Abstract
The adoption of low-carbon fuels in maritime propulsion requires operational autonomy, material suitability, and compliance with safety standards, making liquid fuels like LNG and LH2 the most viable options. LNG is widely used for reducing GHG, NOx, and SOx emissions, while LH2, though new to the maritime sector, leverages aerospace experience. This paper explores the operational requirements and challenges of LH2 cryogenic handling systems using LNG practices as a reference. Key comparisons are made between LNG and LH2 supply systems, focusing on cryogenic materials, hydrogen embrittlement, and structural integrity under maritime conditions. Most maritime-approved materials are suitable for cryogenic use, and hydrogen embrittlement is less critical at cryogenic temperatures due to reduced atomic mobility. Risk assessments suggest LH2’s safety record stems from limited operational data rather than superior inherent safety. The paper also addresses crucial safety and regulatory considerations for both fuels, underscoring the need for strict adherence to standards to ensure the safe and compliant integration of LH2 in the maritime industry.
1. Introduction
In these days, hydrogen is mostly stored and handled in compressed form, the reason being the simpler technological requirements with respect to liquefied, cryo-compressed, or chemical-based storage (in form of methanol, ammonia, liquid hydrogen carriers, etc.). In some cases, metal hydrides are preferred thanks to the lower pressures required, but in this case the storage is weighty and the adsorption/desorption reactions are energy intensive and require thorough thermal management. In this scope, liquefied hydrogen is deemed among the most suitable forms of physical-based hydrogen storage for maritime applications, thanks to its unmatched gravimetric energy density and no need for chemical conversion prior to utilization in prime movers or power generation systems [1]. LH2 is most commonly used in aerospace applications, where the combination of high energy density and low weight represents the best solution for rockets and space launchers. Therefore, the technological maturity achieved in these fields indicates the potential for technology transfer to more commercially relevant sectors, such as power generation and transports [1].
In the context of the maritime industry, it appears that this is a two-sided issue. While it is imperative that industry and regulatory bodies leverage the extensive knowledge accumulated in the domain of LNG, a sector with a more established presence, it is evident that hydrogen is a distinct molecule with its own physical characteristics. Hence, challenges associated with storage, handling, and safety should be taken into careful consideration and tackled with a quite different procedure, while the measures undertaken with LNG should in fact be regarded just as a mere starting point for future maritime uptake.
Hydrogen is widely explored as an alternative fuel for maritime transport. Thanks to their efficiency, fuel cells are expected to be a key technology in decarbonizing the maritime sector, both in PEMFC (proton exchange membrane fuel cells) and SOFC (solid oxide fuel cells) architectures [2]. An extensive number of projects is currently dealing with fuel cell integrations onboard, especially using PEMFCs coupled with batteries or SOFCs for hoteling or auxiliary power units [2].
Despite being the most abundant element in the universe, hydrogen must be obtained from molecules at a cost in terms of both energy and emissions. Methane reforming is the most common hydrogen production method: Out of 97 Mt total production in 2023, more than 80% was sourced from high-carbon processes (more than 60% from unabated methane reforming and around 20% from coal gasification) [3] at an average SEC (specific energy consumption) of 46.2 kWhth/kg [4]. Water electrolysis is considered the benchmark technology for zero-emission production. However, the total installed electrolyzer capacity is a mere 1.4 GW worldwide, of which less than 20% is actually operational [3]. It is expected that the ongoing research and commitment to sustainable energy production will increase the share of alternative fuels obtained from green sources.
This paper investigates the suitability of liquefied natural gas (LNG) and liquid hydrogen (LH2) for maritime propulsion, with the aim of providing a comparison of their technical performance, operational challenges, material compatibility, and safety challenges in order to assess their roles in the sector’s transition toward low-carbon shipping.
2. Overview of Cryogenic Fuel Utilization in Maritime Applications
A general state-of-the-art assessment for LNG and LH2 hydrogen handling systems in the marine environment depicts a general two-fold situation.
LNG is a well-established technology in the marine sector from container ships to cruise ships, having a high TRL and being the preferred alternative for short-term decarbonization. Although it is demanding in terms of components and materials for the handling system owing to the cryogenic temperatures, the wider and wider uptake fosters market solidity and availability. Moreover, natural gas-running prime movers count on long time technological maturity, irrespective of the fuel source. Hence, LNG is a ready-to-go alternative for immediate uptake of mass-produced low emission power solutions. Furthermore, established engineering best practices exist for LNG applications [5].
Hydrogen in gaseous form still has limited uptake in the marine sector, and no concrete examples in liquefied form. While conventional fossil fuels still play a key role alongside the newcomer LNG in deep-sea segments thanks to their energy density, short-sea segments may benefit more from hydrogen penetration in the near future. However, despite some prototypes that have been constructed and tested, the Technology Readiness Level (TRL) is low, and components and materials must be designed to withstand extreme conditions such as high pressures in the case of GH2 storage or cryogenic temperatures in the case of liquefied hydrogen (LH2). Hence, the criticalities existing for LNG are even worse for hydrogen, especially when hydrogen storage in liquefied form is considered. Furthermore, achieving equivalent onboard energy storage necessitates significantly increased tank volumes, which introduces constraints on vessel layout and has direct implications for ship design, including reduced payload capacity, altered hull geometry, and complex integration of cryogenic containment systems. Operationally, these storage penalties translate into shorter voyage ranges or the need for more frequent refueling while also introducing safety, boil-off management, and efficiency considerations that are less pronounced in LNG systems.
Despite this evident difference in technological advancement, several analogies can be found between the best practices adopted with the two liquid cryogenic fuels, and the growing experience with LNG may prove crucial to overcome the main challenges associated with onboard cryogenic hydrogen handing.
2.1. Liquefied Natural Gas (LNG)
Natural gas is mainly composed of methane, but in general it is a mixture of hydrocarbons with different LHVs, densities, and boiling points, as the composition varies consistently depending on the geographical zone of extraction. However, a content of ~90% methane is generally considered realistic on average [6]. In Table 1 the thermophysical features of natural gas are reported (pure methane has higher LHV and lower density).

Table 1.
Main thermophysical properties of LNG.
Natural gas in its liquefied form (LNG) represents the current state-of-the-art for deep-sea and cruise ships targeting GHG emissions reduction. As it is clear from Figure 1, most vessels still run on conventional fuels, but LNG is currently the first alternative either for ships already in operation or for new construction. This is a consequence of the maturity of the natural gas market, of the high TRL of LNG production, storage, and utilization and, lately, it also has some geopolitical implications. Moreover, natural gas could even be sourced from biomass valorization. Biomethane has gained more and more attention in transportation thanks to its reduced carbon footprint as compared to natural gas [7], but its utilization on large scale maritime transport is questionable due to its limited supply and the current difficulty of expanding scalability to the same volumes as fossil LNG.
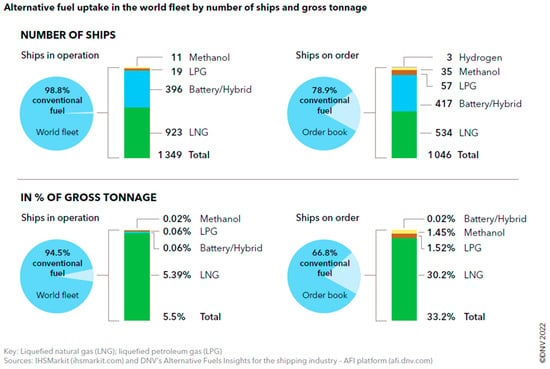
Figure 1.
Alternative fuel uptake in the maritime sector [8].
Today, LNG-powered ships make use of three types of tanks, according to IMO classification (IGC code): Type A, employing a prismatic design and a complete secondary barrier, non-pressurized. These derive directly from standard oil and bulk tankers and are typically shaped as the cross-section of the vessel they equip; Type B, employing spherical (Moss type) or prismatic design and a partial secondary barrier, non-pressurized; Type C, cylindrical-shaped mono-lobed or two-lobed, pressurized.
Type A and Type B designs are used for bulk LNG tankers or generally for bulk transporters, while the third is generally used for smaller vessels with LNG propulsion [8]. Spherical Type B vessels are mostly used by Japanese LNG carriers, while European tankers tend to rely on membrane Type A tank [9]. A visual comparison of the three designs is reported in Figure 2. Comparing Type A and Type C tanks, the former have 30 to 40% greater volume efficiencies [9].
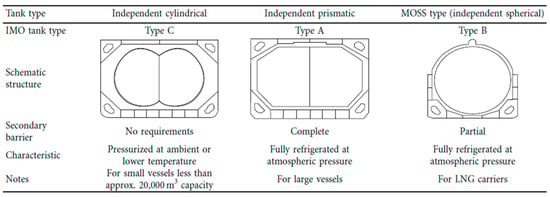
Figure 2.
Outlook of tank types for onboard LNG storage.
LNG tankers sometimes employ membrane tanks, namely a sandwich construction in such a fashion that the ship hull becomes the outer tank, and the insulation is placed in between the hull and the internal membranal liner. Sometimes, a secondary membrane made of composite material is exploited. This design allows for quicker and easier assembly onboard, but it can only suit vessels where the cryogen constitutes the payload.
2.2. Liquefied Hydrogen (LH2)
Hydrogen in cryogenic liquefied form has favorable properties that make it convenient for transportation, a sector in which high energy density is typically required. As opposed to any other hydrogen carrier (ammonia, LOHC etc.), it does not require any conversion before its utilization in a prime mover. However, some serious challenges are associated with cryogenic storage onboard, beginning with evaporation control and boil-off handling. In Table 2 the relevant properties of hydrogen are reported and compared to that of methane.

Table 2.
Thermophysical properties of hydrogen and methane. Obtained from REFPROP [10].
It is evident that methane is around six times denser than hydrogen and easier to handle in cryogenic form thanks to its higher boiling point. Indeed, the lower the boiling point, the more difficult it is to maintain the cryogenic gas in liquefied form, meaning that the evaporated BOG is more relevant in terms of percentage of stored fuel mass (BOR, boil-off rate, has units [kgBOG/kgLH2]).
The utilization of liquefied hydrogen sets an additional challenge. In ambient conditions, a hydrogen molecule consists of 75% ortho-hydrogen (o-H2) and 25% para-hydrogen (p-H2). The latter is also named normal hydrogen. The two forms differ only in their atomic structures; namely, the protons have the same (parallel) or opposite (antiparallel) spin, resulting in higher or lower internal energy, respectively. At equilibrium, LH2 consists of 100% para-hydrogen, p-H2. Ortho-hydrogen, o-H2, is thermodynamically unstable at low temperatures and spontaneously converts to p-H2, releasing 527 kJ/kg of heat [11], which is more than the latent heat of vaporization of LH2 (446 kJ/kg) at ambient pressure. This means that the liquefaction of normal hydrogen results in a spontaneous exothermic conversion of the bulk liquid to para-hydrogen, releasing an amount of heat sufficient to vaporize back some mass. (At equilibrium, the reaction heat would exceed the vaporization heat, resulting in full re-gasification. However, the slow kinetics reduce the effective rate of reaction, limiting the re-vaporization impact). The technical solution consists in promoting ortho- to para-conversion prior to condensation by means of a catalytic metal-based ortho-para reactor inside the liquefaction plant [11]. Despite this clear hindrance to using hydrogen in a liquefied form, it does not represent an issue as long as the bulk liquid is maintained below the para-ortho inversion temperature, which sits around 100 K. On the contrary, this improves dormancy, since LH2 is converted from p-H2 to o-H2 through an endothermic reaction during heating up. However, this is not sufficient to prevent boil-off, as the kinetics of p-H2 to o-H2 conversion are considerably slower than vaporization. Nevertheless, some studies consider a catalytic para-ortho converter as a potential enhancer of thermal insulation for improved dormancy [12,13,14]. Nevertheless, hydrogen reliquefaction is energy-intensive and, especially for transportation, this often proves anti-economical. Specific Energy Consumption (SEC) values obtained from numerical simulations are generally around 6–8 kWh/kgH2, while real plant SEC sits around 12–15 kWh/kgH2, confirming the large discrepancy between simulations and real industrial liquefiers [11,15,16]. However, to prove the interest in such novel fuels, the research is also exploring the techno-economics of LH2 uptake in the maritime sector [17,18]. General results show that such fuel is still far from being economically attractive when compared to other alternatives, further penalizing its current appeal for ship makers. However, it is known that some shipbuilders are actually willing to pursue their commitment towards a hydrogen powered passenger ship [19].
Aside from the maritime sector, LH2 is also an interesting alternative for aircraft applications, since it guarantees the highest range as compared to any other hydrogen storage methods. This interest is confirmed by several projects ongoing on this topic. Airbus is investigating hydrogen powered aircraft within the ZEROe project [20], exploring the feasibility of three different aircraft designs. Furthermore, they have accumulated considerable expertise in the domain of aerospace engineering, evidenced by their involvement in the development of liquid hydrogen fuel systems for spaceflight vehicles. Furthermore, Airbus is also involved in the investigation of modified ground operations, aiming to develop essential technologies and standards for hydrogen refueling at airports [21]. Early findings confirm that a dedicated LH2 refueling area should be provided at airports, rather than having LH2 trucks moving around the apron. Rolls-Royce has partnered with other relevant companies in the sector to develop hydrogen combustion aero engines for both medium and short haul aircraft [22]. In the aviation sector, however, the large volume required by hydrogen storage appears to be the most challenging obstacle, especially for long haul aircraft. Therefore, researchers focus on short-haul aircraft or rotorcraft [23], also highlighting the potential for recuperated cycles to improve specific fuel consumption.
3. Storage and Handling Systems for Maritime Cryogenic Fuels
Conceptually, two alternatives are possible for LNG and cryogenic fuels in general: a conventional pump-driven system or a pump-free layout with a pressure buildup (PBU) unit. In the first case, the liquefied gas is drawn from a pump, usually submerged and insulated, and conveyed to the vaporizer via vacuum-insulated piping. In the second system, the cryogenic tank is pressurized via a pressure buildup system, which regulates the natural boil-off tendency via the thermal load from the PBU heat exchanger. Figure 3 depicts the conceptual scheme of both alternatives. The pump-driven system allows for higher delivery pressures and wider range of deliverable mass flows to the user, but includes a critical, expensive, and low-TRL component like a cryogenic pump. The PBU-driven system offers lower performance in terms of maximum pressure achievable, but it is simpler and allegedly more reliable. In addition, the pressurized layout allows for slightly higher storage temperatures and possibly less stringent insulation, even though saturation temperature and consequently BOG rates only have minor dependence on storage pressure, especially in the case of liquefied hydrogen, as shown in Table 3.
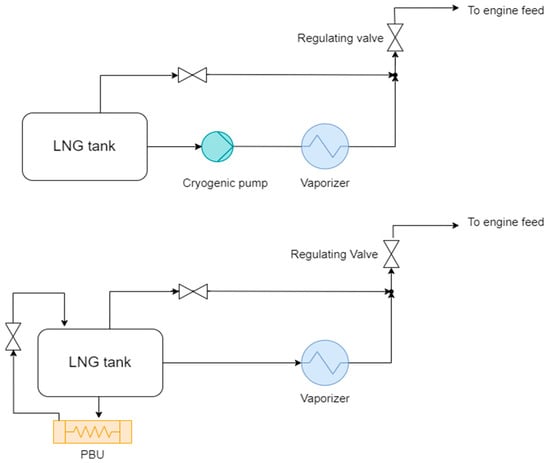
Figure 3.
Conceptual schemes of cryogenic fuel handling layouts: pump-driven (top) and pressure-driven with PBU (bottom) systems.

Table 3.
Hydrogen and natural gas saturation point variation with storage pressure. Obtained from REFPROP [10].
3.1. LH2 System Availability and Comparison with LNG
The distance between LNG and liquid hydrogen for maritime use is net and clear, especially in terms of technology readiness level (TRL), both at the component level and the full system level. On the one hand, maritime LNG components and systems are offered by a number of manufacturers, based on both reciprocating pumps and submerged turbopumps for a wide range of nominal mass flows. For instance, both Wartsila and MAN Cryo offer a complete and scalable assembly with a cryogenic pump and Type C tank up to 9 bar storage pressure in the first case [24] and a PBU configuration in the second case [25].
On the other hand, the availability of LH2 full systems is limited. Nonetheless, there are some products available on the market for LH2. Linde specializes in storage systems and transfer lines for refueling stations, while Cryostar and Nikkiso focus more on end user applications with a wide range of reciprocating pumps and vaporizers [26,27]. Chart Industries also offers a wide range of cryogenic pumps, heat exchangers, and cryogenic storage tanks [28]. At present, Cryostar is the only known manufacturer to be offering submerged transfer pumps for liquefied hydrogen [26], with mass flow ratings up to 500 m3/h (~10 kg/s). It is reported that a superyacht constructed by Feadship will be the first vessel to have a completely below-deck liquid hydrogen storage and vaporization system [29]. Additionally, a group of partners including RINA, Wartsila, and Helbio have studied and proposed a solution for the onboard production of hydrogen based on LNG reforming [30]. The hydrogen from syngas is used in a mix with vaporized natural gas, eliminating the need for storage. The CO2 is liquefied via the cold LNG stream and stored in a tank for shore disposal. Alternatively, it may be used as an inert fluid for onboard purposes [30].
From Table 4, it is evident that a large delay in LH2 technology readiness exists compared to LNG for maritime applications. Simple construction elements, such as piping, heat exchangers, and tanks are unlikely to be significant impediments to the uptake of liquid hydrogen. However, technological challenges, particularly those related to pumps and pipe fittings, are likely to hinder the advancement of this field. DNV has outlined an expected maturation timeline for energy converters and corresponding safety regulations for the onboard use of alternative fuels [31].

Table 4.
Current Technology Readiness Levels (TRL) of the main components in a cryogenic fuel handling system.
3.2. The Problem of Boil-Off (BOG): Handling Methods and Comparison with LNG
Regardless of insulation performance, all gases stored in liquefied form will generate a certain amount of boil-off gas (BOG), building pressure inside the tank. To prevent tank failure, such evaporated fractions must be removed. Therefore, it is crucial to understand the best solutions for making use of the BOG without further affecting conversion efficiency.
Sensitivity assessments on the influence of several parameters in a marine environment highlight that for LNG, ambient temperature and storage size have limited influences on the boil-off rate (BOR), while the LH2 BOR is strongly affected by these parameters [17]. Here, efficiency is defined as the ratio of the output and input energy content in the bulk fuel. Overall, for large volumes pertaining to LH2 bulk carriers rather than LH2 fuel tanks, BOG treatment or utilization allows the recovery of around 4–6% of the energy lost through spontaneous evaporation.
Although it is true that preventing overpressures in cryogenic tanks is desirable from a safety point of view, a small amount of built-up pressure sets evaporation back and reduces the absolute mass of evaporated BOG for the same heat transfer into the cryogenic tank. To date, state-of-the-art automotive and marine LNG and LH2 storage tanks allow for MAWP levels in the range 5–15 bara, with higher levels in the case of PBU-type handling systems.
LNG has a peculiar feature to deal with: since it consists of a mixture of different hydrocarbons with different boiling points, a change in composition occurs during evaporation, leading to an increasingly higher concentration of heavy fractions. This process is referred to as “LNG weathering”. As may be derived from [32], the energy content varies greatly during evaporation, depending on the initial composition and the presence of nitrogen in the gas. Furthermore, BOG also has a very different composition than bulk liquefied natural gas [32], hence its thermophysical properties also change considerably.
To prevent overpressures, the BOG must be handled by means of several alternatives. Continuous venting is clearly too wasteful and is never used in normal operation except for security reasons in the event of an emergency.
Reliquefaction requires an additional system, which typically includes a compressor, a cold source, and an expansion device, as depicted in Figure 4.
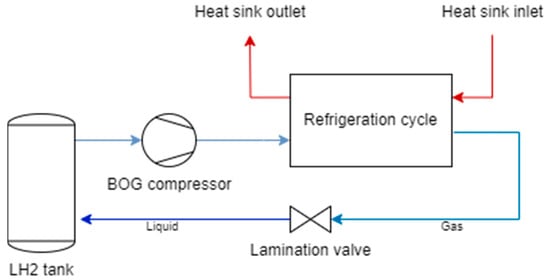
Figure 4.
Conceptual scheme of a reliquefaction system.
The refrigeration cycle displayed in Figure 4 may be either a conventional reverse cycle or a cryocooler. Standard reverse cycles (e.g., chillers, refrigerators, and heat pumps) are recuperative cycles, whereby the working fluid evolves in one direction between two fixed pressure levels. This category includes Joule–Thomson, RBC, and Claude coolers. Conversely, regenerative cycles employ oscillating flow and pressure with complementary phase angles to attain refrigeration at the cold end. Pulse-tube cryocoolers and Stirling chillers are examples of this category. Anyway, BOG pre-compression might be beneficial, at the expense of an additional BOG compressor power in addition to the power consumption of the refrigeration cycle [33].
It must be recalled that BOG reliquefaction is an energy intensive process: for large hydrogen liquefaction plants, numerical estimates from process simulation are between 6 and 8 kWh/kgLH2 [11,15,34], while real SECs are higher. A review of the relevant literature suggests that LNG liquefaction plants typically have SECs that are one order of magnitude lower [34]. When focusing on the maritime sector, where the plant must be more compact and the efficiencies of the components are lower, it can be easily forecast that SEC values will be hardly acceptable, not even for large volumes such as in the Suiso Frontier LH2 tanker [35]. Indeed, during the trip from Australia to Japan, roughly 10% of the loaded LH2 is dispersed through the vent mast. Traditional cryogenic cycles are suitable for large scale land-based gas liquefaction plants, while regenerative cryocoolers are more compact and might be suitable for LH2 reliquefaction for transport. Nevertheless, a study conducted by NASA to assess the potential for a zero boil-off scenario revealed that state-of-the-art cryocoolers necessitate an average power input of 0.45 kW per W of cooling power [36]. In light of this, even though cryocoolers are compact and relatively lightweight, to date BOG reliquefaction does not appear to be an economically viable solution onboard LH2 powered ships.
The solution of direct BOG utilization as fuel is viable and currently employed onboard dual-fuel ships. For the case of a typical LNG carrier, the fuel supply at the maximum rated power is almost equal to the generated BOG quantity [37], while for passenger ships or cruise ships the smaller size of the tank might not create sufficient BOG to run the engines. Regarding LH2, since BOG is significantly higher than LNG and granting that reliquefaction is not considered an option, a part of the stored hydrogen mass is likely to be lost. Finally, since BOG must be heated up to room temperature prior to its use, a significant proportion of available cold exergy from the fuel could be utilized for the purpose of energy storage, with the objective of either cooling/heating [38], power production [39], or as a thermal sink for auxiliary thermal engines [40].
4. Materials for Cryogenic Hydrogen Storage
4.1. Mechanical Properties at Cryogenic Temperatures
Severe cryogenic conditions pose problems in selecting a material capable of withstanding mechanical stress within an acceptable range. In general, exceptionally low temperatures tend to make materials very brittle. In particular, as the Ductile to Brittle Transition Temperature (DBTT) is overcome, the ability of a material to withstand dynamic stresses (i.e., impacts or sudden changes in pressure) is highly reduced.
Metallic materials usually have excellent fracture toughness in ambient conditions. However, only some of those retain acceptable figures in cryogenic conditions and are chosen for such sectors. The most suitable metals for cryogenics are austenitic steels and high-resistance aluminum alloys. Other than that, titanium alloys and nickel alloys show adequate performance as well; however, they are consistently more expensive. At cryogenic temperatures, the material has improved mechanical resistance, but it is also more brittle [41,42]. This is a common trend for most steels, especially martensitic and ferritic, while for austenitic ones such as AISI 316 the deviation is less evident [41].
On the contrary, other metallic alloys display a behavior which is quite different as compared to steels, e.g., 6061-T6 aluminum alloy. Generally, the trend of increasing yield point is conserved but, in this case, the plastic range is also enhanced. At present, aluminum alloys are the main materials for liquid hydrogen tanks for space rockets [43], but titanium alloys are also employed for components (e.g., turbopumps) of rocket engines [43].
Materials that show suitable properties at low temperatures generally also have lower thermal conductivity than other similar metal alloys. This is true for AISI 316 [18] and Ti-6Al-4V [41,42] but also holds for other metallic materials. Although not decisive, this is a clear advantage for cryogenic applications in terms of reduced heat flow to the liquefied gas [43].
Regarding the marine field, the corrosive environment calls for specific characteristics of the material to be utilized. AISI 316 has adequate characteristics at cryogenic temperatures [18]. The Korean Register quantifies that tensile strength is more than twice for AISI 316 and 316L at 20 K with respect to room temperature [44]. This is undoubtedly convenient, since such material has a wide range of established marine-related applications and its cost is still acceptable [45]. The fact that it proves adequate is confirmed on the Suiso Frontier LH2 tanker, where both the inner vessel and the outer containment are realized utilizing austenitic stainless steel [46]. It is also known that nitrogen-enriched stainless steel alloys, commercially known as Nitronic®, offer considerable corrosion resistance and tensile strength thanks to their nitrogen-enhanced austenitic stability, both at very high temperatures and at cryogenic conditions [47,48]. Hence, these might be considered as alternatives to more conventional materials when a very harsh environment is present.
Composite materials have distinctive characteristics. At present, these materials are widely used for high pressure storage tanks and lightweight applications. In [49], the authors report data for various unidirectional epoxy composites, showing that even at temperatures of a few Kelvins, not only do they suffer almost no degradation in tensile strength, they also perform better. As an example of a real world application of composites, the Suiso Frontier employs glass fiber-reinforced plastic (GFRP) for the inner vessel saddles [46].
4.2. Hydrogen Embrittlement
Hydrogen embrittlement refers to the mechanism involving hydrogen penetration into a solid material starting from its surface, an event that can seriously reduce the ductility and load capability of susceptible materials. Hydrogen diffuses into the metal grain boundaries and forms voids, which are preferred nucleation sites for crack propagation under load.
According to NASA [50], hydrogen embrittlement can be classified into three categories:
- Hydrogen Environmental Embrittlement (HEE)
- Hydrogen Internal Embrittlement (IHE)
- Hydrogen Reaction Embrittlement (HRE)
A distinction among these categories based on competing factors is reported in [45]. Usually, this phenomenon is represented in terms of the HEE index, namely a non-dimensional parameter which equals unity when HE has no influence and decreases as much as the material is affected.
As confirmed by NASA [50], hydrogen embrittlement is particularly enhanced under very high pressures, since the natural tendency of hydrogen to escape through the metallic lattice is enhanced by the pushing effect of the pressure and promotes HEE effects at the tip of a propagating crack. For materials exposed in an aqueous environment, a degradation mechanism known as Stress Corrosion Cracking (SCC) may appear [50]. In this phenomenon, material at the crack tip is removed by a corrosive process caused by an aqueous environment or contact with moisture, enhancing crack propagation and possible related failure. In fact, this process is typical of materials with low corrosion resistance, such as aluminum alloys, while steels and corrosion-resistant metals are less prone [50].
The effect of cryogenic temperatures on hydrogen embrittlement is still not completely clear. According to NASA [51], rapid cooling of a metal from higher than ambient to room temperature can result in IHE due to the reduced hydrogen solubility at ambient conditions. This appears to be relevant for LH2, e.g., during bunkering or tank refill. However, it is not clear whether cooling from ambient to cryogenic temperatures enhances HE. Furthermore, in stable cryogenic conditions the material is less prone to hydrogen penetration into the metal lattice due to the reduced mobility of hydrogen [51]. The idea that hydrogen’s mobility is exceptionally low at cryogenic temperatures is shared among other authors [51].
NASA researchers [50] observed that HEE is most severe near room temperature or slightly lower. This can be explained as follows: at very low temperatures, the diffusivity of hydrogen is too sluggish to fill a sufficiently high number of vacancies in the lattice, but at high temperatures, hydrogen mobility is enhanced, and trapping is diminished. For advanced metal alloys, the minimum of the HEE index is moved toward higher temperatures. The aforementioned AISI 316 exhibits a maximum decrease in HEE index (by some 30%) at around −73 °C [50]. Another interesting result is that a precise range of nickel content (13–33%) in the alloy exists for the material to be especially resistant to embrittlement; this is apparently valid for both superalloys and stainless steels [50].
The American Institute of Aeronautics and Astronautics (AIAA) has defined a ranking of metallic materials that best resist hydrogen embrittlement [51]. This ranking also includes some composite materials and polymers. It appears that most of the metal alloys are sufficiently resistant to hydrogen embrittlement, while only a couple of polymers sustain hydrogen-rich environments [51].
In conclusion, aluminum alloys and austenitic stainless steel appear to be sufficiently capable as fuel tank material for cryogenic hydrogen service. The choice between them depends on other parameters such as fatigue resistance, mechanical resistance, and thermal conductivity. With the focus on maritime uses, it is evident that resistance to corrosion is an additional requirement. This might suggest austenitic steels, in particular AISI 316 (or X5CrNiMo17-12-2 as per the European designation) or its low-carbon version (316L or X2CrNiMo17-12-2), which are both already well known to the maritime industry. Alternatively, titanium alloys and nickel alloys are equally suitable for use in cryogenic temperature, but suffer from enhanced hydrogen embrittlement owing to the configuration of their crystal lattice and their cost is significantly higher.
4.3. Insulation of Cryogenic Tanks
Cryogenic tanks require high-performance insulation to prevent heat transfer toward the bulk liquid. Since the TRL of cryogenic tanks is increasing, research pushes toward better and better insulation techniques, as proven by a few European Projects investigating novel solutions for LH2 tank insulation [52]. As a general consideration, every cryogenic tank today available has both active and passive insulation. Active insulation consists of a double liner arrangement in which a vacuum is created in between layers to reduce the thermal conductivity. Since this is hardly sufficient to guarantee acceptable thermal flux, further passive insulation technologies are adopted. In this scope, some methods appear to be the most promising for future technological development [53]. Experimental values for the overall thermal conductivity of passive insulation technologies are found in [54] depending on vacuum pressure; the results were obtained using liquid nitrogen as a cryogen medium. Table 5 contains a summary of the possible insulation methods that are applicable to LH2 tanks.

Table 5.
Overview of the most common insulation methods for liquefied gas tanks.
Multi-layer insulation is undoubtedly the readiest technology for cryogenic tank insulation, and also the most widespread [53]. It also appears to be also the most suitable for maritime fuel tanks containing LH2, considering their good performance, their simplicity, and technological readiness level.
A vapor-cooled shield consists of a thermal shield from the environment created by allowing the cold BOG to flow around the tank. In case of marine applications, and in general wherever BOG may be used for power generation, a VCS could be an interesting solution to simultaneously maintaining the bulk liquid at cold temperatures and exploiting the BOG, possibly in combination with para-ortho conversion [14].
The main concern about glass microspheres is their brittleness, although the spheres do not usually fail due to vibration or thermal cycling [57]. Moreover, their performance is not affected by vacuum loss or vacuum pressure variations [53]. Theoretically, a face-centered cubic arrangement maximizes the insulation potential, since the spheres have the least total contact area per unit volume [57]. In practice, the spheres’ arrangement is random, and there is little chance to control their actual disposition.
Perlite powder is lighter but has a higher thermal conductivity than glass microspheres. It also has increased fragility and is less resistant to thermal cycles [50]. Perlite powder has already some large size applications (>3 m3) for liquid nitrogen [58]. Aerogels have very low density, but their thermal conductivity is somewhat higher than glass microspheres and perlite powder [53] and they are extremely expensive. Today, their application is limited to research scale cryogenic tanks [56].
5. Safety Aspects for LH2: Precautions and Recommendations
Cryo-fuel utilization as alternative marine fuel poses severe safety issues, especially with LH2 as compared to LNG. Land-based liquid hydrogen storage, handling, and utilization is thoroughly regulated by several prominent institutes (ISO, NASA, etc.) and precise prescriptive rules or regulations are in place. Since this is not yet the case for transports in general, and specifically for the maritime sector, there is still a vast space to be filled with standards for the future realization of hydrogen-powered ships. Moreover, timelines of estimated maturity level seem to convey the idea that the biggest hurdle is indeed represented by regulatory immaturity rather than a technological underdevelopment [8].
These days, project approval and certification follows a process based on equivalent risk. This so-called Alternative Design (AD) approach requires the risk to be as low as reasonably possible (ALARP) or, in other words, quantitatively equal to that of ships designed under standard prescriptions [59]. It seems clear that it cannot be employed as a standard for hydrogen-fueled ships, since it would create an unsustainably complicated process (HAZID, HAZOP, explosion analysis, quantitative risk analysis etc.) with low repeatability. However, in a first tentative phase the AD assessment process is more flexible than prescriptive rules. Hence, insofar as the authority’s requirements are met, a certain freedom is left to designers [59]. As previously mentioned, LNG aspects are dealt with through an established set of rules and guidelines. Although it has more than a few similarities to LNG, liquefied hydrogen also presents numerous different and more challenging issues to deal with. Some researchers tried to summarize a comparison between LNG and LH2 in terms of practical aspects, further assessing their main criticalities, as reported in Table 6 [60]. It is confirmed that LH2 still faces major criticalities, and the comparison is unfavorable under many aspects.

Table 6.
Summary comparison between LNG and LH2 in terms of practical feasibility. “LNG”: the given characteristic constitutes an advantage for LNG. “LH2”: the given characteristic constitutes an advantage for LH2. “0”: the challenges are similar for the two fuels. Adapted from [60].
In the scope of the maritime sector, the International Maritime Organization (IMO) has two sets of guidelines that are relevant to LNG and LH2: the IGC-code and the IGF-code. These regulate the design of a ship for carrying liquefied gases in bulk and ships propelled by low-flashpoint fuels, respectively.
In addition to that, since hydrogen is not specifically described as a cargo in the IGC Code, in 2016 IMO issued the Interim Recommendation for Carriage of Liquefied Hydrogen in Bulk, MSC.420 (97) [61]. This document has been updated ever since, and a more recent amendment is expected to be issued as a result of the Committee on Carriage of Cargoes and Containers (CCC) resolution. The main recommendations regard the inerting of hazardous spaces (tank connection space and fuel preparation room), devoting particular attention to allow the ubication of fuel tanks and fuel lines in an enclosed sub-deck space rather than on the open deck. The inerting of enclosed spaces and the purging of the piping after maintenance of the lines are two crucial tasks for the safety of the ship. As discussed in the following, LH2 enclosed storages set a unique condition owing to its extreme thermodynamic conditions.
5.1. Inertization of Enclosed Spaces
It is clear that flammability limits strongly vary with respect to temperature and pressure of the enclosure [62]. However, the available data almost always applies to ambient conditions or high temperatures [63]. However, the following considerations might be drawn:
- A decrease in pressure corresponds to an increase in LFL, a decrease in UFL, and an increase in minimum ignition energy. Thus, sub-atmospheric pressures are beneficial [64], but the effect is not appreciable from a safety point of view.
- Higher temperatures increase the flammability range (lower LFL and higher UFL). The behavior of the H2/air mixture at cryogenic temperatures is still not clear, but the flammability range is expected to reduce. Some authors have concluded that at 100 K (−173 °C) the flammability range of hydrogen is narrower, but again, not appreciably for safety concerns [64]. From this perspective, cryogenic storage does not represent an enhanced safety measure with respect to pressurized gaseous storage.
The inerting of explosive atmospheres is achieved by introducing inert gases such as nitrogen, helium, argon, or carbon dioxide. Several works have explored the effectiveness of different inert gases and concluded that, taking the maximum explosion pressure and maximum rate of pressure rise as indicators, the effectiveness of inert gases on confined hydrogen explosion inhibition ranked from strong to weak is CO2, N2, Ar, and He [51,65,66]. Others confirmed the trend without assessing CO2 but including laminar flame speed influence [67]. In [51], steam is also assessed as inert, but it is straightforward to observe that this is not viable at cryogenic temperatures. Besides, no safety provision even suggests which inerting gas is the most appropriate for marine application, irrespective of the storage conditions. The authors in [51] also report the best diluent for each tube configuration: seemingly, wide tubes and large spaces benefit from CO2 utilization, but no consideration on the thermodynamic conditions is made.
The best theoretical solution from a thermodynamical perspective could be helium: having the lowest boiling point, this substance is gaseous even at −253 °C. However, helium has a higher cost as compared to the others, cannot be produced onboard, and its storage in large volumes may be anti-economical. Inerting hazardous spaces with CO2 causes no issues if the hydrogen is in compressed form and if the inerted spaces are not accessible by personnel [51]. Seemingly, the best choice remains nitrogen even for LH2, provided that condensed N2 is collected in an insulated drip tray, evacuated from the room, and disposed of [51]. It is also observed that nitrogen condensation or solidification can occur only in the event of direct contact with liquefied hydrogen [55], which can occur only in case of a hydrogen leak in liquefied form.
With reference to LH2 leakage, a research study conducted by SANDIA Laboratories discovered that hydrogen escapes as superheated gas only for storage pressures higher than around 7 bar; otherwise, it always consists of a two-phase leak [67]. The affecting parameters are the ubication of the leakage (saturated vapor or saturated liquid) and the local temperature close to the leakage. The leakages which reach the furthest hazardous distance in terms of LFL are the ones originating from liquid bulk at low storage pressure [67]. Some authors theoretically applied the IGF Code’s Alternative Design specifications for a short-sea high speed hydrogen ferry [68] employing CGH2 stored at 250 bar in Type IV cylinders, assessing the risk associated to the following items: storage tank, high pressure piping, low pressure piping, engine, and vent mast. They found that the estimated risk related to the hydrogen system is very low, and much lower than the acceptable risk level related to hydrogen (two orders of magnitude).
5.2. Pre-Normative Research Outcomes and Associated Risk
Extensive work has been conducted in the framework of the PRESLHY project [64,69]. The risk events associated with LH2 system failure can generally be classified based on fire occurrence (ignited/unignited) or immediate/delayed combustion. Releases from an LH2 tank could also be single phase or multi-phase. In the context of maritime operations, it should be pointed out that scenarios involving the release of liquid hydrogen within sub-deck enclosures are likely to be of minimal concern. This is confidently true because such an occurrence is to be avoided in the first place, since the associated loss potential is very high and could possibly lead to an unacceptable risk level.
Table 7 reports the main risk events that an LH2 system can undergo. It appears that the most probable outcomes are either unignited releases, both in form of pool spill and two-phase jets, and ignited events, i.e., pool fire and jet fire. BLEVE is usually evaluated under the assumption of complete burning of the whole tank content, which is overly conservative as demonstrated by experiments [70]. PPP only occurs in enclosed spaces, and only if the H2 leak is sufficiently large [71].

Table 7.
Overview on typical failure consequences of LH2 systems, characteristics, and outcomes.
It is known that there exist a scarcity of data and general experience with hydrogen release, especially LH2. Therefore, it is important to identify what are the most probable incidents or accidents, both in terms of causes and outcomes. In [72] were identified 287 occurrences and it was concluded that:
- Explosion occurs in 96% of GH2 releases and about 50% of LH2 releases.
- Hot surfaces constitute the ignition source in most cases (21% for GH2 and 11% for LH2).
- There were 199 injuries out of 201 GH2 accidents and 10 injuries out of 86 LH2 accidents.
PRESHLY project identified 18 incidents related to LH2 storage and containment systems during transportation and liquefaction/storage [64,69]. The causes and outcomes of such incidents are reported in Table 8 and Table 9, respectively.

Table 8.
Overview of the causes and outcomes of LH2 failure events during transportation. Adapted from [69].

Table 9.
Overview of the causes and outcomes of LH2 failure events during liquefaction and storage. Adapted from [69].
Equipment failure included unexpected burst disk failure, loss of vacuum, or a loose flange connection. Of the five cases during transit, two were related to road traffic accidents and the other three concerned venting due to burst disk failure and/or loss of vacuum. Injury to personnel occurred in 3 (17%) cases, 2 of which were cold burns. Property or equipment damage occurred in 7 (39%) cases.
It was noted that out of six major incidents involving storage vessels, three occurred during decommissioning/commissioning (warm-up/cool-down). There were several incidents where unexpected ignition upon venting occurred, resulting in fire or explosion. Injury occurred in 3 (8%) incidents and non-trivial damage in 23 (59%) cases.
From Table 8 and Table 9 it appears that more failures occur during liquefaction and storage as compared to cryogenic transportation. This could be justified assuming that produced LH2 is not often transported. Moreover, BLEVE seems not to be as typical as suggested by academic/research assessments, which often assume that the whole tank content ignites completely and simultaneously. In reality, even after a tank rupture, LH2 remains in liquid form and the evaporated fraction is cold and concentrated, hence its ignition is unlikely to occur all at once: a small flash fire is a more realistic scenario.
5.3. Open Challenges for Safe Maritime Hydrogen Operation
While in absence of a rigid regulation it is not yet possible to standardize H2-fueled ship design, some considerations on the most relevant challenges to be overcome for a safe hydrogen utilization onboard may be drawn.
- Hydrogen tank collocation. The IGF-code suggests that the tank be placed on the open deck to prevent an explosion to involve the whole hull. However, for some large ships this is troublesome, and an enclosed deck ubication is unavoidable. In the scope of MarHySafe project, a conceptual scheme for enclosed LH2 storage onboard those ships was proposed for all applications where open deck tanks prove anti-economical [59]. It results that open deck tanks are not and probably will not be the standard for deep-sea large cruise vessels.
- Prevention of flammable cloud formation. Directly related to the previous point, an enclosed space is very likely to quickly become an ignitable volume; thus, an adequate ventilation system is necessary. However, the threshold of 30 air changes per hour (ACH) proposed by the IMO’s Interim Guidelines for Safety of Ships Using Hydrogen as Fuel sounds too optimistic, since it is extremely far below the theoretical minimum for hydrogen [87]. Normally, air flow is selected to ensure hydrogen concentration to be less than 25% of the lower flammability limit, that is 1% v/v hydrogen. This is also the typical triggering value for hydrogen sensors. In [88] it is evaluated that a fuel cell enclosure of 3 m × 3 m × 2 m requires around 1500 ACH to stay below a supposedly safe 1% with a full-bore 6.2 g/s leakage from the FC feed line. While it is true that in a large ICE/GT powered ship globally hazardous concentrations are reached in a longer period because of the larger spaces, massflow rates are also larger. To provide a concrete example of a worst-case scenario, it was evaluated that it takes around 80 s to reach a flammable concentration from a full-bore rupture of a 35 MW-fuel pipe inside a 15,000 m3 engine room. To stay under hydrogen LFL, this would require ~45 ACH. If the 1% safety margin scenario is applied, this value skyrockets 4 times higher. For reference, current engine room requirements for a highly hazardous fuel like ammonia are set at 45 ACH [89]. However, the likelihood of such event occurring is very low, thus from a risk assessment perspective it could prove irrelevant.
- Inerting of the enclosures. As mentioned, inerting is crucial for safety reasons. The use of nitrogen is straightforward for CGH2 storage and utilization in enclosed spaces. The problem for LH2 operation about condensation of the inert gas is a fact, but the issue can be overcome quite easily with a collecting drip pan, provided that condensation or solidification does not affect any fuel handling component or normal engine operation. Alternatively, helium can be used as it guarantees a gaseous phase throughout all operating temperatures, but it is costly and cannot be produced onboard as nitrogen. However, more precise prescriptions should be issued regarding the possible need for a complete space inertization.
- Safe venting. Hydrogen storage facilities should be equipped with venting systems for both normal operating requirements and emergency situations. Vent lines for hydrogen (including pressure relief lines and boil-off from cryogenic systems) should be routed to a safe location outside. Vent lines are also used to dispose of hydrogen purged from the system for maintenance. The vent should be designed to prevent moisture or ice from accumulating in the line, to be safely inerted, and should have a cross section large enough to accommodate the vented mass flow without choking occurrences. NFPA 2 establishes that the discharge must be at least 3 m above any adjacent equipment and away from personnel areas, air intakes, and overhangs [88]. According to NASA/AIAA, flaring may be considered for hydrogen vent rates larger than 0.2 kg/s [90].
- Explosion protection. Inside confined spaces, the explosion risk is one of the most relevant. Explosions should be avoided at all times by ensuring a sufficient ventilation that prevents the accumulation of flammable mixtures. In case safety measures are not sufficient to avoid an explosion, the containment should be designed in such a way that the walls are collapsible enough to release the pressure wave toward the outside with no or minimal harm to people.
- Fire protection. Hydrogen fires are very difficult to detect because a hydrogen flame is colorless. For this reason, specific flame sensors and temperature sensors are paramount. However, any ignition source should be eliminated or confined. Walls must be fire-compliant (A-60 class), and an extinguisher fluid must be provided. Given the fast combustion reaction, in event of a hydrogen fire the most effective action is to suppress the hydrogen source rather than suffocate the flame. This is especially true in event of jet fires, while flash fires and BLEVEs are less predictable and easier to extinguish.
6. Conclusions
In the scope of maritime decarbonization through utilization of alternative fuels, it is undoubted that the maturity level of LH2 storage and handling systems is low as compared to its LNG counterpart. Despite the possibility of exploiting the vast experience accumulated with LNG in terms of cryogenic storage, BOG handling, and space arrangement, the practical design of LH2 handling systems requires tailored solutions.
First, the problem of cryogenic storage is addressed, concluding that LH2 requires higher performing insulation and better thermal management than LNG. Furthermore, pressurized liquid storage does not increase remarkably the boiling point, thus boil-off is not reduced significantly. In contrast to LNG, in the framework of a marine application, hydrogen boil-off reliquefaction is still economically unviable, even for large bulk carriers. Hence, from the economic point of view the best solution is the utilization of the BOG for direct power production purposes whenever safely possible.
The review of suitable materials for hydrogen storage concluded that metallic materials, which are already widely employed onboard, may also be effectively used with cryogenic fuels. For instance, aluminum alloys and AISI 316 and 316L stainless steels retain more than sufficient mechanical features even at very low temperatures. On the other hand, the use of aluminum alloys is considered inappropriate due to their limited resistance to the corrosive effects typical of marine environments. The enhanced weight efficiency of aluminum alloys makes them particularly well-suited for applications in aircraft. Moreover, cryogenic conditions seem not to perceivably enhance hydrogen embrittlement on the material, which however remains a serious challenge that hinders hydrogen uptake. Further research is required on composites’ and polymers’ sensitivity to hydrogen embrittlement.
Insulation techniques are advanced enough to guarantee sufficient performance for maritime LH2 fuel tanks, where the need for increased dormancy is not as critical as in the case of large scale land storage. However, some solutions have the potential to mitigate heat dispersion with relatively simple arrangements.
Despite the lack of a large number of test cases, research on safety is providing more and more valuable results, highlighting that only some risk events are really pertaining to actual LH2 equipment. As a general conclusion, behavior of LH2 releases depends on the mass leaked from the tank. If the leaked mass is limited, the leakage tends to evaporate quickly and disperse, possibly igniting in the presence of sufficient energy, while a large mass of liquid is prone to stratify giving rise to pools of slowly evaporating hydrogen. The most probable hazardous events are explosion and delayed flash fire, while BLEVE is much less critical than expected from research outcomes because the actual conditions are generally less severe than physical assumptions.
Safety is allegedly the most relevant concern for hydrogen storage and utilization onboard ships. The aim is to surpass the Alternative Design in favor of a more standardized design process. The main risks are linked to the formation of flammable mixtures in case of a fuel leak, owing to the high tendency of hydrogen gas to escape and quickly fill any enclosed volume. For these reasons, adequate ventilation should be provided, vent circuits must be present, and adequately sized and, as a last option if explosion is unavoidable, walls should oppose the blast with as little resistance as possible.
Finally, notwithstanding the technological maturity of hydrogen-ready maritime systems being at a low level in comparison with traditional propulsion or generation systems, the absence of a developed market with particular regard to LH2 handling systems is another crucial aspect that hinders hydrogen uptake toward zero-emission power generation. It is of the utmost importance that the technical and regulatory advancements in this domain are accompanied by a definitive commitment from all stakeholders toward the development and deployment of zero-emission power systems.
Author Contributions
Conceptualization, M.P. and A.T.; validation, A.T.; formal analysis, investigation, resources, data curation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, A.T.; supervision, M.P.; project administration, A.T.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
Activities described in this paper have been developed within Fincantieri project “Wave 2 the Future” (W2F), funded by the European Union—NextGenerationEU. W2F project is part of IPCEI Hy2Tech.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACH | Air Changes per Hour |
| AD | Alternative Design |
| AIAA | American Institute of Aeronautics and Astronautics |
| ALARP | As Low As Reasonably Possible |
| BOG | Boil-off Gas |
| BOR | Boil-off Rate |
| BLEVE | Boiling Liquid Expanding Vapor Explosion |
| DBTT | Ductile to Brittle Transition Temperature |
| DNB | Departure from Nucleate Boiling |
| DDT | Deflagration to Detonation Transition |
| GT | Gas Turbine |
| GFRP | Glass Fiber-Reinforced Plastic |
| GHG | Greenhouse Gas |
| HGM | Hollow Glass Microspheres |
| HEE | Hydrogen Environmental Embrittlement |
| HRE | Hydrogen Reaction Embrittlement |
| ICE | Internal Combustion Engine |
| IHE | Internal Hydrogen Embrittlement |
| IMO | International Maritime Organization |
| LH2 | Liquefied Hydrogen |
| LNG | Liquefied Natural Gas |
| LOHC | Liquid Organic Hydrogen Carrier |
| LFL | Lower Flammable Limit |
| LHV | Lower Heating Value |
| MAWP | Maximum Allowable Working Pressure |
| MDO | Marine Diesel Oil |
| MGO | Marine Gasoline Oil |
| MLI | Multi-Layer Insulation |
| PBU | Pressure Buildup Unit |
| RBC | Reverse Brayton Cycle |
| SEC | Specific Energy Consumption |
| SOFI | Spray-On Foam Insulation |
| TRL | Technology Readiness Level |
| UFL | Upper Flammable Limit |
| VCS | Vapor Cooled Shield |
References
- Ustolin, F.; Campari, A.; Taccani, R. An Extensive Review of Liquid Hydrogen in Transportation with Focus on the Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1222. [Google Scholar] [CrossRef]
- Elkafas, A.G.; Rivarolo, M.; Gadducci, E.; Magistri, L.; Massardo, A.F. Fuel Cell Systems for Maritime: A Review of Research Development, Commercial Products, Applications, and Perspectives. Processes 2023, 11, 97. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Global Hydrogen Review; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Al-Breiki, M.; Bicer, Y. Liquified Hydrogen vs Liquefied Renewable Methane: Evaluating energy consumption and infrastructure for sustainable fuels. Fuel 2023, 350, 128779. [Google Scholar] [CrossRef]
- Kanbur, B.B.; Xiang, L.; Dubey, S.; Choo, F.H.; Duan, F. Cold utilization systems of LNG: A review. Renew. Sustain. Energy Rev. 2017, 79, 1171–1188. [Google Scholar] [CrossRef]
- Korpys, M.; Wojcik, J.; Synowiec, P. Methods for sweetening natural and shale gas. Chemik 2014, 68, 211–215. [Google Scholar]
- Milojević, S.; Stopka, O.; Orynycz, O.; Tucki, K.; Šarkan, B.; Savić, S. Exploitation and Maintenance of Biomethane-Powered Truck and Bus Fleets to Assure Safety and Mitigation of Greenhouse Gas Emissions. Energies 2025, 18, 2218. [Google Scholar] [CrossRef]
- LNG Containment Systems: Finding the Way for Type A. Available online: https://www.dnv.com/expert-story/maritime-impact/LNG-containment-systems-finding-the-way-for-Type-A.html (accessed on 17 July 2023).
- Mokhatab, S.; Mak, J.Y.; Valappil, J.V.; Wood, D.A. Handbook of Liquefied Natural Gas; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology, Standard Reference Data Program: Gaithersburg, MD, USA, 2018. [Google Scholar] [CrossRef]
- Zhang, T.; Uratani, J.; Huang, Y.; Xu, L.; Griffiths, S.; Ding, Y. Hydrogen liquefaction and storage: Recent progress and perspectives. Renew. Sustain. Energy Rev. 2023, 176, 113204. [Google Scholar] [CrossRef]
- Peng, J.K.; Ahluwalia, R.K. Enhanced dormancy due to para-ortho hydrogen conversion in insulated cryogenic pressure vessels for automotive applications. Int. J. Hydrogen Energy 2013, 38, 13664–13672. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, S.; Wan, C.; Bao, S.; Zhi, X.; Qiu, L.; Wang, K. Performance analysis of vapor-cooled shield insulation integrated with para-ortho hydrogen conversion for liquid hydrogen tanks. Int. J. Hydrogen Energy 2023, 48, 3078–3090. [Google Scholar] [CrossRef]
- Leng, Y.; Zhang, S.; Wang, X.; Pu, L.; Xu, P. Comparative study on thermodynamic performance of liquid hydrogen storage insulation system incorporating vapor-cooled shield with para–ortho hydrogen conversion by one-dimensional and quasi-two-dimensional model. Energy Convers. Manag. 2024, 321, 119068. [Google Scholar] [CrossRef]
- Aasadnia, M.; Mehrpooya, M. Large-scale liquid hydrogen production methods and approaches: A review. Appl. Energy 2018, 212, 57–83. [Google Scholar] [CrossRef]
- Bi, Y.; Ju, Y. Design and analysis of an efficient hydrogen liquefaction process based on Helium reverse Brayton cycle integrating with steam methane reforming and liquefied natural gas cold energy utilization. Energy 2022, 252, 124047. [Google Scholar] [CrossRef]
- Song, Q.; Tinoco, R.R.; Yang, H.; Yang, Q.; Jiang, H.; Chen, Y.; Chen, H. A comparative study on energy efficiency of the maritime supply chains for liquefied hydrogen, ammonia, methanol and natural gas. Carbon Capture Sci. Technol. 2022, 4, 100056. [Google Scholar] [CrossRef]
- Kim, S.; Oh, S.; Kang, S. Techno-economic assessment of liquefied hydrogen tanker ships utilizing various propulsion systems. Energy Convers. Manag. 2025, 336, 119895. [Google Scholar] [CrossRef]
- Fincantieri and Viking Announce the World’s First Hydrogen-Powered Cruise Ship. Available online: https://www.fincantieri.com/en/media/press-releases/2025/fincantieri-and-viking-announce-the-world-s-first-hydrogen-powered-cruise-ship-and-sign-contracts-for-two-new-units (accessed on 1 August 2024).
- Airbus ZEROe: Our Hydrogen Powered Aircraft. Available online: https://www.airbus.com/en/innovation/energy-transition/hydrogen/zeroe-our-hydrogen-powered-aircraft (accessed on 1 August 2024).
- Ground Operation of Liquid Hydrogen Aircraft (GOLIAT). EU Horizon 2020. Available online: https://cordis.europa.eu/project/id/101138379 (accessed on 1 August 2024).
- Rolls Royce, Hydrogen. Available online: https://www.rolls-royce.com/innovation/net-zero/decarbonising-complex-critical-systems/hydrogen.aspx/1000 (accessed on 1 December 2024).
- Zhang, C.; Yu, G.; Liang, Y.; Ling, G. Exploration and performance assessment of recuperated rotorcraft powerplant utilizing hydrogen as fuel. Energy Convers. Manag. 2025, 336, 119872. [Google Scholar] [CrossRef]
- Wartsila Marine Solutions. LNG Shipping Solutions 2 Gas: A Green Solution; Wartsila Marine Solutions: Helsinki, Finland, 2017. [Google Scholar]
- MAN ES. MAN Cryo Cryogenic Solutions for Onshore and Offshore Applications. Available online: https://www.man-es.com/docs/default-source/document-sync-archive/man-cryo-eng.pdf?sfvrsn=f1030ce2_3 (accessed on 8 November 2023).
- Cryostar. Reciprocating Pumps. Available online: https://cryostar.com/reciprocating-pumps/ (accessed on 1 August 2024).
- Nikkiso CEIG. Reciprocating Pump SVG. Available online: https://www.nikkisoceig.com/product/sgv/ (accessed on 1 August 2024).
- CHART Industries. Available online: https://www.chartindustries.com/ (accessed on 1 August 2024).
- MAN Cryo Supplies Fuel System for World’s First Hydrogen-Powered Superyacht. Available online: https://www.man-es.com/docs/default-source/press-releases-new/pr-feadship_h2_en.pdf?sfvrsn=a007b9e0_1 (accessed on 24 July 2024).
- Wartsila. Wartsila and RINA Partner with Stakeholders to Deliver a Viable Hydrogen Solution to Meet IMO 2050 Target. Available online: https://www.wartsila.com/media/news/25-11-2021-wartsila-and-rina-partner-with-other-stakeholders-to-deliver-a-viable-hydrogen-fuel-solution-to-meet-imo-2050-target-3013516 (accessed on 5 December 2023).
- DNV. Maritime Forecast to 2050; DNV: Bærum, Norway, 2022. [Google Scholar]
- Migliore, C.; Tubilleja, C.; Vesovic, V. Weathering prediction model for stored liquefied natural gas (LNG). J. Nat. Gas Sci. Eng. 2015, 26, 570–580. [Google Scholar] [CrossRef]
- Kwak, D.H.; Heo, J.H.; Park, S.H.; Seo, S.J.; Kim, J.K. Energy-efficient design and optimization of boil-off gas (BOG) re-liquefaction process for liquefied natural gas (LNG)-fuelled ship. Energy 2018, 148, 915–929. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- HESC—The Suiso Frontier. Available online: https://www.hydrogenenergysupplychain.com/about-the-pilot/supply-chain/the-suiso-frontier/ (accessed on 22 November 2023).
- Colozza, A.J. Hydrogen Storage for Aircraft Applications Overview; NASA: Brook Park, OH, USA, 2002. Available online: http://www.sti.nasa.gov (accessed on 1 May 2024).
- Kochunni, S.K.; Chowdhury, K. Concept and evaluation of energy-efficient boil-off gas reliquefiers in LNG carrier ships propelled by dual-fuel engines. Cryogenics 2022, 123, 103453. [Google Scholar] [CrossRef]
- Zang, H.; Tang, J.; Qi, M.; He, T. Carnot battery storage system integrated with liquid hydrogen cold energy: Thermodynamics, economic analysis and optimization. Energy Convers. Manag. 2025, 325, 119400. [Google Scholar] [CrossRef]
- Mazzoni, S.; Rajoo, S.; Romagnoli, A. A boil-off gas utilization for improved performance of heavy duty gas turbines in combined cycle. Proc. Inst. Mech. Eng. Part A J. Power Energy 2019, 233, 96–110. [Google Scholar] [CrossRef]
- Bisio, G.; Massardo, A.; Agazzani, A. Combined Helium and Combustion Gas Turbine Plant Exploiting Liquid Hydrogen (LH2) Physical Exergy. ASME J. Eng. Gas Turbines Power 1996, 118, 257–264. [Google Scholar] [CrossRef]
- Duthil, P. Material Properties at Low Temperature. arXiv 2015, arXiv:1501.07100. [Google Scholar] [CrossRef]
- ASM International. Atlas of Stress-Strain Curves; ASM International: Almere, The Netherlands, 2002. [Google Scholar]
- Qiu, Y.; Yang, H.; Tong, L.; Wang, L. Research progress of cryogenic materials for storage and transportation of liquid hydrogen. Metals 2021, 11, 1101. [Google Scholar] [CrossRef]
- Park, J.; Chun, K.; Lee, T.; Kim, Y.; Kim, J. Guidelines for Selection of Metallic Materials of Containment System for Alternative Fuels for Ships; Korean Register: Busan, Republic of Korea, 2022. [Google Scholar]
- Chun, K.W. Technical guide for materials of containment system for hydrogen fuels for ships. J. Adv. Mar. Eng. Technol. 2022, 46, 212–217. [Google Scholar] [CrossRef]
- Muragishi, O.; Inatsu, S.; Uraguchi, R.; Yamashiro, K.; Imai, T.; Ohashi, T.; Shimogaki, T.; Yoshida, T.; Koumoto, T. Hydrogen Transportation-Development of Liquefied Hydrogen Carrier. Hydrog. Transp.–Dev. Liq. Hydrog. Carr. 2021, 182, 35–40. [Google Scholar]
- Nitronic® 40 Stainless Steel. Available online: https://www.electralloy.com/images/pdf/Product_Sheets/Electralloy/Nitronic-40.pdf (accessed on 1 May 2024).
- Nitronic® 60 Stainless Steel. Available online: https://www.electralloy.com/images/pdf/Product_Sheets/Nitronic/Nitronic60_main.pdf (accessed on 1 May 2024).
- Schutz, J.B. Properties of composite materials for cryogenic applications. Cryogenics 1998, 38, 3–12. [Google Scholar] [CrossRef]
- Lee, J.A. Hydrogen Embrittlement; NASA: Huntsville, AL, USA, 2016. Available online: https://ntrs.nasa.gov/api/citations/20160005654/downloads/20160005654.pdf (accessed on 1 May 2024).
- Gregory, F.D. Safety Standards for Hydrogen and Hydrogen Systems; NASA: Washington, DC, USA, 1997.
- NICOLHy. Novel Insulation Concepts for Liquefied Hydrogen Storage Tanks. Available online: https://nicolhy.eu/The-Project/ (accessed on 1 December 2024).
- Yatsenko, E.A.; Goltsman, B.M.; Novikov, Y.V.; Izvarin, A.I.; Rusakevich, I.V. Review on modern ways of insulation of reservoirs for liquid hydrogen storage. Int. J. Hydrogen Energy 2022, 47, 41046–41054. [Google Scholar] [CrossRef]
- Scholtens, B.E.; Fesmire, J.E.; Sass, J.P.; Augustynowicz, S.D.; Heckle, K.W. Cryogenic thermal performance testing of bulk-fill and aerogel insulation materials. In Proceedings of the AIP Conference Proceedings, Chattanooga, TN, USA, 16–20 July 2008; pp. 152–159. [Google Scholar] [CrossRef]
- Jiang, W.B.; Zuo, Z.Q.; Huang, Y.H.; Wang, B.; Sun, P.J.; Li, P. Coupling optimization of composite insulation and vapor-cooled shield for on-orbit cryogenic storage tank. Cryogenics 2018, 96, 90–98. [Google Scholar] [CrossRef]
- Wang, P.; Liao, B.; An, Z.; Yan, K.; Zhang, J. Measurement and calculation of cryogenic thermal conductivity of HGMs. Int. J. Heat Mass Transf. 2019, 129, 591–598. [Google Scholar] [CrossRef]
- Chan, C.K.; Mem, P.; ASME. Conductance of Packed Spheres in Vacuum. 1973. Available online: http://asmedigitalcollection.asme.org/heattransfer/article-pdf/95/3/302/5663791/302_1.pdf (accessed on 1 November 2024).
- Linde, A.G. Datenblatt LITS F2 D; The Linde Group: Woking, UK, 2023. [Google Scholar]
- DNV. Handbook for Hydrogen-Fuelled Vessels; DNV: Bærum, Norway, 2021. [Google Scholar]
- Nerheim, A.R.; Æsøy, V.; Holmeset, F.T. Hydrogen as a maritime fuel–can experiences with LNG be transferred to hydrogen systems? J. Mar. Sci. Eng. 2021, 9, 743. [Google Scholar] [CrossRef]
- International Maritime Organization. Interim Recommendations for Carriage of Liquefied Hydrogen in Bulk. MSC.420(97); International Maritime Organization: London, UK, 2016. [Google Scholar]
- Dwyer, J.; Hansel, J.G.; Philips, T. Temperature Influence on the Flammability Limits of Heat Treating Atmospheres; Air Products and Chemicals, Inc.: Allentown, PA, USA, 2003. [Google Scholar]
- Pio, G.; Salzano, E. Flammability limits of methane (LNG) and hydrogen (LH2) at extreme conditions. Chem. Eng. Trans. 2019, 77, 601–606. [Google Scholar] [CrossRef]
- Verfondern, K.; Cirrone, D.; Molkov, V.; Makarov, D.; Coldrick, S.; Ren, Z.; Wen, J.; Proust, C.; Friedrich, A.; Jordan, T. Handbook of Hydrogen Safety: Chapter on LH2 Safety.—Pre-Normative Research for Safe Use of Liquid Hydrogen (PRES-LHY), Project Deliverable 6.1—FCHJU Horizon 2020; HySafe: Union Grove, WI, USA, 2020. [Google Scholar]
- Li, Y.; Bi, M.; Gan, B.; Yan, C.; Ren, J.; Gao, W. Inhibition of Confined Hydrogen Explosion by Inert Gases. In Proceedings of the International Conference on Hydrogen Safety, Seoul, Republic of Korea, 24–26 September 2019. [Google Scholar]
- Yang, W.; Zheng, L.; Wang, C.; Wang, X.; Jin, H.; Fu, Y. Effect of ignition position and inert gas on hydrogen/air explosions. Int. J. Hydrogen Energy 2021, 46, 8820–8833. [Google Scholar] [CrossRef]
- Winters, W.S. Modeling Leaks from Liquid Hydrogen Storage Systems; OSTI: Albuquerque, NM, USA, 2009. Available online: http://www.ntis.gov/help/ordermethods.asp?loc=7-4-0#online (accessed on 1 December 2024).
- Aarskog, F.G.; Hansen, O.R.; Strømgren, T.; Ulleberg, Ø. Concept risk assessment of a hydrogen Driven high speed Passenger ferry. Int. J. Hydrogen Energy 2020, 45, 1359–1372. [Google Scholar] [CrossRef]
- PRESHLY European Project. Horizon 2020 FCHJU. Available online: https://preslhy.eu/ (accessed on 1 March 2025).
- Willoughby, D.; Royle, M. Releases of Unignited Liquid Hydrogen, HSL Report XS/11/70; Health and Safety Executive: Bootle, UK, 2012.
- Van Wingerden, K.; Kluge, M.; Habib, A.K.; Ustolin, F.; Paltrinieri, N. Medium-scale tests to investigate the possibility and effects of BLEVEs of storage vessels containing liquified hydrogen. Chem. Eng. Trans. 2022, 90, 547–552. [Google Scholar] [CrossRef]
- Brennan, S.; Molkov, V. Pressure Peaking Phenomenon for indoor hydrogen releases. Int. J. Hydrogen Energy 2018, 43, 18530–18541. [Google Scholar] [CrossRef]
- Verfondern, K.; Dienhart, B. Pool spreading and vaporization of liquid hydrogen. Int. J. Hydrogen Energy 2007, 32, 256–267. [Google Scholar] [CrossRef]
- Ustolin, F.; Giannini, L.; Pio, G.; Salzano, E.; Paltrinieri, N. On the Mechanical Energy Involved in the Catastrophic Rupture of Liquid Hydrogen Tanks. Chem. Eng. Trans. 2022, 91, 421–426. [Google Scholar] [CrossRef]
- Cirrone, D.; Makarov, D.; Proust, C.; Molkov, V. Numerical study of the spark ignition of hydrogen-air mixtures at ambient temperatures. Int. J. Hydrogen Energy 2024, 79, 353–363. [Google Scholar] [CrossRef]
- Spiegler, P.; Hopenfeld, J.; Silberberg, M.; Bumpus, C.F.; Norman, A. Onset of stable film boiling and the foam limit. Int. J. Heat Mass Transf. 1963, 6, 987–989. [Google Scholar] [CrossRef]
- Odsæter, L.H.; Skarsvag, H.; Aursand, E.; Ustolin, F.; Reigstad, G.; Paltrinieri, N. Liquid Hydrogen Spills on Water—Risk and Consequences of Rapid Phase Transition. Energies 2021, 14, 4789. [Google Scholar] [CrossRef]
- Friedrich, A.; Breitung, W.; Stern, G.; Veser, A.; Kuznetsov, M.; Fast, G.; Oechsler, B.; Kotchourko, N.; Jordan, T.; Travis, J.R.; et al. Ignition and heat radiation of cryogenic hydrogen jets. Int. J. Hydrogen Energy 2012, 37, 17589–17598. [Google Scholar] [CrossRef]
- Babrauskas, V. Estimating large pool fire burning rates. Fire Technol. 1983, 19, 251–261. [Google Scholar] [CrossRef]
- Panda, P.P.; Hecht, E.S. Ignition and flame characteristics of cryogenic hydrogen releases. Int. J. Hydrogen Energy 2017, 42, 775–785. [Google Scholar] [CrossRef]
- Cirrone, D.; Makarov, D.V.; Molkov, V. Cryogenic hydrogen jets: Flammable envelope size and hazard distances for jet fire. In Proceedings of the International Conference on Hydrogen Safety (ICHS), Adelaide, Australia, 24–26 September 2019. [Google Scholar]
- Zabetakis, M.G. Safety with Cryogenic Fluids; Plenum Press: New York, NY, USA, 1967. [Google Scholar]
- Makarov, D.; Shentsov, V.; Kuznetsov, M.; Molkov, V. Hydrogen Tank Rupture in Fire in the Open Atmosphere: Hazard Distance Defined by Fireball. Hydrogen 2021, 2, 134–146. [Google Scholar] [CrossRef]
- Cirrone, D.; Makarov, D.; Lach, A.W.; Gaathaug, A.V.; Molkov, V. The Pressure Peaking Phenomenon for Ignited Under-Expanded Hydrogen Jets in the Storage Enclosure: Experiments and Simulations for Release Rates of up to 11.5 g/s. Energies 2022, 15, 271. [Google Scholar] [CrossRef]
- Shentsov, V.; Kuznetsov, M.; Molkov, V. The pressure peaking phenomenon: Validation for unignited releases in laboratory-scale enclosure. In Proceedings of the International Conference of Hydrogen Safety, Yokohoma, Japan, 19–21 October 2015; Available online: http://www.ichs2015.com/images/papers/148.pdf (accessed on 1 March 2025).
- Lach, A.W.; Gaathaug, A.V.; Vaagsaether, K. Pressure peaking phenomena: Unignited hydrogen releases in confined spaces—Large scale experiments. Int. J. Hydrogen Energy 2020, 45, 32702–32712. [Google Scholar] [CrossRef]
- Bureau Veritas. Hydrogen Fuelled Ships. Report NR678. 2023. Available online: https://erules.veristar.com/dy/data/bv/pdf/678-NR_2023-11.pdf (accessed on 1 March 2025).
- G-55; Standard for Hydrogen Vent Systems. CGA, 2021. Available online: https://webstore.ansi.org/standards/cga/cga2021-2447147?srsltid=AfmBOop5x3W-PG4Xux-Aj2wAfk6yVZRSrHZ%E2%80%A6 (accessed on 1 November 2024).
- Bureau Veritas. Ammonia-Fuelled Ships—Tentative Rules. Report NR671. 2022. Available online: https://erules.veristar.com/dy/data/bv/pdf/671-NR_2022-07.pdf (accessed on 1 March 2025).
- G095A-2017; Guide to Safety of Hydrogen and Hydrogen Systems. ANSI/AIAA: Reston, VA, USA, 2018.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).