Comparative Study on Fuel Gas Supply Systems for LNG Bunkering Using Carbon Dioxide and Glycol Water
Abstract
1. Introduction
2. Analytical Methods
2.1. Comparing Alternative Heat Sources to Seawater
2.2. Research Area
2.3. Governing Equations and Optimization
3. Results
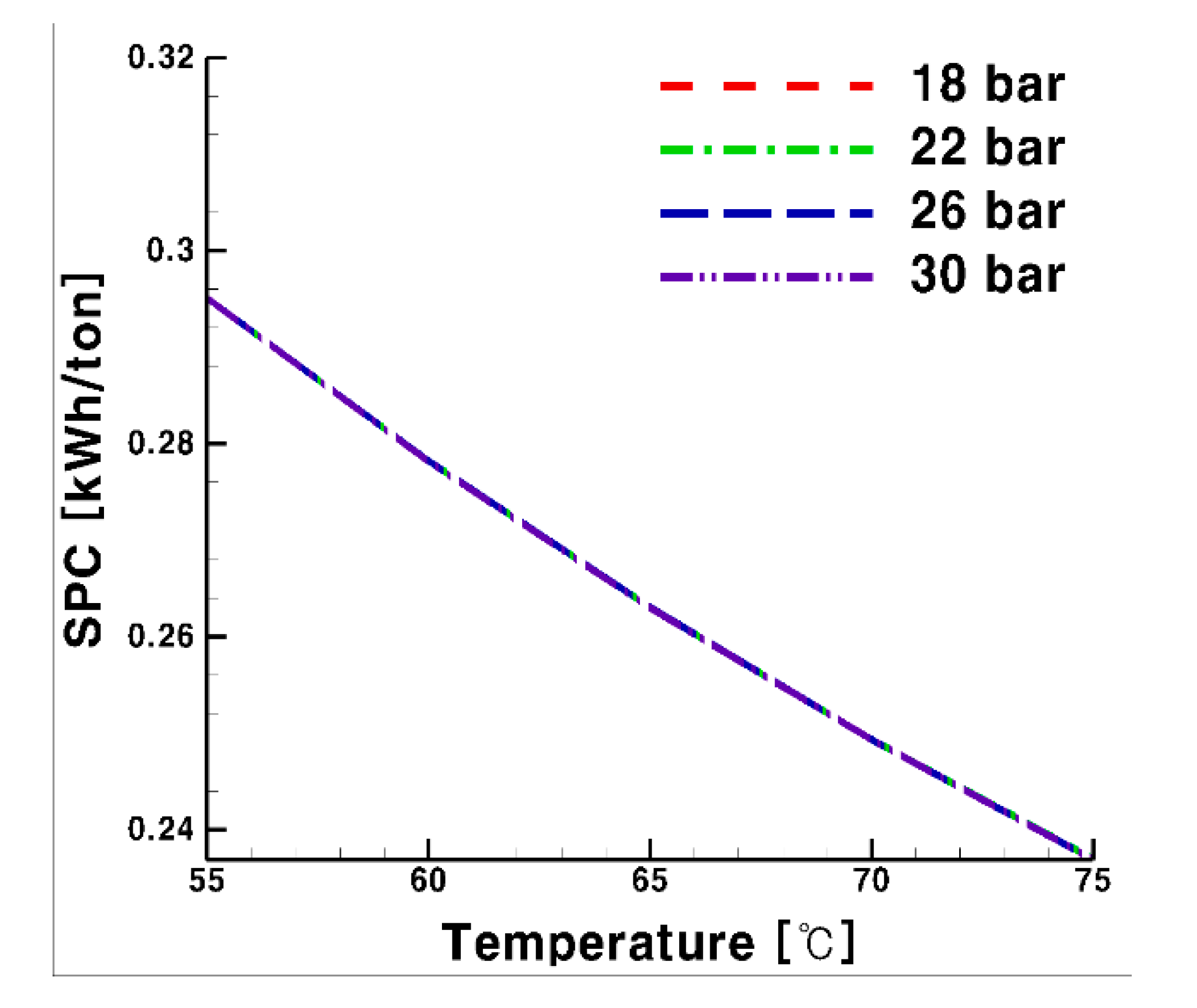
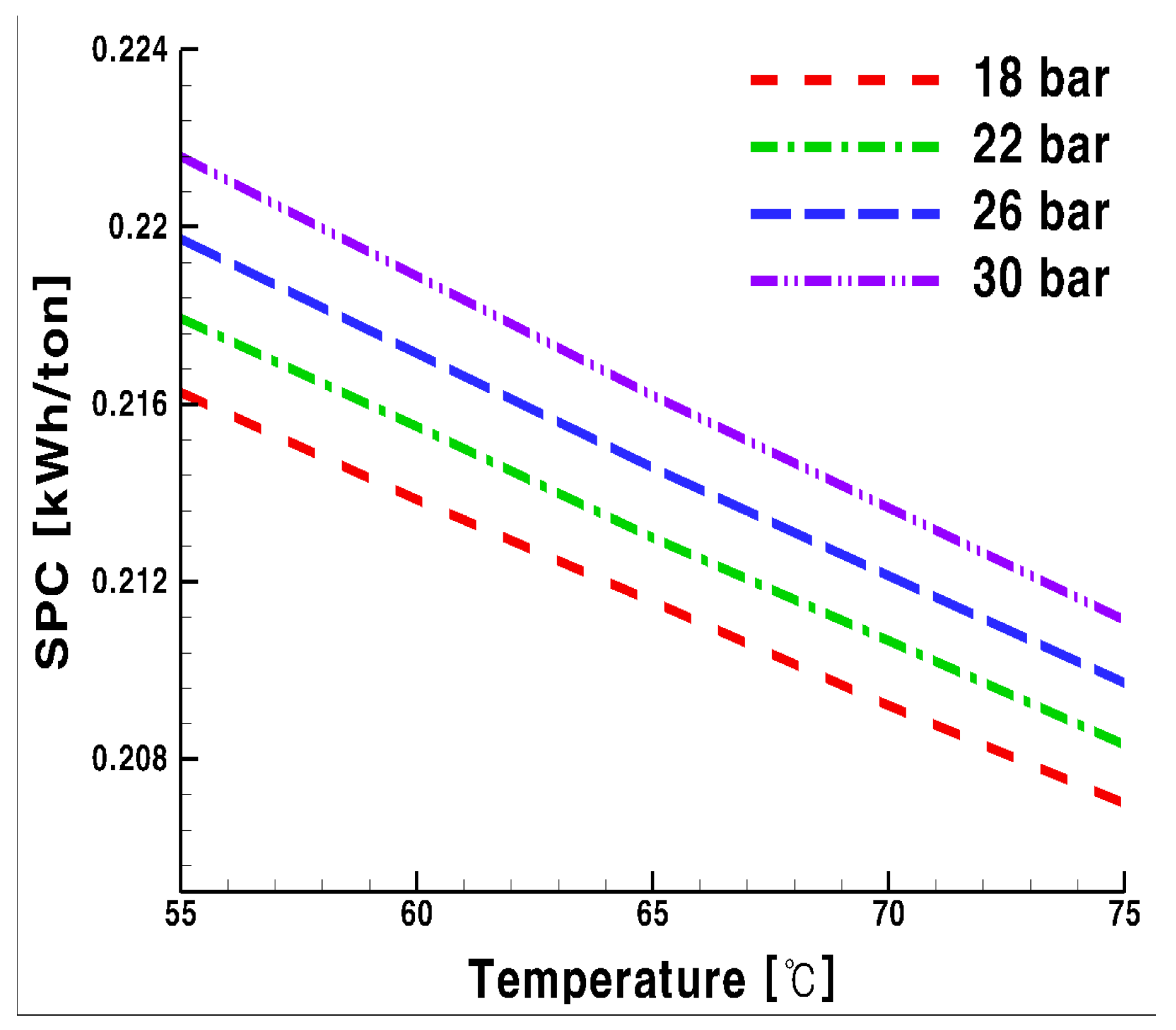
3.1. Specific Power Consumption
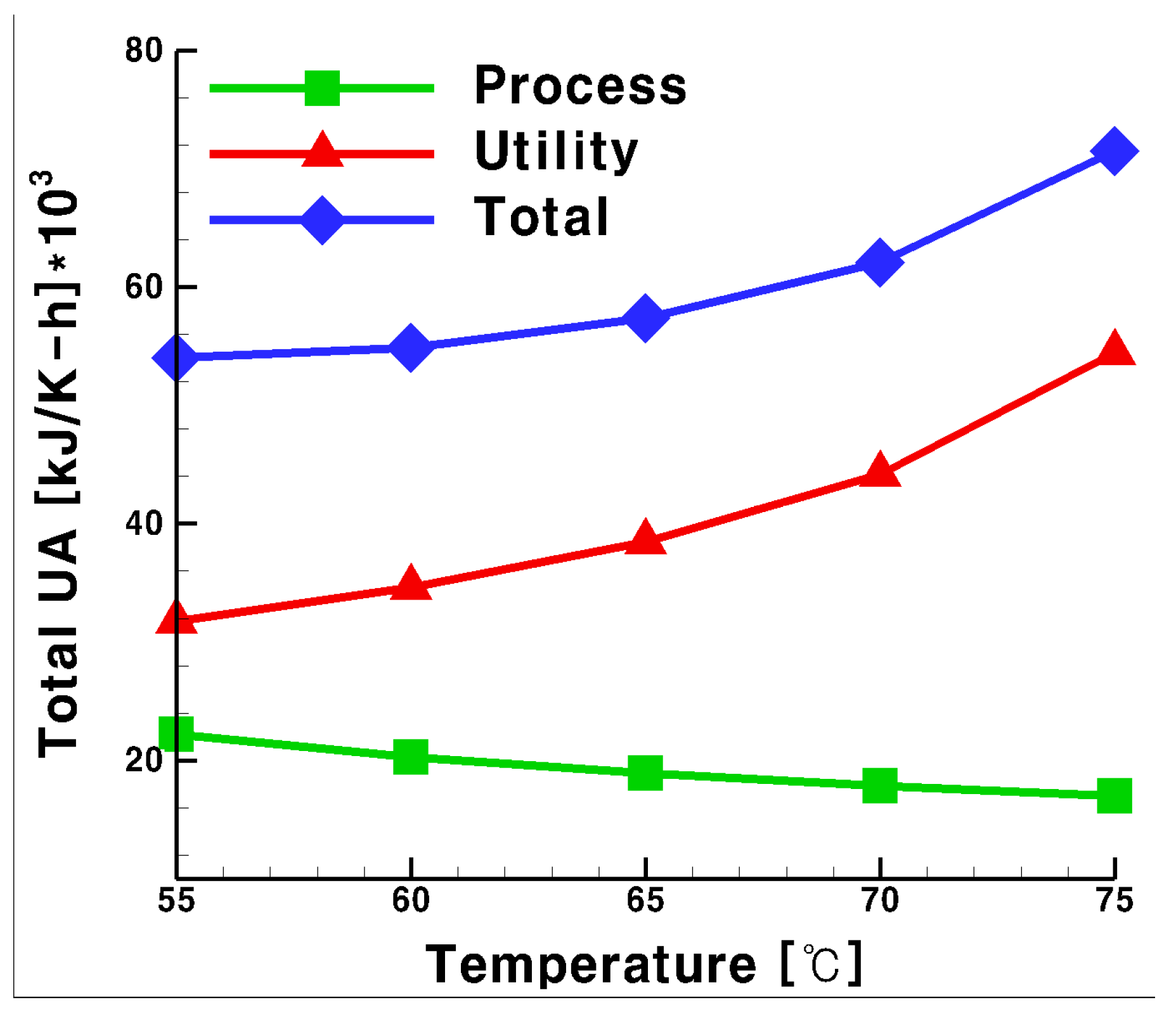
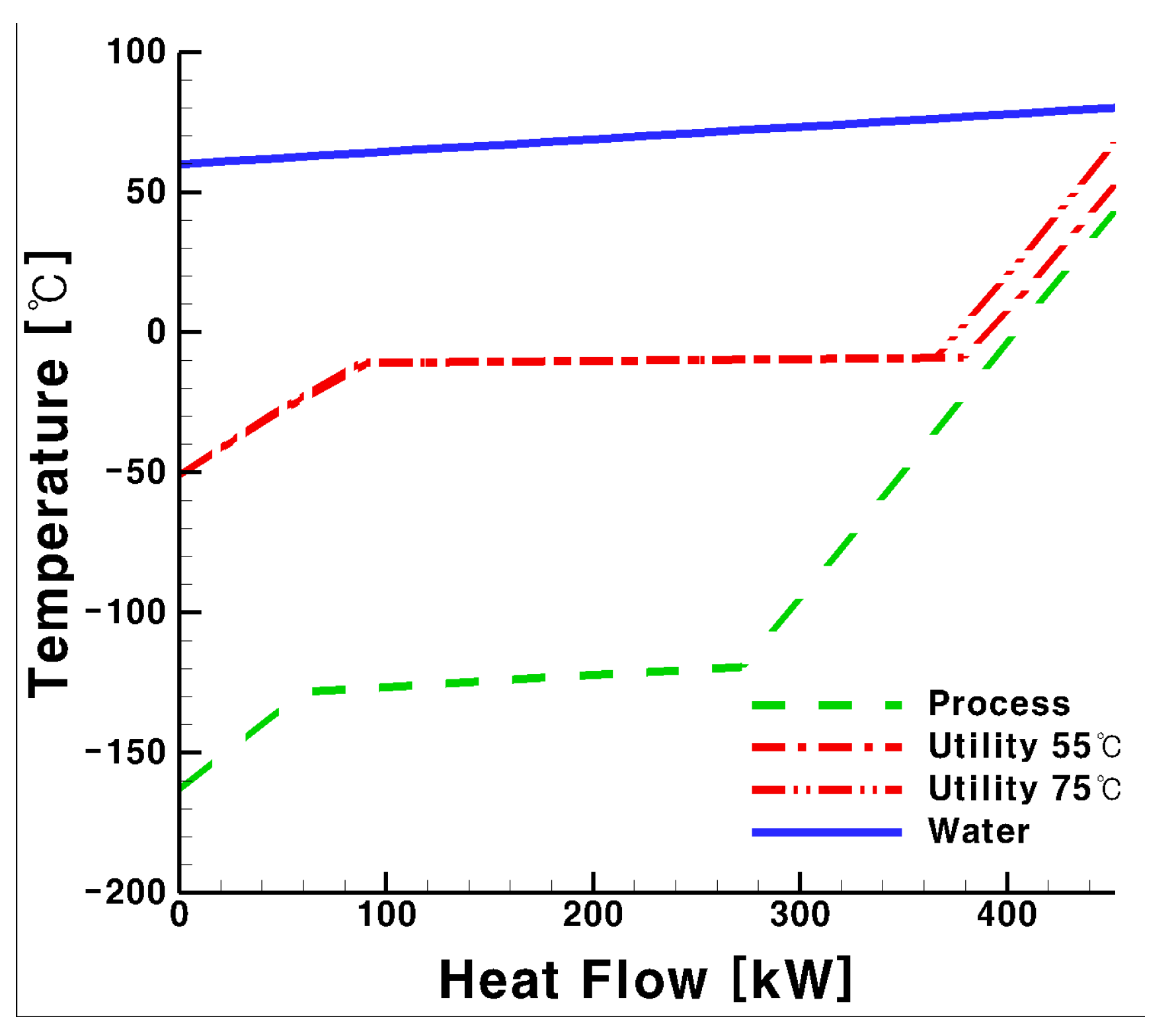
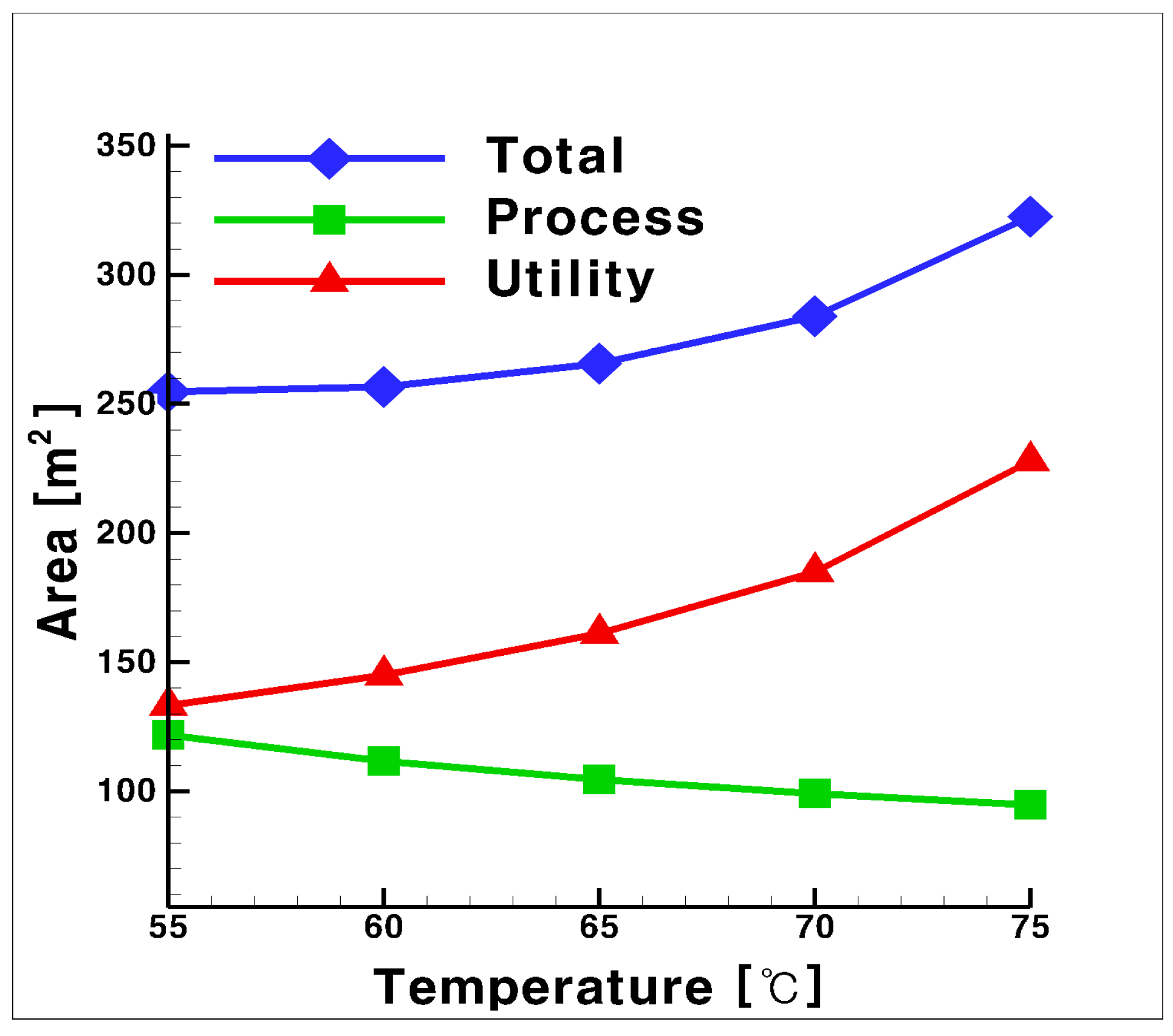
3.2. Total UA for the Process and Utility Groups with Glycol Water as Heat Medium
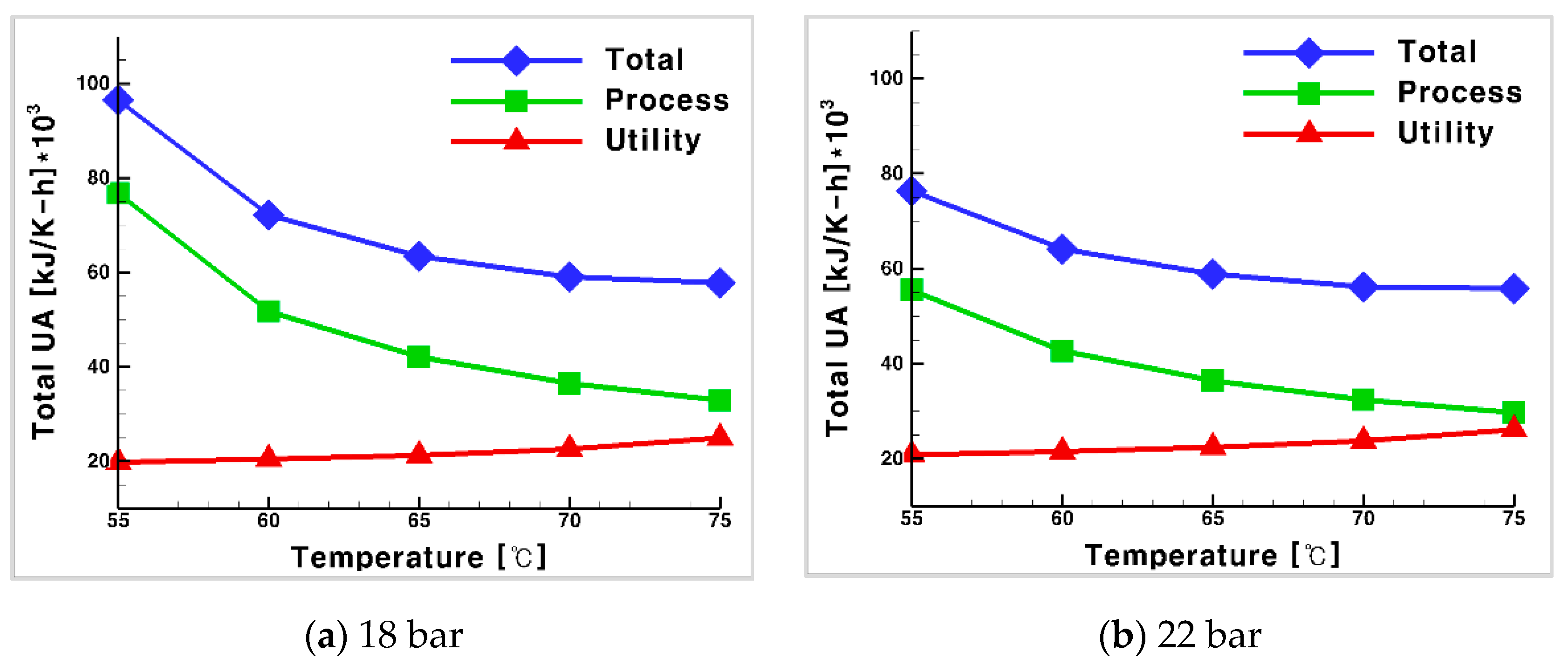
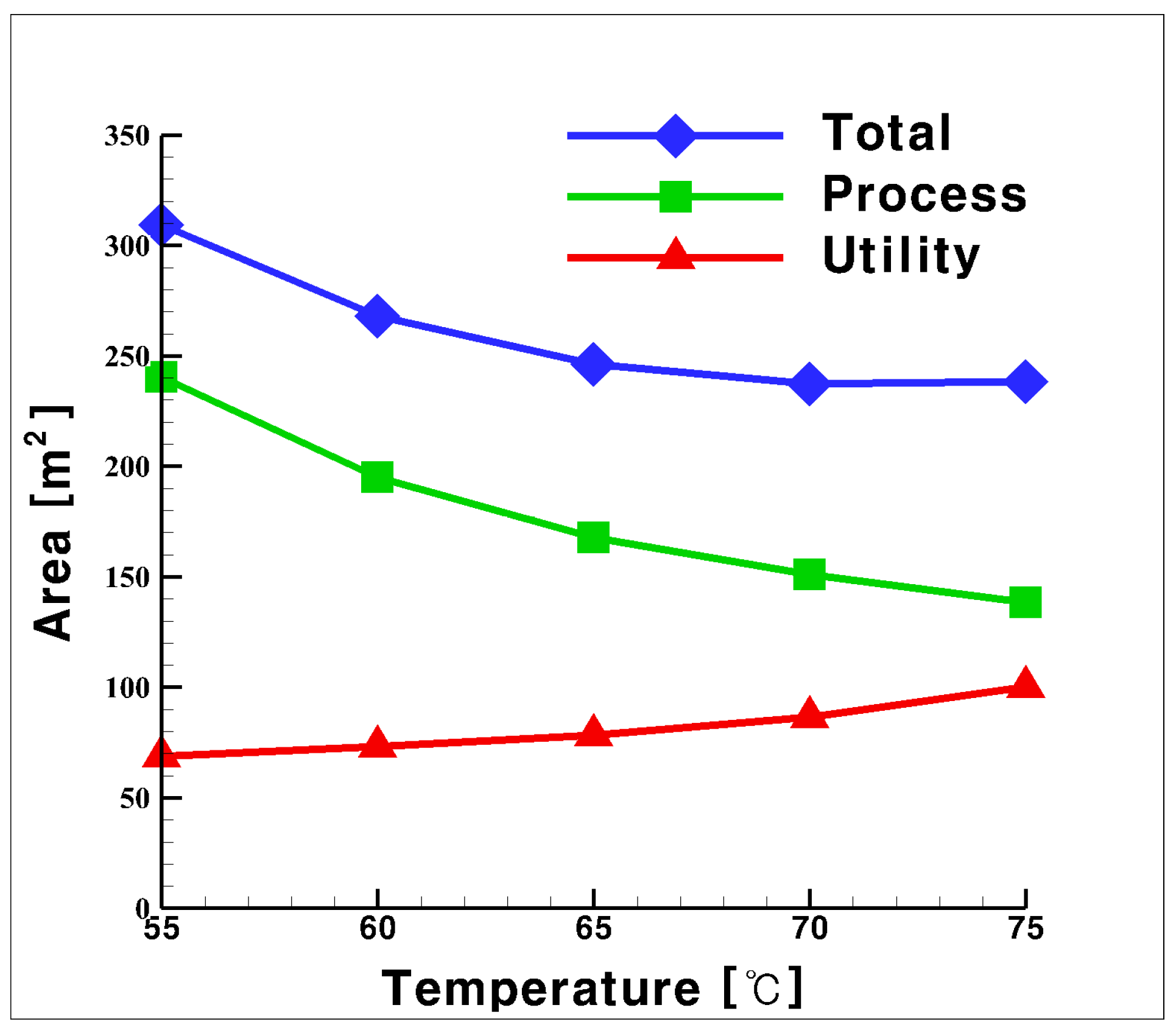
3.3. Total UA for the Process and Utility Groups with CO2 as Heat Medium
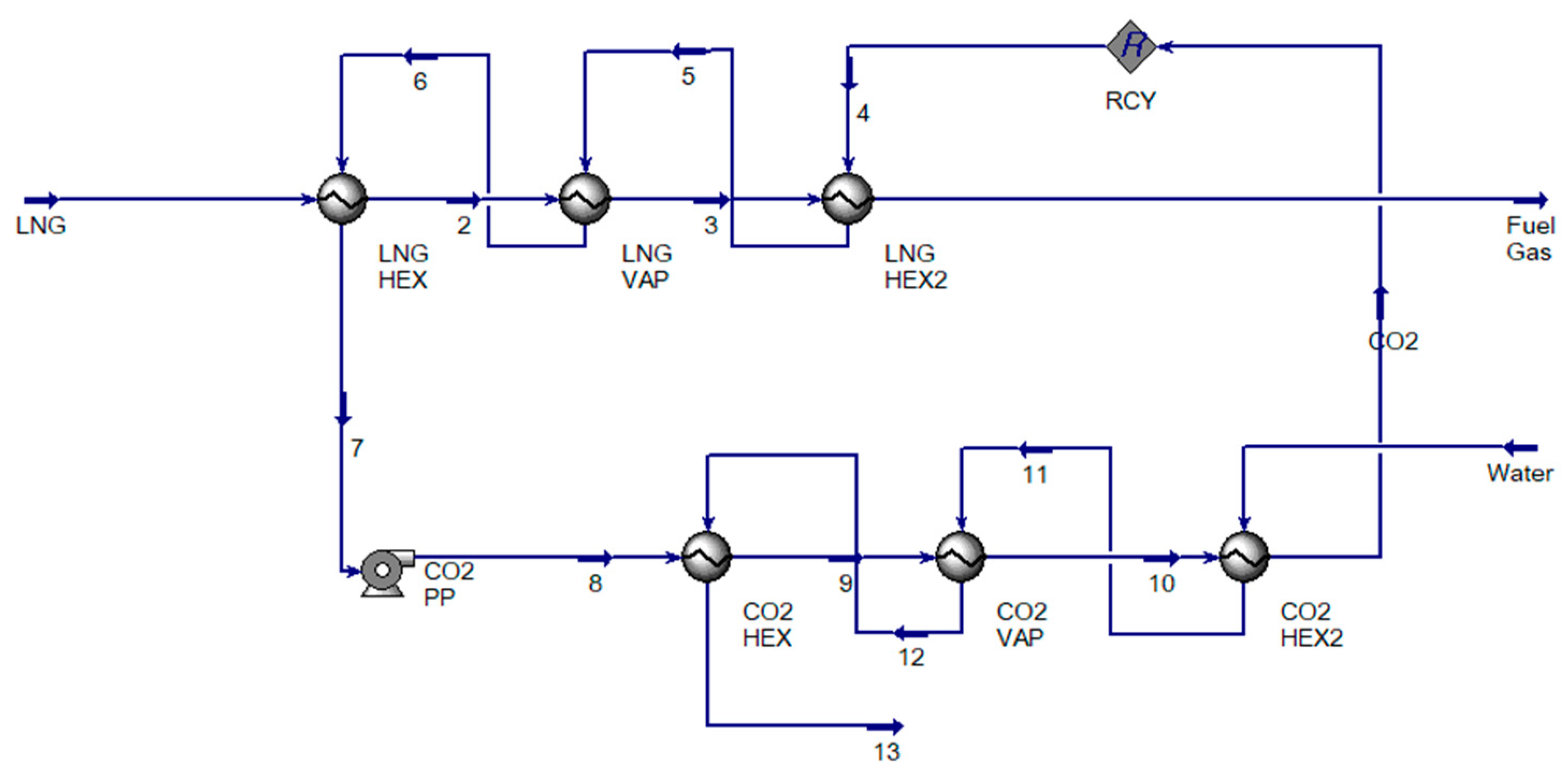
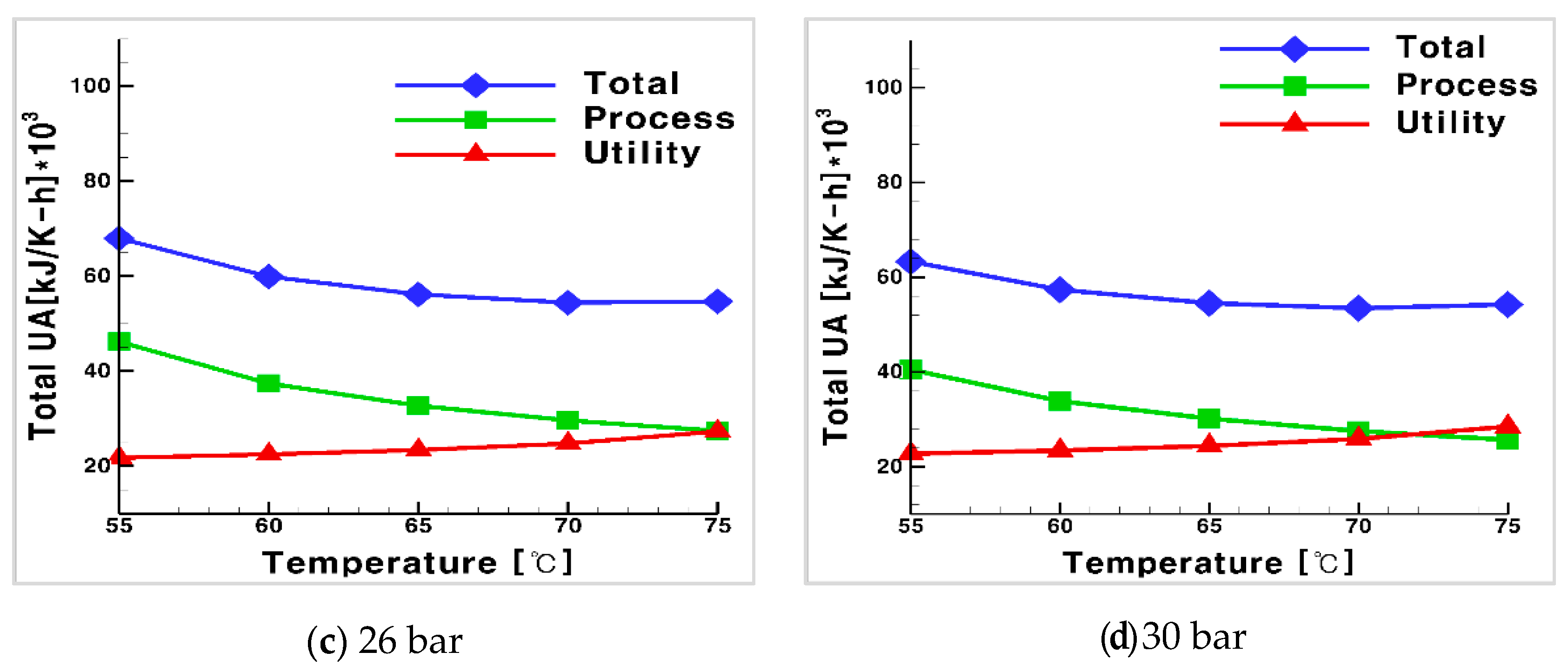
3.4. Process Optimization Using CO2
3.5. Overall Process and Utility Area
4. Conclusions
- (1)
- CO2 is the most advantageous heat medium for the gasification of LNG in terms of performance. CO2 enhanced the efficiency (i.e., SPC) by 14% due to the usage of latent heat of vaporization.
- (2)
- For the system using glycol water, SPC was not affected by pressure change, but lower operating pressure entailed less SPC. For both media, the higher the supply temperature, the lower the required SPC.
- (3)
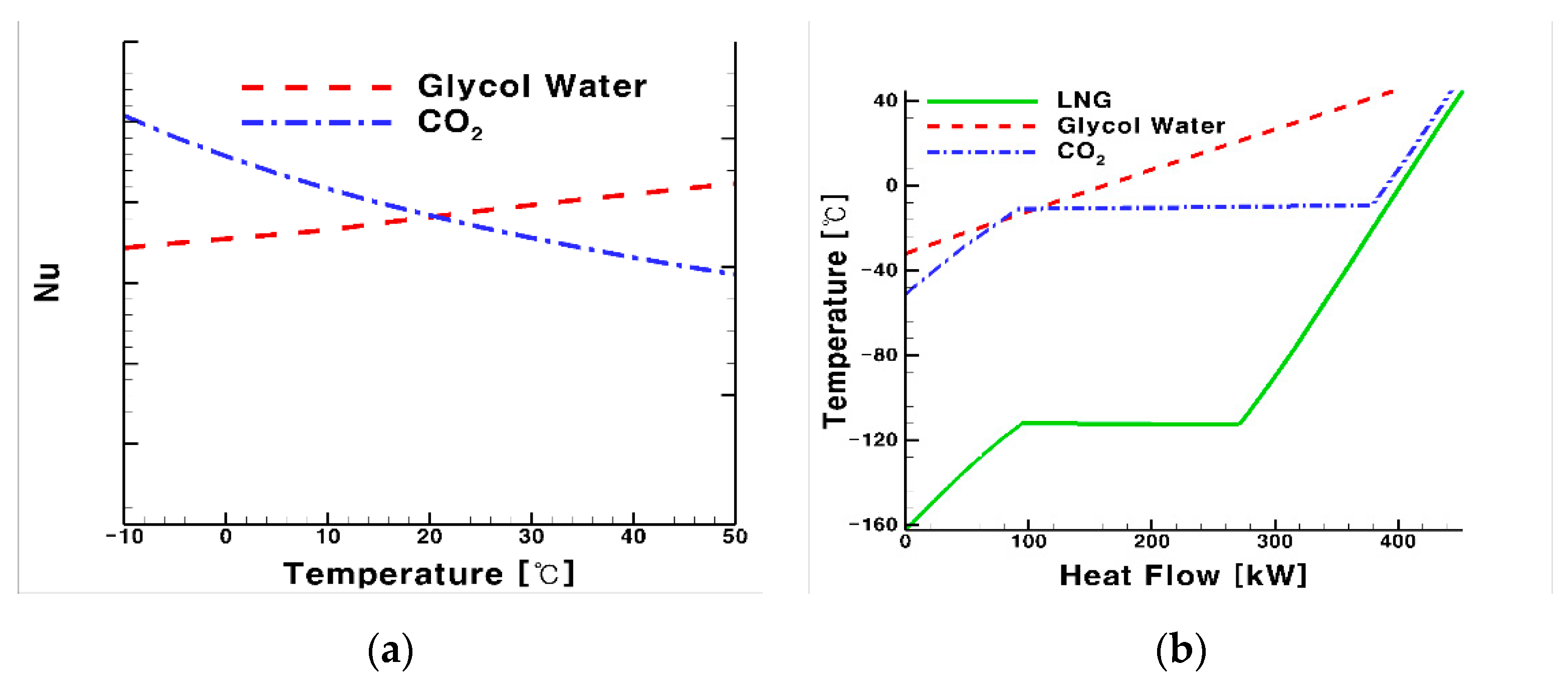
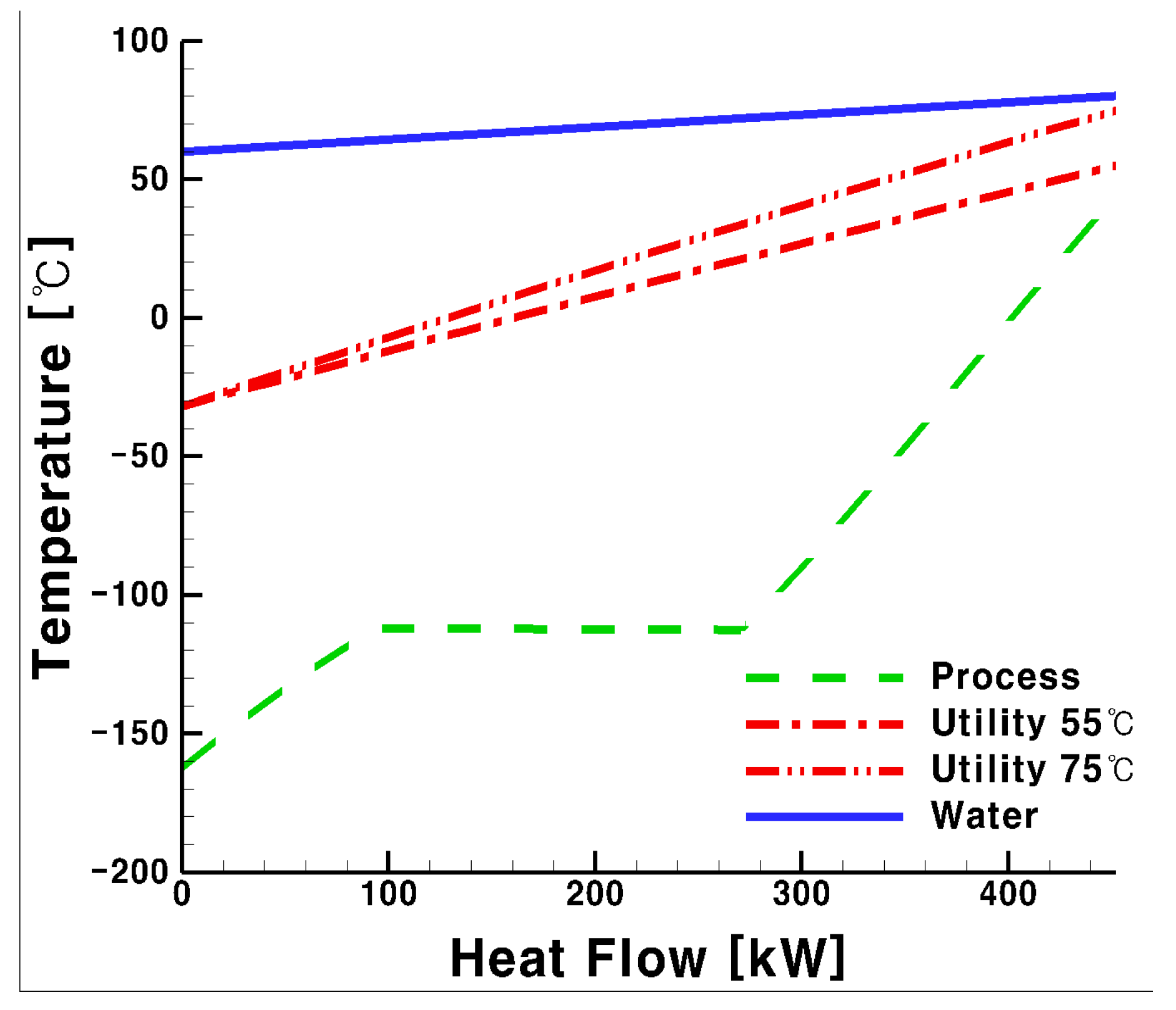
- The higher the supply temperature for the system using CO2, the smaller the heat transfer contact area of the process side heat exchanger, but the corresponding value of the utility side heat exchanger was larger. From the viewpoint of the heat transfer area, as the temperature increases, the heat exchanging area decreases.
- (4)
- Conversely, glycol water exhibited a laminar flow at low temperatures, thereby reducing the heat transfer performance and required a 7% larger heat transfer area for the heat exchanger compared with that using CO2. This result adds support to our conclusion that CO2 is more suitable as a heat medium for the LNG FGS system.
Author Contributions
Funding
Conflicts of Interest
References
- Cheenkachorn, K.; Poompipatpong, C.; Ho, C.G. Performance and emissions of a heavy-duty diesel engine fuelled with diesel and LNG (liquid natural gas). Energy 2013, 53, 52–57. [Google Scholar] [CrossRef]
- Yun, S.K. Characteristics of boil-off-gas partial re-liquefaction systems in LNG ships. J. Korean Soc. Mar. Eng. 2016, 40, 174–179. [Google Scholar] [CrossRef]
- Linde to Supply LNG Fuel Gas System to New High-Speed Ferry. Available online: https://www.linde-engineering.com/en/news_and_media/press_releases/news_20150410.html/ (accessed on 16 June 2019).
- Contributing to Hydrogen Economy Activation. Available online: http://www.planttech.co.kr/archives/10607/ (accessed on 16 June 2019).
- Sun, H.; Zhu, H.; Liu, F.; Ding, H. Simulation and optimization of a novel Rankine power cycle for recovering cold energy from liquefied natural gas using a mixed working fluid. Energy 2014, 70, 317–324. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, Y.T.; Kang, H.K. An analysis on the characteristics of regasification system for gas fueled ship depending on the mixing ratio of eglycol and water. J. Korean Soc. Mar. Eng. 2014, 38, 799–805. [Google Scholar] [CrossRef]
- García, R.F.; Carril, J.C.; Gomez, J.R.; Gomez, M.R. Combined cascaded Rankine and direct expander based power units using LNG (liquefied natural gas) cold as heat sink in LNG regasification. Energy 2016, 105, 16–24. [Google Scholar] [CrossRef]
- Lee, S. Multi-parameter optimization of cold energy recovery in cascade Rankine cycle for LNG regasification using genetic algorithm. Energy 2017, 118, 776–782. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Sharifzadeh, M.M.M.; Zonouz, M.J.; Rosen, M.A. Cost and economic potential analysis of a cascading power cycle with liquefied natural gas regasification. Energy Convers. Manag. 2018, 156, 68–83. [Google Scholar] [CrossRef]
- Shi, X.; Che, D. A combined power cycle utilizing low-temperature waste heat and LNG cold energy. Energy Convers. Manag. 2009, 50, 567–575. [Google Scholar] [CrossRef]
- Lu, T.K.S.W.; Wang, K.S. Analysis and optimization of a cascading power cycle with liquefied natural gas (LNG) cold energy recovery. Appl. Therm. Eng. 2009, 29, 1478–1484. [Google Scholar] [CrossRef]
- Kim, S.; Nam, K.; Kim, J. Analysis on design concept of LNG regasification system. J. Soc. Nav. Archit. Korea 2012, 1680–1683. [Google Scholar]
- Park, S.; Park, C.; Lee, U.; Jung, I.; Na, J.; Kshetrimayum, K.S.; Han, C. Comparative study of process integration and retrofit design of a liquefied natural gas (LNG) regasification process based on exergy analyses: A case study of an LNG regasification process in South Korea. Ind. Eng. Chem. Res. 2014, 53, 14366–14376. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, K. A novel cryogenic power cycle for LNG cold energy recovery. Energy 2011, 36, 2828–2833. [Google Scholar] [CrossRef]
- Gómez, M.R.; Garcia, R.F.; Gómez, J.R.; Carril, J.C. Review of thermal cycles exploiting the exergy of liquefied natural gas in the regasification process. Renew. Sustain. Energy Rev. 2014, 38, 781–795. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.C. Thermodynamic assessment of integrated heat recovery system combining exhaust-gas heat and cold energy for LNG regasification process in FSRU vessel. J. Mech. Sci. Technol. 2016, 30, 1389–1398. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Wang, B.; Dong, J.; Jiang, Y. Thermodynamic analysis on the process of regasification of LNG and its application in the cold warehouse. Therm. Sci. Eng. Prog. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- DaeChul, L.; Afianto, H.; Han-shik, C.; Hyo-min, J. Numerical analysis of LNG vaporizer heat transfer characteristic in LNG fuel ship. J. Korean Soc. Mar. Eng. 2013, 37, 22–28. [Google Scholar] [CrossRef]
- Tomków, Ł.; Cholewiński, M. Improvement of the LNG (liquid natural gas) regasification efficiency by utilizing the cold exergy with a coupled absorption–ORC (organic Rankine cycle. Energy 2015, 87, 645–653. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Q.; Sun, N.; Li, Y. Enhancement of energy performance in a boil-off gas re-liquefaction system of LNG carriers using ejectors. Energy Convers. Manag. 2016, 126, 875–888. [Google Scholar] [CrossRef]
- Bao, J.; Lin, Y.; Zhang, R.; Zhang, N.; He, G. Strengthening power generation efficiency utilizing liquefied natural gas cold energy by a novel two-stage condensation Rankine cycle(TCRC). Energy Convers. Manag. 2017, 143, 312–325. [Google Scholar] [CrossRef]
- Lee, U.; Mitsos, A. Optimal multicomponent working fluid of organic Rankine cycle for exergy transfer from liquefied natural gas regasification. Energy 2017, 17, 489–501. [Google Scholar] [CrossRef]
- Lim, T.W.; Lee, C.K. Theoretical analysis of ORC (organic Rankine cycle) system to recover waste heat of marine engine. J. Fish. Mar. Sci. Educ. 2017, 29, 1522–1530. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, Y.H.; Kang, H.K.; Lim, T.W. Performance analysis of the organic Rankine cycle using LNG cold energy in LNG-fueled ships. J. Koren Soc. Mar. Eng. 2018, 42, 524–530. [Google Scholar] [CrossRef]
- Plus, A. Aspen Plus Documentation Version V7. 3; Aspen Tech: Cambridge, MA, USA, 2011. [Google Scholar]
- Peng, D.Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Dauber, F.; Span, R. Influence of Thermodynamic Property Models on the Simulation of LNG Evaporation and Liquefaction Processes; IGRC: Paris, France, 2008. [Google Scholar]
- Yuan, Z.; Cui, M.; Xie, Y.; Li, C. Design and analysis of a small-scale natural gas liquefaction process adopting single nitrogen expansion with carbon dioxide pre-cooling. Appl. Therm. Eng. 2014, 64, 139–146. [Google Scholar] [CrossRef]

| Advantage | Disadvantage | Operating Temperature (°C) | Risk | |
|---|---|---|---|---|
| Glycol Water | High Specific heat | Corrosion | (−30) to 106 | Toxicity |
| Pure CO2 | Affordable | Icing | (−78.5) to 31.4 | PEL 1: 5000 ppm |
| Process | ||||
|---|---|---|---|---|
| Shell | Tube | Shell | Tube | |
| Feed Medium | Glycol water | LNG | CO2 | LNG |
| Pr | 744.6 | 2.1 | 2.5 | 2.1 |
| Re | 91.6 | 12,168.9 | 78,714.2 | 123,138.5 |
| Nu | 50.81 | 53.32 | 315.39 | 53.19 |
| Composition | Mole Fraction (%) | ||
|---|---|---|---|
| Feed | LNG | CH4 | 100 |
| Heat Medium | Glycol water | C2H4OH2 | 45 |
| H2O | 55 | ||
| Pure CO2 | CO2 | 100 |
| Name | Glycol Water U (kJ/K·h·m2) | CO2 U (kJ/K·h·m2) |
|---|---|---|
| LNG HEX | 117 | 308 |
| LNG VAP | 226 | 280 |
| LNG HEX2 | 191 | 127 |
| HEX | 238 | 452 |
| VAP | - | 414 |
| HEX 2 | - | 191 |
| Glycol Water | Pure CO2 | Ratio (%) | |
|---|---|---|---|
| SPC Operating Point | 18 bar, 75 °C | - | |
| SPC (kWh/ton) | 0.24 | 0.207 | 13.75 |
| UA Operating Point | 18 bar, 55 °C | 30 bar, 75 °C | - |
| UA (kJ/K·h) | 53,863 | 53,390 | 0.88 |
| Area Operating Point | 18 bar, 55 °C | 30 bar, 75 °C | - |
| Area (m2) | 255 | 238 | 6.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Park, E.-Y. Comparative Study on Fuel Gas Supply Systems for LNG Bunkering Using Carbon Dioxide and Glycol Water. J. Mar. Sci. Eng. 2019, 7, 184. https://doi.org/10.3390/jmse7060184
Choi J, Park E-Y. Comparative Study on Fuel Gas Supply Systems for LNG Bunkering Using Carbon Dioxide and Glycol Water. Journal of Marine Science and Engineering. 2019; 7(6):184. https://doi.org/10.3390/jmse7060184
Chicago/Turabian StyleChoi, Jungho, and Eun-Young Park. 2019. "Comparative Study on Fuel Gas Supply Systems for LNG Bunkering Using Carbon Dioxide and Glycol Water" Journal of Marine Science and Engineering 7, no. 6: 184. https://doi.org/10.3390/jmse7060184
APA StyleChoi, J., & Park, E.-Y. (2019). Comparative Study on Fuel Gas Supply Systems for LNG Bunkering Using Carbon Dioxide and Glycol Water. Journal of Marine Science and Engineering, 7(6), 184. https://doi.org/10.3390/jmse7060184





