Abstract
Thermal tolerance tests on Acropora millepora, a common Indo-Pacific hard coral, have shown that adult corals can acquire increased thermal tolerance by shuffling existing type C to type D Symbiodinium zooxanthellae when subjected to increased seawater temperatures. We report here dimethylsulphoniopropionate (DMSP) concentrations in A. millepora and examine links between DMSP concentrations, zooxanthellae clade, and bleaching tolerance. DMSP analysis on native and transplanted corals from three locations in the Great Barrier Reef indicated that the lower thermal tolerance in type C zooxanthellae coincided with variable DMSP concentrations, whilst the more thermal tolerant type D zooxanthellae had more stable areal DMSP concentrations as seawater temperatures increased. Our results suggest this increased thermal tolerance in type D zooxanthellae may reflect the ability of these coral symbionts to conserve their antioxidant DMSP levels to relatively constant concentrations, enabling the coral to overcome the build-up of oxygen free radicals in the cytoplasm of A. millepora. A conceptual diagram illustrates how the antioxidants DMS (P) participate in the bleaching process by scavenging oxygen free radicals and form DMSO, thus moderating coral bleaching and increasing thermotolerance.
1. Introduction
Coral bleaching has been more regularly observed in the Great Barrier Reef (GBR) GBR over more than two decades [1,2,3,4,5] with bleaching events becoming more severe in the mid-1980s and late 1990s, culminating in two extreme mass coral bleaching episodes in 1998 and 2002 severely affecting hundreds of coral reefs and potentially threatening the health and integrity of coral reefs around the world [1,6,7]. It is thought that climate change is the main cause of these mass coral bleaching episodes, but human impacts such as increased nutrient levels, suspended sediments from land runoff and dredging can exacerbate the bleaching response. The bleaching process is initiated by a stress (usually abiotic), which often causes the expulsion of algal pigments or entire zooxanthellae, leaving empty tissue that creates the result of a pale or bleached host [7,8,9]. Light and temperature stress appear to induce bleaching by creating oxygen toxicity pathways. Photosynthesis in any plant cell including zooxanthellae reduces molecular oxygen to water and in the process oxygen free-radicals and other cellular intermediates are produced [10,11]. The radicals superoxide (O2−), hydroperoxyl (HO2), hydroxyl (OH−) and the intermediate hydrogen peroxide (H2O2) are formed in a stepwise fashion during the last stage of photosynthesis (reactions 2–5; [10]). Superoxide is generated in the mitochondria, nuclei and chloroplasts of plant cells [10,12]. This radical causes lipid peroxidation, mitochondria dysfunction, ATP depletion, membrane damage and leakage, essentially resulting in cell death [10,11]. Although this process occurs naturally, temperature and light stress can exacerbate the rate of radical formation [7,13]. Lesser (1996) found high concentrations of intracellular superoxide and hydrogen peroxide when he exposed cultured zooxanthellae to elevated temperatures and ultra violet radiation (UVR) [14]. Downs et al. (2002) studied a depth transect in the Florida Keys after a period of elevated sea surface temperatures (SST) and found strong correlations between oxidative damage products and coral bleaching, suggesting that the two processes are closely related [15].
Early studies that focused on the areas damaged by oxygen radicals in zooxanthellae have suggested that the light reactions of photosystem II (PSII) in the chloroplast are the primary damage site from high temperatures and UVR [13,14]. Jones et al. (1998) have reported that the primary damage site to zooxanthellae chloroplasts is the Calvin cycle (dark reactions) as they are not able to cope with the over-production of excitation energy from increased light [16]. Jones et al. (1998) suggested that the buildup of energy in the light reactions causes over-reduction and instead of producing molecular oxygen, oxygen-free radicals are produced [16]. This study was very important with respect to the biochemical processes involved in bleaching as it resolved that the first step is heat stress, which causes a change to the electron flow pathway. When this scenario occurs in conjunction with high light intensities, over-reduction of the light reactions occur initiating oxygen toxicity pathways and subsequent bleaching [16,17].
Until recent genetic advances, symbiotic zooxanthellae were classified as a single species, Symbiodinium microadriaticum Freudenthal (reviewed by Hoegh-Guldberg, 1999, [7]). Presently there are nine known “clades” or genetically different strains of zooxanthellae that have been identified using the Polymerase Chain Reaction (PCR) and RNA sequencing techniques developed by Rowan and Powers (1991), [18]. The authors analysed a host of cnidarians for zooxanthellae genotype distribution and suggested that zooxanthellae diversity is greatest between hosts rather than within a host, and a single host is more likely to harbour closely related zooxanthellae rather than distant relatives. Loh et al. (1997) collected seven Acropora species from One Tree Island (GBR) and found that six species contained predominately clade C while one species contained clades A and C [19]. Some researchers have reported microhabitat partitioning, as a single coral colony can harbour multiple zooxanthellae clades, all occupying distinct zones [20,21]. These studies suggest that there may be multiple influences on zooxanthellae distribution. Although clade identification is being increasingly used to genotype coral around the world, there is still a high level of uncertainty of the exact benefits provided by the different clades. Loh et al. (1997) suggested that further analysis of clade response to physiological stressors may reveal more about the characteristics of each clade [19].
Coral transplant experiments have been extremely useful in deciphering the importance of corals to host stress-tolerant zooxanthellae. Baker (2001) identified that upwards (depth) transplanted coral underwent changes in their zooxanthellae clade compositions after they bleached, suggesting that bleaching allowed the coral to ultimately survive better by acquiring more suitable zooxanthellae [20]. Berkelmans and van Oppen (2006) [22] observed that corals, which changed from clade C to D during the transplant were more tolerant to thermal stress than those corals that did not change their clade type, a result confirmed by other researchers [23]. These studies supported the Adaptive Bleaching Hypothesis of Buddemeier and Fautin (1993) [24] that bleaching provides an opportunity for coral to acquire more suitable or stress-tolerant algal partners, but the exact physiological mechanism by which this occurs is not clear.
In a recent study by Sunda et al. (2002) [25], it has been found that marine phytoplankton provide their own antioxidant defense by utilising the sulphur substances dimethylsulphoniopropionate (DMSP), dimethylsulphide (DMS), dimethylsulphoxide (DMSO), acrylic acid and methanesulphinic acid (MSNA). These five sulphur compounds may function individually or simultaneously as an efficient antioxidant system to scavenge the harmful oxygen free-radicals produced during elevated stress [25]. According to this study DMSP, DMS and acrylate scavenge hydroxyl radicals (OH) and produce DMSO. DMSO may then further react with hydroxyl radicals to produce MSNA, which also scavenges hydroxyl radicals [25,26]. Corals contain exceptionally high levels of DMSP in their zooxanthellae [27,28,29], and produce coral mucus that contains the highest levels of DMS and DMSP (i.e., DMS (P)) of any marine environment [30]. Staghorn coral or Acropora species produce the greatest amounts of DMS for exchange to the atmosphere [31,32].
Oxidation of atmospheric DMS produces a sulphate aerosol which can potentially form cloud condensation nuclei leading to low level cloud development [33,34,35,36]. An increasing amount of evidence now suggests that DMS emitted from coral reefs could keep SSTs cooler in the Western Pacific, including the Great Barrier Reef, through this reef produced low level cloud climate feedback [32,33]. The production of these natural sulphur substances from coral reefs; their effect on regional climate, bird, bacterial and fish behaviour, and possible use as Polynesian navigational aids are reviewed, providing evidence of a very valuable and important ecosystem service not previously described [33].
Marked intra-specific differences in zooxanthellar or cellular DMSP have been reported in two morphologically identical, adjacent colonies of A. formosa at Magnetic Island [27]. One of the colonies bleached to a light tan colour during a bleaching event in January 1994, while no detectable colour change was observed in the other colony. In the colonies that had bleached DMSP concentrations were 436 fmol DMSP zooxanthellae−1, whilst unbleached colonies had a concentration of 171 fmol DMSP zooxanthellae−1, suggesting host production of DMSP in bleached corals [37]. The link between coral DMSP, sulphur substances, such as DMS, DMSO, and the antioxidant capacity (AOC) of Acropora species, has been investigated in more detail by exposing A. aspera to a range of natural environmental stressors (temperature, light, salinity, and air exposure) that lead to oxidative stress in the coral holobiont [31]. Enhanced DMS-DMSP-DMSO production occurred in A. aspera during changes in these natural stressors, indicating that reduced sulphur production (DMS-DMSP-DMSO) and turnover in Acropora corals undergo different biochemical pathways depending on the type and severity of the environmental stress. Decreased salinity and light depletion led to an up-regulation of the coral AOC that was correlated with a significant increase in DMSO [31]. These results, combined with a positive correlation between the AOC and DMSO concentrations suggested that the DMSP-based antioxidant system (DMSP-DMS-DMSO) is involved in the overall antioxidant regulation of the coral holobiont. Enhanced DMS production under increased temperature indicated that thermal stress triggers DMS-DMSP-DMSO formation in coral tissue [31]. The intra-specific differences in adjacent coral colonies now observed by many researchers suggests physiological differences between zooxanthellae and suggested that there may be differences in DMSP concentrations in different clades of zooxanthellae [38,39].
This led us to ask, what is the role of DMSP in coral tissue, and in particular what is the role of DMSP in corals that experience bleaching or high SSTs? The aim of this study was to investigate if a relationship exists between coral DMSP concentrations and coral bleaching tolerance in Acropora millepora by observing how tissue DMSP concentrations vary with different clades of zooxanthellae that are subjected to varying thermal stress from elevated seawater temperatures. Samples from the experiment by Berkelmans and van Oppen (2006) [22] provided a unique opportunity to investigate changes in DMSP in different clades of zooxanthellae that were subjected to increasing SSTs in situ and in the laboratory, and are reported here.
2. Experimental Section
2.1. Coral Collection and Transplantation
Acropora millepora colonies (22) were collected from a cool central offshore reef (Davies Reef), and a cool southern inshore reef (North Keppel Island) in the GBR, Australia, from December 2001 to February 2002 (Figure 1), and transplanted to a warm inshore bay (Nelly Bay) in the central GBR (Magnetic Island) [22]. Average summer (December to February) seawater temperatures at Magnetic Island of 29.2 ± 0.45 °C are 0.9 °C warmer than those at Davies Reef (28.3 ± 0.50 °C), while those at Davies Reef are 1.3 °C warmer than North Keppel Island (Keppels) (27.0 ± 0.50 °C) [22,40]. A further 22 colonies from each of Magnetic Island, Davies Reef and the Keppels were kept at their respective native reefs until their thermal tolerance limits could be experimentally tested, together with the transplanted colonies, which were kept at Magnetic Island for 9 (Keppels Transplant) and 14 months (Davies Transplant). Corals were kept on mesh racks at approximately the same depth as they were collected (2–4 m). The zooxanthellae of these colonies were brought back to the laboratory at AIMS and genotyped [22] and their thermal tolerance limits were tested against A. millepora collected fresh from the field (“control” treatments). Just before the thermal tolerance experiment commenced the zooxanthellae were genotyped again [22].
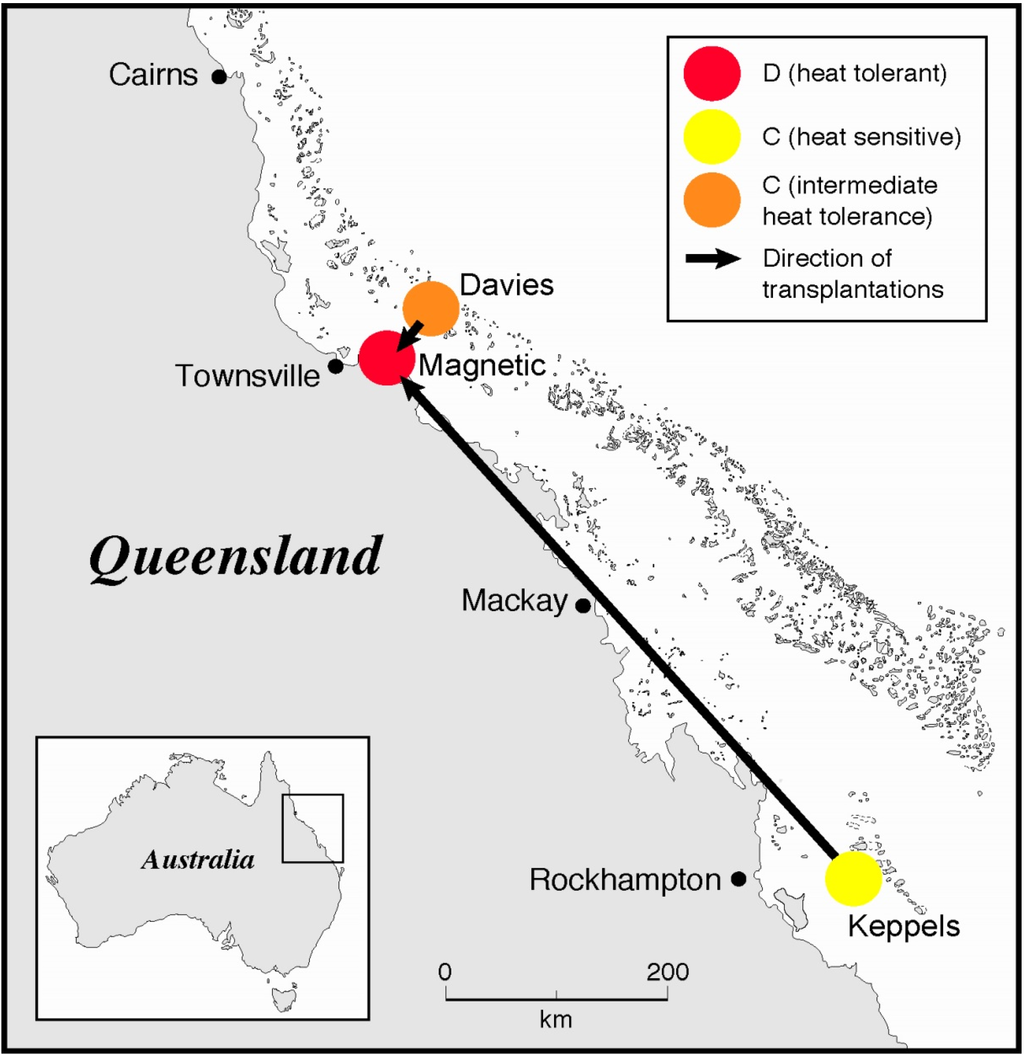
Figure 1.
Location map of coral transplants from the cooler waters of the southern inshore GBR (Keppels) and central offshore waters of the GBR (Davies Reef), to the warmer inshore waters of the central GBR (Magnetic Island) (adapted from [22], with permission from © 2006 The Royal Society).
2.2. Temperature Experiment
Twelve nubbins (3–5 cm long) were cut from six adult A. millepora colonies from each of the five experimental populations (Magnetic Island, Davies Reef, North Keppel Island, Davies Transplant, and Keppels Transplant) (Figure 2). The nubbins were equally distributed in three experimental tanks (A–C), which were set at the five temperature treatments (before, the non-bleaching control (27.5 °C), 30 °C, 31 °C, and 32 °C). The nubbins were conditioned in the experimental tanks for 10 days then exposed to treatment temperatures for 15 days. Tanks were supplied with fresh unfiltered seawater via a flow-through system at a rate of 1.5 L min−1.
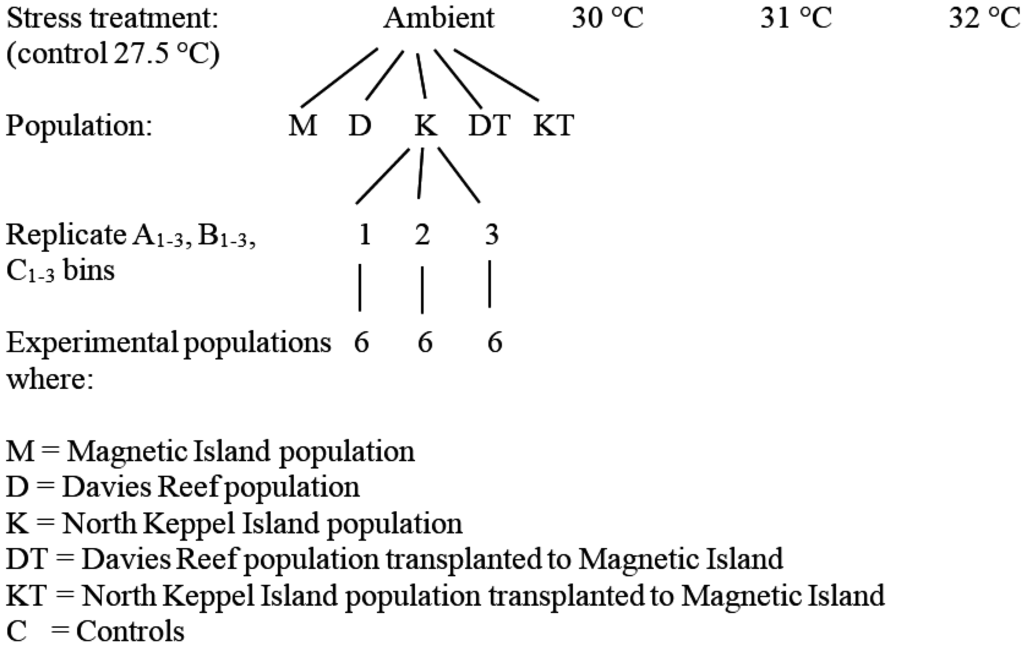
Figure 2.
Experimental set-up devised by Berkelmans and van Oppen (2006) [22]. (Source Ray Berkelmans).
Temperature of the supply water was computer-controlled to 0.2–0.5 °C over the 15 days [41]. Ambient temperatures at the start of the experiment were 27.5 °C. Light was provided by 10 × 400 W metal halide lamps (10,000° K, BLV Germany) with a spectral quality suitable for coral photosynthesis at an average underwater intensity of 190–280 μmole photons m−2 s−1 for 12 h per day [22]. Corals were kept at the above stress temperatures for 15 days. During this time the photosynthetic yield of the corals was measured every second day. The condition of the corals and the detailed change in PAM (Pulse Amplitude Modulated) fluorometry yields are given in Berkelmans and van Oppen (2006) [22], only results for PAM yields at t = 0 and t = 15 days are given here. The within-population notation in the data files was A1, B1, C1, etc.; and enabled the identity of each individual coral nubbin to be determined. After the experiment, the nubbins were frozen in liquid nitrogen and stored at −80 °C until the samples were processed. The base replication rate was, thus, 6 per treatment combination (Figure 2), but for the KT population it was 8.
2.3. Zooxanthellae Genotyping
Zooxanthellae were identified by Berkelmans and van Oppen using the nuclear ribosomal DNA Internal Transcriber Spacer 1 (ITS1) using a Single Stranded Conformation Polymorphism and sequencing analysis [42,43]. This marker and analysis has a lower detection limit of 5%–10% in the relative abundance of multiple zooxanthellae strains [44].
2.4. Zooxanthellae Counts and Coral Surface Area
Coral tissue was stripped from the skeletons by placing each nubbin in a plastic bag containing 10 mL of 0.2 μm filtered seawater. An air gun was used to blast the tissue off the skeleton and a small blender was used to homogenize the tissue (final volume 25–30 mL). A 9 mL sample was drawn off and preserved with 1 mL of formalin (32% w/w). Eight independent drops from each sample were counted using a Neubauer haemocytometer. Zooxanthellae densities were normalized to coral surface area, using the wax method of Stimson and Kinzie (1991) [45].
2.5. Photosynthetic Fitness of the Zooxanthellae
The fluorescence yield as a measure of zooxanthellae fitness in the corals (dark-adapted) was measured over the 15 days of temperature treatments [22] using a Diving-PAM (Waltz, Effeltrich, Germany) after at least 8 h of darkness, followed by fluorescent light conditions of less than 2 μmole photons m−2 s−1. Results recorded here are for the fluorescence yield at day 15 for the four temperature treatments, together with final coral DMSP concentrations.
2.6. Visual Observations of Bleaching
Berkelmans and van Oppen (2006) [22] state that of the Keppels native colonies transplanted to Magnetic Island in July 2002 all bleached pale to white and seven colonies containing type C zooxanthellae died during summer 2003. By April 2003, all surviving colonies had regained their coloration and contained only type D zooxanthellae, including those that were originally dominated by C2 (Table 1).

Table 1.
Dominant zooxanthellae clade type for different coral colonies during and after transplantation.
| Dominant Zooxanthellae Clade Type | ||
|---|---|---|
| Coral Colony | During Transplant | After Transplant |
| Magnetic Island | D | D |
| Keppel Transplant | C2 (80%, sensu) | D |
| D (20%) | ||
| Davies Transplant | C2* | C2* |
Source: Berkelmans and van Oppen (2006) [22].
2.7. DMSP Analysis
A 2 mL aliquot of coral blastate was sub-sampled for DMSP analysis and stored by fixing to pH less than 2 by adding two drops of hydrochloric acid (10%, final volume 2.5 mL). Storage tests show that no changes occur to DMSP for up to one year if fixed with acid [46]. The samples were stored in amber vials for DMSP analysis. The homogenised coral tissue samples were analysed for DMSP concentration using a purge and trap gas chromatographic method whereby DMSP is alkali cleaved to DMS in a 1:1 ratio and quantified using a DMSP standard [27,31,38,46]. Prior to analysis each sample vial was shaken to thoroughly mix the tissue homogenate. Glass microlitre syringes (250 μL to 1000 μL) were used to extract a volume of coral homegenate (usually 500 μL). Each sample was injected in to a glass purge chamber containing 5 mL of 10 M sodium hydroxide (NaOH). High purity nitrogen gas (250 mL/min) was then passed through a glass frit in the bottom of the purge chamber and the liberated DMS generated from alkali cleavage of the DMSP was purged through a moisture trap (K2CO3) and into a Teflon loop that was immersed in a small Dewar of liquid nitrogen (−196 °C). After 10 min the Teflon loop was removed from the liquid nitrogen and placed in a water bath at room temperature. The volatilized DMS was carried by high purity helium (15 mL min−1) onto a stainless steel column packed with Porapak Q at 160 °C. DMS was detected using a flame photometric detector specific for sulphur. Area counts of the DMS peaks generated were made using a data handling software package on the gas chromatograph. The DMSP in the coral tissue was quantified using DMSP standards that were treated exactly the same as the samples and run daily. All samples were analysed randomly.
2.8. Statistical Analysis
Two-way Analysis of Variance (ANOVA) using the averages from the replication tanks (A–C) was used to determine statistical significance in DMSP concentrations between the five colonies and four temperature treatments. The null hypotheses for two-way ANOVA were:
H0:β = 0. The DMSP concentrations of all temperature treatments are equal or there is no effect of increased temperature on zooxanthellar or areal DMSP concentrations. H0:τ = 0. The DMSP concentration means of all colonies are equal or there is no effect of location on zooxanthellar or areal DMSP concentrations. When any significant differences were found within the above two conditions, a matrix was devised to compare each treatment using Fisher’s Least Significant Difference (LSD) to determine statistical significance between individual treatments. Statistically significant correlations were tested using a Pearson’s Product Linear Correlation analysis (parametric, normally distributed) with significance determined at the 0.01 or 0.001 level.
3. Results
3.1. Zooxanthellae Densities
Zooxanthellae densities for the five discrete colonies are shown in Figure 3. Higher zooxanthellae densities generally occurred in the “before” transplanted colonies, compared with the control colonies (see control, Figure 3). There was an overall trend of decreasing zooxanthellae densities as temperatures increased. Keppels, Davies and Davies Transplant colonies had very similar zooxanthellae densities (range 2193–1,837,021 zooxanthellae cm−2, 967–1,140,811 zooxanthellae cm−2, and 898–1,444,368 zooxanthellae cm−2, respectively). Magnetic and Keppels Transplant colonies had overall higher zooxanthellae densities compared to colonies from the other three locations (Figure 3), particularly at 32 °C where the average densities of these two colonies were 650,645 zooxanthellae cm−2 and 369,734 zooxanthellae cm−2, respectively [22]. The lowest zooxanthellae densities occurred in the severely bleached Davies and Davies Transplanted colonies at 32 °C (i.e., 322 zooxanthellae cm−2 and 3357 zooxanthellae cm−2, respectively). For control colonies and 30 °C treatments no statistical difference occurred in zooxanthellae densities between before and after treatments (p > 0.05), with the exception of Keppels Transplant colonies (p < 0.05). At 31 °C there was a significant difference between before and after treatments for all sites (p < 0.001), except Magnetic Island, whilst at 32 °C there was a highly significant difference between before and after treatments (p < 0.001) for all sites.
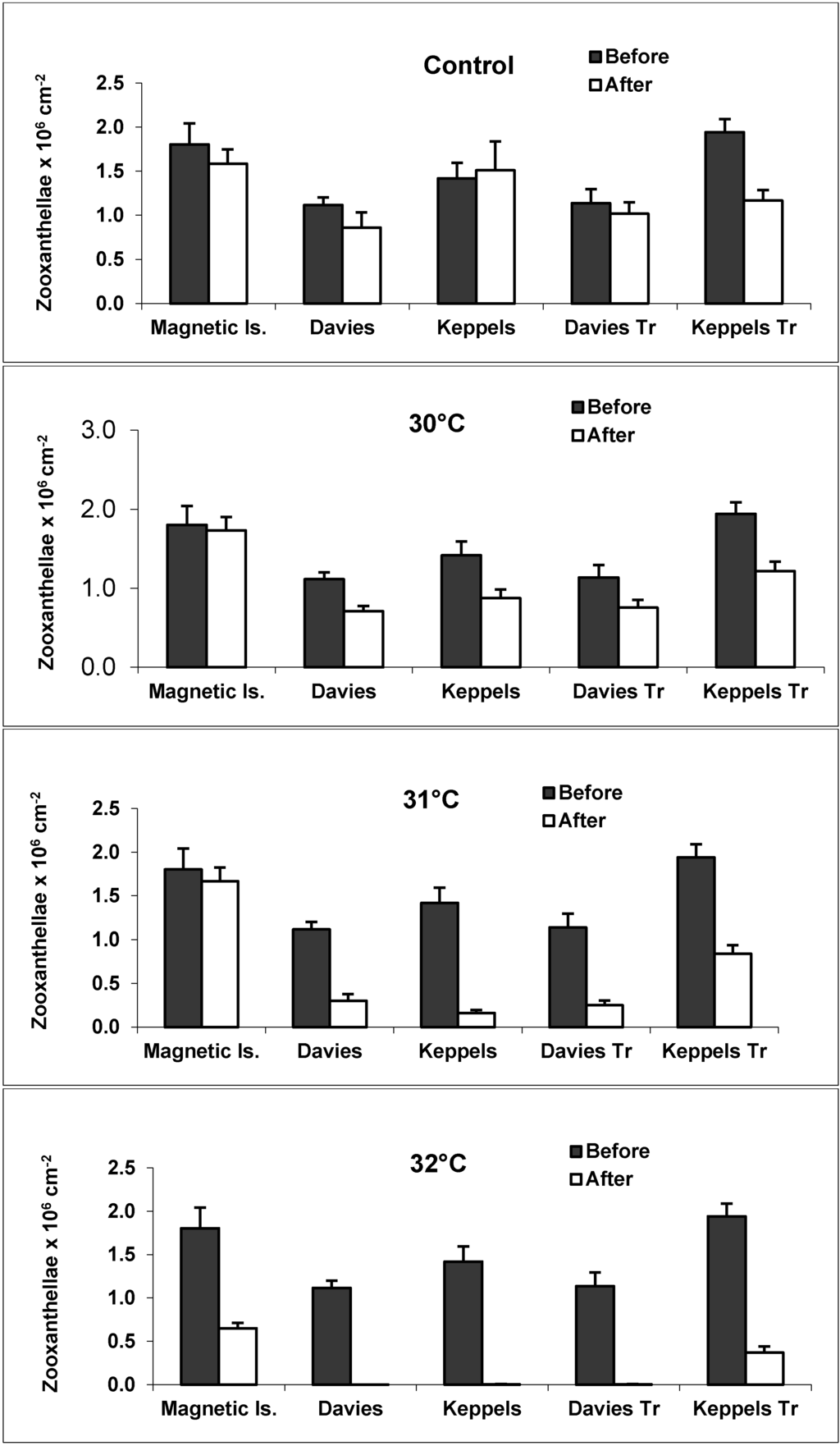
Figure 3.
Zooxanthellae density ± SE (bars) of coral nubbins (n = 18 per temperature treatment) before and after 15-d of heat stress at control (27.5 °C), 30, 31, and 32 °C in each of five experimental populations from the five sites: Maggie (Magnetic Island); North Keppel Island native; Keppel Transplants; Davies Reef native; and Davies Transplants. (Source: Ray Berkelmans). (See Figure 3 in [22] for details of healthy, pale, white, dead zooxanthellae).
3.2. Bleaching Observations and Zooxanthellae Clade Changes
The following information is taken from Berkelmans and van Oppen (2006) [22]. At the time of transplantation (July 2002), all colonies from the Keppels site contained type C2 (sensu) [43] zooxanthellae with about 80% of A. millepora colonies dominant in this type (Table 1). The remaining colonies were dominated by type D zooxanthellae but also contained type C2 in lower abundance [22]. Of the 22 Keppels colonies transplanted to Magnetic Island in July 2002, all bleached white and seven colonies containing type C zooxanthellae died during the warm 2003 Austral summer. By April 2003 all surviving colonies had regained their colouration and contained only type D zooxanthellae, including those that were originally dominated by C2 (Table 1). Of the 22 Davies Reef colonies transplanted to Magnetic Island in late February 2002, 13 survived the 2003 summer, most bleaching pale to white at this time. All of these colonies contained only type C2 zooxanthellae at the time of transplantation and, in contrast to the Keppels colonies, recovered with the same strain. Thus, of the transplanted corals, only the Keppel corals changed zooxanthellae type, most likely as a direct result of temperature-induced bleaching they underwent during the warm 2003 summer at Magnetic Island [22]. The C2 zooxanthellae harboured by A. millepora colonies at Davies Reef differed in ribosomal DNA ITS1 sequence from the C2 zooxanthellae of their conspecifics from the Keppels site [22]. Hence, these zooxanthellae represent a distinct strain and were referred to as type C2*. The native Davies Reef population harboured only type C2* zooxanthellae unchanged over time. Transplanted Davies Reef corals, having failed to alter zooxanthellae type even after 14 months at Magnetic Island performed as if they had never been transplanted [22]. All 22 native Magnetic Island colonies transplanted onto racks at the same location in late February 2002 survived the 2003 summer without bleaching and consistently harboured only type D zooxanthellae through time. The native Keppels population collected were dominant in type C2 zooxanthellae at the time of the temperature experiments [22].
3.3. Tissue DMSP Concentrations
When coral tissue DMSP concentrations were normalized to zooxanthellae densities there was a general trend of increasing cellular DMSP with increasing temperatures for Keppels and Davies native colonies, Davies Transplant and Keppels Transplant colonies (Table 2). Statistical analysis of the cellular DMSP concentrations by two-way ANOVA with replication yielded statistical significance (p < 0.05) within temperature treatments (df = 4, Fcalc =6.89, Fcrit =2.56, p = 0.0002) and for the interaction of location and temperature (p < 0.05) (df = 16, Fcalc = 2.01, Fcrit = 1.85, p = 0.0312), but not for all temperature treatments. At 32 °C cellular concentrations of DMSP in thermally stressed colonies from Keppels, Davies and Davies Transplant colonies were exceptionally high (pmols zooxanthellae−1 versus fmol zooxanthellae−1) and significantly different from controls (df = 16, p < 0.001) (Table 2). Zooxanthellar DMSP concentrations in Keppels, Davies Transplant and Davies colonies at 32 °C were 521 pmol zooxanthellae−1, 80 pmol zooxanthellae−1 and 1760 pmol zooxanthellae−1, respectively (Table 2), a factor of 100 to 1100 times greater concentrations than the cellular DMSP concentrations of their respective controls at 27.5 °C (Table 2). Zooxanthellae densities were very low at 32 °C at these sites (Figure 3), reflecting the severe bleaching of these colonies. The abnormally high cellular DMSP concentrations at 32 °C are more likely a reflection of DMSP production from the host rather than enhanced DMSP production from the very low numbers of zooxanthellae [37]. Keppels Transplant colonies with mixed clades (C2 and D) at the beginning of the experiment (Table 1), and clade D zooxanthellae after transplantation, also showed this general trend of increasing cellular DMSP levels with increasing temperature. These colonies had higher zooxanthellae densities at 32 °C and higher thermotolerance (Figure 3). For Magnetic Island corals with clade D zooxanthellae, cellular DMSP levels did not vary greatly in controls at 27.5 °C and in corals kept at 30 °C and 31 °C (means 663–900 fmol DMSP zooxanthellae−1), but increased markedly to 1554 fmol zooxanthellae−1 at 32 °C, and did not bleach, having the highest densities of zooxanthellae at the end of the 15 days of temperature stress (Figure 3, Table 2).

Table 2.
Cellular DMSP concentrations (fmol zooxanthellae−1) in colonies of native and transplanted Acropora millepora colonies exposed to before, control, 30 °C, 31 °C and 32 °C treatments in order to measure their contrasting thermal tolerances.
| Coral Colony | Before | Control | 30 °C | 31 °C | 32 °C |
|---|---|---|---|---|---|
| Magnetic Island | 449 ± 161 | 900 ± 99 | 882 ± 93 | 663 ± 70 | 1554 ± 263 # |
| Keppels Transplant | 326 ± 229 | 1204 ± 512 | 956 ± 217 | 1503 ± 504 | 3835 ± 1339 ** |
| Keppels | 520 ± 197 | 833 ± 404 | 1532 ± 323 | 6878 ± 322 | 521 ± 221 *# |
| Davies | 1553 ± 431 | 2262 ± 497 | 3163 ± 805 | 4634 ± 1876 | 1761 ± 2081 *# |
| Davies Transplant | 893 ± 218 | 791 ± 194 | 1886 ± 968 | 5068 ± 1657 | 80 ± 30 *# |
* Cellular DMSP concentrations in pmols zooxanthellae−1; # Significant difference between controls and temperature treatments (p < 0.001) and ** significant at p < 0.05.
As the coral host can also produce DMSP, in addition to production by coral zooxanthellae when stressed by elevated temperatures [37], we calculated the areal (coral) concentrations of DMSP (nM cm−2), as this index does not have the confounding problem of whether the extremely high cellular values in Table 2 at 32 °C are the result of coral host or coral symbiont production leading to atypical cellular DMSP concentrations [39]. Areal DMSP concentrations in the “before” treatments for Magnetic Island, Keppels and Keppels Transplanted corals were closely similar (634–809 nM cm−2) (Table 3), but appreciably higher than unbleached Acropora corals collected in the GBR (Table 4) [31,37,47]. In “before” treatments for Davies and Davies Transplant corals DMSP concentrations (1734 and 1016 nM cm−2) were higher than Magnetic Island, Keppels and Keppels Transplant corals (Table 3). These high “before” values, possibly reflect the mass coral bleaching event that occurred across the whole length of the GBR in February 2002 when the corals were transplanted to the Magnetic Island site, and as a consequence increased coral DMSP concentrations [39]. On 13 July 2002 DMSP concentrations in control colonies, taken just before the laboratory thermal stress experiments, were higher than their “before” DMSP concentration for Magnetic Island (p < 0.001), Keppels and
Keppels Transplanted (p < 0.001) corals (Table 3), possibly reflecting the severity of the 2002 mass bleaching event, and other stressors at the Magnetic Island site [39]. No significant difference (p > 0.05) occurred in areal DMSP concentrations between “before” and control treatments for Davies and Davies Transplanted corals, with control levels for Davies corals, relative to control concentrations in Magnetic Island, Keppels and Keppels Transplanted corals. During the thermal stress experiments areal DMSP concentrations in Magnetic Island, Keppels and Keppels Transplanted corals were closely similar to their control concentrations (Table 3), whilst DMSP concentrations in thermally stressed Davies and Davies Transplanted corals were much more variable (Standard deviations for clade D populations were ~10%–20%; whilst clade C and mixed clade populations often had standard deviations over 50%). DMSP concentrations in Davies Transplanted corals decreased dramatically to 270 nM cm−2 when these corals were stressed at 32 °C (Table 3), and were heavily bleached. These corals contained only 322 zooxanthellae cm−2, compared to DMSP concentrations of 1266 nM cm−2 when Davies Transplanted corals were stressed at 31 °C (249,797 zooxanthellae cm−2).

Table 3.
Areal DMSP concentrations (nmol cm−2) in colonies of native and transplanted Acropora millepora colonies exposed to before, control, 30 °C, 31 °C and 32 °C treatments.
| Coral Colony | Before | Control | 30 °C | 31 °C | 32 °C |
|---|---|---|---|---|---|
| Magnetic Island | 809 ± 290 | 1426 ± 157 | 1529 ± 161 | 1106 ± 116 | 1011 ± 171 |
| Keppels Transplant | 634 ± 444 | 1404 ± 198 | 1165 ± 264 | 1264 ± 424 | 1418 ± 495 |
| Keppels | 738 ± 280 | 1258 ± 610 | 1342 ± 283 | 1103 ± 536 | 1283 ± 777 |
| Davies | 1734 ± 481 | 1455 ± 803 | 2244 ± 575 | 1393 ± 564 | 567 ± 67 * |
| Davies Transplant | 1016 ± 248 | 1100 ± 605 | 1424 ± 731 | 1266 ± 414 | 270 ± 99 * |
* Significant difference between controls and temperature treatments at 32 °C (p < 0.001).
The wide variation in tissue DMSP concentrations in corals (Table 4), also highlighted in Deschaseaux et al. (2014a) [31] and Jones et al. (2014) [39], seems to reflect increasing stress on corals in the GBR from elevated SSTs and solar radiation [32], and often reflect bleached corals. This wide variation in DMSP concentration also seems to reflect changes in different clades of zooxanthellae. What is also highlighted in this study is that control corals can also be “stressed” in the field, and so care is needed when comparing DMSP concentrations in controls, with thermally stressed corals in the laboratory.
3.4. Photosynthetic Yield and DMSP Concentrations
Photosynthetic yields of the Keppel Transplants were not significantly different to those of the native Magnetic Island populations, for control corals and corals at any of the treatment temperatures (30–32 °C) [22]. These two populations had positive Fv/Fm ratios at 32 °C, whilst clade C populations had Fv/Fm ratios of zero (Figure 4a–e). Photosynthetic yield and zooxanthellae densities of transplanted and native Davies populations were not significantly different (p > 0.05) from each other at any of the treatment temperatures or time points during the experiment [22], although Davies and Davies transplant corals bleached at 32 °C, whilst Keppels native corals bleached at 31 °C, and all three populations had very low areal concentrations of DMSP when they bleached (Figure 4c–e), compared with Magnetic Island and Keppels transplanted corals.
When DMSP concentrations, expressed as fmol zooxanthellae−1 and nmol cm−2 are plotted against seawater temperatures we can see that for clade D zooxanthellae from Magnetic Island colonies cellular concentrations of DMSP (Figure 5a) are in relatively narrower concentration range from 27.5 °C to 31 °C, and then increase markedly at 32 °C (p > 0.05). In contrast the areal DMSP concentrations for Magnetic Island and Keppels Transplant clade D corals decrease linearly over this temperature range (Figure 5b) (p < 0.10). Clade C zooxanthellae for Keppels, Davies and Davies Transplant colonies (clade C) increase their cellular DMSP concentrations exponentially (Figure 5c, p < 0.01) from 30 to 31 °C and then bleach severely at 32 °C. In contrast areal DMSP concentrations in clade C populations (Figure 5d) decrease linearly from 30 to 32 °C (p < 0.10).

Table 4.
Cellular DMSP and acrylate concentrations (nmol cm−2) in different coral species collected from different locations and different times from the Great Barrier Reef.
| Region | Date Collected | Species | DMSP Concentration (nmol cm−2) | Author |
|---|---|---|---|---|
| Magnetic Is. Nelly Bay, GBR | 1992 | A. formosa (unbleached) | 237 | Broadbent et al. (2002) [27] |
| A. formosa (bleached) | 572 | |||
| Acropora palifera | 3842 ± 1237 | |||
| Heron Island GBR | 2012 | A. aspera (unbleached) | 97 ± 39 | Deschaseaux et al. (2014a) [31] |
| A. aspera * (bleached-heat stress) | 941 ± 121 | |||
| A. aspera ** (bleached-direct sunlight) | 1820 ± 168 | |||
| Davies Reef, GBR | A. millepora | 247 | Tapiolas et al. (2013) [47] | |
| Trunk Reef, GBR | A. millipora | 2720–4260 *** | Tapiolas et al. (2013) [47] | |
| Magnetic Island | 2011 | A. millipora | 51 ± 4.1 # | Deschaseaux et al. (2014b) [38] |
| Nelly Bay, GBR | A. tenuis (axenic cultures) | 63 ± 4.7 ## | ||
| Heron Island | 2001 | A. intermedia | 124 ± 21 | Jones et al. (2014) [39] |
| Heron Island | 2002 | A. intermedia ### | 544 ± 194 | Jones et al. (2014) [39] |
| Magnetic Island Geoffrey Bay GBR | 2002 | A. millipora (control) | 1426 ± 157 | This study |
* = + 3 °C temperature increase; ** = increased solar radiation; *** = acrylate concentrations (as DMSP is enzymatically cleaved to DMS and acrylic acid in a 1:1 ratio, value quoted could reflect DMSP concentrations); # = axenic cultures of Symbiodinium, clade C (low thermal tolerance); ## = axenic cultures of Symbiodinium, clade D (high thermal tolerance); ### = coral bleaching event (February 2002) and rainfall event (September 2002).
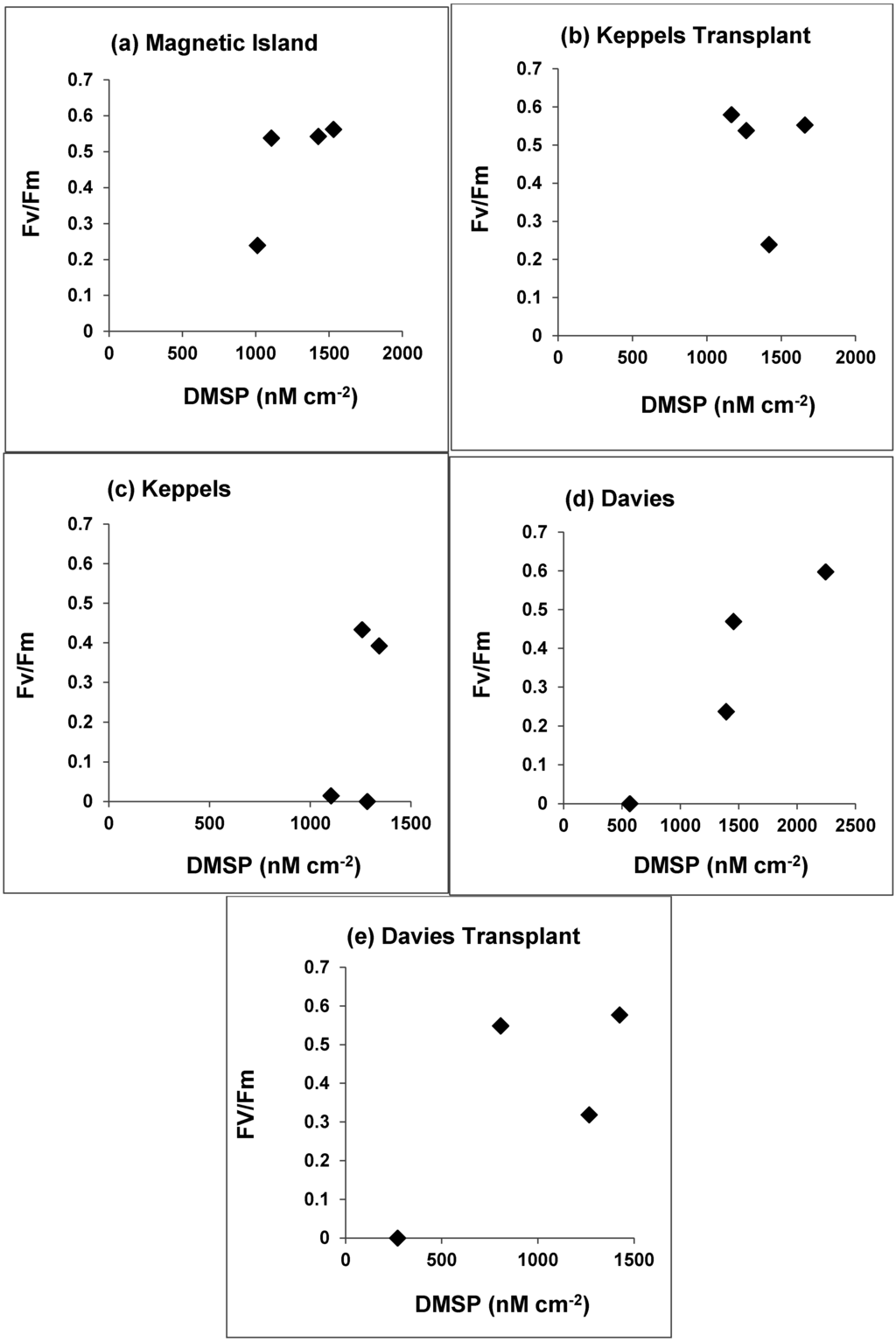
Figure 4.
PAM yield of dark-adapted Acropora millepora colonies and DMSP concentrations of the five coral populations at the end of the time course of the experiment for the four temperature treatments. (Measurements of Fv/Fm are taken from Berkelmans and van Oppen (2006), [22]).
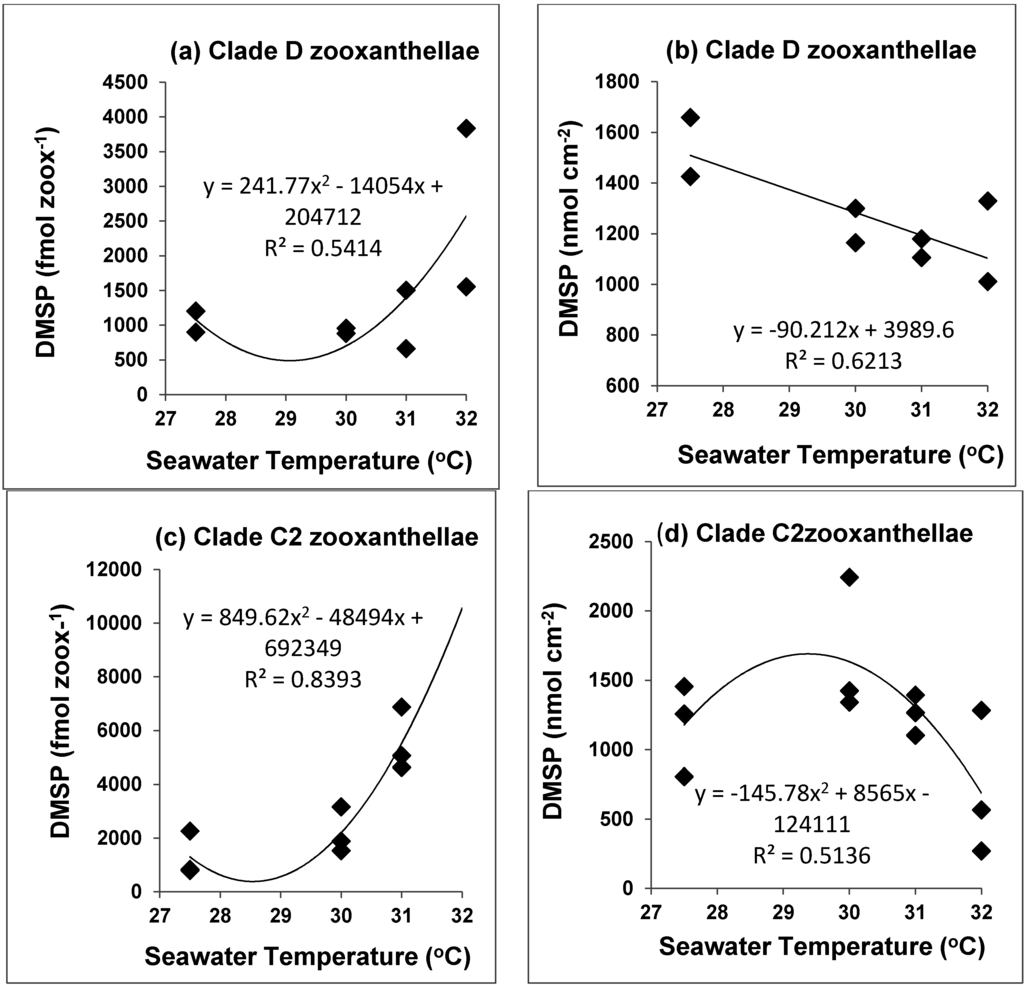
Figure 5.
Change in cellular (fmol zooxanthellae−1) and areal (nM cm−2) DMSP concentrations in (a) Acropora millepora clade D colonies from Magnetic Island and (b) Keppels Transplant colonies; and in clade C zooxanthellae in (c,d) from Keppels, Davies and Davies Transplant colonies at different seawater temperatures.
4. Discussion
4.1. Zooxanthellae Density as a Thermal Stress Indicator
Increased seawater temperature had a more severe effect on Davies, Davies Transplant and Keppels colonies, and a less severe effect on Magnetic Island and Keppels Transplant colonies (see Figure 3). Davies, Davies Transplant and Keppels colonies had the lowest zooxanthellae densities at elevated temperatures, particularly at 32 °C. Magnetic and Keppels Transplant colonies had the highest zooxanthellae densities at 32 °C and the highest survival rate at this elevated temperature [22]. Stimson et al. (2002) [48] and Strychar et al. (2004) [49] have suggested that coral species with naturally high zooxanthellae densities survive bleaching events better than species with low densities, and expelled zooxanthellae are photosynthetically competent [50]. These results suggest similar trends in A. millepora colonies from Magnetic Island and Keppels Transplant colonies which had the highest zooxanthellae densities in the “before” treatments (Figure 3), and had higher overall survivorship when thermally stressed up to 32 °C.
4.2. Photosynthetic Yield and DMSP Concentrations
Thermally induced coral bleaching has been reported to correlate not only with a decrease in zooxanthellae density and/or pigment content [8], but also with declining Fv/Fm values [51]. Healthy corals often have an Fv/Fm ratio varying from 0.5 to 0.6, which decreases during coral bleaching. Berkelmans and van Oppen (2006) [22] state that the photosynthetic yield of the C2 Keppels population diverged and was significantly different from the D and C2* populations (Magnetic Island, Keppel Transplants, Davies native and Davies Transplants) after 11 days at 30 °C, and after seven days at 31 °C (see their Figure 4 and Table 2). They state further that similarly, the C2* Davies Reef corals were more sensitive than the D-dominant Magnetic Island and Keppels Transplant corals, with photosynthetic yield significantly different after seven days at 31 °C, and after three days at 32 °C. In our study Magnetic Island and Keppels Transplant corals after 15 days, always had positive Fv/Fm ratios at 32 °C and high DMSP concentrations (Figure 4a,b), whilst Fv/Fm ratios for clade C populations were zero at 32 °C and displayed much lower DMSP concentrations than clade D zooxanthellae (Figure 4c–e).
4.3. DMSP and Different Symbiodinium Clades
Magnetic Island, Keppels Transplant and Keppels native colonies generally had similar areal DMSP concentrations across all treatments (Table 3), with the former two sites having the highest number of healthy cells across all temperature treatments (Figure 3), and the greatest thermotolerance. The Magnetic Island population was the only population that always possessed clade D zooxanthellae, whilst Keppels Transplanted colonies changed to clade D after transplantation (Table 1, [22]). Conversely, Davies and Davies Transplant populations had significantly different (p < 0.001) lower areal DMSP concentrations at 32 °C compared with controls (Table 3). The Davies colonies had clade C2, whilst Davies Transplant colonies contained C2* zooxanthellae, with these colonies having the lower survivorship when thermally stressed [22].
4.4. DMSP as a Thermal Stress Indicator
The relatively stable areal DMSP concentrations in Magnetic Island, Keppels, Keppels Transplanted coral colonies for control, 30–32 °C treatments (Table 3) may suggest that clade D zooxanthellae are more able to maintain relatively constant concentrations of DMSP, as areal DMSP concentrations decreased by only ~90 nmol DMSP cm−2 °C−1 (Figure 5b), compared with 146 nmol cm−2 °C−1 for clade C zooxanthellae (Figure 5d). During the linear decrease in DMSP from 30 to 32 °C, DMSP decreased by 482 nmol cm−2 °C−1 for clade C zooxanthellae (Figure 5d). Consequently clade D zooxanthellae, and possibly mixed clade populations of zooxanthellae (C and D), may be better able to scavenge the build-up of oxygen free radical concentrations in the coral tissue as temperatures increase to 32 °C, as their total areal (coral) DMSP concentrations do not change appreciably over this temperature range. Our results strongly suggest that corals with clade D zooxanthellae are able to keep DMSP concentrations fairly constant, possibly by enhanced host production of DMSP when the coral is thermally stressed [37], and this seems to increase the corals thermal tolerance. The correlations for cellular DMSP concentrations versus seawater temperatures (Figure 5a,c) suggests that the exceptionally high cellular concentrations at 32 °C could reflect host production of DMSP for both clade D and clade C zooxanthellae. Clearly care is needed in selecting the right cellular or tissue index when ascribing changes in coral tissue DMSP [31,39].
4.5. DMSP as an Antioxidant
There is evidence from this study to further support the role of DMSP as an antioxidant in the context of thermal bleaching of corals [31,39]. For Magnetic Island and Keppels Transplant corals there was no significant difference (p > 0.05) in the areal DMSP concentrations in control corals and the thermally stressed corals at 30–32 °C. At 32 °C when corals normally bleach the zooxanthellae in these two populations were 650,645 zooxanthellae cm−2 and 369,734 zooxanthellae cm−2, respectively (Figure 3). At 32 °C areal DMSP concentrations for Keppels, Davies and Davies Transplant corals containing clade C2 zooxanthellae were generally higher and more variable than areal DMSP concentrations in Magnetic Island and Keppels Transplant colonies across all treatments. This variability may have reflected the mixed populations of clade C and clade D zooxanthellae in these coral colonies and the different concentrations of DMSP in clade C and D zooxanthellae [38], and possible variations in mucus production, which has extremely high DMSP levels in stressed corals [30].
The overall increase in zooxanthellar DMSP within the five coral populations at 31° and 32 °C suggests that DMSP could perform antioxidant functions during bleaching at these temperatures. Increasing the seawater temperature from “control” (27.5 °C) to 32 °C most likely increased the production of oxygen free radicals in the coral tissues. The marked increase in cellular DMSP in clade C2 zooxanthellae from Davies, Davies Transplant and Keppels corals may have been a stress response due to the build-up of oxygen free-radicals (Sunda et al. 2002) [25]. The more constant and less variable areal DMSP concentrations at elevated temperatures for Magnetic and Keppels Transplant colonies, could suggest that as clade D zooxanthellae produces more DMSP under stress these symbionts are better able to scavenge the build-up of oxygen free-radicals at high temperatures of 32 °C.
A conceptual diagram demonstrating the biochemical processes thought to be involved with coral bleaching and antioxidant quenching from DMSP is shown in Figure 6. Based on the findings of Jones et al. (1998) [16] an overdose of heat and light from increased radiation causes an over production of energy (ATP and NADPH reductase) by the light reactions in the grana (shown as multiple arrows in Figure 6) and which are precursors of DMSP production [52]. These energy products cannot be reduced quickly enough by the dark reactions of the Calvin cycle, causing it to shut down, which then creates a buildup of energy [16] (Figure 6). This causes the light reactions to create an over-reduced environment and rather than producing molecular oxygen, oxygen free radicals are formed (Figure 6). As ATP and NADPH reductase levels build up, this could increase the production of DMSP [52]. Normally, organelle degeneration would follow but according to the hypotheses of Sunda et al. (2002) [25] DMSP and DMS scavenge the radicals, oxidizing these reduced S-compounds to DMSO (Figure 6), a result highlighted by Deschaseaux et al. (2014a) [31]. However, why are clade D zooxanthellae more thermotolerant than clade C2 zooxanthellae. The answer may be related to the lower rates of DMSP oxidation in clade D zooxanthellae, compared to clade C zooxanthellae, when exposed to increased seawater temperatures. Accumulating evidence suggests that chloroplast heat-shock proteins (Hsps) are also involved in photosynthetic and PS11 thermotolerance [53], and these proteins are rich in methionine, a precursor for the production of DMSP [52]. Hsps can be induced by manipulating N availability, and are positively correlated with increased thermotolerance of PS11 [53]. The inducement of these Hsps could be occurring in native colonies of A millepora in the nitrogen-rich waters of Magnetic Island (total nitrogen levels are almost double those reported for unpolluted reef sites) [54], but further studies are necessary to confirm this. Clearly further studies are also necessary on areal and zooxanthellar DMSP in different coral colonies containing different clades, as well as on corals from nitrogen-rich and nitrogen poor waters; and how areal and zooxanthellar DMSP and extracellular production of DMS and DMSO change, when corals are thermally stressed.
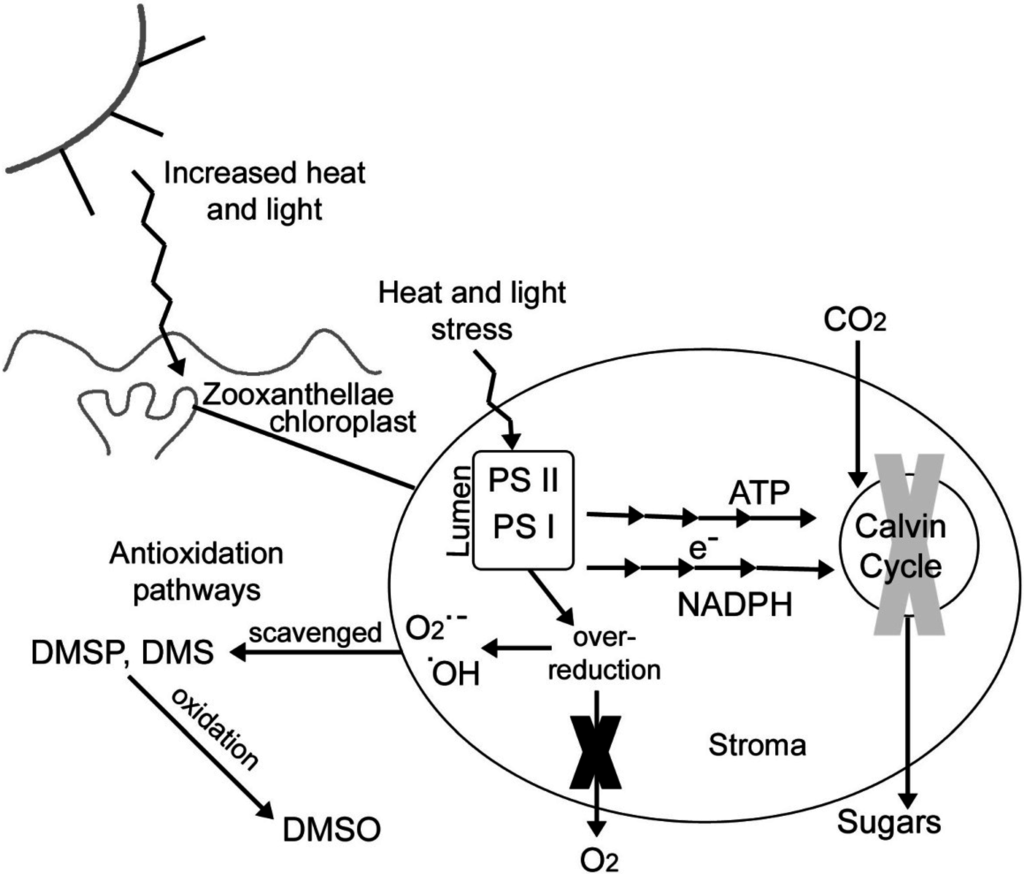
Figure 6.
Conceptual diagram of oxidative stress processes and possible antioxidant roles of DMS, DMSP and DMSO in coral.
Acknowledgments
We would like to thank Ray Berkelmans at AIMS for preserving and sending the coral samples to Southern Cross University (SCU) for DMSP analysis and providing the zooxanthellae counts for Figure 3. Darren Fortescue of the Environmental Analytical Laboratory (EAL) at SCU is also thanked for assistance during this study. We thank the reviewers of this manuscript for their constructive comments.
Author Contributions
Main text paragraph (M_Text). G. B. Jones conceived the project. S. King analysed the coral samples by gas chromatography. G.B. Jones and S. King wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berkelmans, R.; Oliver, J.K. Large-scale bleaching of corals on the Great Barrier Reef. Coral Reefs 1999, 18, 55–60. [Google Scholar] [CrossRef]
- Harriot, V.J. Mortality rates of scleractinian corals before and during a mass bleaching event. Mar. Ecol. Prog. Ser. 1985, 21, 81. [Google Scholar] [CrossRef]
- Jones, R.J. Changes in zooxanthellar densities and chlorophyll concentrations in corals during and after a bleaching event. Mar. Ecol. Prog. Ser. 1997, 158, 51–59. [Google Scholar] [CrossRef]
- Jones, R.J. Zooxanthellae loss as a bioassay for assessing stress in corals. Mar. Ecol. Prog. Ser. 1997, 149, 163–171. [Google Scholar] [CrossRef]
- Jones, R.J.; Berkelmans, R.; Oliver, J.K. Recurrent bleaching of corals at Magnetic Island (Australia) relative to air and seawater temperature. Mar. Ecol. Prog. Ser. 1997, 158, 289–292. [Google Scholar] [CrossRef]
- Berkelmans, R.; De’ath, G.; Kininmonth, S.; Skirving, W.J. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: Spatial correlation, patterns, and predictions. Coral Reefs 2004, 23, 74–83. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Brown, B.E.; le Tissier, M.D.A.; Bythell, J.C. Mechanisms of bleaching deduced from histological studies of reef corals sampled during a natural bleaching event. Mar. Biol. 1995, 122, 655–663. [Google Scholar] [CrossRef]
- Douglas, A.E. Coral bleaching-how and why? Mar. Poll. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Ahern, H.; Ahmad, S.; Birnbaum, M.R.; Cadenas, E.; Chen, Y.; Cunningham, R.P.; Dalton, D.A.; Felton, G.W.; Grisham, M.B.; Kalyanaraman, B.; et al. Oxidative Stress and Antioxidant Defenses in Biology; Chapman & Hall: New York, NY, USA, 1995. [Google Scholar]
- Fridovich, I. The biology of oxygen radicals. Science 1978, 201, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Shick, J.M. Oxygen production by endosymbiotic algae controls superoxide dismutase activity in their animal host. Nature 1982, 297, 579–580. [Google Scholar] [CrossRef]
- Franklin, D.J.; Hoegh-Guldberg, O.; Jones, R.J.; Berge’s, J.A. Cell death and degeneration in the symbiotic dinoflagellates of the coral Stylophora pistillata during bleaching. Mar. Ecol. Prog. Ser. 2004, 272, 117–130. [Google Scholar] [CrossRef]
- Lesser, M.P. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 1996, 41, 271–283. [Google Scholar] [CrossRef]
- Downs, C.A.; Fauth, J.E.; Halas, J.C.; Dustan, P.; Bemiss, J.; Woodley, C.M. Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 2002, 33, 533–543. [Google Scholar] [CrossRef]
- Jones, R.J.; Hoegh-Guldberg, O.; Larkum, A.W.D.; Schreiber, U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 1998, 21, 1219–1230. [Google Scholar] [CrossRef]
- Lesser, M.P.; Stochaj, W.R.; Tapley, D.W.; Shick, J.M. Bleaching in coral reef anthozoans: Effect of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 1990, 8, 225–232. [Google Scholar] [CrossRef]
- Rowan, R.; Powers, D.A. Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar. Ecol. Prog. Ser. 1991, 71, 65–73. [Google Scholar] [CrossRef]
- Loh, W.; Carter, D.; Hoegh-Guldberg, O. Diversity of zooxanthellae from scleractinian corals of One Tree Island (The Great Barrier Reef). In Proceedings of the Australian Coral Reef Society 75th Anniversary Conference, School of Marine Science, University of Queensland, Brisbane, Heron Island, Austrilia, October 1997; Greenwood, J.G., Hall, N.J., Eds.; pp. 141–151.
- Baker, A. Reef corals bleach to survive change. Nature 2001, 411, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Rowan, R.; Knowlton, N.; Baker, A.; Jara, J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 1997, 388, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Berkelmans, R.; van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: A “nugget of hope” for coral reefs in an era of climate change. Proc. R. Soc. B 2006, 273, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Lam, K.K.; Nakano, Y.; Tsai, W.S. A stable association of the stress-tolerant zooxanthellae, Symbiodinium clade D, with the low-temperature-tolerant coral, Oulastrea crispate (Scleractinia: Faviidae) in subtropical non-reefal coral communities. Zool. Stud. 2003, 42, 540–550. [Google Scholar]
- Buddemeier, R.W.; Fautin, D.G. Coral bleaching as an adaptive mechanism: A testable hypothesis. Bioscience 1993, 43, 320–326. [Google Scholar] [CrossRef]
- Sunda, W.; Keiber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; de Mora, S.J. Intracellular dimethylsulfoxide (DMSO) in unicellular marine algae: Speculations on its origin and possible biological role. J. Phycol. 1999, 35, 8–18. [Google Scholar] [CrossRef]
- Broadbent, A.D.; Jones, G.B.; Jones, R.J. DMSP in corals and benthic algae from the Great Barrier Reef. Estuar. Coast. Shelf Sci. 2002, 55, 547–555. [Google Scholar] [CrossRef]
- Hill, R.W.; Dacey, J.W.H.; Krupp, D.A. Dimethylsulfoniopropionate in Reef Corals. B Mar. Sci. 1995, 57, 489–494. [Google Scholar]
- Jones, G.B.; Curran, M.A.J.; Broadbent, A.D. Dimethylsulphide in the South Pacific. In Recent Advances in Marine Science and Technology; Bellwood, O., Choat, H., Saxena, N., Eds.; Pacon International and James Cook University of North Queensland: Townsville, Australia, 1994; pp. 183–190. [Google Scholar]
- Broadbent, A.D.; Jones, G.B. DMS and DMSP in mucus ropes, coral mucus, surface films and sediment pore waters from coral reefs in the Great Barrier Reef. Mar. Freshw. Res. 2004, 55, 849–855. [Google Scholar] [CrossRef]
- Deschaseaux, E.S.M.; Jones, G.B.; Deseo, M.A.; Shepherd, K.M.; Kiene, R.P.; Swan, H.B.; Harrison, P.L.; Eyre, B.D. Effects of environmental factors on dimethylated sulfur compounds and their potential role in the antioxidant system of the coral holobiont. Limnol. Oceanogr. 2014, 59, 758–768. [Google Scholar] [CrossRef]
- Fischer, E.; Jones, G.B. Atmospheric dimethysulphide production from corals in the Great Barrier Reef and links to solar radiation, climate and coral bleaching. Biogeochemistry 2012, 110, 31–46. [Google Scholar] [CrossRef]
- Jones, G.B. The ethnobiology of coral and coral reefs. In The Reef Sulphur Cycle: Influence on Climate and Ecosystem Services; Narchi, N., Price, L., Eds.; Springer: Berlin, Germany, 2015. (in press) [Google Scholar]
- Jones, G.B.; Trevena, A.J. The influence of coral reefs on atmospheric dimethylsulphide over the Great Barrier Reef, Coral Sea, Gulf of Papua, Solomon and Bismarck Seas. Mar. Freshw. Res. 2005, 56, 85–93. [Google Scholar] [CrossRef]
- Modini, R.L.; Ristovski, Z.D.; Johnson, G.R.; He, C.; Surawski, N.; Morawska, L.; Suni, T.; Kulmala, M. New particle formation and growth at a remote, sub-tropical coastal location. Atmos. Chem. Phys. Discuss. 2009, 9, 12101–12139. [Google Scholar] [CrossRef]
- Swan, H.; Jones, G.; Deschaseaux, E. Dimethylsulfide and coral reef ecosystems. In Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, 9–13 July 2012.
- Raina, J.B.; Tapiolas, D.M.; Forêt, S.; Lutz, A.; Abrego, D.; Ceh, J.; Seneca, F.O.; Clode, P.L.; Bourne, D.G.; Willis, B.L.; et al. DMSP biosynthesis by an animal and its role in coral thermal stress response. Nature 2013, 502, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Deschaseaux, E.S.M.; Beltran, V.H.; Jones, G.B.; Deseo, M.A.; Swan, H.B.; Harrison, P.L.; Eyre, B.D. Comparative response of DMS and DMSP concentrations in Symbiodinium clades C1 and D1 under thermal stress. J. Exp. Mar. Biol. Ecol. 2014, 459, 181–189. [Google Scholar] [CrossRef]
- Jones, G.B.; Fischer, E.; Deschaseaux, E.S.M.; Harrison, P.L. The effect of coral bleaching on the cellular concentration of dimethylsulphoniopropionate in reef corals. J. Exp. Mar. Biol. Ecol. 2014, 460, 19–31. [Google Scholar] [CrossRef]
- Berkelmans, R. Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 229, 73–82. [Google Scholar] [CrossRef]
- Turner, P.; Berkelmans, R.; Brodie, M. Precise set-point control of temperature for coral bleaching experiments. Mar. Technol. Soc. J. 2002, 36, 70–75. [Google Scholar] [CrossRef]
- Ulstrup, K.E.; van Oppen, M.J.H. Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol. Ecol. 2003, 12, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, M.J.H.; Palstra, F.P.; Piquet, A.M.-T.; Miller, D.J. Patterns of coral-dinoflagellate associations in Acropora: Significance of local availability and physiology of Symbiodinium strands and host-symbiont selectivity. Proc. R. Soc. Lond. B 2001, 268, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Fabricus, K.E.; Mieog, J.C.; Colin, P.L.; Idip, D.; van Oppen, M.J.H. Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol. Ecol. 2004, 13, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Stimson, J.; Kinzie, R.A., III. The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen enrichment and control conditions. J. Exp. Mar. Biol. Ecol. 1991, 153, 63–74. [Google Scholar] [CrossRef]
- Curran, M.A.J.; Jones, G.B.; Burton, H. Spatial distribution of DMS and DMSP in the Australasian sector of the Southern Ocean. In: Special Issue on the Southern Hemisphere Marine Aerosol Characterisation Experiment (ACE 1). J. Geophys. Res. 1998, 103, 16677–16689. [Google Scholar] [CrossRef]
- Tapiolas, D.M.; Raina, J.B.; Lutz, A.; Willis, B.L.; Motti, C.A. Direct measurement of dimethylsulphoniopropionate (DMSP) in reef-building corals using quantitative nuclear magnetic resonance (qNMR) spectroscopy. J. Exp. Mar. Biol. Ecol. 2013, 443, 85–89. [Google Scholar] [CrossRef]
- Stimson, J.; Sakai, K.; Sembali, H. Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs 2002, 21, 409–421. [Google Scholar]
- Strychar, K.B.; Coates, M.; Sammarco, P.W. Loss of Symbiodinium from bleached Australian scleractinian corals (Acropora hyacinthus, Favites complanata and Porites solida). Mar. Freshw. Res. 2004, 55, 135–144. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R.; Larkum, A.W.D. Zooxanthellae expelled from bleached corals at 33 degrees C are photosynthetically competent. Mar. Ecol. Prog. Ser. 2001, 220, 163–168. [Google Scholar] [CrossRef]
- Jones, R.J.; Hoegh-Guldberg, O. Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: Photoprotection, photo-inactivation and the relationship to coral bleaching. Plant Cell Environ. 2001, 24, 89–99. [Google Scholar] [CrossRef]
- Andreae, M.O. Ocean-atmosphere interactions in the global biogeochemical sulphur cycle. Mar. Chem. 1990, 30, 1–29. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Downs, C.A.; Sharkey, T.D.; Coleman, J.S. The small, methionine-rich chloroplast heat-shock protein protects photosystem 11 electron transport during heat stress. Plant Physiol. 1998, 116, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Muslim, I.; Jones, G.B. The seasonal variation of dissolved nutrients, chlorophyll a and suspended sediments at Nelly Bay, Magnetic Island. Estuar. Coast. Shelf Sci. 2003, 57, 445–455. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).