Community Structure, Health Status and Environmental Drivers of Coral Reefs in Koh Seh Island of the Kep Archipelago, Cambodia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Design and Data Collection
2.1.1. Substrate and Coral Cover
2.1.2. Physical-Chemical Variables and Sedimentation Rate Calculation
2.1.3. Data Analysis
3. Results
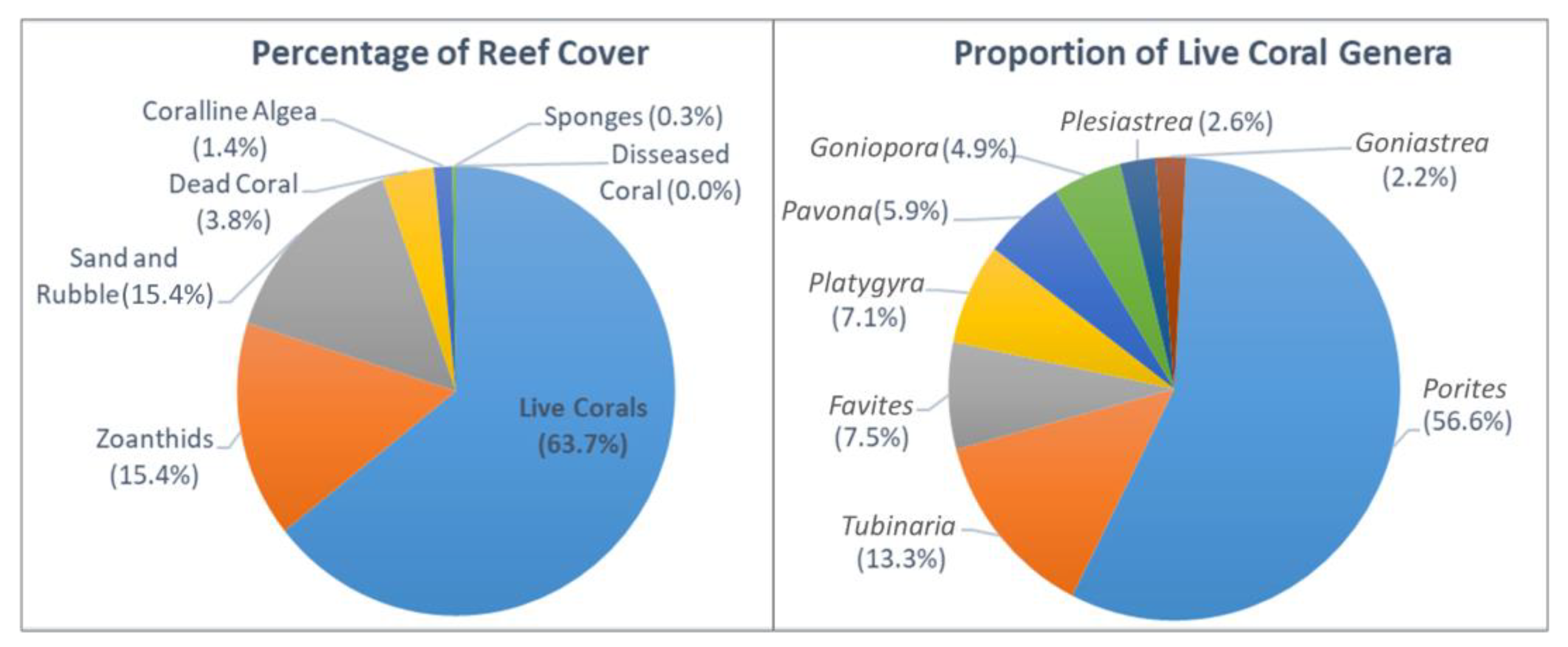
3.1. Substrate Covers and Coral Community Organization
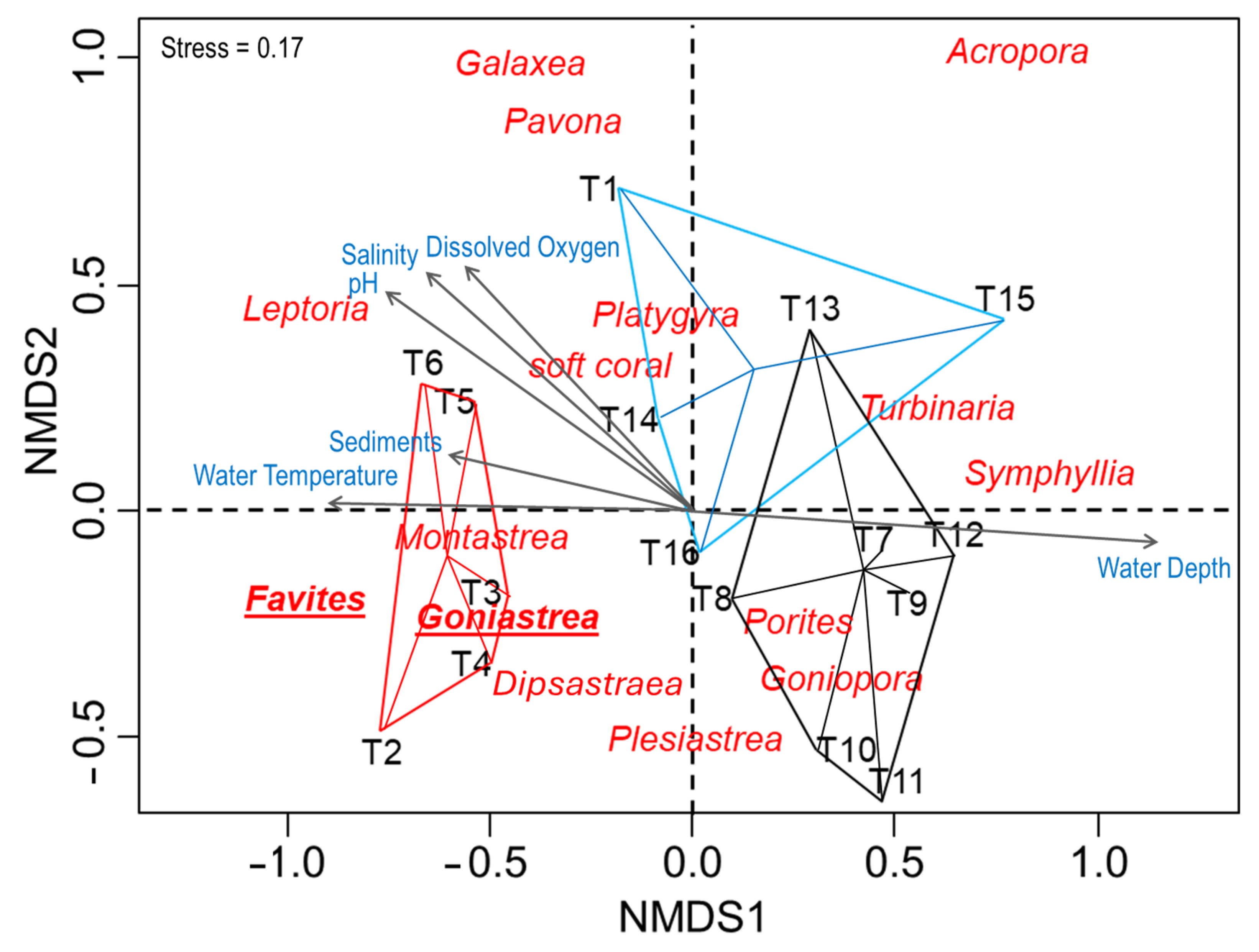
3.2. Health Status and Environmental Characterization of Coral Communities
4. Discussion
4.1. Reef Cover and Coral Community Organization
4.2. Health Status and Environmental Drivers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reaka-Kudla, M.L. The global biodiversity of coral reefs: A comparison with rain forests. Biodivers. II Underst. Prot. Our Biol. Resour. 1997, 2, 551. [Google Scholar]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- McMannus, J.W. Coral Reefs. In Encyclopedia of Ocean Sciences; Academic Press: Cambridge, MA, USA, 2001; Volume 1, pp. 524–534. [Google Scholar] [CrossRef]
- Fisheries-Refugia. Establishment of Fisheries Refugia in Cambodia: Background and Situation Analysis to Support. 2025. Available online: https://fisheries-refugia.org/country-activities/cambodia/background/status-and-trends.html (accessed on 20 July 2025).
- Lyna, K.; Mara, O. The Socio-Economic Contributions Made by Coral Reefs: Research findings from Koh Sdech Commune in Kirisakor District, Koh Kong Province, Cambodia. In Natural Resource Governance in Cambodia; Royal University of Phnom Penh: Phnom Penh, Cambodia, 2015; Volume 2, pp. 1–16. Available online: https://www.rupp.edu.kh/fds/dnrmd/documents/9_NRMDPaper-natural%20resource%20governmance%20in%20Cambodia_vol2.pdf#page=5 (accessed on 20 July 2025).
- Ellis, J.I.; Jamil, T.; Anlauf, H.; Coker, D.J.; Curdia, J.; Hewitt, J.; Jones, B.H.; Krokos, G.; Kürten, B.; Hariprasad, D.; et al. Multiple stressor effects on coral reef ecosystems. Glob. Change Biol. 2019, 25, 4131–4146. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.E.A.; Haissoune, A.; Ferber, P. The status of coral reefs and seagrass meadows in the Kep Archipelago. Cambodian J. Nat. Hist. 2019, 1, 24–39. [Google Scholar]
- MCC (Marine Conservation Cambodia). IUU and MCS in Cambodia. 2016. Available online: https://marineconservationcambodia.org/2016/03/23/mcs-and-iuu/ (accessed on 20 July 2025).
- Thorne, B.V.; Mulligan, B.; Mag Aoidh, R.; Longhurst, K. Current status of coral reef health around the Koh Rong Archipelago, Cambodia. Cambodian J. Nat. Hist. 2015, 2015, 98–113. [Google Scholar]
- Hamilton, M. Perceptions of fishermen towards marine protected areas in Cambodia and the Philippines. Biosci. Horiz. 2012, 5, hzs007. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology-Developments in Environmental Modelling, 3rd ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Oksanen, J. Vegan: An Introduction to Ordination. 2025. Available online: http://cran.r-project.org/web/packages/vegan/vignettes/intro-vegan.pdf (accessed on 20 July 2025).
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R.; Springer Science & Business Media: New York, NY, USA, 2011; p. 2011. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Sor, R. Modelling Spatio-Temporal Changes of Benthic Macroinvertebrate Communities in Asian and European Rivers. Doctoral Dissertation, Université Paul Sabatier-Toulouse III, Toulouse, France, Universiteit Gent, Ghent, Belgium, 2017. [Google Scholar]
- Team, R.C. Language and Environment for Statistical Computing: R Foundation for Statistical Computing, Vienna, Autriche; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Rogers, C.S. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 1990, 62, 185–202. [Google Scholar] [CrossRef]
- Browne, N.K. Spatial and temporal variations in coral growth on an inshore turbid reef subjected to multiple disturbances. Mar. Environ. Res. 2012, 77, 71–83. [Google Scholar] [CrossRef]
- Savage, J.M.; Osborne, P.E.; Hudson, M.D.; Knapp, M.; Budello, L. A current status assessment of the coral reefs in the Koh Sdach Archipelago, Cambodia. Cambodian J. Nat. Hist. 2014, 2014, 47–54. [Google Scholar]
- van Bochove, J.W.; McVee, M.; Ioannou, N.; Raines, P. Cambodia Coral Reef Conservation Project—Year 1 Report. No. February 2010. 2011. Available online: https://paperzz.com/doc/8523192/cambodia-coral-reef-conservation-project (accessed on 20 July 2025).
- Perry, C.; Morgan, K.M.; Smithers, S.G.; Johnson, J.A.; Daniell, J. Evidence of extensive reef development and high coral cover in nearshore environments: Implications for understanding coral adaptation in turbid settings. Sci. Rep. 2016, 6, 29616. [Google Scholar] [CrossRef] [PubMed]
- Primov, K.D.; Burdick, D.R.; Lemer, S.; Forsman, Z.H.; Combosch, D.J. Genomic data reveals habitat partitioning in massive Porites on Guam, Micronesia. Sci. Rep. 2024, 14, 17107. [Google Scholar] [CrossRef] [PubMed]
- Edinger, E.N.; Risk, M.J. Reef classification by coral morphology predicts coral reef conservation value. Biol. Conserv. 2000, 92, 1–13. [Google Scholar] [CrossRef]
- Samperiz, A.; Sosdian, S.; Hendy, E.; Johnson, K.; John, E.H.; Jupiter, S.D.; Albert, S. Coastal seawater turbidity and thermal stress control growth of reef-building Porites spp. corals in Fiji. Sci. Rep. 2025, 15, 17172. [Google Scholar] [CrossRef]
- Hennige, S.J.; Smith, D.J.; Walsh, S.J.; McGinley, M.P.; Warner, M.E.; Suggett, D.J. Acclimation and adaptation of scleractinian coral communities along environmental gradients within an Indonesian reef system. J. Exp. Mar. Biol. Ecol. 2010, 391, 143–152. [Google Scholar] [CrossRef]
- van Bochove, J.W.; Ioannou, N.; McVee, M.; Raines, P. Evaluating the status of Cambodia’s coral reefs through baseline surveys and scientific monitoring. Cambodian J. Nat. Hist. 2011, 2011, 114–121. [Google Scholar]
- Haas, A.F.; Smith, J.E.; Thompson, M.; Deheyn, D.D. Effects of reduced dissolved oxygen concentrations on physiology and fluorescence of hermatypic corals and benthic algae. PeerJ 2014, 2, e235. [Google Scholar] [CrossRef]
- Kuanui, P.; Chavanich, S.; Viyakarn, V.; Omori, M.; Lin, C. Effects of temperature and salinity on survival rate of cultured corals and photosynthetic efficiency of zooxanthellae in coral tissues. Ocean. Sci. J. 2015, 50, 263–268. [Google Scholar] [CrossRef]
- Guadayol, Ò.; Silbiger, N.J.; Donahue, M.J.; Thomas, F.I. Patterns in temporal variability of temperature, oxygen and pH along an environmental gradient in a coral reef. PLoS ONE 2014, 9, e85213. [Google Scholar] [CrossRef]
- MCC. Strategic Environmental Assessment of the Proposed Marine Protected Area, Kep Archipelago, Cambodia. 2015. Available online: https://marineconservationcambodia.org/wp-content/uploads/2022/09/Strategic-environmental-assessment-of-the-proposed-marine-protected-area-Kep-Archipelago-Cambodia.pdf (accessed on 20 July 2025).
- Browne, N.; Braoun, C.; McIlwain, J.; Nagarajan, R.; Zinke, J. Borneo coral reefs subject to high sediment loads show evidence of resilience to various environmental stressors. PeerJ 2019, 7, e7382. [Google Scholar] [CrossRef]
- Lee, Y.L.; Mohamed, M.I.; Bujang, J.S.; Ali, J.H. Ecology of Scleractinian Corals in the Waters of Port Dickson and Their Tolerance to Sedimentation. Doctoral Dissertation, Universiti Putra Malaysia, Serdang, Malaysia, 2005. [Google Scholar]
- Cruz, I.C.S.; Meira, V.H.; de Kikuchi, R.K.P.; Creed, J.C. The role of competition in the phase shift to dominance of the zoanthid Palythoa cf. variabilis on coral reefs. Mar. Environ. Res. 2016, 115, 28–35. [Google Scholar] [CrossRef]
- Anthony, K.R.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef]
- Kubicek, A.; Breckling, B.; Hoegh-Guldberg, O.; Reuter, H. Climate change drives trait-shifts in coral reef communities. Sci. Rep. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Janßen, A.; Wizemann, A.; Klicpera, A.; Satari, D.Y.; Westphal, H.; Mann, T. Sediment Composition and Facies of Coral Reef Islands in the Spermonde Archipelago, Indonesia. Front. Mar. Sci. 2017, 4, 144. [Google Scholar] [CrossRef]
- Wakwella, A.; Mumby, P.J.; Roff, G. Sedimentation and overfishing drive changes in early succession and coral recruitment. Proc. R. Soc. B 2020, 287, 20202575. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Harrison, P.L.; Brooks, L.O. Reduced salinity decreases the fertilization success and larval survival of two scleractinian coral species. Mar. Environ. Res. 2013, 92, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Boufadel, M.C.; Jackson, N.L. Evidence of salt accumulation in beach intertidal zone due to evaporation. Sci. Rep. 2016, 6, 31486. [Google Scholar] [CrossRef]
- Moberg, F.; Nyström, M.; Kautsky, N.; Tedengren, M.; Jarayabhand, P. Effects of reduced salinity on the rates of photosynthesis and respiration in the hermatypic corals Porites lutea and Pocillopora damicornis. Mar. Ecol. Prog. Ser. 1997, 157, 53–59. [Google Scholar] [CrossRef]
- Cowling, A. Spatial methods for line transect surveys. Biometrics 1998, 54, 828–839. [Google Scholar] [CrossRef]
- Bozec, Y.M.; O’Farrell, S.; Bruggemann, J.H.; Luckhurst, B.E.; Mumby, P.J. Tradeoffs between fisheries harvest and the resilience of coral reefs. Proc. Natl. Acad. Sci. USA 2016, 113, 4536–4541. [Google Scholar] [CrossRef]

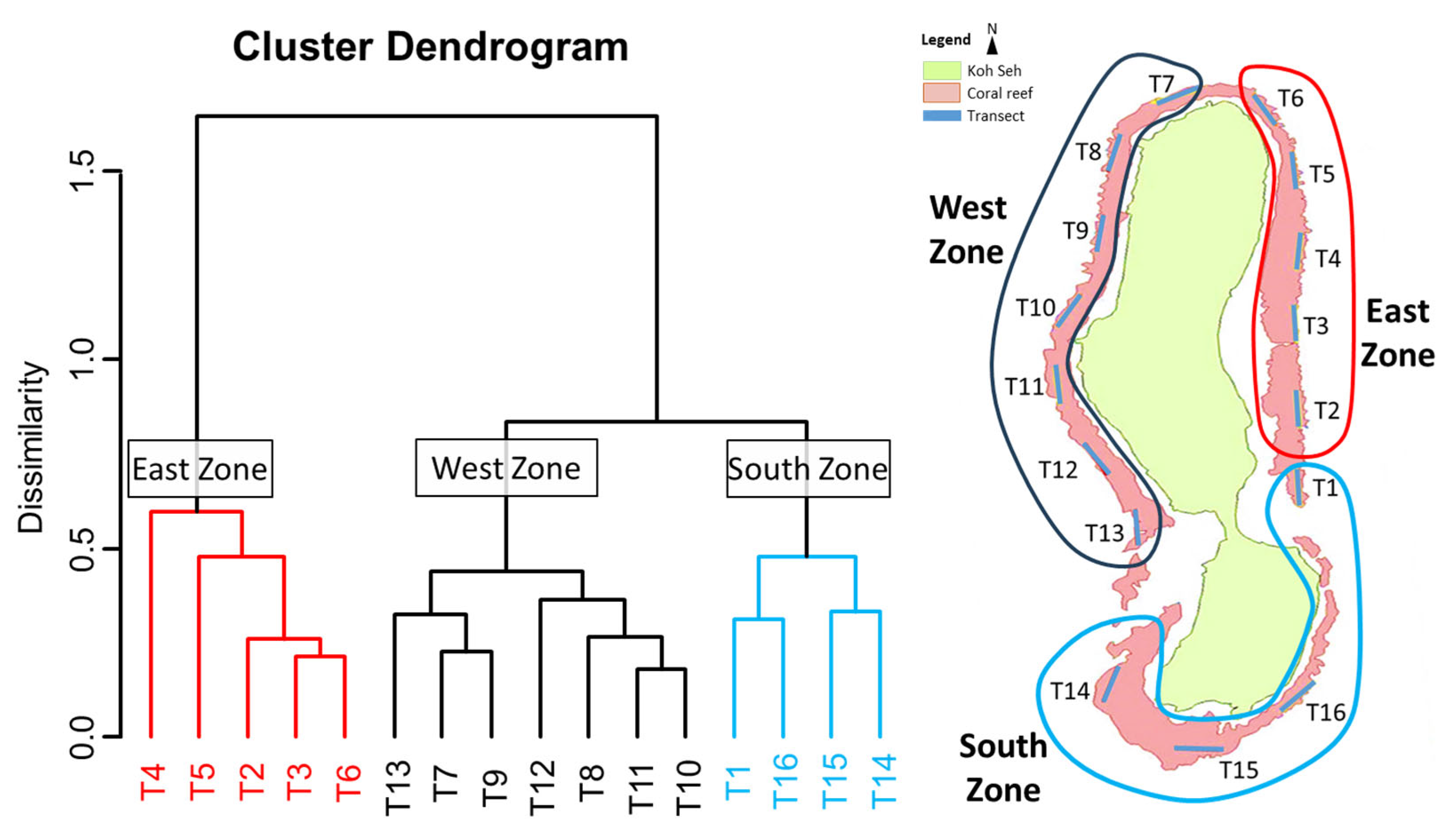
| Cluster or Zone [n] | |||||||
|---|---|---|---|---|---|---|---|
| Variables | East Zone [5] | South Zone [4] | West Zone [7] | p-Value | |||
| Coral Community | |||||||
| Richness | 7.2 | ±2.4 | 6.8 | ±2.1 | 6.1 | ±1.9 | 0.673 |
| Abundance | 28.8 | ±10.8 | 23.0 | ±8.8 | 24.9 | ±3.3 | 0.351 |
| Diversity H | 1.7 | ±0.3 | 1.6 | ±0.3 | 1.3 | ±0.3 | 0.129 |
| Benthic Reef Cover (%) | |||||||
| Live hard corals | 51.0 | ±34.4 | 63.0 | ±24.1 | 73.2 | ±22.7 | 0.417 |
| Sponges | 0.0 | ±0.0 | 0.0 | ±0.0 | 0.6 | ±1.5 | 0.525 |
| Zoanthids | 27.5 | ±21.7 | 19.5 | ±18.6 | 5.8 | ±9.5 | 0.147 |
| Dead coral | 5.5 | ±10.4 | 3.0 | ±6.0 | 2.9 | ±7.6 | 0.643 |
| Coralline algae | 0.0 | ±0.0 | 5.5 | ±11.0 | 0.0 | ±0.0 | 0.223 |
| Sand and Rubble | 16.0 | ±17.2 | 9.0 | ±6.2 | 17.5 | ±19.3 | 0.929 |
| Cluster [n] or Zone | ||||||
|---|---|---|---|---|---|---|
| Variables | East Zone [5] | South Zone [4] | West Zone [7] | |||
| DO (%) *** | 96.5 | ±1.9 a | 97.2 | ±1.4 a | 88.5 | ±9.0 b |
| Temperature (°C) * | 30.0 | ±0.7 a | 29.5 | ±0.6 a | 29.0 | ±0.0 b |
| pH * | 8.2 | ±0.1 a | 8.1 | ±0.1 a | 7.7 | ±0.4 b |
| Salinity (ppt) | 33.3 | ±0.7 | 33.3 | ±0.5 | 28.3 | ±5.1 |
| Sediment (g) * | 26.8 | ±37.9 a | 14.9 | ±8.3 a | 14.6 | ±32.2 b |
| Water Depth (m) *** | 1.0 | ±0.4 a | 2.0 | ±0.2 a | 2.5 | ±0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ith, S.O.; Haissoune, A.; Reid, A.; Sor, R. Community Structure, Health Status and Environmental Drivers of Coral Reefs in Koh Seh Island of the Kep Archipelago, Cambodia. J. Mar. Sci. Eng. 2025, 13, 1644. https://doi.org/10.3390/jmse13091644
Ith SO, Haissoune A, Reid A, Sor R. Community Structure, Health Status and Environmental Drivers of Coral Reefs in Koh Seh Island of the Kep Archipelago, Cambodia. Journal of Marine Science and Engineering. 2025; 13(9):1644. https://doi.org/10.3390/jmse13091644
Chicago/Turabian StyleIth, Srey Oun, Amick Haissoune, Alex Reid, and Ratha Sor. 2025. "Community Structure, Health Status and Environmental Drivers of Coral Reefs in Koh Seh Island of the Kep Archipelago, Cambodia" Journal of Marine Science and Engineering 13, no. 9: 1644. https://doi.org/10.3390/jmse13091644
APA StyleIth, S. O., Haissoune, A., Reid, A., & Sor, R. (2025). Community Structure, Health Status and Environmental Drivers of Coral Reefs in Koh Seh Island of the Kep Archipelago, Cambodia. Journal of Marine Science and Engineering, 13(9), 1644. https://doi.org/10.3390/jmse13091644








