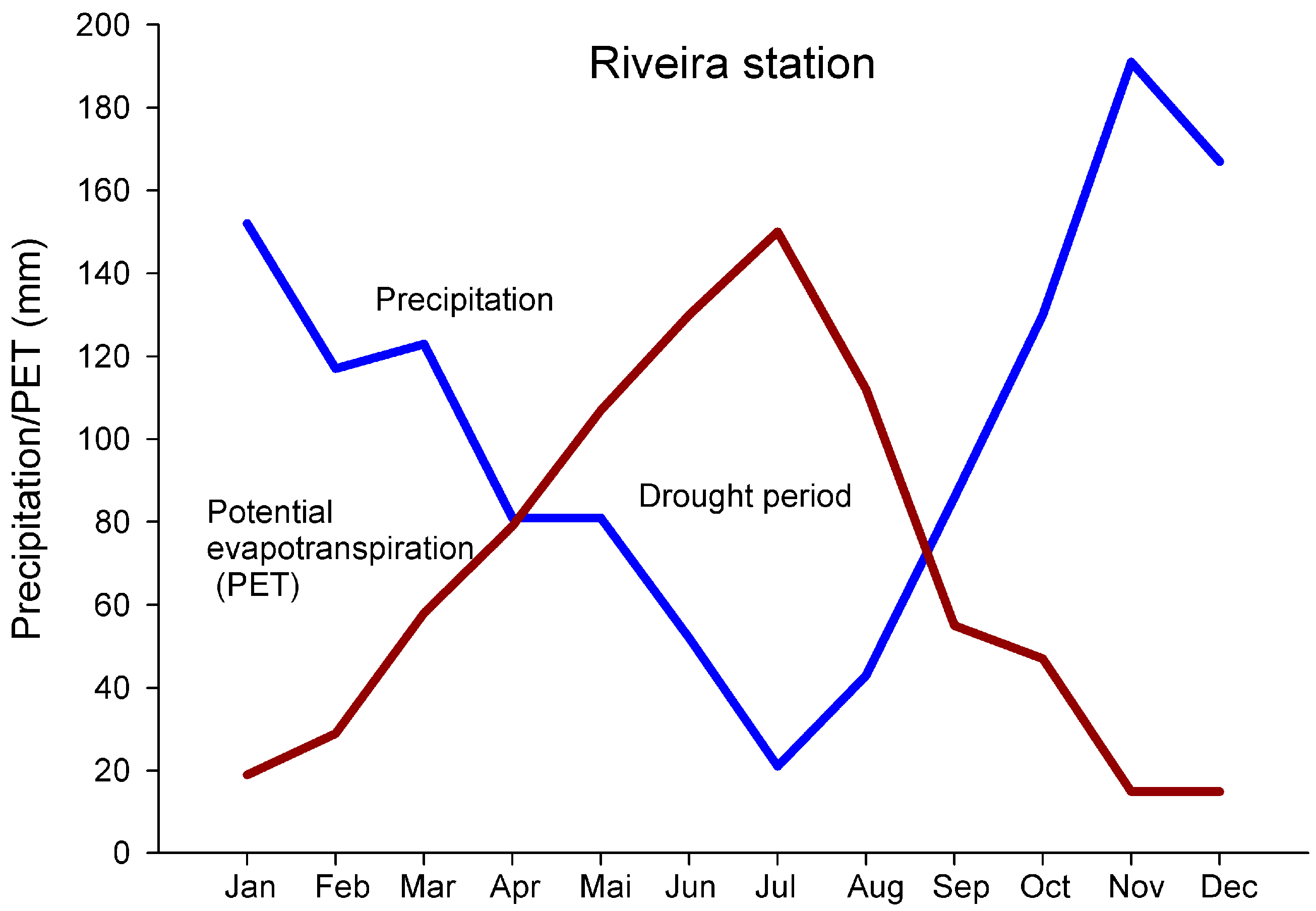
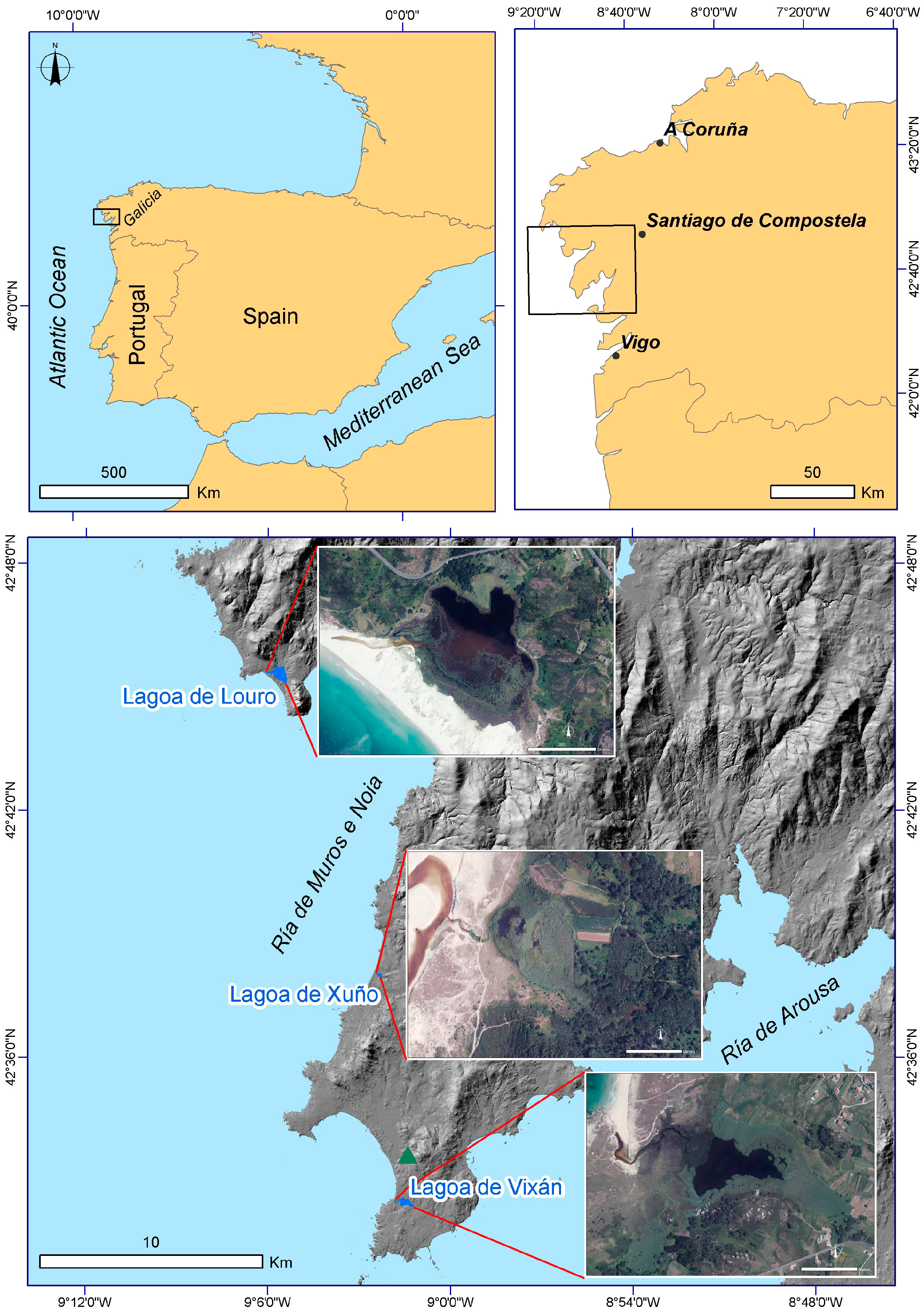
2.1. Environmental Setting
2.2. Lagoon Delimitation and Zoning
- Free water: area permanently covered by a water sheet.
- Phragmites: area colonized by the community defined by Phragmites australis reed beds, characterized by the highest freshwater influence.
- Juncus/Scirpus: area with a higher seawater influence, occupied by Juncus maritimus, Juncus acutus, Scirpus maritimus, and S. lacustris, which often appear interspersed in the study areas.
- Sand or dune area: sedimentary area, either forming dunes or intertidal flats.
- Grass: herbaceous vegetation dominated by Paspalum vaginatum.
- Aquatic vegetation: floating freshwater plants such as Nymphaea alba, Utricularia australis, Potamogeton trichoides, and Littorela uniflora.
2.3. Geochemical Analysis of Water and Sediments
3. Results
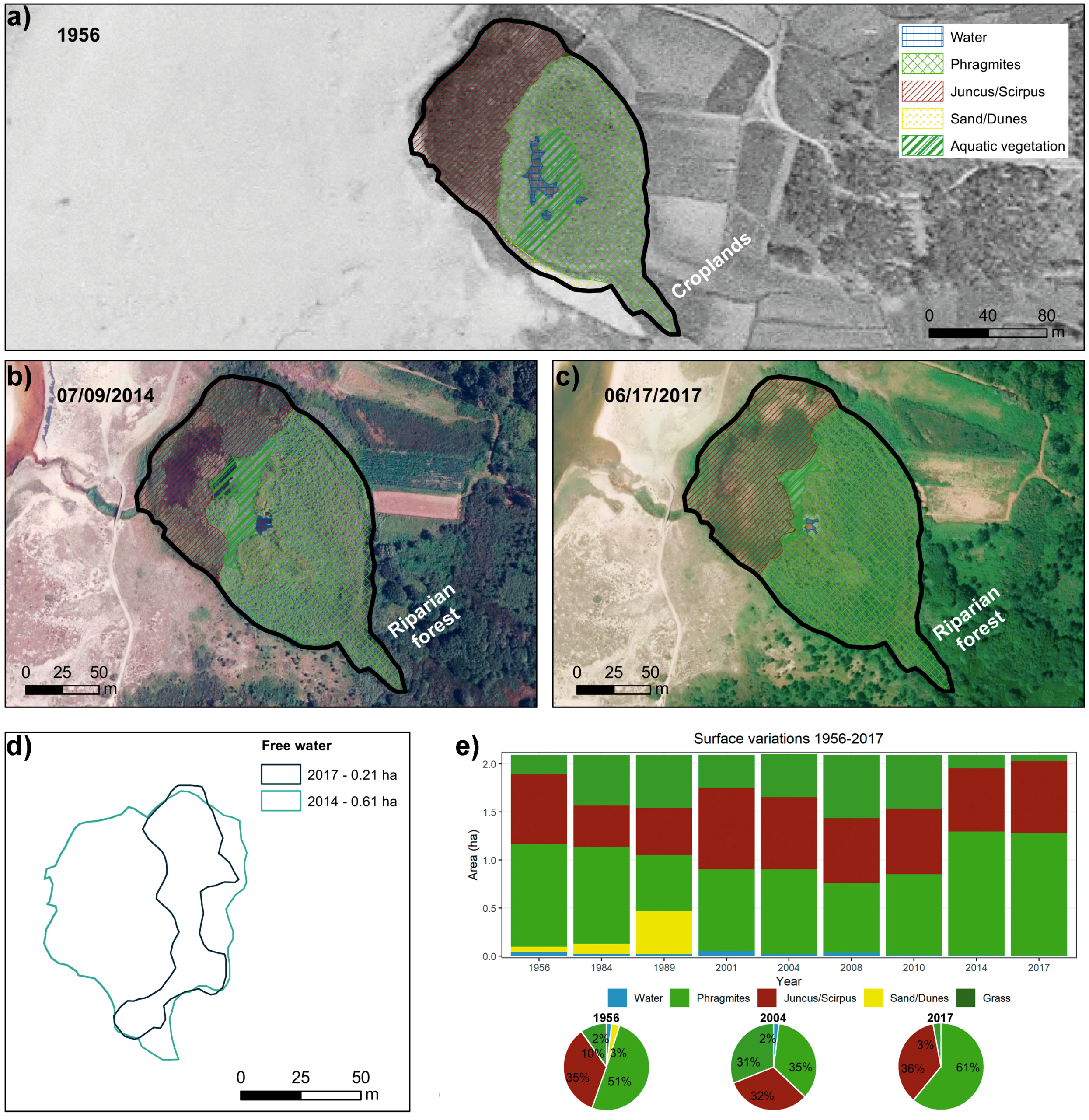
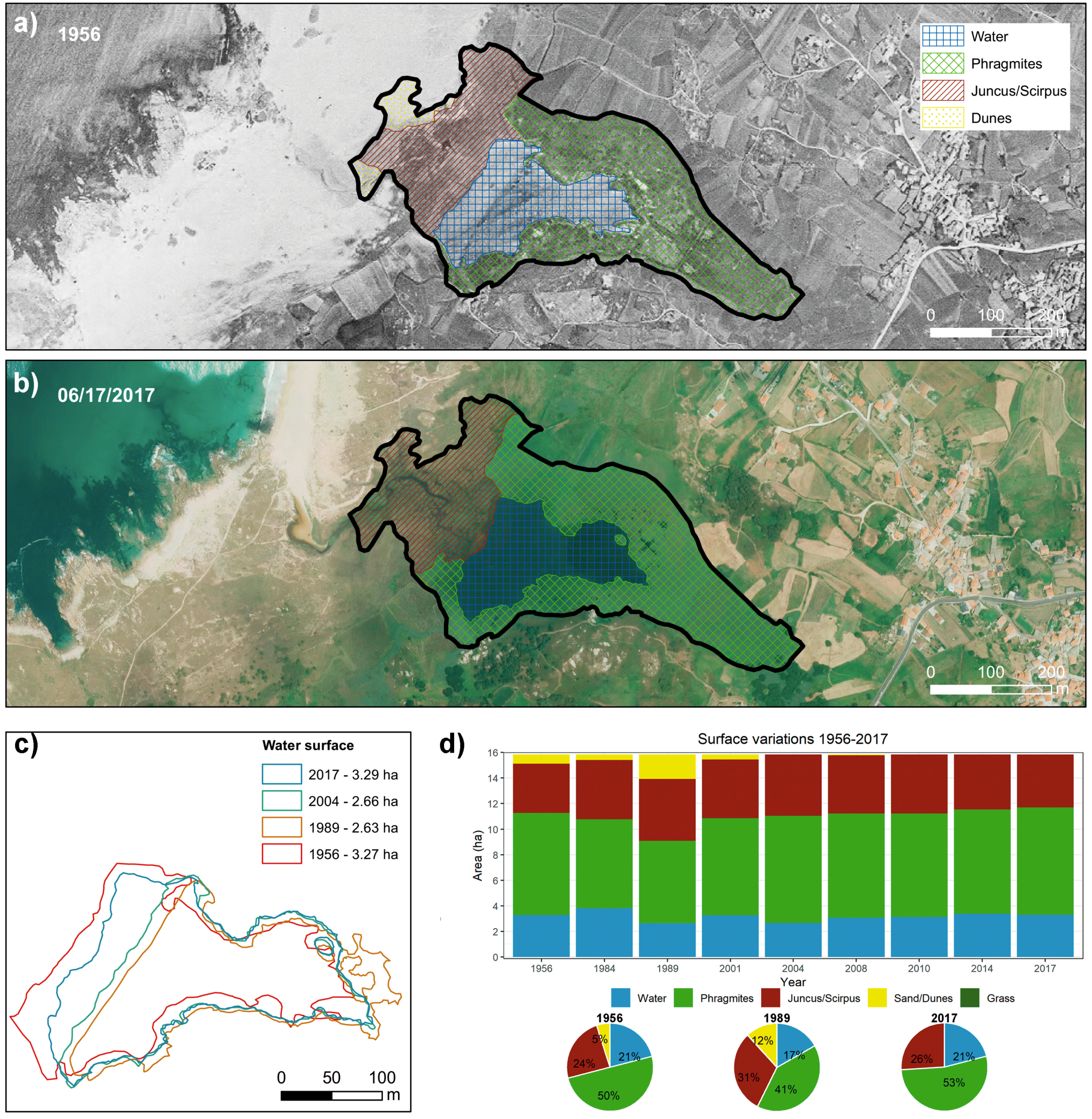
3.1. Delimitation and Evolution of the Lagoons in the Last 60 Years
3.2. Geochemical Characterization of Water and Sediment
3.2.1. Physicochemical Characterization and Composition of Water
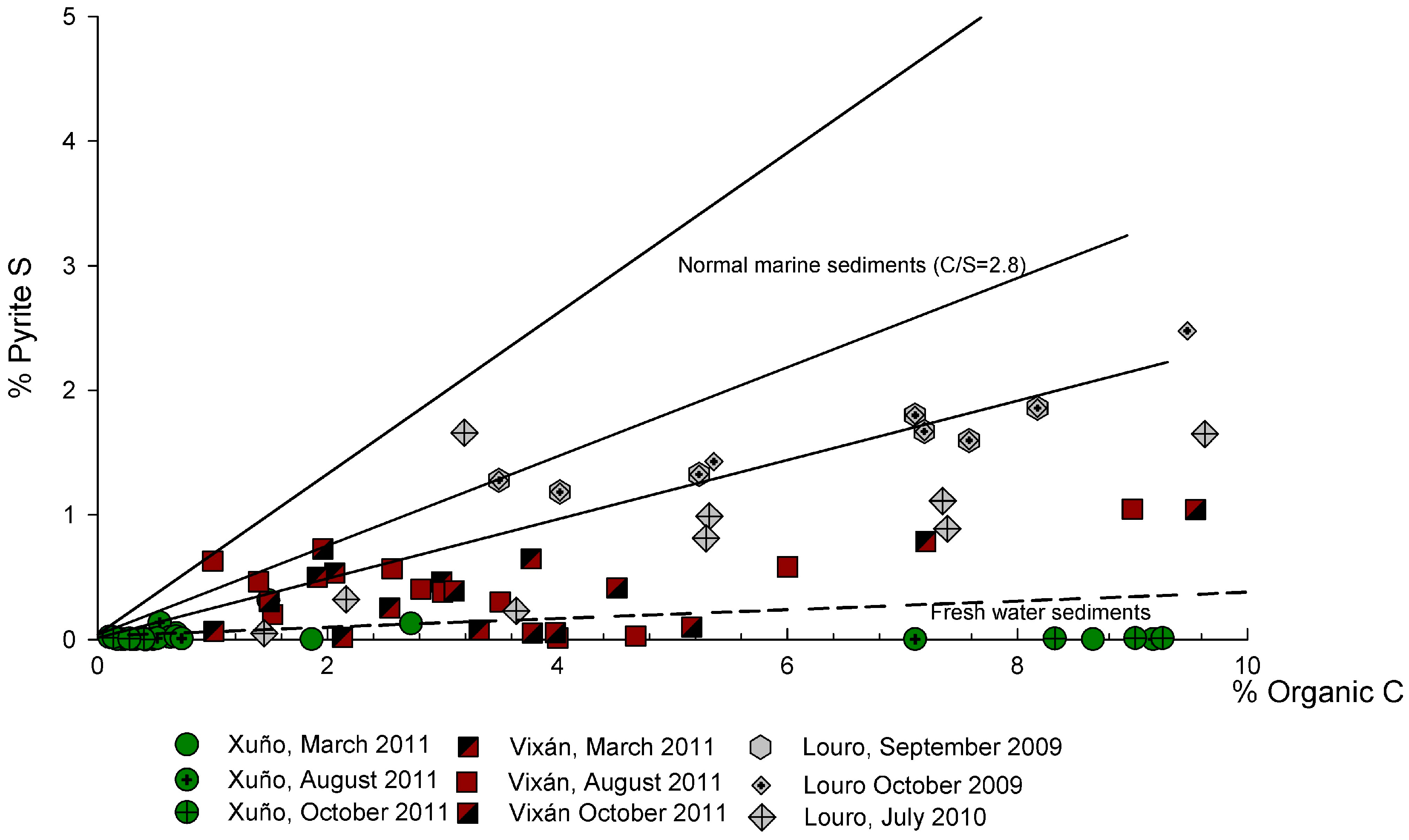
3.2.2. Organic C/Pyritic S Ratio in Sediments
4. Discussion
4.1. Types of Lagoons According to Their Location and Geochemistry (Coastal Lagoons and Natural Eutrophic Lakes)
4.2. Evolution and Environmental Implications of the Reduction in the Free Water Body and of Changes in the Areas Colonized by Vegetation
5. Conclusions
- The three lagoons are experiencing a progressive siltation and eutrophication process, which decreases water quality and substantially deteriorates their environmental function. The progressively increasing coverage by macrophytic communities at the expense of the area covered by free water body and quality is compromising the lagoons’ capacity to host stable populations of aquatic and marsh bird species.
- An adequate management of these spaces requires, first and foremost, a thorough understanding of their origin and dynamics. Knowledge of these aspects is currently incomplete, which has led to their incorrect classification in terms of habitat type, according to directive 92/43/CEE.
- The allocation of lagoons and lakes to the categories listed in Annex I of the Habitats Directive is very complex and requires a multidisciplinary approach.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar] [CrossRef]
- Davidson, N.C.; Laffoley, D.; Doody, J.P.; Way, L.; Gordon, J.; Key, R.; Drake, C.M.; Pienkowski, M.; Mitchell, R.M.; Duff, K.L. Nature Conservation and Estuaries in Great Britain; Nature Conservancy Council: Peterborough, UK, 1991. [Google Scholar]
- Dahl, T.E. Wetlands Losses in the United States 1780’s to 1980’s; U.S. Department of the Interior: Washington, DC, USA; U.S. Fish and Wildlife Service: Washington, DC, USA, 1990.
- Kotze, D.C.; Breen, C.M.; Quinn, N. Wetland Losses in South Africa. In ‘Wetlands of South Africa’; Department of Environmental Affairs and Tourism: Pretoria, South Africa, 1995. [Google Scholar]
- An, S.; Li, H.; Guan, B.; Zhou, C.; Wang, Z.; Deng, Z.; Zhi, Y.; Liu, Y.; Xu, C.; Fang, S.; et al. China’s Natural Wetlands: Past Problems, Current Status, and Future Challenges. AMBIO J. Hum. Environ. 2007, 36, 335–342. [Google Scholar] [CrossRef]
- Kennish, M.J.; Paerl, H.W. Coastal Lagoons. Critical Habitats of Environmental Change; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781138111844. [Google Scholar]
- Cohen, J.E.; Small, C.; Mellinger, A.; Gallup, J.; Sachs, J. Estimates of Coastal Populations. Science 1997, 278, 1209–1213. [Google Scholar] [CrossRef]
- McGranahan, G.; Balk, D.; Anderson, B. The rising tide: Assessing the risks of climate change and human settlements in low elevation coastal zones. Environ. Urban. 2007, 19, 17–37. [Google Scholar] [CrossRef]
- Perez-Alberti, A.; Gomez-Pazo, A. The Rocky Coasts of Northwest Spain. In The Spanish Coastal Systems; Springer: Berlin, Germany, 2019; pp. 27–47. ISBN 9783319931692. [Google Scholar]
- POLGalicia. Plan de Ordenación do Litoral de Galicia; Consellaría de Medio Ambiente, Territorio e Infraestruturas: Santiago de Compostela, Spain, 2010. [Google Scholar]
- Schwartz, M.L. Encyclopedia of Coastal Science; Schwartz, M.L., Ed.; Springer: Dordrecht, The Netherlands, 2005; ISBN 978-1-4020-1903-6. [Google Scholar]
- Carballeira, A.; Devesa, C.; Retuerto, R.; Santillán, E.; Ucieda, F. Bioclimatología de Galicia; Fundación Pedro Barrié de la Maza, Conde de Fenosa: A Coruña, Spain, 1983; ISBN 84-85728-27. [Google Scholar]
- Oertel, G.F. Coastal Lakes and Lagoons. In Encyclopedia of Coastal Science; Schwartz, M.L., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 263–266. ISBN 978-1-4020-3880-8. [Google Scholar]
- Oertel, G.F. The barrier island system. Mar. Geol. 1985, 63, 1–18. [Google Scholar] [CrossRef]
- Sáez, A.; Carballeira, R.; Pueyo, J.J.; Vázquez-Loureiro, D.; Leira, M.; Hernández, A.; Valero-Garcés, B.L.; Bao, R. Formation and evolution of back-barrier perched lakes in rocky coasts: An example of a Holocene system in north-west Spain. Sedimentology 2018, 65, 1891–1917. [Google Scholar] [CrossRef] [Green Version]
- Del Río, L.; Gracia, F.J. Error determination in the photogrammetric assessment of shoreline changes. Nat. Hazards 2013, 65, 2385–2397. [Google Scholar] [CrossRef]
- Leventhal, J.S. Carbon-sulfur plots to show diagenetic and epigenetic sulfidation in sediments. Geochim. Cosmochim. Acta 1995, 59, 1207–1211. [Google Scholar] [CrossRef]
- Berner, R.A. Burial of organic carbon and pyrite sulfur in the modem ocean: Its geochemical and environmental significance. Am. J. Sci. 1982, 282, 451–473. [Google Scholar] [CrossRef]
- Calvert, S.E.; Karlin, R.E. Relationships between sulphur, organic carbon, and iron in the modern sediments of the Black Sea. Geochim. Cosmochim. Acta 1991, 55, 2483–2490. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.A.; Morse, J.W. A quantitative method for determination of trace metal concentrations in sedimentary pyrite. Mar. Chem. 1990, 29, 119–144. [Google Scholar] [CrossRef]
- Otero, X.L.; Ferreira, T.O.; Huerta-Díaz, M.A.; Partiti, C.S.M.; Souza, V.; Vidal-Torrado, P.; Macías, F. Geochemistry of iron and manganese in soils and sediments of a mangrove system, Island of Pai Matos (Cananeia—SP, Brazil). Geoderma 2009, 148, 318–335. [Google Scholar] [CrossRef]
- Otero Pérez, X.L. Monitorización de Los Procesos Biogeoquímicos en Las Lagunas Litorales en Relación Con su Calidad Ambiental y Respuesta al Cambio Climático; Scientific Report; Xunta de Galicia: Santiago de Compostela, Spain, 2011. [Google Scholar]
- Berner, R.A.; Raiswell, R. C/S method for distinguishing freshwater from marine sedimentary rocks. Geology 1984, 12, 365. [Google Scholar] [CrossRef]
- Bao, R.; Alonso, A.; Delgado, C.; Pagés, J.L. Identification of the main driving mechanisms in the evolution of a small coastal wetland (Traba, Galicia, NW Spain) since its origin 5700 cal yr BP. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 247, 296–312. [Google Scholar] [CrossRef] [Green Version]
- González-Villanueva, R.; Pérez-Arlucea, M.; Costas, S.; Bao, R.; Otero, X.L.; Goble, R. 8000 years of environmental evolution of barrier–lagoon systems emplaced in coastal embayments (NW Iberia). Holocene 2015, 25, 1786–1801. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Hill, D.A. Managing Habitats for Conservation; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521447763. [Google Scholar]
- Vymazal, J. Types of constructed wetlands for wastewater treatment: Their potential for nutrient removal. In Transformations of Nutrients in Natural and Constructed Wetlands; Backhuys: Leiden, The Netherlands, 2001. [Google Scholar]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment: Five Decades of Experience. Environ. Sci. Technol. 2011, 45, 61–69. [Google Scholar] [CrossRef]
- Frédette, C.; Grebenshchykova, Z.; Comeau, Y.; Brisson, J. Evapotranspiration of a willow cultivar (Salix miyabeana SX67) grown in a full-scale treatment wetland. Ecol. Eng. 2019, 127, 254–262. [Google Scholar] [CrossRef]
- Álvarez-Rogel, J.; del Carmen Tercero, M.; Arce, M.I.; Delgado, M.J.; Conesa, H.M.; González-Alcaraz, M.N. Nitrate removal and potential soil N2O emissions in eutrophic salt marshes with and without Phragmites australis. Geoderma 2016, 282, 49–58. [Google Scholar] [CrossRef]
- García, G.; Muñoz-Vera, A. Characterization and evolution of the sediments of a Mediterranean coastal lagoon located next to a former mining area. Mar. Pollut. Bull. 2015, 100, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Del Carmen Tercero, M.; Álvarez-Rogel, J.; Conesa, H.M.; Párraga-Aguado, I.; González-Alcaraz, M.N. Phosphorus retention and fractionation in an eutrophic wetland: A one-year mesocosms experiment under fluctuating flooding conditions. J. Environ. Manag. 2017, 190, 197–207. [Google Scholar] [CrossRef]
- Serrano, L.; Reina, M.; Quintana, X.D.; Romo, S.; Olmo, C.; Soria, J.M.; Blanco, S.; Fernández-Aláez, C.; Fernández-Aláez, M.; Caria, M.C.; et al. A new tool for the assessment of severe anthropogenic eutrophication in small shallow water bodies. Ecol. Indic. 2017, 76, 324–334. [Google Scholar] [CrossRef]
- Foley, B.; Jones, I.D.; Maberly, S.C.; Rippey, B. Long-term changes in oxygen depletion in a small temperate lake: Effects of climate change and eutrophication. Freshw. Biol. 2012, 57, 278–289. [Google Scholar] [CrossRef]

| Lagoon | Date | pH | Eh | Elec. Cond. | Salinity | NH4+ | NO3− | PO43− | Cl− |
|---|---|---|---|---|---|---|---|---|---|
| mV | dS m−1 | ‰ | --------------------mg L−1----------------- | ||||||
| Louro | Oct. 2009 | 8.8 | 246 | 16.0 | 9 | <0.05 | 2.83 | 0.12 | 4764 |
| Jan. 2010 | 7.9 | 399 | 32.0 | 23 | <0.05 | 0.41 | <0.05 | 6412 | |
| Jun. 2010 | 7.8 | 442 | 1.6 | 21 | <0.05 | <0.05 | 0.06 | 11065 | |
| Oct. 2010 | 8.2 | 388 | 34.0 | 39 | 0.48 | <0.05 | 0.08 | 19450 | |
| Jan. 2011 | 7.9 | 359 | 10.7 | na | 0.23 | <0.05 | 0.06 | 9284 | |
| Sep. 2011 | 9.8 | 383 | 21.3 | 15 | <0.05 | <0.05 | na | na | |
| Vixán | Oct. 2009 | 8.8 | 273 | 12.0 | 12 | <0.05 | 1.63 | <0.05 | 4061 |
| Jan. 2010 | 8.1 | 386 | 3.5 | 2 | <0.05 | 4.75 | <0.05 | 746 | |
| Jun. 2010 | 8.1 | 365 | 1.5 | 2 | 0.09 | 2.05 | 0.06 | 424 | |
| Oct. 2010 | 7.8 | 390 | 19.0 | 19 | 0.06 | 0.07 | 0.09 | 9564 | |
| Jan. 2011 | 7.7 | 414 | 2.8 | 1.5 | <0.05 | 5.55 | 0.07 | 785 | |
| Sep. 2011 | 8.9 | 380 | 7.0 | 3 | <0.05 | 40.8 | <0.05 | 2507 | |
| Xuño | Oct. 2009 | 6.7 | 249 | 0.60 | <1 | 0.37 | 0.11 | <0.05 | 134 |
| Jan. 2010 | 7.3 | 405 | 0.27 | <1 | <0.05 | 4.65 | <0.05 | 47 | |
| Jun. 2010 | 6.9 | 362 | 0.28 | <1 | <0.05 | 1.00 | 0.45 | 63 | |
| Oct. 2010 | 6.1 | 488 | 0.35 | <1 | <0.05 | 0.06 | 0.09 | 97 | |
| Jan. 2011 | na | 335 | 0.44 | <1 | 0.32 | <0.05 | 0.14 | 134 | |
| Sep. 2011 | na | 213 | 0.88 | <1 | <0.05 | 0.70 | <0.05 | 228 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga-Santiago, P.; Gómez-Pazo, A.; Pérez-Alberti, A.; Montero, P.; Otero Pérez, X.L. Trends in the Recent Evolution of Coastal Lagoons and Lakes in Galicia (NW Iberian Peninsula). J. Mar. Sci. Eng. 2019, 7, 272. https://doi.org/10.3390/jmse7080272
Fraga-Santiago P, Gómez-Pazo A, Pérez-Alberti A, Montero P, Otero Pérez XL. Trends in the Recent Evolution of Coastal Lagoons and Lakes in Galicia (NW Iberian Peninsula). Journal of Marine Science and Engineering. 2019; 7(8):272. https://doi.org/10.3390/jmse7080272
Chicago/Turabian StyleFraga-Santiago, Pedro, Alejandro Gómez-Pazo, Augusto Pérez-Alberti, Pablo Montero, and Xose Lois Otero Pérez. 2019. "Trends in the Recent Evolution of Coastal Lagoons and Lakes in Galicia (NW Iberian Peninsula)" Journal of Marine Science and Engineering 7, no. 8: 272. https://doi.org/10.3390/jmse7080272
APA StyleFraga-Santiago, P., Gómez-Pazo, A., Pérez-Alberti, A., Montero, P., & Otero Pérez, X. L. (2019). Trends in the Recent Evolution of Coastal Lagoons and Lakes in Galicia (NW Iberian Peninsula). Journal of Marine Science and Engineering, 7(8), 272. https://doi.org/10.3390/jmse7080272








