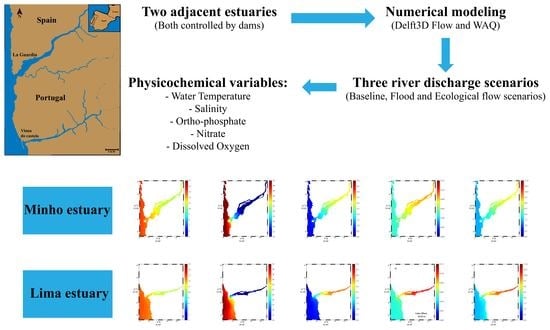
Modeling the Impact of Extreme River Discharge on the Nutrient Dynamics and Dissolved Oxygen in Two Adjacent Estuaries (Portugal)
Abstract
1. Introduction
2. Materials and Methods
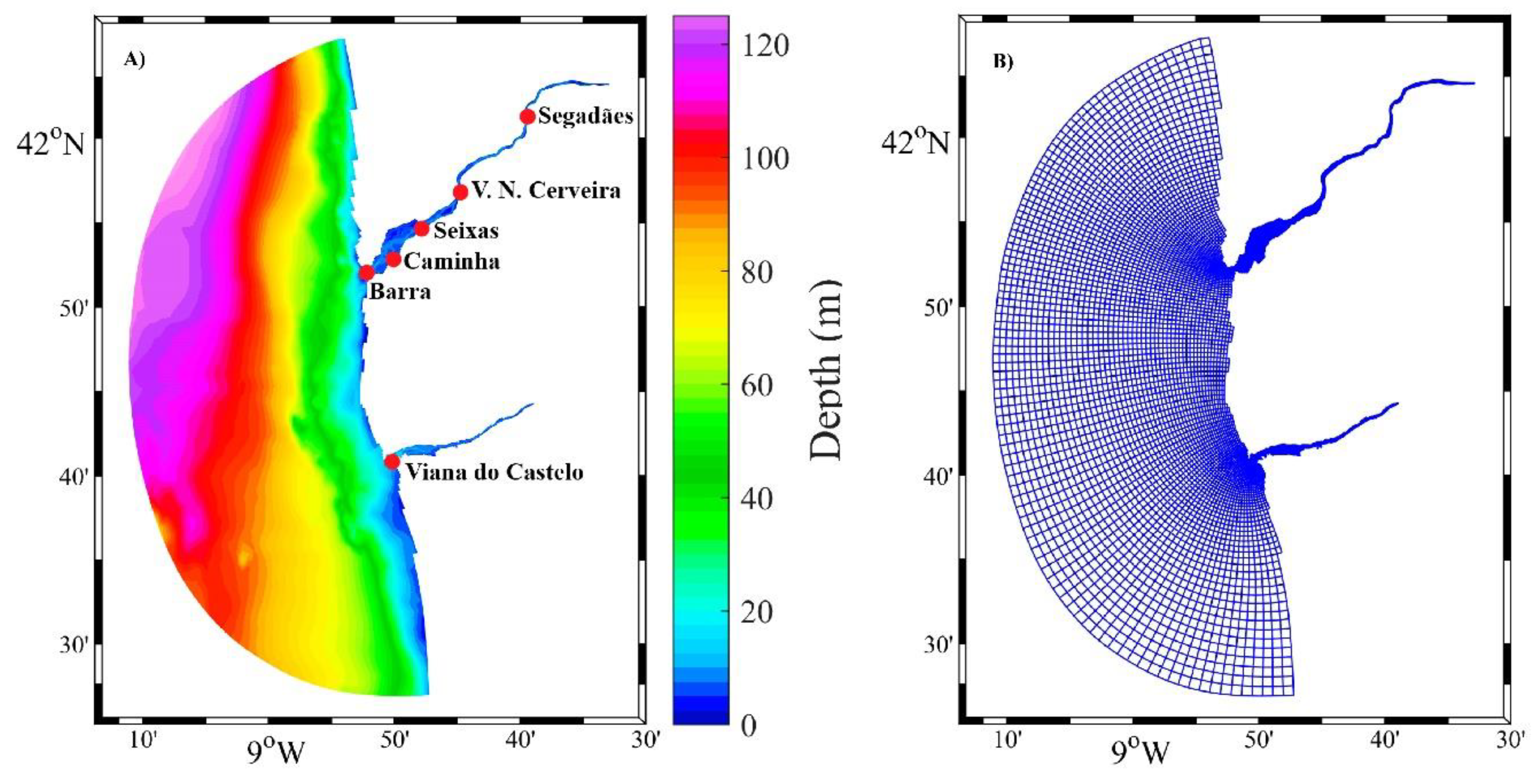
2.1. Study Area
2.2. In Situ Data Collection
2.3. Model Implementation
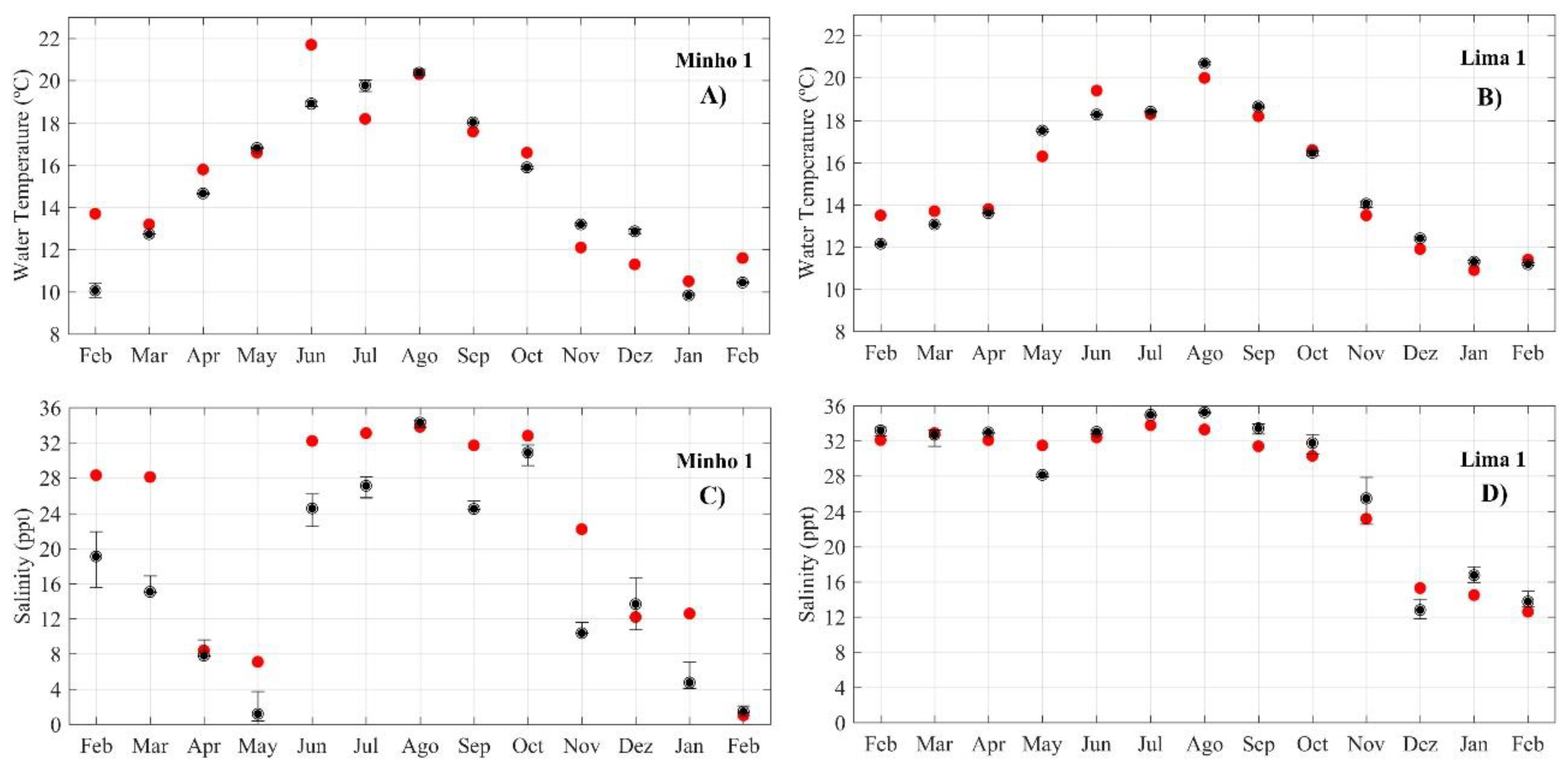
2.4. Model Calibration and Validation
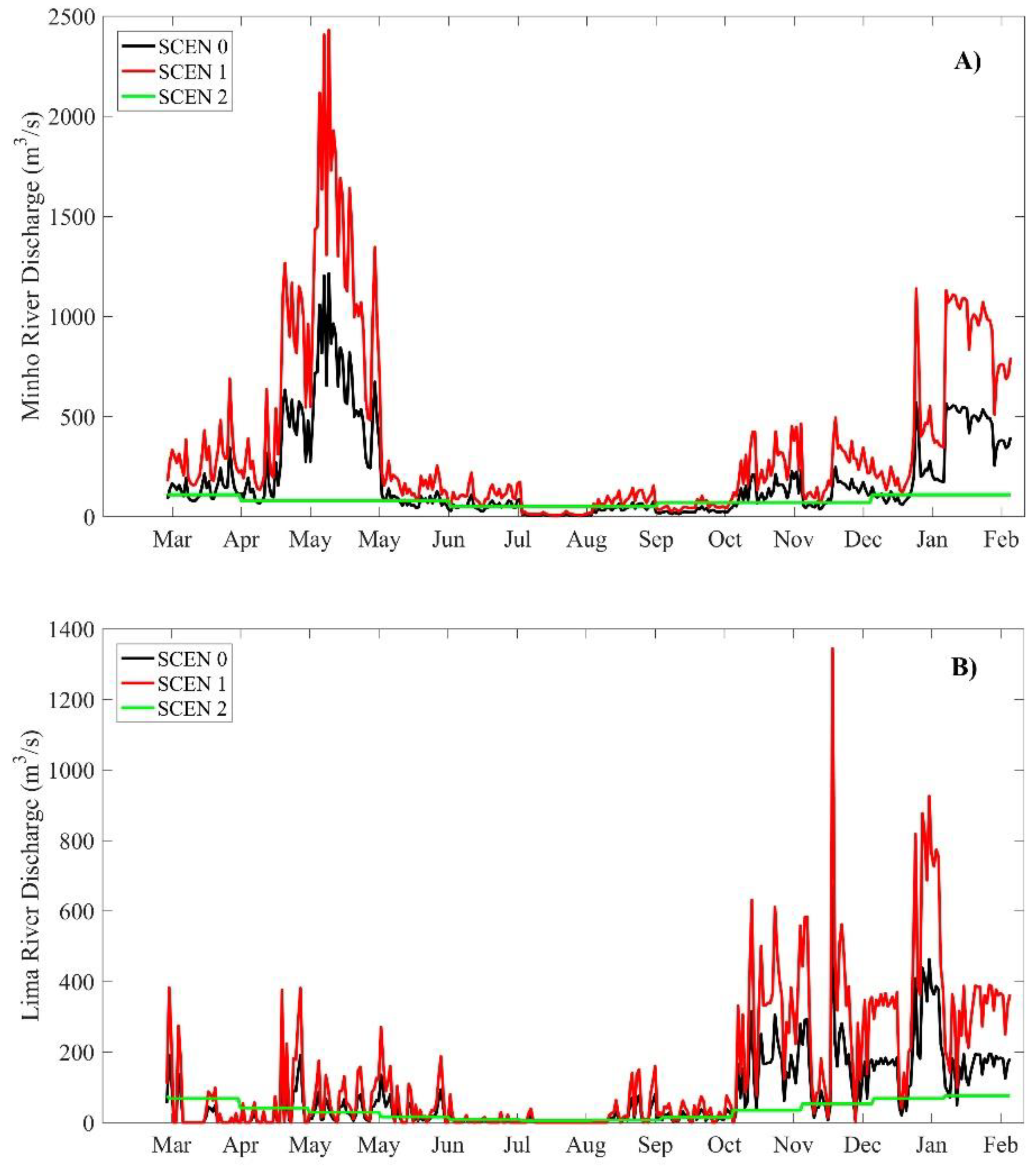
2.5. Model Scenarios
3. Results
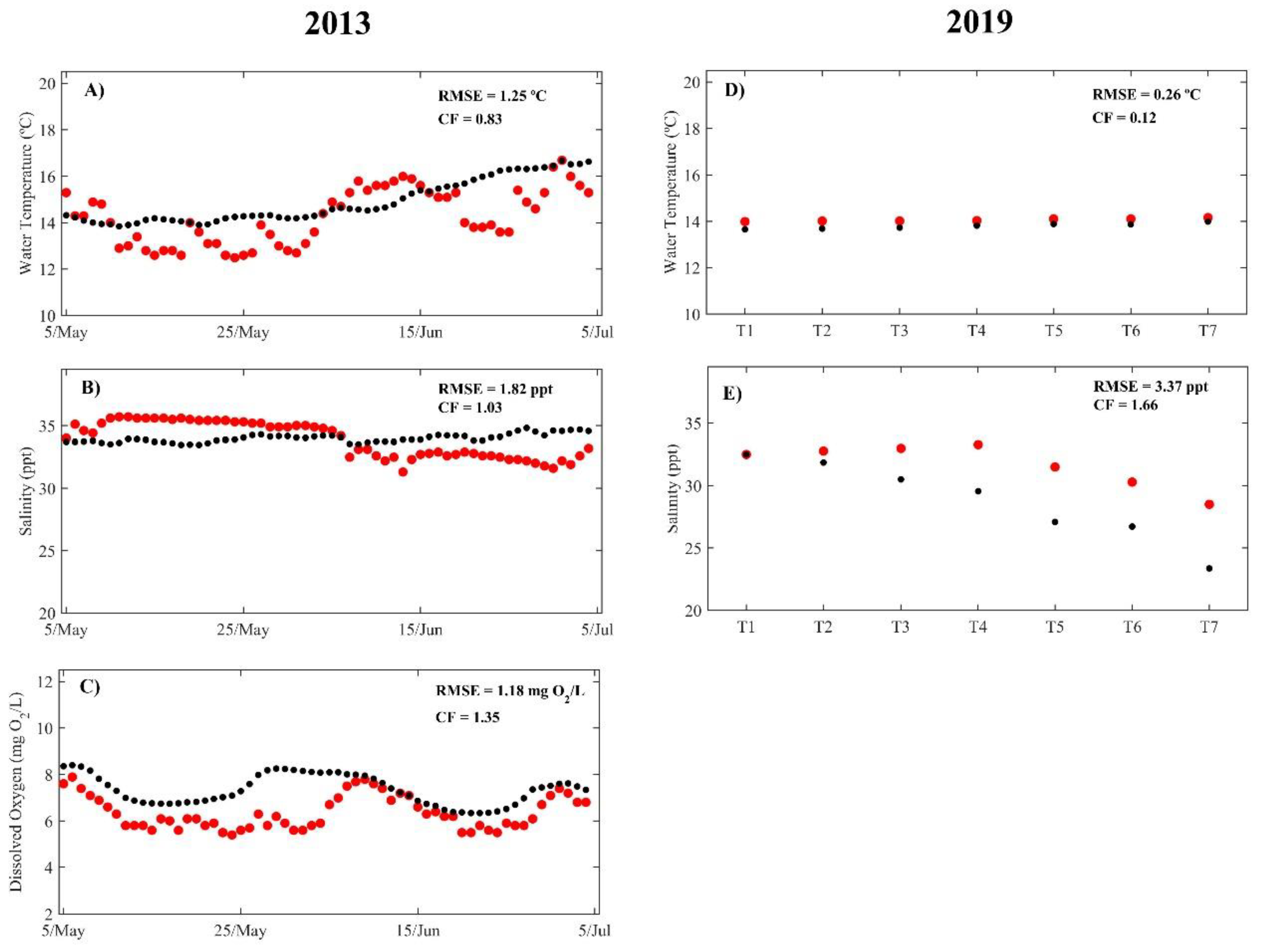
3.1. Model Calibration and Validation
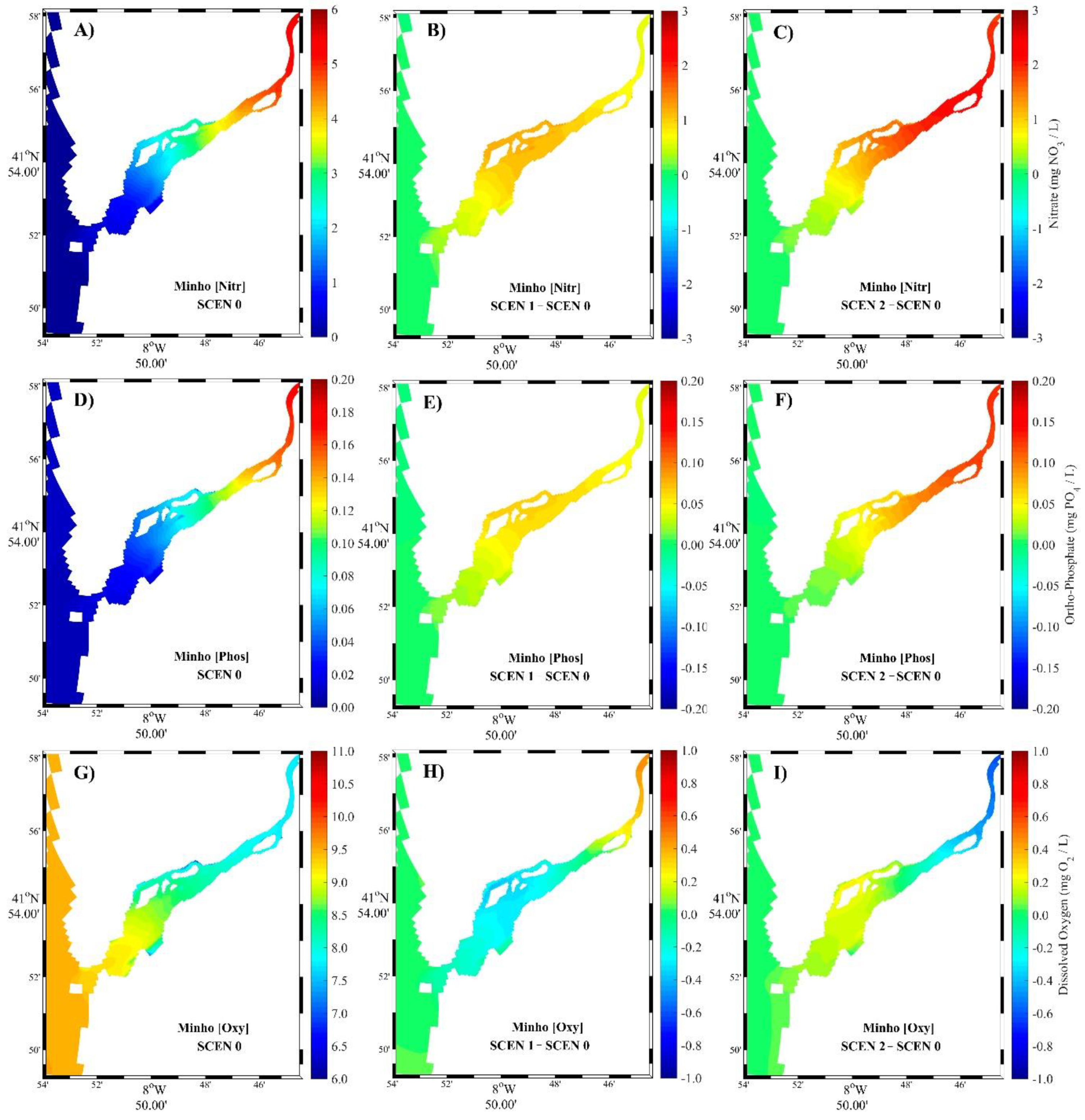
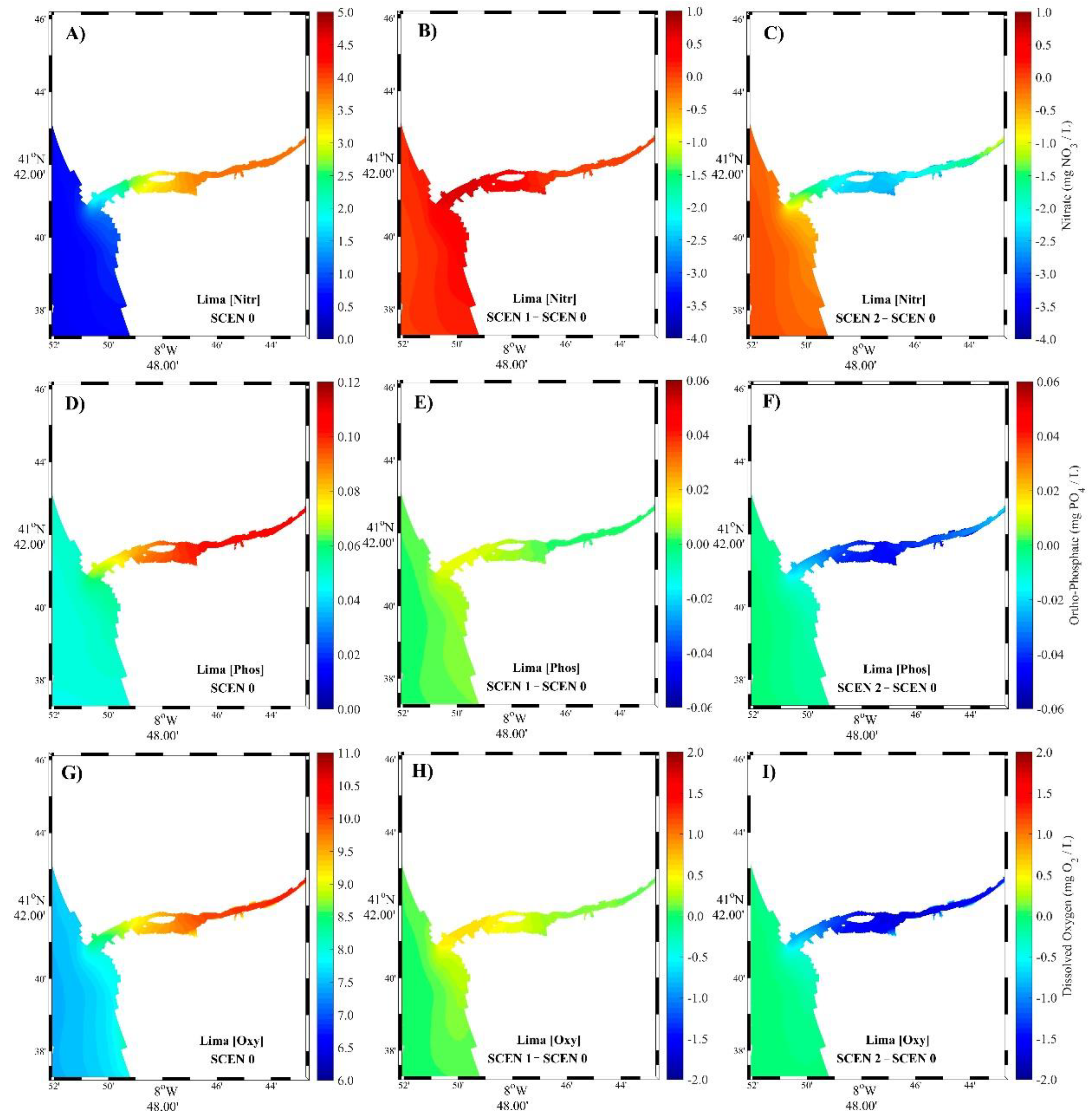
3.2. Model Scenarios
3.2.1. Summer Season
3.2.2. Winter Season
4. Discussion
4.1. Model Calibration and Validation
4.2. Model Scenarios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glamore, W.C.; Rayner, D.S.; Rahman, P.F. Estuaries and Climate Change; Technical Monograph Prepared for the National Climate Change Adaptation Research Facility; Water Research Laboratory of the School of Civil and Environmental Engineering, UNSW: Sydney, Australia, 2016. [Google Scholar]
- Miranda, L.; Castro, B.; Kjerfve, B. Principios de Oceanografia Física de Estuários; Technical Report; Universidade de São Paulo: São Paulo, Brazil, 2002; 427p. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Nixon, S.W.; Ammerman, J.W.; Atkinson, L.P.; Berounsky, V.M.; Billen, G.; Boicourt, W.C.; Boynton, W.R.; Church, T.M.; Ditoro, D.M.; Elmgren, R.; et al. The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 1996, 35, 141–180. [Google Scholar] [CrossRef]
- Boynton, W.R.; Hagy, J.D.; Cornwell, J.C.; Kemp, W.M.; Greene, S.M.; Owens, M.S.; Baker, J.E.; Larsen, R.K. Nutrient Budgets and Management Actions in the Patuxent River Estuary, Maryland. Estuaries Coasts 2008, 31, 623–651. [Google Scholar] [CrossRef]
- Bukaveckas, P.A.; Isenberg, W.N. Loading, Transformation, and Retention of Nitrogen and Phosphorus in the Tidal Freshwater James River (Virginia). Estuaries Coasts 2013, 36, 1219–1236. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Talley, L.D. Climate change impacts on marine ecosystems. Estuaries 2002, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.E.; Smith, L.; Qian, T.; Dai, A.; Fasullo, J. Estimates of the Global Water Budget and Its Annual Cycle Using Observational and Model Data. J. Hydrometeorol. 2007, 8, 758–769. [Google Scholar] [CrossRef]
- Joehnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, M.P.; Stroom, M.J. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Jones, A.; Haywood, J.; Boucher, O.; Kravitz, B.; Robock, A. Geoengineering by stratospheric SO2 injection: Results from the met Office HadGEM2 climate model and comparison with the Goddard Institute for Space Studies ModelE. Atmos. Chem. Phys. 2010, 10, 5999–6006. [Google Scholar] [CrossRef]
- IPCC. Part A: Global and Sectoral Aspects Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Rosenzweig, C.; Casassa, G.; Karoly, J.D.; Imeson, A.; Liu, C.; Menzel, A.; Rawlins, S.; Root, L.T.; Seguin, B.; Tryjanowski, P. Assessment of observed changes and responses in natural and managed systems. Climate Change 2007: Impacts, Adaptation and Vulnerability. In Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, L.M., Canziani, F.O., Palutikof, P.J., an der Linden, J.P., Hanson, E.C., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 79–131. [Google Scholar]
- Van Vliet, M.T.H.; Zwolsman, J.J.G. Impact of summer droughts on the water quality of the Meuse river. J. Hydrol. 2008, 353, 1–17. [Google Scholar] [CrossRef]
- Forbes, K.A.; Kienzle, S.W.; Coburn, C.A.; Byrne, J.M.; Rasmussen, J. Simulating the hydrological response to predicted climate change on a watershed in southern Alberta, Canada. Clim. Chang. 2011, 105, 555–576. [Google Scholar] [CrossRef]
- Falloon, P.D.; Betts, R.A. The impact of climate change on global river flow in HadGEM1 simulations. Atmos. Sci. Lett. 2006, 7, 62–68. [Google Scholar] [CrossRef]
- Xu, H.; Luo, Y. Climate change and its impacts on river discharge in two climate regions in China. Hydrol. Earth Syst. Sci. 2015, 19, 4609–4618. [Google Scholar] [CrossRef]
- Kennedy, V. Anticipated Effects of Climate Change on Estuarine and Coastal Fisheries. Fisheries 1990, 15, 16–24. [Google Scholar] [CrossRef]
- Ray, G.C.; Hayden, B.P.; Bulger, A.J.; McCormick-Ray, M.G. Effects of global warming on the biodiversity of coastal-marine zones. In Global Warming and Biological Diversity; Peters, R.L., Lovejoy, T.E., Eds.; Yale University Press: New Haven, CT, USA, 1992; pp. 91–104. [Google Scholar]
- Schwartz, F.J. Fishes affected by freshwater inflows and/or marine intrusions in North Carolina. J. Elisha Mitchell Sci. Soc. 1998, 114, 173–189. [Google Scholar]
- Wetz, M.S.; Yoskowitz, D.W. An ‘extreme’ future for estuaries? Effects of extreme climatic events on estuarine water quality and ecology. Mar. Pollut. Bull. 2013, 69, 7–18. [Google Scholar] [CrossRef]
- Hickel, W.; Mangelsdorf, P.; Berg, J. The human impact in the German Bight: Eutrophication during three decades (1962–1991). Helgoländer Meeresunters. 1993, 47, 243–263. [Google Scholar] [CrossRef]
- Voynova, Y.G.; Sharp, J.H. Anomalous Biogeochemical Response to a Flooding Event in the Delaware Estuary: A Possible Typology Shift Due to Climate Change. Estuaries Coasts 2012, 35, 943–958. [Google Scholar] [CrossRef]
- Wiltshire, K.H.; Manly, B.F.J. The warming trend at Helgoland Roads, North Sea: Phytoplankton response. Helgol. Mar. Res. 2004, 58, 269–273. [Google Scholar] [CrossRef]
- Luterbacher, J.; Werner, J.P.; Smerdon, J.E.; Fernández-Donado, L.; González-Rouco, F.J.; Barriopedro, D.; Ljungqvist, F.C.; Büntgen, U.; Zorita, E.; Wagner, S.; et al. European summer temperatures since Roman times. Environ. Res. Lett. 2016, 11, 024001. [Google Scholar] [CrossRef]
- Statham, P.J. Nutrients in estuaries—An overview and the potential impacts of climate change. Sci. Total Environ. 2012, 434, 213–227. [Google Scholar] [CrossRef]
- Amon, R.M.W.; Benner, R. Photochemical and microbial consumption of dissolved organic carbon and dissolved oxygen in the Amazon River system. Geochim. Cosmochim. Acta 1996, 60, 1783–1792. [Google Scholar] [CrossRef]
- Lange, R.; Staaland, H.; Mostad, A. The effect of salinity and temperature on solubility of oxygen and respiratory rate in oxygen-dependent marine invertebrates. J. Exp. Mar. Biol. Ecol. 1972, 9, 217–229. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Kattwinkel, M.; Kühne, J.-V.; Foit, K.; Liess, M. Climate change, agricultural insecticide exposure, and risk for freshwater communities. Ecol. Appl. 2011, 21, 2068–2081. [Google Scholar] [CrossRef]
- Hooper, M.J.; Ankley, G.T.; Cristol, D.A.; Maryoung, L.A.; Noyes, P.D.; Pinkerton, K.E. Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ. Toxicol. Chem. 2013, 32, 32–48. [Google Scholar] [CrossRef]
- Preece, R.M.; Jones, H.A. The effect of Keepit Dam on the temperature regime of the Namoi River, Australia. River Res. Appl. 2002, 18, 397–414. [Google Scholar] [CrossRef]
- Cumming, G.S. The impact of low-head dams on fish species richness in Wisconsin, USA. Ecol. Appl. 2004, 14, 1495–1506. [Google Scholar] [CrossRef]
- Bredenhand, E.; Samways, M.J. Impact of a dam on benthic macroinvertebrates in a small river in a biodiversity hotspot: Cape Floristic Region, South Africa. J. Insect Conserv. 2009, 13, 297–307. [Google Scholar] [CrossRef]
- Wei, G.; Yang, Z.; Cui, B.; Li, B.; Chen, H.; Bai, J.; Dong, S. Impact of Dam Construction on Water Quality and Water Self-Purification Capacity of the Lancang River, China. Water Resour. Manag. 2009, 23, 1763–1780. [Google Scholar] [CrossRef]
- Fagherazzi, S.; Kirwan, M.L.; Mudd, S.M.; Guntenspergen, G.R.; Temmerman, S.; D’Alpaos, A.; van de Koppel, J.; Rybczyk, J.M.; Reyes, E.; Craft, C.; et al. Numerical models of salt marsh evolution: Ecological, geomorphic, and climatic factors. Rev. Geophys. 2012, 50. [Google Scholar] [CrossRef]
- Mieszkowska, N.; Firth, L.; Bentley, M. Impacts of climate change on intertidal habitats. MCCIP Sci. Rev. 2013, 2013, 180–192. [Google Scholar]
- Vargas, C.I.C.; Vaz, N.; Dias, J.M. An evaluation of climate change effects in estuarine salinity patterns: Application to Ria de Aveiro shallow water system. Estuar. Coast. Shelf Sci. 2017, 189, 33–45. [Google Scholar] [CrossRef]
- Mateus, M.; Vaz, N.; Neves, R. A process-oriented model of pelagic biogeochemistry for marine systems. Part II: Application to a mesotidal estuary. J. Mar. Syst. 2012, 94, 90–101. [Google Scholar] [CrossRef]
- Sousa, M.C. Modelling the Minho River Plume Intrusion into the Rias Baixas; Aveiro University and Porto University: Aveiro, Portugal, 2013. [Google Scholar]
- Pan, G.; Chai, F.; Tang, D.; Wang, D. Marine phytoplankton biomass responses to typhoon events in the South China Sea based on physical-biogeochemical model. Ecol. Modell. 2017, 356, 38–47. [Google Scholar] [CrossRef]
- Mattern, J.P.; Song, H.; Edwards, C.A.; Moore, A.M.; Fiechter, J. Data assimilation of physical and chlorophyll a observations in the California current system using two biogeochemical models. Ocean Model. 2017, 109, 55–71. [Google Scholar] [CrossRef]
- Neves, R. Numerical models as decision support tools in coastal areas. In Assessment of the Fate and Effects of Toxic Agents on Water Resources; Springer: Dordrecht, The Netherlands, 2007; pp. 171–195. [Google Scholar]
- APA Plano de Gestão da Região Hidrográfica do Minho e Lima—RH1—Relatório técnico—Comissão Europeia; Technical Report; Agência Portuguesa do Ambiente: Amadora, Portugal, 2012; 178p.
- PBH. Plano de Bacia Hidrográfica do Rio Minho—Relatório Final; Technical Report; Ministério do Ambiente e do Ordenamento do Território, Instituto da Água, Instituto Português: Lisboa, Portugal, 2001; 1704p. [Google Scholar]
- Delgado, A.; Taveira-Pinto, F.; Silva, R. Hydrodynamic and morphodynamic preliminary simulation of river Minho estuary. In Proceedings of the 6a Jornadas de Hidráulica, Recursos Hídricos e Ambiente; Faculty of Engineering, Uiversity of Porto: Porto, Portugal, 2011; pp. 113–126. [Google Scholar]
- Sousa, R.; Guilhermino, L.; Antunes, C. Molluscan fauna in the freshwater tidal area of the river Minho estuary, NW of Iberian Peninsula. Ann. Limnol. Int. J. Limnol. 2005, 41, 141–147. [Google Scholar] [CrossRef]
- Freitas, V.; Costa-Dias, S.; Campos, J.; Bio, A.; Santos, P.; Antunes, C. Patterns in abundance and distribution of juvenile flounder, Platichthys flesus, in Minho estuary (NW Iberian Peninsula). Aquat. Ecol. 2009, 43, 1143. [Google Scholar] [CrossRef]
- Azevedo, D.A.; Lacorte, S.; Viana, P.; Barceló, D. Occurrence of Nonylphenol and Bisphenol-A in Surface Waters from Portugal. J. Braz. Chem. Soc. 2001, 12, 532–537. [Google Scholar] [CrossRef]
- Castro, M.; Santos, M.M.; Monteiro, N.M.; Vieira, N. Measuring lysosomal stability as an effective tool for marine coastal environmental monitoring. Mar. Environ. Res. 2004, 58, 741–745. [Google Scholar] [CrossRef]
- Santos, S.; Vilar, V.J.P.; Alves, P.; Boaventura, R.A.R.; Botelho, C. Water quality in Minho/Miño River (Portugal/Spain). Environ. Monit. Assess. 2013, 185, 3269–3281. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Evaluation of distribution, mobility and binding behaviour of heavy metals in surficial sediments of Louro river (Galicia, Spain) using chemometric analysis: A case study. Sci. Total Environ. 2004, 330, 115–129. [Google Scholar] [CrossRef] [PubMed]
- SNIRH Sistema Nacional de Informação de Recursos Hídricos. Available online: www.snirh.pt (accessed on 20 January 2018).
- Vale, L.M.; Dias, J.M. The effect of tidal regime and river flow on the hydrodynamics and salinity structure of the Lima estuary: Use of a numerical model to assist on estuary classification. J. Coast. Res. 2011, 64, 1604–1608. [Google Scholar]
- Barbosa, A.M.; Lousada, S.; Haie, N. Análise da Qualidade das Águas Superficiais de Ponte de Lima; Technical Report; Universidade do Minho: Braga, Portugal, 2004; 14p. [Google Scholar]
- Guimarães, L.; Gravato, C.; Santos, J.; Monteiro, L.S.; Guilhermino, L. Yellow eel (Anguilla anguilla) development in NW Portuguese estuaries with different contamination levels. Ecotoxicology 2009, 18, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Cairrão, E.; Couderchet, M.; Soares, A.M.V.M.; Guilhermino, L. Glutathione-S-transferase activity of Fucus spp. as a biomarker of environmental contamination. Aquat. Toxicol. 2004, 70, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Zacarias, N. Influência da Batimetria e do Caudal Fluvial na Propagação da Maré no Estuário do rio Minho; Technical Report; Universidade de Évora: Évora, Portugal, 2007; 81p. [Google Scholar]
- Vieira, L.R.; Guilhermino, L.; Morgado, F. Zooplankton structure and dynamics in two estuaries from the Atlantic coast in relation to multi-stressors exposure. Estuar. Coast. Shelf Sci. 2015, 167, 347–367. [Google Scholar] [CrossRef]
- Lesser, G.R.; Roelvink, J.A.; van Kester, J.A.T.M.; Stelling, G.S. Development and validation of a three-dimensional morphological model. Coast. Eng. 2004, 51, 883–915. [Google Scholar] [CrossRef]
- Bonamano, S.; Madonia, A.; Borsellino, C.; Stefanì, C.; Caruso, G.; De Pasquale, F.; Piermattei, V.; Zappalà, G.; Marcelli, M. Modeling the dispersion of viable and total Escherichia coli cells in the artificial semi-enclosed bathing area of Santa Marinella (Latium, Italy). Mar. Pollut. Bull. 2015, 95, 141–154. [Google Scholar] [CrossRef]
- Gils, J.; Ouboter, M.R.L.; De Rooij, N.M. Modelling of water and sediment quality in the Scheldt estuary. Neth. J. Aquat. Ecol. 1993, 27, 257–265. [Google Scholar] [CrossRef]
- Deltares. Simulation of Multi-Dimensional Hydrodynamic Flows and Transport Phenomena, Including Sediments [User Manual]; Deltares: Delft, The Netherlands, 2016; 702p. [Google Scholar]
- Deltares. Water Quality and Aquatic Ecology [User Manual]; Deltares: Delft, The Netherlands, 2016; 394p. [Google Scholar]
- Macmillan, D.S.; Beckley, B.D.; Fang, P. Monitoring the TOPEX and Jason-1 microwave tadiometers with GPS and VLBI Wet Zenith path delays. Mar. Geod. 2004, 27, 703–716. [Google Scholar] [CrossRef]
- Whitehead, P.; Bussi, G.; Hossain, M.A.; Dolk, M.; Das, P.; Comber, S.; Peters, R.; Charles, K.J.; Hope, R.; Hossain, M.S. Restoring water quality in the polluted Turag-Tongi-Balu river system, Dhaka: Modelling nutrient and total coliform intervention strategies. Sci. Total Environ. 2018, 631–632, 223–232. [Google Scholar] [CrossRef]
- Tomić, A.Š.; Antanasijević, D.; Ristić, M.; Perić-Grujić, A.; Pocajt, V. A linear and non-linear polynomial neural network modeling of dissolved oxygen content in surface water: Inter- and extrapolation performance with inputs’ significance analysis. Sci. Total Environ. 2018, 610–611, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, C.; Kuang, C.; Kolditz, O.; Shao, H.; Zhang, J.; Liu, H. A water quality model applied for the rivers into the Qinhuangdao coastal water in the Bohai Sea, China. J. Hydrodyn. Ser. B 2016, 28, 905–913. [Google Scholar] [CrossRef]
- Kori, B.B.; Manojkumar, B.; Mise, S.R. Application of MIXPIPOX Model to Karanja River Water Quality. Procedia Earth Planet. Sci. 2015, 11, 260–265. [Google Scholar] [CrossRef][Green Version]
- Kroeze, C.; Gabbert, S.; Hofstra, N.; Koelmans, A.A.; Li, A.; Löhr, A.; Ludwig, F.; Strokal, M.; Verburg, C.; Vermeulen, L.; et al. Global modelling of surface water quality: A multi-pollutant approach. Curr. Opin. Environ. Sustain. 2016, 23, 35–45. [Google Scholar] [CrossRef]
- Terry, J.A.; Sadeghian, A.; Baulch, H.M.; Chapra, S.C.; Lindenschmidt, K.-E. Challenges of modelling water quality in a shallow prairie lake with seasonal ice cover. Ecol. Modell. 2018, 384, 43–52. [Google Scholar] [CrossRef]
- Bui, H.H.; Ha, N.H.; Nguyen, T.N.D.; Nguyen, A.T.; Pham, T.T.H.; Kandasamy, J.; Nguyen, T.V. Integration of SWAT and QUAL2K for water quality modeling in a data scarce basin of Cau River basin in Vietnam. Ecohydrol. Hydrobiol. 2019, 19, 210–223. [Google Scholar] [CrossRef]
- Cebe, K.; Balas, L. Water quality modelling in kaş bay. Appl. Math. Model. 2016, 40, 1887–1913. [Google Scholar] [CrossRef]
- Doan, Q.T.; Nguyen, T.M.L.; Quach, T.T.T.; Tran, A.P.; Nguyen, C.D. Assessment of water quality in coastal estuaries under the impact of an industrial zone in Hai Phong, Vietnam. Phys. Chem. Earth Parts A/B/C 2019. [Google Scholar] [CrossRef]
- Feng, T.; Wang, C.; Hou, J.; Wang, P.; Liu, Y.; Dai, Q.; Yang, Y.; You, G. Effect of inter-basin water transfer on water quality in an urban lake: A combined water quality index algorithm and biophysical modelling approach. Ecol. Indic. 2018, 92, 61–71. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Keupers, I.; Willems, P. Conceptual river water quality model with flexible model structure. Environ. Model. Softw. 2018, 104, 102–117. [Google Scholar] [CrossRef]
- Salleh, S.H.M.; Ahmad, A.; Mohtar, W.H.M.W.; Lim, C.H.; Maulud, K.N.A. Effect of projected sea level rise on the hydrodynamic and suspended sediment concentration profile of tropical estuary. Reg. Stud. Mar. Sci. 2018, 24, 225–236. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; He, Z.; Kalhoro, N.A.; Xu, D. Numerical modelling study of seawater intrusion in Indus River Estuary, Pakistan. Ocean Eng. 2019, 184, 74–84. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, Y.; Sun, T.; Yang, Z.; Hao, Y. Coupled hydrodynamic and ecological simulation for prognosticating land reclamation impacts in river estuaries. Estuar. Coast. Shelf Sci. 2018, 202, 290–301. [Google Scholar] [CrossRef]
- Dias, J.M.; Lopes, J.F. Implementation and assessment of hydrodynamic, salt and heat transport models: The case of Ria de Aveiro lagoon (Portugal). Environ. Model. Softw. 2006, 21, 1–15. [Google Scholar] [CrossRef]
- Dias, J.M.; Sousa, M.C.; Bertin, X.; Fortunato, A.B.; Oliveira, A. Numerical modeling of the impact of the Ancão inlet relocation (Ria Formosa, Portugal). Environ. Model. Softw. 2009, 24, 711–725. [Google Scholar] [CrossRef]
- Atwater, M.A.; Ball, J.T. Computation of IR sky temperature and comparison with surface temperature. Sol. Energy 1978, 21, 211–216. [Google Scholar] [CrossRef]
- Radach, G.; Moll, A. Review of three-dimensional ecological modeling related to the North Sea shelf system. Part II: Model validation and data needs. Oceanogr. Mar. Biol. 2006, 44, 1–60. [Google Scholar]
- Oliveira, V.H. Coupled Modelling of the Minho and Lima Estuaries: Biochemical Response to Extreme Events and Interaction of Plumes Estuarine. Master’s Thesis, Aveiro University, Aveiro, Portugal, 2018; 132p. [Google Scholar]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.; Engelbrecht, F.; et al. Chapter 3: Impacts of 1.5 °C global warming on natural and human systems. In Global Warming of 1.5 °C; Geneva, Switzerland, 2018; pp. 175–311. Available online: http://pure.iiasa.ac.at/15518 (accessed on 13 November 2019).
- APA. Parte 2—Caracterização e Diagnóstico (Anexos)—Plano de Gestão da Região Hidrográfica do Minho e Lima (RH1); Technical Report; Agência Portuguesa do Ambiente: Amadora, Portugal, 2015; 92p. [Google Scholar]
- BOE. Anexo III. Plan Hidrológico de la Parte Española de la DH del MIÑO-Sil (2015–2021); Technical Report; Boletín Oficial del Estado: Catalonia, Spain, 2009; 28p. [Google Scholar]
- Reis, J.L.; Martinho, A.S.; Pires-Silva, A.; Silva, A.J. Assessing the influence of the river discharge on the Minho estuary tidal regime. J. Coast. Res. 2009, II, 1405–1409. [Google Scholar]
- Nagisetty, R.M.; Flynn, K.F.; Uecker, D. Dissolved oxygen modeling of effluent-dominated macrophyte-rich Silver Bow Creek. Ecol. Modell. 2019, 393, 85–97. [Google Scholar] [CrossRef]
- Suarez, V.V.C.; Brederveld, R.J.; Fennema, M.; Moreno-Rodenas, A.; Langeveld, J.; Korving, H.; Schellart, A.N.A.; Shucksmith, J. Evaluation of a coupled hydrodynamic-closed ecological cycle approach for modelling dissolved oxygen in surface waters. Environ. Model. Softw. 2019, 119, 242–257. [Google Scholar] [CrossRef]
- Vaz, L.; Mateus, M.; Serôdio, J.; Dias, J.M.; Vaz, N. Primary production of the benthic microalgae in the bottom sediments of Ria de Aveiro lagoon. J. Coast. Res. 2016, 75, 178–182. [Google Scholar] [CrossRef]
- Wangersky, P.J. Estuaries; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Abrahams, C.; Brown, L.; Dale, K.; Edwards, F.; Jeffries, M.; Klaar, M.; Ledger, M.; May, L.; Milner, A.; Murphy, J.; et al. The Impact of Extreme Events on Freshwater Ecosystems. In Ecological Issues; Jones, I., Ed.; British Ecological Society: London, UK, 2013; p. 68. [Google Scholar]
- Bejda, A.J.; Studholme, A.L.; Olla, B.L. Behavioral responses of red hake,Urophycis chuss, to decreasing concentrations of dissolved oxygen. Environ. Biol. Fishes 1987, 19, 261–268. [Google Scholar] [CrossRef]
- Roman, M.R.; Gauzens, A.L.; Rhinehart, W.K.; White, J.R. Effects of low oxygen waters on Chesapeake Bay zooplankton. Limnol. Oceanogr. 1993, 38, 1603–1614. [Google Scholar] [CrossRef]
- Breitburg, D.L. Behavioral response of fish larvae to low dissolved oxygen concentrations in a stratified water column. Mar. Biol. 1994, 120, 615–625. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Adamack, A.; Rose, K.A.; Kolesar, S.E.; Decker, B.; Purcell, J.E.; Keister, J.E.; Cowan, J.H. The pattern and influence of low dissolved oxygen in the Patuxent River, a seasonally hypoxic estuary. Estuaries 2003, 26, 280–297. [Google Scholar] [CrossRef]
- Lloyd, R. Effect of Dissolved Oxygen Concentrations on the Toxicity of Several Poisons to Rainbow Trout (Salmo Gairdnerii Richardson). J. Exp. Biol. 1961, 38, 447–455. [Google Scholar]
- Dauer, D.M.; Rodi, A.J.; Ranasinghe, J.A. Effects of low dissolved oxygen events on the macrobenthos of the lower Chesapeake Bay. Estuaries 1992, 15, 384–391. [Google Scholar] [CrossRef]
- Smith, K.J.; Able, K.W. Dissolved oxygen dynamics in salt marsh pools and its potential impacts on fish assemblages. Mar. Ecol. Prog. Ser. 2003, 258, 223–232. [Google Scholar] [CrossRef]
- Stevens, P.W.; Blewett, D.A.; Casey, J.P. Short-term effects of a low dissolved oxygen event on estuarine fish assemblages following the passage of hurricane Charley. Estuaries Coasts 2006, 29, 997–1003. [Google Scholar] [CrossRef]





| Substances | Processes |
|---|---|
| Algae [Non-Diatoms] | Potential minimum dissolved oxygen [OXYMin] |
| Net primary production and mortality [GroMrt_Gre] | |
| Dissolved Oxygen [OXY] | Potential minimum dissolved oxygen [OXYMin] |
| Uptake of nutrients by growth of alga [NutUpt_alg] | |
| Variation of primary production within day [VAROXY] | |
| Horizontal dispersion velocity dependence [HDisperVel] | |
| Denitrification in water column [Denwat_NO3] | |
| Nitrification of ammonium [Nitrif_NH4] | |
| Reaeration of oxygen [RearOXY] | |
| Composition [Compos] | |
| Ammonium [NH4] | Uptake of nutrients by growth of alga [NutUpt_alg] |
| Nitrification of ammonium [Nitrif_NH4] | |
| Composition [Compos] | |
| Nitrate [NO3] | Uptake of nutrients by growth of alga [NutUpt_alg] |
| Denitrification in water column [DenWat_NO3] | |
| Nitrification of ammonium [Nitrif_NH4] | |
| Composition [Compos] | |
| Orthophosphate [PO4] | Uptake of nutrients by growth of alga [NutUpt_alg] |
| Ad(de)sorption of orthophosphorus to inorganic matter | |
| Composition [Compos] |
| Estuary | Stations | Root-Mean-Square Error (RMSE) (m) | SKILL | Bias (m) |
|---|---|---|---|---|
| Minho | Barra | 0.111 | 0.994 | −0.0068 |
| Caminha | 0.072 | 0.997 | −8.8287 × 10−4 | |
| Seixas | 0.201 | 0.974 | 0.0066 | |
| Vila Nova de Cerveira | 0.048 | 0.998 | −4.203 × 10−4 | |
| Segadães | 0.056 | 0.997 | −4.2299 × 10−4 | |
| Lima | Viana do Castelo | 0.046 | 0.999 | −1.1767 × 10−4 |
| Estuary | Variable | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSE | CF | RMSE | CF | RMSE | CF | RMSE | CF | RMSE | CF | RMSE | CF | RMSE | CF | ||
| Minho | Water Temperature | 1.34 | 0.30 | 1.00 | 0.21 | 0.81 | 0.20 | 0.87 | 0.17 | 0.78 | 0.17 | 1.01 | 0.25 | 1.45 | 0.26 |
| Salinity | 4.12 | 0.27 | 1.16 | 0.05 | 0.54 | 0.02 | 0.37 | 0.02 | 0.27 | 0.01 | 0.40 | 0.02 | 0.59 | 0.04 | |
| Dissolved Oxygen | 1.72 | 3.04 | 1.57 | 2.75 | 1.42 | 2.69 | 1.58 | 2.35 | 1.58 | 1.88 | 1.40 | 1.73 | 1.52 | 1.81 | |
| Nitrate | 1.03 | 1.58 | 0.91 | 1.05 | 0.82 | 0.97 | 0.97 | 1.08 | 1.20 | 1.50 | 0.98 | 1.64 | 0.92 | 1.58 | |
| Orthophosphate | 0.05 | 0.66 | 0.09 | 0.83 | 0.14 | 0.80 | 0.13 | 0.73 | 0.12 | 0.73 | 0.09 | 0.57 | 0.08 | 0.75 | |
| Lima | Water Temperature | 0.63 | 0.17 | 0.74 | 0.20 | 0.56 | 0.12 | 0.65 | 0.16 | 0.65 | 0.15 | 0.77 | 0.19 | 0.96 | 0.24 |
| Salinity | 1.17 | 0.10 | 1.68 | 0.16 | 1.71 | 0.13 | 1.99 | 0.16 | 2.73 | 0.30 | 2.93 | 0.40 | 2.60 | 0.28 | |
| Dissolved oxygen | 1.68 | 2.65 | 1.42 | 2.13 | 1.52 | 2.36 | 1.43 | 1.75 | 1.46 | 1.65 | 1.39 | 1.69 | 1.32 | 1.34 | |
| Nitrate | 0.73 | 1.98 | 0.81 | 2.02 | 0.84 | 1.86 | 0.97 | 1.33 | 1.08 | 2.11 | 1.51 | 1.69 | 1.05 | 2.01 | |
| Orthophosphate | 0.05 | 1.09 | 0.05 | 1.10 | 0.05 | 1.07 | 0.17 | 0.56 | 0.20 | 0.71 | 0.17 | 0.80 | 0.13 | 0.98 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, V.H.; Sousa, M.C.; Morgado, F.; Dias, J.M. Modeling the Impact of Extreme River Discharge on the Nutrient Dynamics and Dissolved Oxygen in Two Adjacent Estuaries (Portugal). J. Mar. Sci. Eng. 2019, 7, 412. https://doi.org/10.3390/jmse7110412
Oliveira VH, Sousa MC, Morgado F, Dias JM. Modeling the Impact of Extreme River Discharge on the Nutrient Dynamics and Dissolved Oxygen in Two Adjacent Estuaries (Portugal). Journal of Marine Science and Engineering. 2019; 7(11):412. https://doi.org/10.3390/jmse7110412
Chicago/Turabian StyleOliveira, Vítor H., Magda C. Sousa, Fernando Morgado, and João M. Dias. 2019. "Modeling the Impact of Extreme River Discharge on the Nutrient Dynamics and Dissolved Oxygen in Two Adjacent Estuaries (Portugal)" Journal of Marine Science and Engineering 7, no. 11: 412. https://doi.org/10.3390/jmse7110412
APA StyleOliveira, V. H., Sousa, M. C., Morgado, F., & Dias, J. M. (2019). Modeling the Impact of Extreme River Discharge on the Nutrient Dynamics and Dissolved Oxygen in Two Adjacent Estuaries (Portugal). Journal of Marine Science and Engineering, 7(11), 412. https://doi.org/10.3390/jmse7110412