Benthic Nutrient Fluxes from Mangrove Sediments of an Anthropogenically Impacted Estuary in Southern China
Abstract
:1. Introduction
2. Materials and Methods
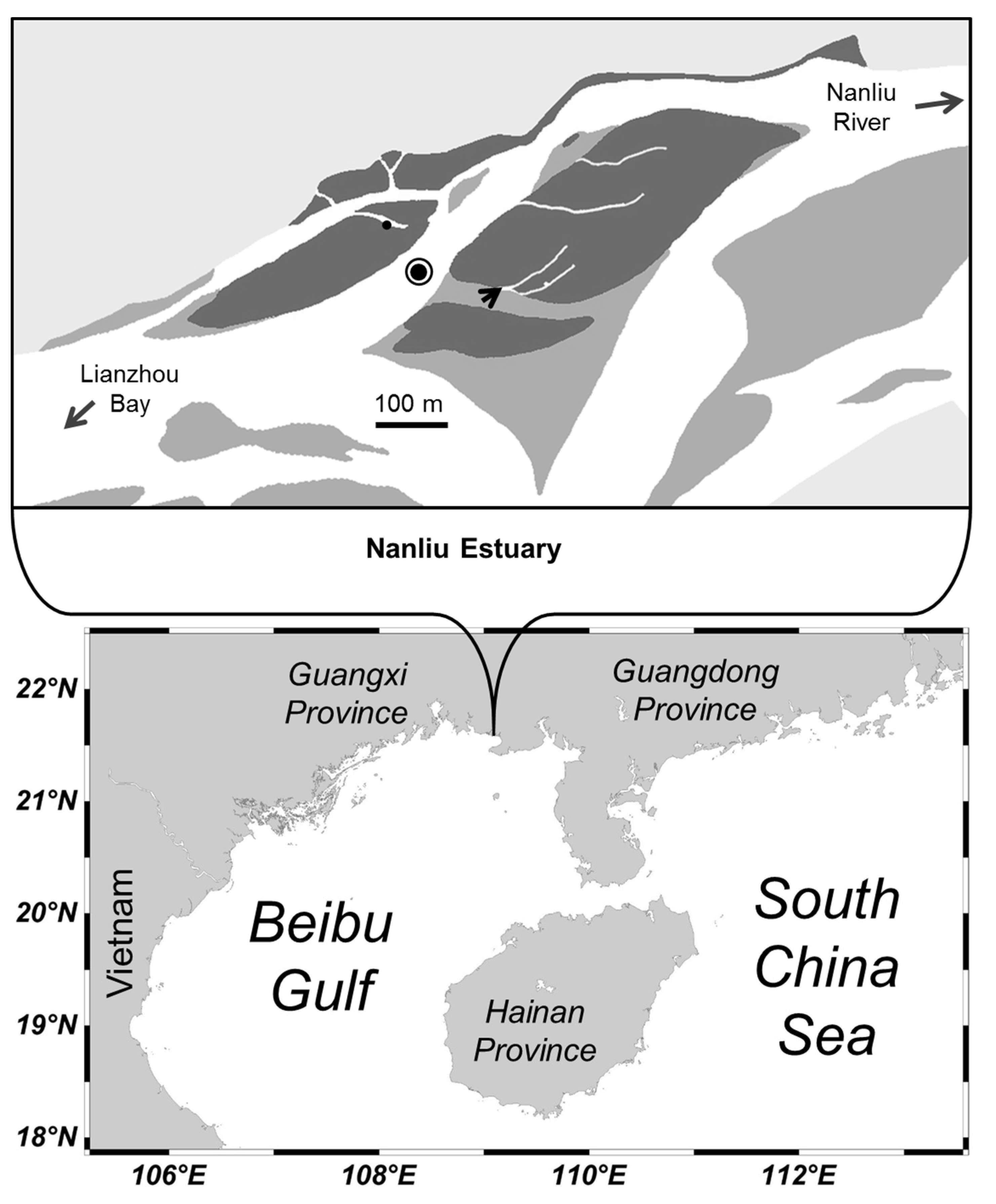
2.1. Study Site
2.2. Field Sampling
2.3. Core Incubation Experiment
| Initial Concentrations [μM] | Temperature [°C] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Date | NO3− | NO2− | NH4+ | PO43− | Si | DOC | O2 | Monthly Average | During Incubation |
| 16 March 2011 | 197.0 | 5.4 | 9.0 | 2.8 | 76.5 | 383.0 | 308.5 | 15.3 | 16.0 |
| 3 October 2011 | 84.9 | 4.3 | 19.1 | 1.5 | 94.2 | 371.5 | 223.1 | 25.6 | 25.0 |
2.4. Sample Analysis
3. Results
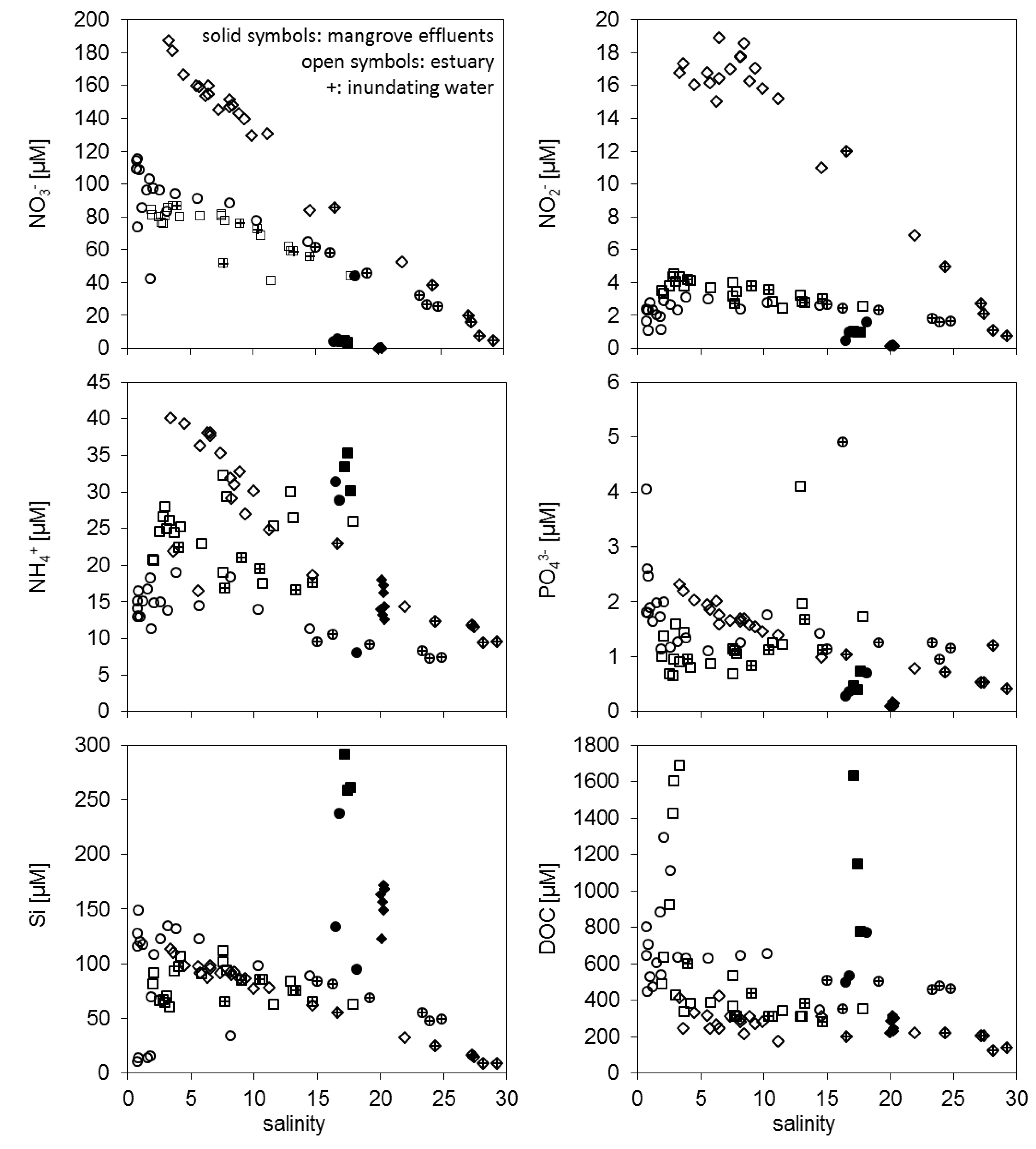
3.1. Concentration Differences between Estuarine Water and Mangrove Effluents
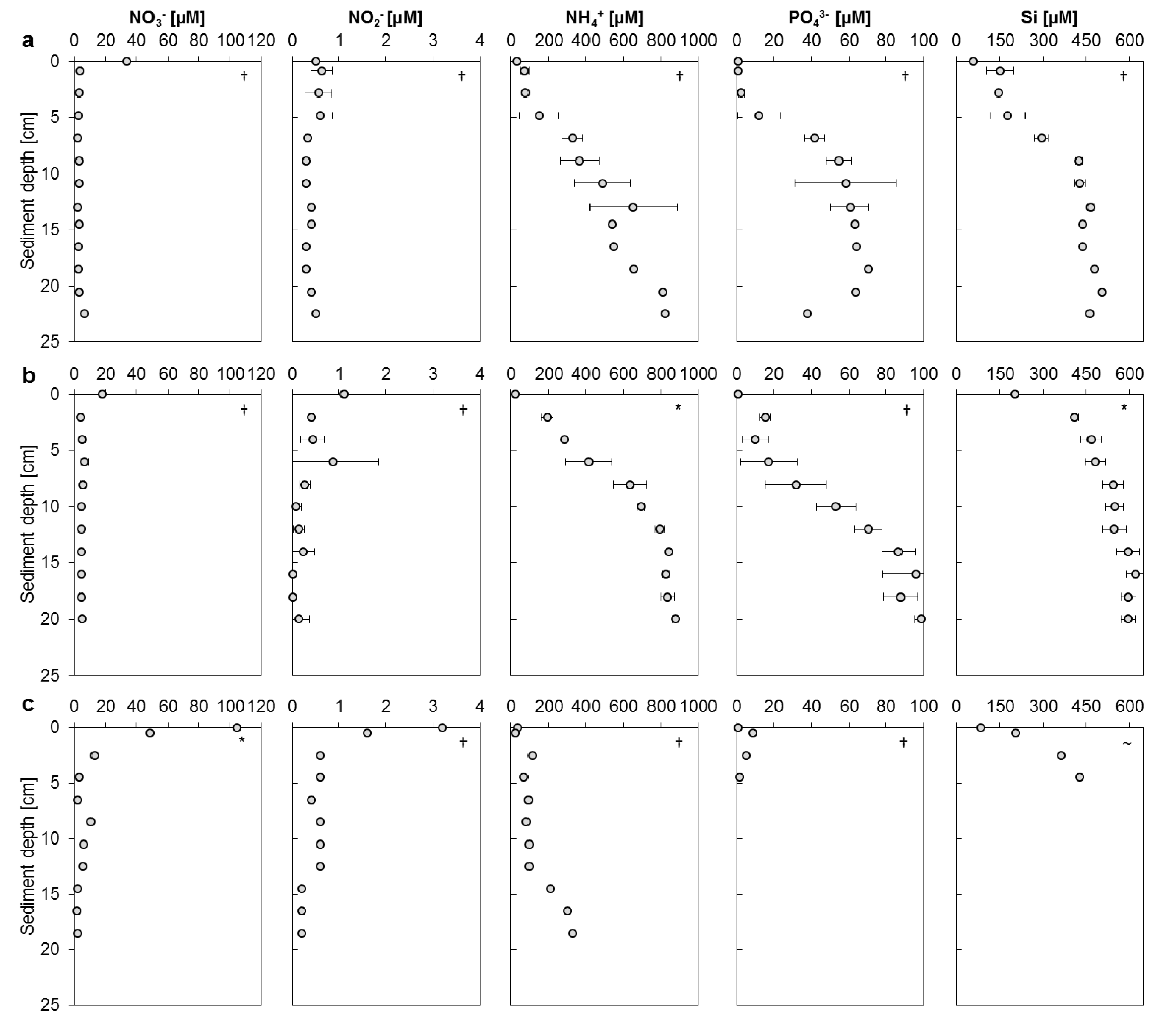
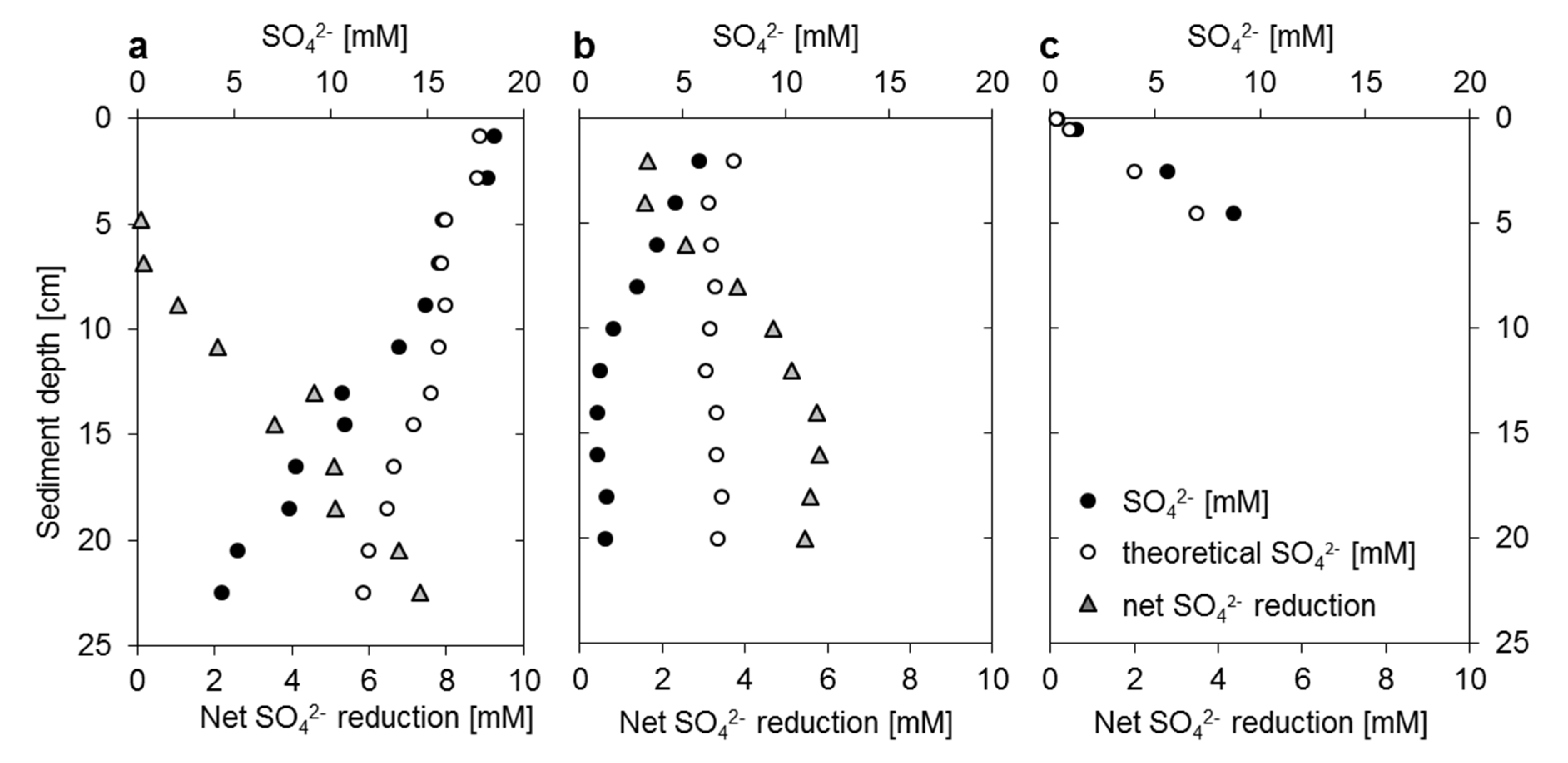
3.2. Mangrove Sediment Pore Water Profiles
3.3. Mangrove Sediment–Water Fluxes
3.3.1. Dissolved Oxygen
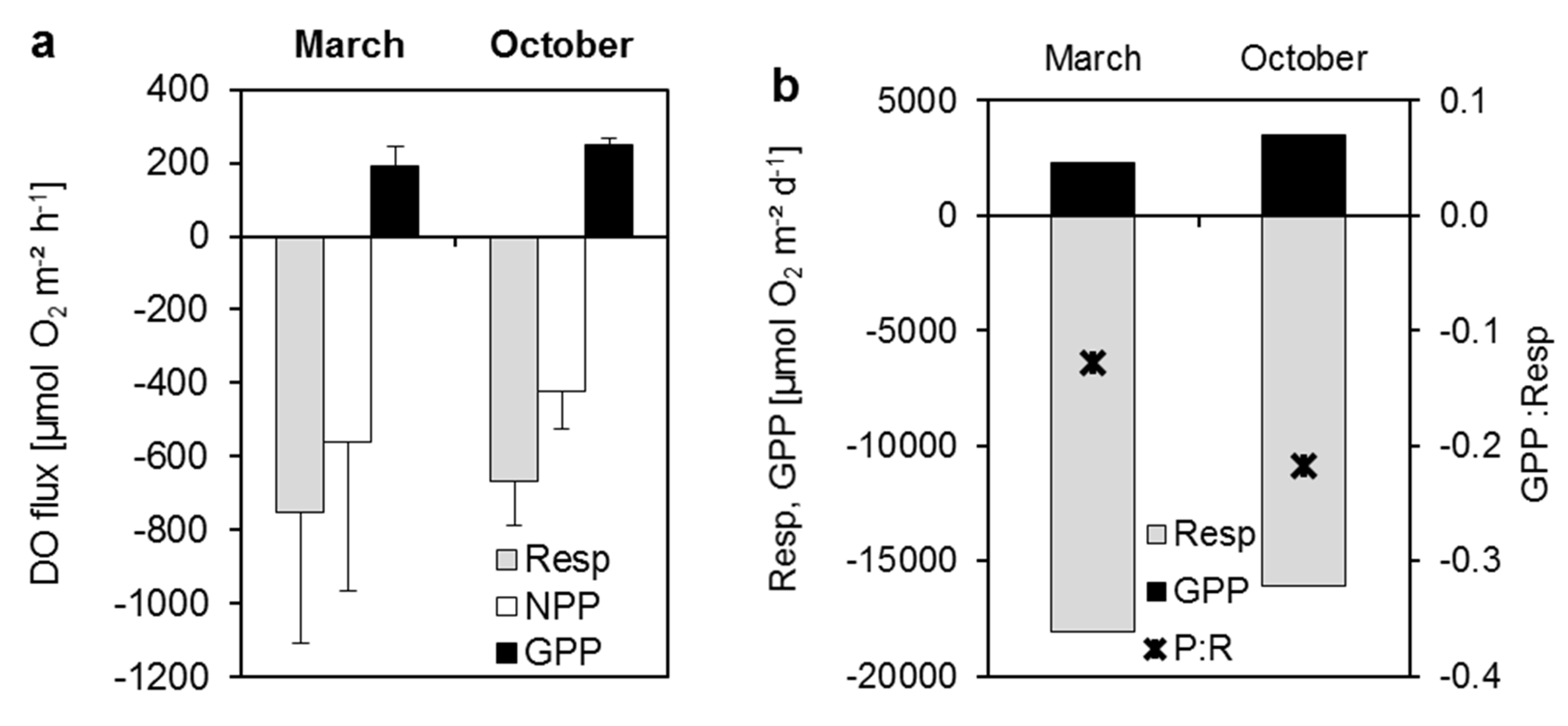
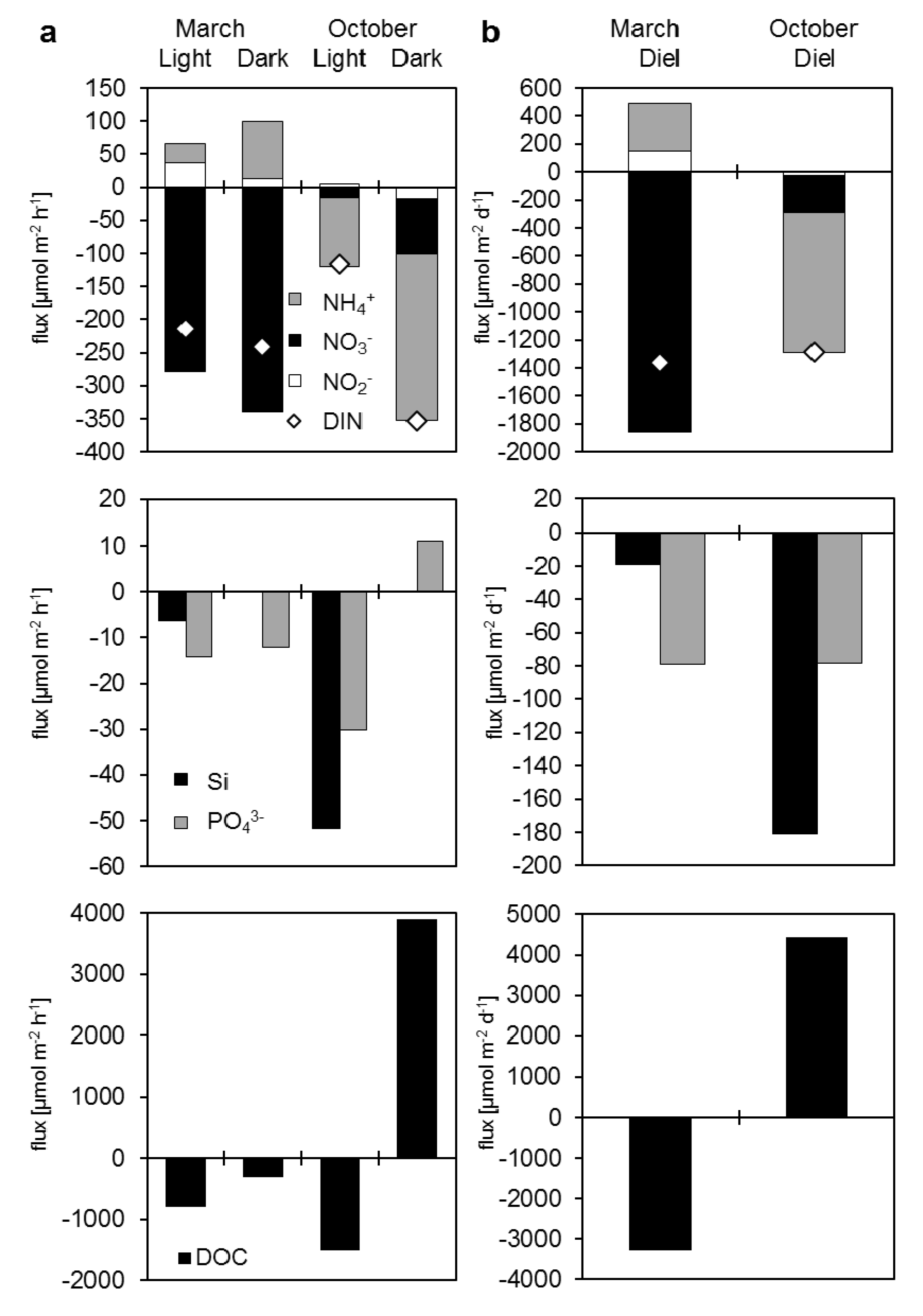
3.3.2. Dissolved Nutrient and Organic Carbon Fluxes
| Hourly flux [μmol m−2 h−1] | Daily flux [μmol m−2 day−1] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | Spring | Summer | Spring | Summer | ||||||
| Respiration | −499.4 | −669.3 | −11985.1 | −16062.2 | ||||||
| NPP | −270.5 | −420.8 | −9238.4 | −12583.2 | ||||||
| GPP | 228.9 | 248.5 | 2746.7 | 3478.9 | ||||||
| CO2 efflux | 499.4 | 669.3 | 11985.1 | 16062.2 | ||||||
| CO2 influx | −228.9 | −248.5 | −2746.7 | −3478.9 | ||||||
| CO2 net flux | 384.9 | 524.3 | 9238.4 | 12583.2 | ||||||
| N demand | 22.9 | 24.8 | 274.7 | 347.9 | ||||||
| b | ||||||||||
| Measured fluxes | Light | Dark | Light | Dark | Diel | Diel | ||||
| NO3− | −279.1 | −339.6 | −16.3 | −83.9 | −1856.1 | −266.7 | ||||
| NO2− | 37.0 | 13.1 | 4.5 | −17.1 | 150.4 | −27.1 | ||||
| NH4+ | 28.4 | 85.9 | −104.3 | −251.8 | 342.9 | −994.3 | ||||
| DIN | −213.7 | −240.6 | −116.1 | −352.7 | −1362.7 | −1288.1 | ||||
| PO43− | −14.3 | −12.1 | −30.2 | 11.1 | −79.1 | −78.0 | ||||
| Si | −6.3 | 0.0 | −51.7 | 0.0 | −19.0 | −181.1 | ||||
| DOC | −790.5 | −305.6 | −1518.4 | 3900.7 | −3288.3 | 4437.3 | ||||
4. Discussion
4.1. Sediment-Dissolved Oxygen Fluxes
4.2. Organic Carbon and Nutrient Fluxes
4.3. Mangrove System Nutrient Dynamics
4.4. Nutrient Filtration by the Mangrove System
| River Discharge | Shrimp Pond Effluents | Mangrove Filtration | ||
|---|---|---|---|---|
| NO3− | [103 mol·day−1] | 2007.8 | 5.3 | −9.6 to −1.4 |
| % of total input | 99.7 | 0.3 | −0.1 to −0.5 | |
| PO43− | [103 mol·day−1] | 33.3 | 0.9 | −0.41 to −0.40 |
| % of total input | 97.4 | 2.6 | −1.2 to −1.2 |
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Conley, D.J.; Schelske, C.L.; Stoermer, E.F. Modification of the Biogeochemical Cycle of Silica with Eutrophication. Mar. Ecol. Prog. Ser. 1993, 101, 179–192. [Google Scholar] [CrossRef]
- Hodgkiss, I.; Ho, K. Are changes in N:P ratios in coastal waters the key to increased red tide blooms? Hydrobiologia 1997, 352, 141–147. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Officer, C.B.; Ryther, J.H. The Possible Importance of Silicon in Marine Eutrophication. Mar. Ecol. Prog. Ser. 1980, 3, 83–91. [Google Scholar] [CrossRef]
- Paerl, H.W. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnol. Oceanogr. 1997, 42, 1154–1165. [Google Scholar] [CrossRef]
- Selman, M.; Greenhalgh, S.; Diaz, R.; Sugg, Z. Eutrophication and Hypoxia in Coastal Areas: A Global Assessment of the State of Knowledge. WRI Policy Note Eutrophication Hypoxia Coast. Areas 2008, 1, 1–6. [Google Scholar]
- Turner, R.E.; Qureshi, N.; Rabalais, N.N.; Dortch, Q.; Justic, D.; Shaw, R.F.; Cope, J. Fluctuating silicate:nitrate ratios and coastal plankton food webs. Proc. Natl. Acad. Sci. USA 1998, 95, 13048–13051. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.C.; Webb, R.M.T. Potential Effects of Runoff, Fluvial Sediment, and Nutrient Discharges on the Coral Reefs of Puerto Rico. J. Coast. Res. 2009, 189–208. [Google Scholar] [CrossRef]
- Corredor, J.E.; Howarth, R.W.; Twilley, R.R.; Morell, J.M. Nitrogen cycling and anthropogenic impact in the tropical interamerican seas. Biogeochemistry 1999, 46, 163–178. [Google Scholar] [CrossRef]
- Burford, M.A.; Alongi, D.M.; McKinnon, A.D.; Trott, L.A. Primary production and nutrients in a tropical macrotidal estuary, Darwin Harbour, Australia. Estuar. Coast. Shelf Sci. 2008, 79, 440–448. [Google Scholar] [CrossRef]
- Valiela, I.; Bowen, J.L.; York, J. Mangrove Forests: One of the World’s Threatened Major Tropical Environments. BioScience 2001, 51, 807–815. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Lee, S.Y. Mangroves of China: A brief review. For. Ecol. Manag. 1997, 96, 241–259. [Google Scholar] [CrossRef]
- Terada, K.; Koibuchi, Y.; Koibuchi, M. Comparison of material fluxes based on observations in different types of estuaries including mangrove area. In Proceedings of the 2010 AGU Ocean Sciences Meeting, Portland, OR, USA, 22–26 February 2010.
- Adame, M.F.; Virdis, B.; Lovelock, C.E. Effect of geomorphological setting and rainfall on nutrient exchange in mangroves during tidal inundation. Mar. Freshw. Res. 2010, 61, 1197–1206. [Google Scholar] [CrossRef]
- Valiela, I.; Cole, M.L. Comparative Evidence that Salt Marshes and Mangroves May Protect Seagrass Meadows from Land-derived Nitrogen Loads. Ecosystems 2002, 5, 92–102. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Tu, Z.; Gao, X.; Wang, W. Maintenance of estuarine water quality by mangroves occurs during flood periods: A case study of a subtropical mangrove wetland. Mar. Pollut. Bull. 2010, 60, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chung, A.; Tam, N.F.Y.; Pi, N.; Wong, M.H. Constructed mangrove wetland as secondary treatment system for municipal wastewater. Ecol. Eng. 2008, 34, 137–146. [Google Scholar] [CrossRef]
- Wu, Y.; Tam, N.F.Y.; Wong, M.H. Effects of salinity on treatment of municipal wastewater by constructed mangrove wetland microcosms. Mar. Pollut. Bull. 2008, 57, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Monroy, V.H.; Torres, L.A.; Bahamon, N.; Newmark, F.; Twilley, R.R. The Potential Use of Mangrove Forests as Nitrogen Sinks of Shrimp Aquaculture Pond Effluents: The Role of Denitrification. J. World Aquac. Soc. 1999, 30, 12–25. [Google Scholar] [CrossRef]
- Robertson, A.I.; Phillips, M.J. Mangroves as filters of shrimp pond effluent: Predictions and biogeochemical research needs. Hydrobiologia 1995, 295, 311–321. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, Y.S. Retention of nutrients and heavy metals in mangrove sediment receiving wastewater of different strengths. Environ. Technol. 1993, 14, 719–729. [Google Scholar] [CrossRef]
- Ye, Y.; Tam, N.F.Y.; Wong, Y.S. Livestock Wastewater Treatment by a Mangrove Pot-cultivation System and the Effect of Salinity on the Nutrient Removal Efficiency. Mar. Pollut. Bull. 2001, 42, 512–520. [Google Scholar] [CrossRef]
- Jørgensen, B. Bacteria and Marine Biogeochemistry. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 169–206. [Google Scholar]
- Mortimer, R.J.G.; Krom, M.D.; Watson, P.G.; Frickers, P.E.; Davey, J.T.; Clifton, R.J. Sediment–Water Exchange of Nutrients in the Intertidal Zone of the Humber Estuary, UK. Mar. Pollut. Bull. 1999, 37, 261–279. [Google Scholar] [CrossRef]
- Nixon, S. Remineralization and nutrient cycling in coastal marine ecosystems. In Estuaries and Nutrients; Neilson, B.J., Cronin, L.E., Eds.; Humana Press: Clifton, NJ, USA, 1981; pp. 111–138. [Google Scholar]
- Alongi, D.M.; Ramanathan, A.L.; Kannan, L.; Tirendi, F.; Trott, L.A.; Bala Krishna Prasad, M. Influence of human-induced disturbance on benthic microbial metabolism in the Pichavaram mangroves, Vellar–Coleroon estuarine complex, India. Mar. Biol. 2005, 147, 1033–1044. [Google Scholar] [CrossRef]
- Berg, P.; Risgaard-Petersen, N.; Rysgaard, S. Interpretation of Measured Concentration Profiles in Sediment Pore Water. Limnol. Oceanogr. 1998, 43, 1500–1510. [Google Scholar] [CrossRef]
- Hansen, K.; Kristensen, E. The impact of the polychaete Nereis diversicolor and enrichment with macroalgal (Chaetomorpha linum) detritus on benthic metabolism and nutrient dynamics in organic-poor and organic-rich sediment. J. Exp. Mar. Biol. Ecol. 1998, 231, 201–223. [Google Scholar] [CrossRef]
- Kristensen, K.; Hansen, K. Transport of carbon dioxide and ammonium in bioturbated (Nereis diversicolor) coastal, marine sediments. Biogeochemistry 1999, 45, 147–168. [Google Scholar] [CrossRef]
- Nielsen, O.I.; Kristensen, E.; Macintosh, D.J. Impact of fiddler crabs (Uca spp.) on rates and pathways of benthic mineralization in deposited mangrove shrimp pond waste. J. Exp. Mar. Biol. Ecol. 2003, 289, 59–81. [Google Scholar] [CrossRef]
- Nordhaus, I.; Wolff, M.; Diele, K. Litter processing and population food intake of the mangrove crab Ucides cordatus in a high intertidal forest in northern Brazil. Estuar. Coast. Shelf Sci. 2006, 67, 239–250. [Google Scholar] [CrossRef]
- Xin, P.; Jin, G.; Li, L.; Barry, D.A. Effects of crab burrows on pore water flows in salt marshes. Adv. Water Resour. 2009, 32, 439–449. [Google Scholar] [CrossRef]
- Alongi, D.M.; Boto, K.G.; Tirendi, F. Effect of exported mangrove litter on bacterial productivity and dissolved organic carbon fluxes in adjacent tropical nearshore sediments. Mar. Ecol. Prog. Ser. 1989, 56, 133–144. [Google Scholar] [CrossRef]
- Bartoli, M.; Nizzoli, D.; Viaroli, P. Microphytobenthos activity and fluxes at the sediment-water interface: Interactions and spatial variability. Aquat. Ecol. 2003, 37, 341–349. [Google Scholar] [CrossRef]
- Lerat, Y.; Lasserre, P.; Corre, P. le Seasonal changes in pore water concentrations of nutrients and their diffusive fluxes at the sediment-water interface. J. Exp. Mar. Biol. Ecol. 1990, 135, 135–160. [Google Scholar] [CrossRef]
- Wolfe, C.I.; Hammond, D.E.; Schwartz, R.J. Evaluating the accuracy of core incubations. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2009; Volume 1, p. 1184. [Google Scholar]
- Ishii, Y.; Yabe, T.; Nakamura, M.; Amano, Y.; Komatsu, N.; Watanabe, K. Effect of Nitrate on Phosphorus Mobilization from Bottom Sediment in Shallow Eutrophic Lakes. J. Water Environ. Technol. 2009, 7, 163–176. [Google Scholar] [CrossRef]
- An, S.; Gardner, W.S. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Mar. Ecol. Prog. Ser. 2002, 237, 41–50. [Google Scholar] [CrossRef]
- Denis, L.; Grenz, C.; Alliot, É.; Rodier, M. Temporal variability in dissolved inorganic nitrogen fluxes at the sediment–water interface and related annual budget on a continental shelf (NW Mediterranean). Oceanol. Acta 2001, 24, 85–97. [Google Scholar] [CrossRef]
- Rowe, G.T.; Boland, G.S.; Phoel, W.C.; Anderson, R.F.; Biscaye, P.E. Deep-sea floor respiration as an indication of lateral input of biogenic detritus from continental margins. Deep Sea Res. Part II Top. Stud. Oceanogr. 1994, 41, 657–668. [Google Scholar] [CrossRef]
- Orihel, D.M.; Rooney, R.C. A field-based technique for sediment incubation experiments. J. Limnol. 2012, 71, e25. [Google Scholar] [CrossRef]
- Cathalot, C.; Rabouille, C.; Pastor, L.; Deflandre, B.; Viollier, E.; Buscail, R.; Grémare, A.; Treignier, C.; Pruski, A. Temporal variability of carbon recycling in coastal sediments influenced by rivers: Assessing the impact of flood inputs in the Rhône River prodelta. Biogeosciences 2010, 7, 1187–1205. [Google Scholar] [CrossRef]
- Adame, M.; Lovelock, C. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 2011, 663, 23–50. [Google Scholar] [CrossRef]
- Bouillon, S.; Dehairs, F.; Velimirov, B.; Abril, G.; Borges, A.V. Dynamics of organic and inorganic carbon across contiguous mangrove and seagrass systems (Gazi Bay, Kenya). J. Geophys. Res. 2007, 112, G02018. [Google Scholar] [CrossRef]
- Bouillon, S.; Middelburg, J.J.; Dehairs, F.; Borges, A.V.; Abril, G.; Flindt, M.R.; Ulomi, S.; Kristensen, E. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosci. Discuss. 2007, 4, 317–348. [Google Scholar] [CrossRef]
- Dittmar, T.; Lara, R.J. Driving Forces Behind Nutrient and Organic Matter Dynamics in a Mangrove Tidal Creek in North Brazil. Estuar. Coast. Shelf Sci. 2001, 52, 249–259. [Google Scholar] [CrossRef]
- Committee of Annals of Chinese Estuaries. Annals of Chinese Estuaries; Ocean Press: Beijing, China, 1998; Volume 14. [Google Scholar]
- Kaiser, D.; Unger, D.; Qiu, G.; Zhou, H.; Gan, H. Natural and human influences on nutrient transport through a small subtropical Chinese estuary. Sci. Total Environ. 2013, 450–451, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Unger, D.; Qiu, G. Particulate organic matter dynamics in coastal systems of the northern Beibu Gulf. Cont. Shelf Res. 2014, 82, 99–118. [Google Scholar] [CrossRef]
- Mortazavi, B.; Riggs, A.A.; Caffrey, J.M.; Genet, H.; Phipps, S.W. The Contribution of Benthic Nutrient Regeneration to Primary Production in a Shallow Eutrophic Estuary, Weeks Bay, Alabama. Estuar. Coasts 2012, 35, 862–877. [Google Scholar] [CrossRef]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis. Second, Revised and Extended Edition; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie: Deerfield Beach, FL, USA, 1983; Volume 2. [Google Scholar]
- Kérouel, R.; Aminot, A. Fluorometric determination of ammonia in sea and estuarine waters by direct segmented flow analysis. Mar. Chem. 1997, 57, 265–275. [Google Scholar] [CrossRef]
- Alongi, D.M.; Sasekumar, A.; Tirendi, F.; Dixon, P. The influence of stand age on benthic decomposition and recycling of organic matter in managed mangrove forests of Malaysia. J. Exp. Mar. Biol. Ecol. 1998, 225, 197–218. [Google Scholar] [CrossRef]
- Alongi, D.M. Zonation and Seasonality of Benthic Primary Production and Community Respiration in Tropical Mangrove Forests. Oecologia 1994, 98, 320–327. [Google Scholar] [CrossRef]
- Boucher, G.; Clavier, J.; Garrigue, C. Oxygen and carbon dioxide fluxes at the water-sediment interface of a tropical lagoon. Mar. Ecol. Prog. Ser. 1994, 107, 185–193. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Pfitzner, J.; Tirendi, F.; Zagorskis, I.; Brunskill, G.J.; Davidson, A.; Clough, B.F. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Mar. Geol. 2001, 179, 85–103. [Google Scholar] [CrossRef]
- Alongi, D.M.; Christoffersen, P.; Tirendi, F. The influence of forest type on microbial-nutrient relationships in tropical mangrove sediments. J. Exp. Mar. Biol. Ecol. 1993, 171, 201–223. [Google Scholar] [CrossRef]
- Holmer, M.; Andersen, F.Ø.; Holmboe, N.; Kristensen, E.; Thongtham, N. Spatial and temporal variability in benthic processes along a mangrove-seagrass transect near the Bangrong Mangrove, Thailand. Wetl. Ecol. Manag. 2001, 9, 141–158. [Google Scholar] [CrossRef]
- Kristensen, E.; Andersen, F.Ø.; Kofoed, L.H. Preliminary assessment of benthic community metabolism in a south-east Asian mangrove swamp. Mar. Ecol. Prog. Ser. 1988, 48, 137–145. [Google Scholar] [CrossRef]
- Kristensen, E.; Andersen, F.; Holmboe, N.; Holmer, M.; Thongtham, N. Carbon and nitrogen mineralization in sediments of the Bangrong mangrove area, Phuket, Thailand. Aquat. Microb. Ecol. 2000, 22, 199–213. [Google Scholar] [CrossRef]
- Kristensen, E.; Devol, A.H.; Ahmed, S.I.; Saleem, M. Preliminary study of benthic metabolism and sulfate reduction in a mangrove swamp of the Indus Delta, Pakistan. Mar. Ecol. Prog. Ser. 1992, 90, 287–297. [Google Scholar] [CrossRef]
- Kristensen, E.; Holmer, M.; Bussarawit, N. Benthic Metabolism and Sulfate Reduction in a Southeast Asian Mangrove Swamp. Mar. Ecol. Prog. Ser. 1991, 73, 93–103. [Google Scholar] [CrossRef]
- Trott, L.A.; McKinnon, A.D.; Alongi, D.M.; Davidson, A.; Burford, M.A. Carbon and nitrogen processes in a mangrove creek receiving shrimp farm effluent. Estuar. Coast. Shelf Sci. 2004, 59, 197–207. [Google Scholar] [CrossRef]
- Gocke, K.; Vitola, M.; Rojas, G. Oxygen consumption patterns in a mangrove swamp on the Pacific coast of Costa Rica. Rev. Biol. Trop. 1981, 29, 143–154. [Google Scholar]
- Golley, F.; Odum, H.T.; Wilson, R.F. The Structure and Metabolism of a Puerto Rican Red Mangrove Forest in May. Ecology 1962, 43, 9–19. [Google Scholar] [CrossRef]
- Alongi, D.M.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Klumpp, D.W. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Alongi, D.M.; Carvalho, N.A.; Amaral, A.L.; Costa, A.; da Trott, L.; Tirendi, F. Uncoupled surface and below-ground soil respiration in mangroves: Implications for estimates of dissolved inorganic carbon export. Biogeochemistry 2011, 109, 151–162. [Google Scholar] [CrossRef]
- Alongi, D.M. The role of bacteria in nutrient recycling in tropical mangrove and other coastal benthic ecosystems. Hydrobiologia 1994, 285, 19–32. [Google Scholar] [CrossRef]
- Boynton, W.R.; Kemp, W.M. Nutrient regeneration and oxygen consumption by sediments along an estuarine salinity gradient. Mar. Ecol. Prog. Ser. 1985, 23, 45–55. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Rees, G.N.; Bowen, P.M.; Watson, G.O. Variability in benthic respiration in three southeastern Australian lowland rivers. River Res. Appl. 2005, 21, 1147–1156. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Benner, R. Denitrification, nutrient regeneration and carbon mineralization in sediments of Galveston Bay, Texas, USA. Mar. Ecol. Prog. Ser. 1994, 114, 275–288. [Google Scholar] [CrossRef]
- Al-Raei, A.; Bosselmann, K.; Böttcher, M.; Hespenheide, B.; Tauber, F. Seasonal dynamics of microbial sulfate reduction in temperate intertidal surface sediments: Controls by temperature and organic matter. Ocean Dyn. 2009, 59, 351–370. [Google Scholar] [CrossRef]
- Pamatmat, M.M. Ecology and Metabolism of a Benthic Community on an Intertidal Sandflat. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1968, 53, 211–298. [Google Scholar] [CrossRef]
- Pomeroy, L.R. Algal productivity in salt marshes of Georgia. Limnol. Oceanogr. 1959, 386–397. [Google Scholar] [CrossRef]
- Guillén, J.; Bourrin, F.; Palanques, A.; Durrieu de Madron, X.; Puig, P.; Buscail, R. Sediment dynamics during wet and dry storm events on the Têt inner shelf (SW Gulf of Lions). Mar. Geol. 2006, 234, 129–142. [Google Scholar] [CrossRef]
- Smith, T.J.; Anderson, G.H.; Tiling, G. A tale of two storms: Surges and sediment deposition from Hurricanes Andrew and Wilma in Florida’s southwest coast mangrove forests. Sci. Storms USGS Response Hurric. 2005 2005, 1, 169–174. [Google Scholar]
- Souza, M.; Gomes, V.; Freitas, S.; Andrade, R.; Knoppers, B. Net Ecosystem Metabolism and Nonconservative Fluxes of Organic Matter in a Tropical Mangrove Estuary, Piauí River (NE of Brazil). Estuar. Coasts 2009, 32, 111–122. [Google Scholar] [CrossRef]
- Jørgensen, B.B. Bacterial sulfate reduction within reduced microniches of oxidized marine sediments. Mar. Biol. 1977, 41, 7–17. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, 1–12. [Google Scholar] [CrossRef]
- Maher, D.T.; Eyre, B.D. Benthic fluxes of dissolved organic carbon in three temperate Australian estuaries: Implications for global estimates of benthic DOC fluxes. J. Geophys. Res. 2010, 115, G04039. [Google Scholar] [CrossRef]
- Stanley, S.O.; Boto, K.G.; Alongi, D.M.; Gillan, F.T. Composition and bacterial utilization of free amino acids in tropical mangrove sediments. Mar. Chem. 1987, 22, 13–30. [Google Scholar] [CrossRef]
- Ziegler, S.; Benner, R. Dissolved organic carbon cycling in a subtropical seagrass-dominated lagoon. Mar. Ecol. Prog. Ser. 1999, 180, 149–160. [Google Scholar] [CrossRef]
- Alongi, D.M. The dynamics of benthic nutrient pools and fluxes in tropical mangrove forests. J. Mar. Res. 1996, 54, 123–148. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Davis, S., III; Childers, D.; Day, J.; Rudnick, D.; Sklar, F. Wetland-water column exchanges of carbon, nitrogen, and phosphorus in a southern Everglades dwarf mangrove. Estuar. Coasts 2001, 24, 610–622. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Day, J.W.; Twilley, R.R.; Vera-Herrera, F.; Coronado-Molina, C. Flux of Nitrogen and Sediment in a Fringe Mangrove Forest in Terminos Lagoon, Mexico. Estuar. Coast. Shelf Sci. 1995, 40, 139–160. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Twilley, R.R. The Relative Role of Denitrification and Immobilization in the Fate of Inorganic Nitrogen in Mangrove Sediments (Terminos Lagoon, Mexico). Limnol. Oceanogr. 1996, 41, 284–296. [Google Scholar] [CrossRef]
- Rivera-Monroy, V.H.; Twilley, R.R.; Boustany, R.G.; Day, J.W.; Vera-Herrera, F.; del Carmen Ramirez, M. Direct denitrification in mangrove sediments in Terminos Lagoon, Mexico. Mar. Ecol. Prog. Ser. 1995, 126, 97–109. [Google Scholar] [CrossRef]
- Lorenzen, J.; Larsen, L.H.; Kjær, T.; Revsbech, N.P. Biosensor Determination of the Microscale Distribution of Nitrate, Nitrate Assimilation, Nitrification, and Denitrification in a Diatom-Inhabited Freshwater Sediment. Appl. Environ. Microbiol. 1998, 64, 3264–3269. [Google Scholar] [PubMed]
- Fernandes, S.O.; Michotey, V.D.; Guasco, S.; Bonin, P.C.; Loka Bharathi, P.A. Denitrification prevails over anammox in tropical mangrove sediments (Goa, India). Mar. Environ. Res. 2012, 74, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.L.; Allen, D.E.; Schmidt, S. Nitrification and denitrification as sources of sediment nitrous oxide production: A microsensor approach. Mar. Chem. 2008, 110, 68–76. [Google Scholar] [CrossRef]
- Cornwell, J.C.; Kemp, W.M.; Kana, T.M. Denitrification in coastal ecosystems: Methods, environmental controls, and ecosystem level controls, a review. Aquat. Ecol. 1999, 33, 41–54. [Google Scholar] [CrossRef]
- Deek, A.; Emeis, K.; van Beusekom, J. Nitrogen removal in coastal sediments of the German Wadden Sea. Biogeochemistry 2012, 108, 467–483. [Google Scholar] [CrossRef]
- Gardner, W.; McCarthy, M. Nitrogen dynamics at the sediment–water interface in shallow, sub-tropical Florida Bay: Why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 2009, 95, 185–198. [Google Scholar] [CrossRef]
- Bellos, D.; Sawidis, T.; Tsekos, I. Nutrient chemistry of River Pinios (Thessalia, Greece). Environ. Int. 2004, 30, 105–115. [Google Scholar] [CrossRef]
- Bu, H.; Meng, W.; Zhang, Y. Nitrogen pollution and source identification in the Haicheng River basin in Northeast China. Sci. Total Environ. 2011, 409, 3394–3402. [Google Scholar] [CrossRef] [PubMed]
- Falco, S.; Niencheski, L.F.; Rodilla, M.; Romero, I.; Gonzáez del Río, J.; Sierra, J.P.; Mösso, C. Nutrient flux and budget in the Ebro estuary. Estuar. Coast. Shelf Sci. 2010, 87, 92–102. [Google Scholar] [CrossRef]
- Liu, S.M.; Li, R.H.; Zhang, G.L.; Wang, D.R.; Du, J.Z.; Herbeck, L.S.; Zhang, J.; Ren, J.L. The impact of anthropogenic activities on nutrient dynamics in the tropical Wenchanghe and Wenjiaohe Estuary and Lagoon system in East Hainan, China. Mar. Chem. 2011, 125, 49–68. [Google Scholar] [CrossRef]
- Meybeck, M.; Dürr, H.H.; Vörösmarty, C.J. Global coastal segmentation and its river catchment contributors: A new look at land-ocean linkage. Glob. Biogeochem. Cycles 2006, 20, GB1S90. [Google Scholar] [CrossRef]
- Brunet, R.C.; Garcia-Gil, L.J. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiol. Ecol. 1996, 21, 131–138. [Google Scholar] [CrossRef]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Chong, L.S.; Prokopenko, M.G.; Berelson, W.M.; Townsend-Small, A.; McManus, J. Nitrogen cycling within suboxic and anoxic sediments from the continental margin of Western North America. Mar. Chem. 2012, 128–129, 13–25. [Google Scholar] [CrossRef]
- Gardner, W.S.; McCarthy, M.J.; An, S.; Sobolev, D.; Sell, K.S.; Brock, D. Nitrogen Fixation and Dissimilatory Nitrate Reduction to Ammonium (DNRA) Support Nitrogen Dynamics in Texas Estuaries. Limnol. Oceanogr. 2006, 51, 558–568. [Google Scholar] [CrossRef]
- Dong, L.F.; Sobey, M.N.; Smith, C.J.; Rusmana, I.; Phillips, W.; Stott, A.; Osborn, A.M.; Nedwella, D.B. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnol. Oceanogr. 2011, 51, 279–291. [Google Scholar] [CrossRef]
- Pérez-Villalona, H.; Cornwell, J.C.; Ortiz-Zayas, J.R.; Cuevas, E. Sediment Denitrification and Nutrient Fluxes in the San José Lagoon, a Tropical Lagoon in the Highly Urbanized San Juan Bay Estuary, Puerto Rico. Estuar. Coasts 2015. [Google Scholar] [CrossRef]
- Kartal, B.; Kuypers, M.M.M.; Lavik, G.; Schalk, J.; Op den Camp, H.J.M.; Jetten, M.S.M.; Strous, M. Anammox bacteria disguised as denitrifiers: Nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ. Microbiol. 2007, 9, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rütting, T.; Boeckx, P.; Müller, C.; Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosci. Discuss. 2011, 8, 1169–1196. [Google Scholar] [CrossRef]
- Rysgaard, S.; Risgaard-Petersen, N.; Sloth, N.P. Nitrification, denitrification, and nitrate ammonification in sediments of two coastal lagoons in Southern France. Hydrobiologia 1996, 329, 133–141. [Google Scholar] [CrossRef]
- Callender, E. Benthic phosphorus regeneration in the Potomac River Estuary. Hydrobiologia 1982, 91–92, 431–446. [Google Scholar] [CrossRef]
- Howarth, R.W. Nutrient Limitation of Net Primary Production in Marine Ecosystems. Annu. Rev. Ecol. Syst. 1988, 19, 89–110. [Google Scholar] [CrossRef]
- Froelich, P.N. Kinetic Control of Dissolved Phosphate in Natural Rivers and Estuaries: A Primer on the Phosphate Buffer Mechanism. Limnol. Oceanogr. 1988, 33, 649–668. [Google Scholar] [CrossRef]
- Guangxi Mangrove Research Center. Review of China National Data and Information—Final Report; Guangxi Mangrove Research Center: Beihai, China, 2004; p. 76. [Google Scholar]
- Ullman, W.J.; Sandstrom, M.W. Dissolved nutrient fluxes from the nearshore sediments of Bowling Green Bay, central Great Barrier Reef Lagoon (Australia). Estuar. Coast. Shelf Sci. 1987, 24, 289–303. [Google Scholar] [CrossRef]
- Bauer, A.; Radziejewska, T.; Liang, K.; Kowalski, N.; Dellwig, O.; Bosselmann, K.; Stark, A.; Xia, Z.; Harff, J.; Böttcher, M.E.; et al. Regional differences of hydrographical and sedimentological properties in Beibu Gulf, South China Sea. J. Coast. Res. 2013. [Google Scholar] [CrossRef]
- Henry, K.M.; Twilley, R.R. Exploring the effects of black mangrove (Avicennia germinans) expansions on nutrient cycling in smooth cordgrass (Spartina alterniflora) marsh sediments of southern Louisiana, USA. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2011; Volume 1, p. 0441. [Google Scholar]
- Dittmar, T.; Lara, R.J.; Kattner, G. River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters. Mar. Chem. 2001, 73, 253–271. [Google Scholar] [CrossRef]
- Boto, K.G.; Wellington, J.T. Seasonal variations in concentrations and fluxes of dissolved organic and inorganic materials in a tropical, tidally-dominated, mangrove waterway. Mar. Ecol. Prog. Ser. 1988, 50, 151–160. [Google Scholar] [CrossRef]
- Amatya, I.M.; Kansakar, B.R.; Tare, V.; Fiksdal, L. Impact of temperature on biological denitrification process. J. Inst. Eng. 2009, 7, 121–126. [Google Scholar] [CrossRef]
- Goñi, M.A.; Gordon, E.S.; Monacci, N.M.; Clinton, R.; Gisewhite, R.; Allison, M.A.; Kineke, G. The effect of Hurricane Lili on the distribution of organic matter along the inner Louisiana shelf (Gulf of Mexico, USA). Cont. Shelf Res. 2006, 26, 2260–2280. [Google Scholar] [CrossRef]
- Porter, R.P.; Mason, E.T.P.R.P.; Sanford, L.P. Effect of tidal resuspension on benthic–pelagic coupling in an experimental ecosystem study. Mar. Ecol. Prog. Ser. 2010, 413, 33–53. [Google Scholar] [CrossRef]
- Kaiser, D. Leibniz Center for Tropical Marine Ecology, Bremen, Germany. Unpublished work. 2011. [Google Scholar]
- Ashton, E.C.; Hogarth, P.J.; Ormond, R. Breakdown of mangrove leaf litter in a managed mangrove forest in Peninsular Malaysia. Hydrobiologia 1999, 413, 77–88. [Google Scholar] [CrossRef]
- Moll, R. Impact of Mangroves and an Agriculture-Dominated Hinterland on the Carbon and Nutrient Biogeochemistry in the Segara Anakan Lagoon, Java, Indonesia. Ph.D. Thesis, University Bremen, Bremen, Germany, 2011. [Google Scholar]
- Tam, N.F.Y.; Wong, Y.S.; Lan, C.Y.; Wang, L.N. Litter production and decomposition in a subtropical mangrove swamp receiving wastewater. J. Exp. Mar. Biol. Ecol. 1998, 226, 1–18. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Vrijmoed, L.L.P.; Wong, Y.S. Nutrient Dynamics Associated with Leaf Decomposition in a Small Subtropical Mangrove Community in Hong Kong. Bull. Mar. Sci. 1990, 47, 68–78. [Google Scholar]
- Vouvé, F.; Guiraud, G.; Marol, C.; Girard, M.; Richard, P.; Laima, M.J.C. NH4+ turnover in intertidal sediments of Marennes-Oléron Bay (France): Effect of sediment temperature. Oceanol. Acta 2000, 23, 575–584. [Google Scholar] [CrossRef]
- Chu, H.Y.; Tam, N.F.Y.; Lam, S.K.S.; Wong, Y.S. Retention of Pollutants by Mangrove Soil and the Effects of Pollutants on Kandelia Candel. Environ. Technol. 2000, 21, 755–764. [Google Scholar] [CrossRef]
- Feller, I.C.; McKee, K.L.; Whigham, D.F.; O’Neill, J.P. Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 2002, 62, 145–175. [Google Scholar] [CrossRef]
- Feller, I.C.; Whigham, D.F.; O’Neill, J.P.; McKee, K.L. Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 1999, 80, 2193–2205. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, Y.S. Retention of Wastewater-borne Nitrogen and Phosphorus in Mangrove Soils. Environ. Technol. 1996, 17, 851–859. [Google Scholar] [CrossRef]
- Jiang, L.M.; Chen, B.; Qiu, S.F. The Relationship of Silt Transportation and Ocean Dynamical Condition in Delta of Lianzhou Bay. J. Guangxi Acad. Sci. 2008, 24, 25–28. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, D.; Kowalski, N.; Böttcher, M.E.; Yan, B.; Unger, D. Benthic Nutrient Fluxes from Mangrove Sediments of an Anthropogenically Impacted Estuary in Southern China. J. Mar. Sci. Eng. 2015, 3, 466-491. https://doi.org/10.3390/jmse3020466
Kaiser D, Kowalski N, Böttcher ME, Yan B, Unger D. Benthic Nutrient Fluxes from Mangrove Sediments of an Anthropogenically Impacted Estuary in Southern China. Journal of Marine Science and Engineering. 2015; 3(2):466-491. https://doi.org/10.3390/jmse3020466
Chicago/Turabian StyleKaiser, David, Nicole Kowalski, Michael E. Böttcher, Bing Yan, and Daniela Unger. 2015. "Benthic Nutrient Fluxes from Mangrove Sediments of an Anthropogenically Impacted Estuary in Southern China" Journal of Marine Science and Engineering 3, no. 2: 466-491. https://doi.org/10.3390/jmse3020466
APA StyleKaiser, D., Kowalski, N., Böttcher, M. E., Yan, B., & Unger, D. (2015). Benthic Nutrient Fluxes from Mangrove Sediments of an Anthropogenically Impacted Estuary in Southern China. Journal of Marine Science and Engineering, 3(2), 466-491. https://doi.org/10.3390/jmse3020466





