Abstract
Marine reserves are key instruments for the conservation of biodiversity; however, benthic biodiversity studies often lack comprehensive data on species distribution and richness. The Punta Coles Natural Reserve (PCNR), located on the southern coast of Peru within the Humboldt Current System, represents a highly productive marine ecosystem, but information on its biodiversity is limited. The present study examines the benthic community of the hard substrate in the area of the PCNR via censuses by semiautonomous diving “Hookah” at depths between 1 and 15 m to provide baseline information to support its ecosystem management. Using NMDS and PERMANOVAs, we confirmed significant differences in species composition among depth strata, underscoring the role of depth as a key factor driving variability and species distribution in shallow zones. The community structure varies both spatially within the reserve and as a function of depth and is determined by the presence and distribution of key habitat-structuring organisms, as well as the configuration of the seabed. Our study highlights the ecological value of the PCNR, improves regional scientific knowledge, provides a useful baseline against which future anthropogenic pressures can be evaluated, and proposes the integration of subtidal kelp forests (Lessonia trabeculata), mussel beds (Aulacomya atra) and sea squirt (Pyura chilensis) network aggregations into conservation strategies to contribute to best management practices for PCNR.
1. Introduction
Marine reserves have been designed as tools to guarantee the conservation of certain species, communities, habitats or ecosystems [1,2,3]; however, their establishment presents complex and dynamic challenges, particularly in regard to balancing conservation goals with extractive activities [4,5,6,7]. Its design and implementation require not only participatory governance models and adaptive management strategies but also solid ecological knowledge that guarantees its long-term sustainability [8,9]. In countries such as Peru, the effectiveness of these areas depends on participatory governance frameworks, adaptive management schemes and a solid scientific basis.
The province of Ilo, Moquegua Region of Peru, is located in the PCNR (Punta Coles Natural Reserve), a protected area of great relevance for the conservation of marine species communities, habitats and ecosystems directly influenced by the upwelling system associated with the Humboldt Current, which plays a determining role in the ecological dynamics of the Peruvian Pacific coast [10,11]. This upwelling is characterized by the emergence of deep waters rich in nutrients, which favor the development of phytoplankton and support highly productive trophic chains [12]. The PCNR is considered one of the most productive protected natural areas in southern Peru [13,14]. This protected area supports the high diversity and abundance of birds and mammals protected by Peruvian and international regulations [15,16]. It also houses habitat—structuring species with fundamental ecosystem functions [17,18] and, at the same time, constitutes one of the main fishing areas for the artisanal fleet of Moquegua.
The PCNR was initially established as a protection area for guano birds for guano exploitation purposes; its inclusion since 2009 within the Reserva Nacional Sistema de Islas, Islotes y Puntas Guaneras (RNSIIPG) (Supreme Decree No. 024—2009-MINAM) has been in charge of the Servicio Nacional de Áreas Naturales Protegidas (SERNANP), restricting access to extractive activity in the area and thus creating tension between conservation and exploitation interests. Since 2015, SERNANP has promoted sustainable management strategies for commercial resources in accordance with the Master Plan of the National Research Council of the PCNR [19]. In 2010, the area was closed to extractive activities, and, beginning in 2020, a comanagement model was implemented to allow for the sustainable harvesting of Octopus mimus. This model was extended in 2023 to include the management of (Loxechinus albus) (Ministerial Resolution N° 308—2020-PRODUCE and N° 254—2023-PRODUCE).
Despite the high ecological importance of the PCNR, scientific research in this area has focused mainly on intensive monitoring of populations of benthic species of commercial interest and fauna associated with coastal edges. Studies, conducted mostly by the Instituto del Mar del Perú (IMARPE) [18,20] and SERNANP, have contributed significantly to the knowledge of coastal population dynamics [14,16,21]. However, the information generated presents an indefinite periodicity, which limits its comparative value and ability to identify long-term ecological trends.
Even more worrisome is the scarce information available on the composition and abundance of benthic biota, especially in the middle and deep strata of the PCNR, which provides evidence of a critical gap in the scientific knowledge of key components of the marine ecosystem. This knowledge gap is particularly relevant considering that the primary objective of these actions is biodiversity conservation. This limited information is critical considering the susceptibility of these systems to the environmental variability associated with El Niño and the Southern Oscillation (ENSO) [22,23,24], as well as the anthropic pressures of illegal extraction, in addition to the limited capacity of the actors to implement adequate monitoring and inspection programs, compromising the effective management of the PCNR [13].
In this context, there is a clear need to strengthen integrated and systematic research approaches that allow for a continuous and representative evaluation of the marine biota, aligned with ecological dynamics, as well as with decision-making processes in the adaptive management of the reserve.
Therefore, in this study, we examine the subtidal benthic biodiversity in the area of the PCNR to inform conservation strategies by combining the census and sampling of organisms with observations in the field to (i) characterize the composition and structure of the benthic community; (ii) document spatial patterns of diversity, abundance, and distribution of organisms; and (iii) provide a solid and reliable baseline that contributes to improving the design of conservation strategies and management actions aimed at comprehensive and ecosystem management in the area.
2. Materials and Methods
2.1. Study Area
This study was carried out in the PCNR, which is located seven km south of the city of Ilo, in the Moquegua region, Peru, at 17°42′ south latitude and 71°22′ west longitude (Figure 1). The PCNR comprises a marine and terrestrial area of 3000 hectares, where it harbors coastal diversity represented by guano birds (Phalacrocorax bougainvillii, Sula variegate, and Pelecanus thagus), Humboldt penguins (Spheniscus humboldti), South American sea lions (Otaria flavescens), and marine otters (Lontra felina), which use the area as a refuge, feeding ground, breeding site, and resting place [21] (Figure 1). In addition, different habitat—forming organisms can be found, among which subtidal kelp forests (Lessonia trabeculata), mussel beds (Aulacomya atra) and red sea squirt (Pyura chilensis) stand out and are distributed mainly at depths of less than 20 m [17]. These organisms are part of the natural banks of benthic resources in the PCNR. Similarly, Punta Coles is one of the main fishing areas for the artisanal fishing fleet of the Moquegua region, as commercial resources are found there, such as false abalone (Concholepas concholepas), red sea urchin (L. albus), octopus (O. mimus), and limpet (Fissurella latimarginata) [18,19].
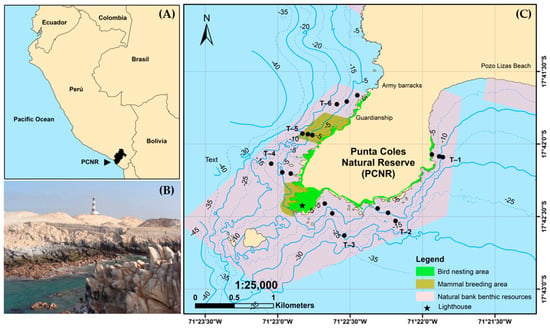
Figure 1.
(A) Location of the study area, (B) landscape photograph and (C) exploration stations. The black circles indicate the stations selected.
This ecosystem is highly productive because of the presence of permanent upwelling centers near the southern coast of Peru and is predominantly influenced by southwest winds with speeds of 3.8–5.2 m s−1 [20,25]. The sea surface temperature (SST) fluctuates between 15.5 °C (autumn) and 19.0 °C (spring) [20], with a salinity of 34.80–35.00 UPS and an average dissolved oxygen content of approximately 5.0 mL L−1 [20,26].
2.2. Field Sampling
The sampling was carried out in April 2024, which corresponds to southern autumn, a season characterized by stable local environmental conditions in southern Peru; this period was selected because of its documented ecological representation of the area [17,18]. The information was collected by diving via the “hookah” system (that is, air supply from the surface) with the support of an artisanal fishing vessel equipped with an echo sounder (Garmin ECHOMAP Plus). The methodology was based on the benthic biodiversity sampling protocol developed by IMARPE (unpublished protocol), which adapts guidelines from the international protocols NAGISA (Natural Geography in Shore Areas) [27] and PISCO (Partnership for Interdisciplinary Studies of Coastal Oceans) [28]. For this study, the ecological and environmental conditions previously recorded in the area were considered [14,17,18].
Punta de Coles, with a shoreline of 1960 m and steep rocky slopes, exhibits a complex submarine morphology [19]. In this context, sampling stations were selected on the basis of historical sites previously monitored by IMARPE to ensure continuity in the data series and enable comparative assessments of biodiversity over time. To determine bathymetric and spatial trends within the PCNR, a stratified sampling design was implemented. Six transects were established perpendicular to the coastline and were evenly distributed between two sectors (north and south). Each transect was subdivided into three depth strata: stratum I (1–5 m), stratum II (5–10 m), and stratum III (10–15 m). The in situ depth was recorded via a digital computer, allowing accurate allocation of samples to the corresponding stratum.
For each transect × stratum combination, three replicates (n = 3) were established. These replicates corresponded to discrete sampling points spaced approximately 5 m apart to reduce observational variability. For each replicate, coverage assessments and organism collection were carried out as described below.
2.3. Sample Collection
For the in situ assessment of coverage-forming organisms (macroalgae, sponges, bryozoans, bivalves, barnacles, sedentary polychaetes, tunicates, etc.), a 0.25 m2 quadrat (0.5 m × 0.5 m) equipped with an internal grid of 100 subdivisions (each 0.05 m × 0.05 m) was used. Coverage was recorded by visually estimating the percentage of each taxon within each subdivision [29,30,31,32]. Only highly aggregated organisms with apparent sizes greater than 2 cm were included.
For the in situ evaluation of megabenthos (organisms > 1 cm), a 1 m2 square frame (1 m × 1 m) was placed adjacent to each coverage quadrat. This sampling allowed for the census of larger benthic organisms such as crustaceans, echinoderms, bivalves, ascidians, and anemones, among others. For both coverage-forming organisms and megabenthos, voucher specimens and photographic records were collected for taxa that could not be reliably identified in the field. In addition, detailed descriptions of the associated habitat types were recorded to support ecological interpretation.
For the sampling of macrobenthos (defined in this study as organisms between 500 µm and approximately 2 cm in body length), a 0.0625 m2 quadrat (0.25 m × 0.25 m) and a metal spatula were used to delineate the sampling area and extract individuals. When macrofauna or megafauna (>2 cm) were present within the macrobenthic quadrat, they were not removed or excluded unless they physically obstructed the sampling of the sediment surface. In such cases, these individuals were carefully relocated and recorded as part of the megabenthos survey. No effort was made to avoid areas of faunal aggregation or to selectively exclude larger individuals to preserve the natural heterogeneity and ecological representativeness of the benthic assemblage. Finally, the samples were placed in labeled bags, preserved in 95% ethanol, and cataloged onboard for subsequent analysis at the Biodiversity Laboratory of the National University of Moquegua (UNAM). The specimens were identified to the lowest possible taxonomic level. For each species, the number of individuals was counted, and their total wet weight (g) was measured via an analytical balance with 0.01 g precision. These data were subsequently used in the statistical analysis. Taxonomic identifications were complemented by a review of the specialized literature: [30,31,32] for polychaetes; [33,34,35] for mollusks; and [36,37,38] for crustaceans.
2.4. Statistical Analysis
To characterize the coverage of sessile organisms and the composition of the megabenthic community, variations in percent cover (%), total abundance, and species richness were evaluated. The influences of the factors “depth” (three levels: 1–5 m, 5–10 m, and 10–15 m) and “sector” (two levels: North and South of the reserve, according to transect location) were analyzed. To assess statistically significant differences among the groups defined by these factors, a two-way analysis of variance (ANOVA) was applied. Prior to this, assumptions of normality (Shapiro-Wilk test) and homoscedasticity (Levi’s test) were verified. The data were square-root transformed (sqrt(x)) to improve compliance with these assumptions. When assumptions were not satisfactorily met—even after transformation (in highly skewed variables)—nonparametric tests, such as the Kruskal-Wallis test, were used to compare differences between factor levels independently.
For the macrobenthic community, variability in abundance, biomass, species richness (s), and community structure was formally evaluated via univariate permutational analysis of variance (PERMANOVA) [39,40,41,42,43]. This was based on a Bray-Curtis similarity matrix of square-root transformed data implemented in PRIMER v6 [44,45,46] with PERMANOVA. and the Vegan [40] and ggplot2 [41] packages from R 4.1.2 software [42,43] were used. The fixed model (two levels) considered the factors “depth”, “sector”, and their interaction, with statistical significance set at p < 0.05, on the basis of 9999 permutations.
To visualize macrobenthic community associations in relation to the factors (“depth” and “sector”), a nonmetric multidimensional scaling (NMDS) ordination [47] was performed via the Bray-Curtis abundance matrix with square-root transformed data. The analysis was exploratory, and no a priori group classifications were imposed; the clustering patterns observed in the ordination reflect natural gradients in species composition across samples. On the basis of individual abundance records, univariate ecological indices were calculated by factors via pooled data. These indices included Simpson’s index (1–λ), Pielou’s evenness (J’), Margalef’s richness index (d), the Shannon diversity index (H’) (calculated via log base e), and the observed species richness (s). Finally, similarity percentage analysis (SIMPER) [48] was conducted to identify the contribution of dominant species to overall community differentiation across significant factors via square-root transformed data.
3. Results
3.1. Morphological and Biological Characterization of the Shallow Subtidal Habitat
The seabed in the PCNR exhibited a predominantly flat and irregular morphology, dominated by a consolidated rocky substrate and rock blocks of varying sizes, with structural characteristics that changed with depth. In stratum I, bedrock and large boulders (>50 cm in diameter) were predominant, indicating greater structural homogeneity in shallow waters. In contrast, strata II and III featured large boulders, medium-sized rock fragments, cobbles, and coarse gravel interspersed with sandy grooves and shell deposits, contributing to increased geomorphological heterogeneity. However, in other sectors of stratum III (stations T-2 and T-3), the substrate was mainly composed of solid rock. Beyond 20 m depth, the dominant substrate shifted to primarily sandy sediments.
Coverage values varied among phyla across depth strata, reflecting both habitat preferences and depth-related zonation. A total of 13 species belonging to multiple phyla were recorded. Rhodophyta (red algae) was the most consistently dominant group across all strata, with particularly high cover in the shallowest stratum (~50%). However, its contribution decreased with depth. Annelida exhibited an increasing trend in coverage with depth. While contributing 12.7% to the shallowest layer, its presence nearly doubled at greater depths (29.6% at 5–10 m and 28.3% at 10–15 m). Chordata was restricted to the upper two strata (1–5 m: 15.0%; 5–10 m: 16.9%). Molluska was exclusively dominant in the deepest stratum, indicating that this group prefers deeper strata. Bryozoa was absent in the shallowest stratum but became one of the dominant groups at 5–10 m (53.7%) and 10–15 m (47.5%), suggesting a strong affinity for deeper, possibly more stable substrates. Porifera (sponges) and Cnidaria were present only in the two upper strata, with low but consistent coverage (~5%), suggesting a preference for shallower hard substrates and possibly greater sensitivity.
In terms of spatial distribution, the northern sector presented a greater representation of colonial organisms and red algae. Coverage was dominated by Bryozoa (33.7%) and Rhodophyta (24.1%), followed by Ochrophyta (18.1%) and Chordata (10.8%). Other groups, such as Annelida, Cnidaria, Molluska, Chlorophyta, and Porifera, contributed in lower proportions (<7%). In the southern sector, the assemblage was clearly dominated by Molluska (38.9%), followed by Annelida (15.6%), Rhodophyta (22.1%), and Chlorophyta (13.4%). In contrast to those in the northern sector, Bryozoa and Cnidaria were absent, and Ochrophyta was recorded with minimal coverage (0.96%) (Figure 2A,B).
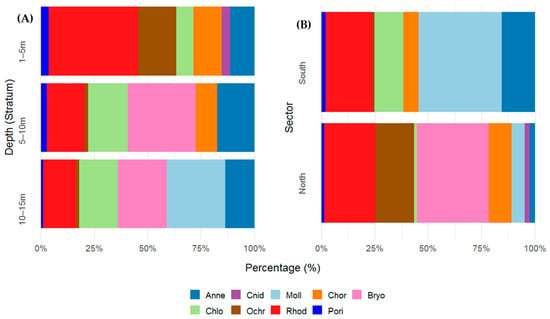
Figure 2.
(A) Percentage (%) cover of benthic taxonomic groups (phylum level) across three depth strata (1–5 m, 5–10 m, and 10–15 m) and (B) spatial variation across different sectors (B) in the Punta Coles Natural Reserve. Annelida (Anne), Bryozoa (Bryo), Chlorophyta (Chlo), Chordata (Chor), Cnidaria (Cnid), Molluska (Moll), Ochrophyta (Ochr), Porifera (Pori), and Rhodophyta (Rhod).
The calcareous alga Lithothamnium sp. was identified as a key species, being present across all depth strata and showing greater coverage in the shallower layers (49.3% ± 6.12% in stratum I and 34.2% ± 4.66% in stratum II). In stratum I, the community was dominated by Rhodymenia corallina (41.5% ± 6.04), P. chilensis (15.0% ± 3.50), and Dictyota kunthii (20.3% ± 5.09%). In addition, Palmophyllum sp., L. trabeculata, and the cnidarian Anemonia alicemartinae were present but presented cover values below 5%. The intermediate layer (stratum II) presented the highest species diversity, with substantial coverage by Bryozoa (53.7% ± 1.66), Phragmatopoma moerchi (29.6% ± 4.79), Chlorophyta sp. (32.0% ± 5.95), and Palmophyllum sp. (30.5% ± 3.83), along with P. chilensis (16.9% ± 3.57) and other associated species. In contrast, stratum III was characterized by the dominance of mussel A. atra (56.5% ± 7.95), which was exclusively found at this depth. Other common taxa included P. moerchi (28.3% ± 5.64%), Bryozoa (47.5% ± 1.50%), and L. trabeculata.
The sectoral distribution analysis revealed that the red alga Lithothamnium sp. had significantly greater coverage in the southern sector (47.60% ± 4.41) than in the northern sector (29.00% ± 2.92), whereas R. corallina exhibited the opposite dominance pattern. Pterosiphonia sp. was detected exclusively in South China (1.50% ± 0.17%). A. atra reached its highest coverage in South China (67.70% ± 5.96), decreasing markedly in North China (23.00% ± 0.00), in contrast to P. chilensis, which showed a more homogeneous distribution between sectors (14.40% ± 2.59 in South China vs. 15.10% ± 2.83 in North China). Chlorophyta sp. and Palmophyllum sp. showed high cover in South China (31.40% ± 6.13 and 30.50% ± 3.13, respectively), while Palmophyllum sp. barely reached 5.00% ± 0.00 in North China, and Chlorophyta sp. was not recorded in that sector. Finally, Bryozoa, Demospongiae (Porifera), and A. alicemartinae (Cnidaria) presented relatively high coverage in North China (51.20% ± 1.31, 3.50% ± 0.33, and 4.50% ± 0.41, respectively), with null or very low coverage in South China (Supplementary Material S1; Figure 3A–F).

Figure 3.
Representative images of the subtidal zone in the Punta Coles Natural Reserve, illustrating dominant biological coverage across depth. Stratum I (A,B) includes Dictyota kunthii (Dk), Pyura chilensis (Py) and Anemonia alicemartinae (Aa). Stratum II (C,D) features the kelp forest Lessonia trabeculata (Lt) and the calcareous alga Lithothamnium sp. (Lh). Stratum III (E,F) is characterized by mussel beds dominated by Aulacomya atra (Au) and Bryozoa (Bry).
For the cover community, species richness exhibited a marginally nonsignificant response to depth (F = 3.55, p = 0.062), suggesting a possible trend of depth–related variation that did not reach statistical significance. Richness was not significantly affected by sector (F = 1.98, p = 0.184) or by the depth × sector interaction (F = 0.40, p = 0.681). In terms of abundance, depth had no significant effect (F = 1.03, p = 0.386), whereas sector significantly influenced abundance (F = 5.44, p = 0.038), indicating spatial differences in the density of coverage-forming organisms across sectors. The interaction effect between depth and sector was not significant (F = 0.93, p = 0.420), suggesting that the observed variation in abundance was driven primarily by differences between sectors rather than depth-dependent patterns (Table 1).

Table 1.
Results of two-way ANOVA testing the effects of depth, sector, and their interaction on the species richness and abundance of coverage-forming and megabenthic communities.
3.2. Community Composition and Structure
3.2.1. Megabenthos
A total of 14 species, representing five major taxonomic groups, were recorded. Among these, the phyla Echinodermata, Molluska, and Cnidaria contributed significantly to the species composition. Echinodermata exhibited notable spatial dominance. The red sea urchin (L. albus) was the most abundant species overall, with mean densities of 7.20 ± 1.21 ind./m2 in stratum I and 5.00 ± 1.41 ind./m2 in stratum II. Its absence from deeper waters (stratum III) indicates a marked preference for shallower habitats. Similarly, the sun star (Heliaster helianthus) and the black sea urchin (Arbacia nigra) were widely distributed across all strata. Notably, A. nigra reached its highest abundance in stratum I (4.00 ± 0.53 ind./m2), with densities declining at greater depths. In contrast, Arbacia spatuligera was recorded exclusively in deeper strata (stratum III).
The sea star (Stichaster striatus) was observed only in the upper strata and at low densities (maximum of 2.00 ± 0.24 ind./m2 in stratum II). The crab (Romaleon setosum) was exclusively recorded in stratum III along transect 1, where it reached a mean density of 6.00 ± 0.85 ind./m2. In commercial Molluska, false abalone (C. concholepas) and black limpet (F. latimarginata) were found in shallower strata. While C. concholepas was limited to stratum I (1.00 ± 0.00 ind./m2), F. latimarginata appeared in both stratum I and III, with comparable densities (2.00 ± 0.24 and 1.00 ± 0.00 ind./m2, respectively). In contrast, Thaisella chocolata exhibited a depth-related distribution, being absent from stratum I and more abundant in deeper strata—reaching 2.00 ± 0.24 ind./m2 in stratum II and 2.67 ± 0.49 ind./m2 in stratum III.
Among the Cnidaria, the red anemone (A. alicemartinae) was predominantly found in strata I and II, with consistent densities (~5 ind./m2), and was absent from stratum III. Other cnidarians, such as Phymanthea pluvial and Antholoba achates, are rare, with very low densities and sporadic occurrence limited to strata I–II P. pluvia and stratum III A. achates.
A comparison between sectors revealed that L. albus was significantly more abundant in the southern sector (7.64 ± 1.25 ind./m2) than in the northern sector (4.46 ± 0.87 ind./m2). A. nigra and H. helianthus presented relatively homogeneous distributions. S. striatus showed greater density in North (1.50 ± 0.14 ind./m2), whereas A. spatuligera and L. magellanica maintained similar values across both sectors.
The mollusks T. chocolata and F. latimarginata were more abundant in North, especially T. chocolata (3.00 ± 0.33 ind./m2 vs. 1.00 ± 0.00 ind./m2 in South), whereas C. concholepas was recorded only in North (1.00 ± 0.00 ind./m2) (Supplementary Material S2; Figure 4A–F).

Figure 4.
Megabenthic communities identified along the Punta Coles Natural Reserve. (A) Red sea urchin Loxechinus albus aggregations, (B) black sea urchin Arbacia nigra, (C) Arbacia spatuligera, (D) sea sun Heliaster helianthus, (E) Phymanthea pluvia and (F) false abalone Concholepas concholepas.
Finally, the commercial brown alga L. trabeculata showed a relatively uniform distribution across all depth strata, with a slight increase in density toward deeper areas, from 3.54 ± 0.76 ind./m2 in stratum I to 3.77 ± 0.93 ind./m2 in stratum III. This pattern may reflect tolerance to a broad depth range. Spatially, L. trabeculata recorded higher densities in the northern sector (4.71 ± 0.70 ind./m2) than in the southern sector (1.57 ± 0.16 ind./m2).
Analysis of variance (ANOVA) revealed that neither depth nor sector nor their interaction had a significant effect on the richness or abundance of the megabenthic community. Specifically, richness showed no response to depth (F = 0.07, p = 0.932) or sector (F = 1.24, p = 0.287), and the interaction between these factors was also nonsignificant (F = 1.69, p = 0.226). Similarly, abundance did not differ significantly with depth (F = 0.63, p = 0.548), sector (F = 0.09, p = 0.769), or their interaction (F = 0.39, p = 0.684). These results suggest a spatially homogeneous distribution of megabenthic taxa across the surveyed area (Table 1).
3.2.2. Macrobenthos
A total of 101 macrobenthic species distributed across eight taxonomic groups were recorded (Supplementary Material S3). The phyla Annelida, Molluska, and Arthropoda stand out for their significant contributions to species composition and organism abundance, underscoring their ecological relevance in the study area.
Macrobenthic density showed marked variation across depth strata and taxonomic groups. Annelida was the most abundant group, with a clear decline in abundance with increasing depth (1–5 m: 44.4%; 5–10 m: 31.9%; 10–15 m: 12.7%), suggesting a preference for shallow substrates. Arthropoda maintained high densities in the upper strata (1–5 m: 23%; 5–10 m: 26.6%) but sharply declined at 10–15 m (5%), indicating a more restricted distribution to shallower zones. Chordata were scarce and limited to shallow strata (1–5 m: 3.07%; 5–10 m: 4.77%). In contrast, Echinodermata was most abundant in the deepest stratum (10–15 m: 67.4%), with much lower occurrence rates at shallower depths (1–5 m: 4.18%; 5–10 m: 8.21%). Molluska showed relatively stable abundances across depth strata (range: 13–24%), indicating a broad distribution without strong bathymetric affinity. Nematoda, Cnidaria, and Nemertea were infrequent and were primarily restricted to shallow zones, with no consistent records in deeper waters.
In terms of spatial distribution, the northern sector was dominated by Arthropoda (36.1%) and Annelida (32.3%), which together represented more than 68% of the total community. They were followed by Molluska (15.3%) and Nematoda (5.6%), while other groups, such as Cnidaria, Chordata, Echinodermata and Nemertea, had low representation (<5%). In contrast, the southern sector was characterized by greater contributions from Echinodermata (32.5%) and Annelida (30.5%). Molluska also showed a considerable presence (22.1%), whereas Arthropoda sharply decreased in this sector (10.1%) compared with its dominance in North. The remaining groups accounted for less than 3% (Figure 5A,B).
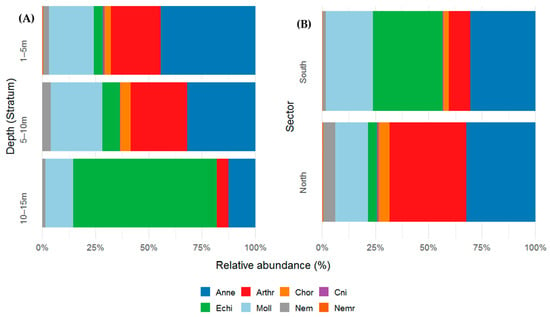
Figure 5.
(A) Relative abundance (percentage %) of macrobenthic taxonomic groups (phylum level) across three depth strata (1–5 m, 5–10 m, and 10–15 m) and (B) spatial variation across different sectors (B) in the Punta Coles Natural Reserve. Annelida (Anne), Arthropoda (Art), Chordata (Chor), Cnidaria (Cnid), Echinodermata (Echi), Molluska (Moll), Nematoda (Nem) and Nemertea (Nemr).
In terms of representative species, A. atra was recorded exclusively in stratum III, where it exhibited high abundance (1524.0 ± 221.62 ind./m2). The family Eatoniellidae predominated in strata I (1168.0 ± 327.43 ind./m2) and strata II (1225.60 ± 466.69 ind./m2) but was absent in stratum III. Ophiactis kroeyeri showed a marked increase in abundance with depth and was most abundant in stratum III (3090.29 ± 1073.61 ind./m2). In contrast, the polychaetes P. moerchi and Syllis sp. 1 were more abundant in stratum I (2832.00 ± 1063.29 ind./m2 and 725.71 ± 209.56 ind./m2, respectively), with a progressive decrease toward deeper strata.
Similarly, the results revealed differences in the abundances of representative species between sectors. A. atra was considerably more abundant in the southern sector (1845 ± 162 ind./m2) than in the northern sector (560 ± 30.1 ind./m2). The families Eatoniellidae and O. kroeyeri also presented higher abundances in South China (1452 ± 319 ind./m2 and 1958 ± 670 ind./m2, respectively) than in North China (32 ± 4.35 ind./m2 and 129 ± 26.2 ind./m2, respectively). The polychaete P. moerchi followed this same pattern, with significantly higher densities in South China (2559 ± 672 ind./m2) than in North China (297 ± 89.4 ind./m2). In contrast, Amphipoda sp. 1 and Syllis sp. 1 were more abundant in the northern sector (586 ± 167 ind./m2 and 574 ± 147 ind./m2, respectively) than in the southern sector (198 ± 38.8 ind./m2 and 315 ± 65.3 ind./m2, respectively). The remaining species exhibited variable abundance patterns depending on stratum and sector (Figure 6A,B).
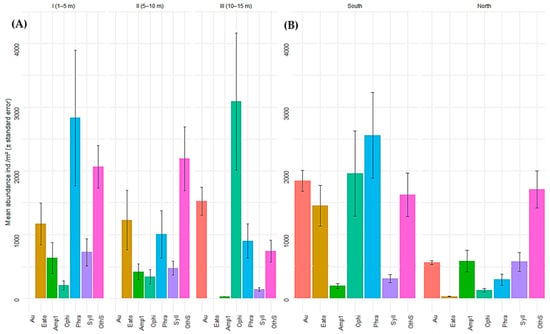
Figure 6.
(A) Mean abundance (ind./m2 ± standard error) of representative macrobenthic species across three depth strata (1–5 m, 5–10 m, and 10–15 m) and (B) across different sectors within the Punta Coles Natural Reserve. Aulacomia atra (Au), Eatoniellidae (Eato), Amphipoda sp. 1 (Amp1), Ophiactis kroeyeri (Ophi), Phragmatopoma moerchi (Phra), Syllis sp. 1 (Syll), other species (OthS).
Species richness (S) was highest in the intermediate stratum (II: 5–10 m), with a total of 75 recorded species, indicating greater taxonomic richness at this depth. However, both the Shannon diversity index (H’ = 3.21) and Pielou’s evenness index (J’ = 0.75) reached their highest values in stratum III, suggesting a more equitable distribution of individuals among species and greater overall diversity in this stratum. Similarly, the Simpson index (1–λ = 0.94) was greater in this stratum, reinforcing the notion of reduced dominance by a few species and a more structurally balanced community.
Sector-level analysis indicated that species richness was greater in the southern sector (S = 86), than in the northern sector (S = 73). Nevertheless, both the Shannon index (H’ = 3.20) and Pielou’s evenness index (J’ = 0.74) were greater in the northern sector. Moreover, the Simpson index (1–λ = 0.93) was also higher in this sector, indicating a more equitable community structure with less dominance by a few species (Table 2).

Table 2.
Species diversity indices across three depth strata and different sectors in the Punta Coles Natural Reserve.
Nonmetric multidimensional scaling (NMDS) analysis, which is based on the Bray-Curtis dissimilarity matrix, revealed a compositional gradient in the macrobenthic community structure that was associated primarily with depth. The stress value of 0.14 indicated an acceptable two-dimensional ordination. Along axis 1, a clear segregation pattern was observed, with deeper stations generally distributed on the left side of the diagram and shallower stations on the right, suggesting that depth functions as a dominant environmental gradient. Although some spatial variation between sectors is visible, the differentiation is not pronounced, reinforcing the idea that the environmental factors associated with depth exert a stronger influence on community structure than does geographic location. The variation observed along axis 2 may reflect differences in biological substrate composition (e.g., presence of macroalgae or tube-dwelling polychaetes), which increase habitat heterogeneity and may influence local assemblage patterns [28,29,30] (Figure 7A,B).
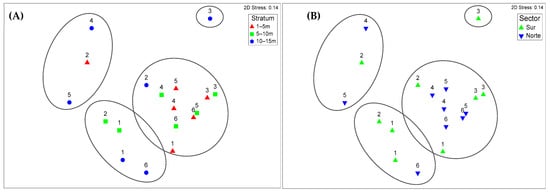
Figure 7.
Nonmetric multidimensional scaling (NMDS) ordination plots of macrobenthic community composition in the Punta Coles Natural Reserve based on Bray–Curtis dissimilarity of square-root transformed abundance data. (A) Ordination by depth stratum (1–5 m: red triangles; 5–10 m: green squares; 10–15 m: blue circles). (B) Ordination by sector (south: green upward triangles; north: blue downward triangles). The ellipses indicate the groupings formed in the analysis.
Univariate PERMANOVA revealed a significant effect of depth on the total abundance of macrobenthic organisms (p = 0.0142), suggesting that differences in abundance are driven primarily by depth. In contrast, no effect was detected for the spatial factor (sector) or for the interaction between depth and sector. In terms of total biomass, significant differences were found for both depth (p = 0.0339) and sector (p = 0.0035), indicating that both factors contribute to the variability in community biomass. However, their interaction was not significant (p = 0.0821). With respect to species richness, the only significant effect was associated with the interaction between depth stratum and sector (p = 0.016), suggesting that differences in species richness are not solely due to depth or sector independently but rather to the combination of both. Finally, the multivariate analysis of community structure also revealed significant effects of depth (p = 0.0024), sector (p = 0.0008), and their interaction (p = 0.0235), highlighting that community composition varies along both the depth gradient and between sectors and that complex interactions further influence community organization (Table 3).

Table 3.
Results of PERMANOVA tests to examine variability between “depth” and “sector” for (a) total abundance, (b) total biomass, (c) total richness and (d) community structure.
SIMPER analysis revealed marked differences in species composition among depth strata and between sectors (Table 4). The comparison between the extreme strata (1–5 m and 10–15 m) revealed the highest average dissimilarity (89.28%), indicating strong community turnover associated with the bathymetric gradient. Among the species contributing most to this dissimilarity was P. moerchi, accounting for 17.85% of the total. O. kroeyeri and Syllis sp. 1 also made significant contributions (9.99% and 9.40%, respectively). In the comparison between adjacent strata (1–5 m and 5–10 m), the average dissimilarity was also moderately high (79.17%), again dominated by P. moerchi (21.41%), followed by Syllis sp. 1 and Eatoniellidae, reflecting a gradual community shift with increasing depth. The comparison between strata 5–10 m and 10–15 m also revealed high dissimilarity (86.37%). In this case, in addition to P. moerchi (17.75%), O. kroeyeri (12.00%) and Syllis sp. 1 (7.15%) were important contributors, suggesting greater community heterogeneity between the middle and deeper zones.

Table 4.
Percentage (%) dissimilarity (SIMPER) of macrobenthic communities by stratum in the Punta Coles Natural Reserve.
At the spatial level (between sectors), the average dissimilarity was 85.33%, indicating substantial community turnover even within the same depth stratum. P. moerchi was again the most influential species (19.53%), followed by O. kroeyeri (10.42%) and Syllis sp. 1 (8.17%).
4. Discussion
This research represents one of the first efforts to characterize the subtidal benthic heterogeneity of the PCNR, a marine protected area on the southern Peruvian coast recognized for its remarkable coastal biodiversity [14,17,21]. Our study revealed several key habitat-forming organisms, such as subtidal kelp forests (Lessonia trabeculata), mussel beds (Aulacomya atra), and red sea squirt (Pyura chilensis). Additionally, other relevant habitat-forming species, including seaweeds, calcareous algae, tube-dwelling polychaetes, sponges, and other benthic groups, were identified. The observed diversity patterns exhibited distinctive spatial and bathymetric complexities. While the richness of cover species showed no significant trend with depth, abundance did vary significantly between sectors, suggesting that the distribution of habitat-forming organisms is more strongly influenced by spatial factors than by depth alone.
The total richness identified—13 cover species, 14 megabenthic species, and 101 macrobenthic species—underscores the ecological importance of the PCNR as a marine biodiversity hotspot along the southern Peruvian coast. This diversity was greater than that in other protected areas within the RNSIIPG, such as Isla Lobos de Tierra [49,50,51] and Bahía Paracas [52], and comparable to that in other complex marine systems, such as the Chilean Fjords [53]. The dominance of consolidated rocky substrates—evidenced by the prevalence of the calcareous alga Lithothamnium sp. and the widespread presence of L. trabeculata across all depth strata—provides the structural complexity necessary to support high biodiversity. These species, together with the high coverage of P. chilensis in shallow strata (0–10 m) and the mussel beds of A. atra in deeper zones (>10 m), act as ecosystem engineers by modifying the habitat and facilitating the establishment and refuge of a wide range of other organisms [54]. This likely explains the high macrobenthic richness observed in the reserve [27,55,56].
The megabenthic community included both commercially valuable and noncommercial but ecologically relevant species. The edible sea urchin Loxechinus albus was the most abundant species, predominating at depths shallower than 10 m. As a generalist herbivore, it primarily consumes fleshy and calcareous algae [57,58], and its spatial distribution appears to be closely tied to the availability of food sources such as L. trabeculata and Lithothamnium sp. Although these algae were widely distributed across the PCNR, they presented lower cover in areas where L. albus was particularly abundant, possibly due to intense herbivory. While no significant differences were detected in megabenthic composition between sectors and depth strata, emerging patterns of bathymetric and spatial zonation were observed for several species. For example, the vertical distributions of the sea urchins L. albus, A. nigra, and A. spatuligera differ notably and are likely influenced by habitat characteristics, ecological preferences [58,59,60,61], and movement patterns [62,63].
The presence of commercially valuable species such as Concholepas concholepas, Thaisella chocolata, and Fissurella latimarginata [19], although at low densities, highlights the importance of the reserve in safeguarding marine resources. Our study underscores the need for continuous monitoring to assess the impacts of potential anthropogenic pressures, particularly during the comanagement phase. Although comanagement typically focuses on target species, there is a risk of unauthorized extraction of other commercially valuable resources. This situation is exacerbated by institutional limitations in enforcing effective oversight [13], which could undermine the conservation objectives of the reserve.
PERMANOVA confirmed the significant effects of depth and sector on the macrobenthic community, as well as a complex interaction between them, indicating that these communities are structured by a combination of bathymetric gradients and spatial heterogeneity. Similar patterns have been described in studies conducted in Chilean fjords [53] and in previous IMARPE research [64,65]. This differentiated distribution appears to be driven by the presence of various habitat-forming organisms in the PCNR, which, through their structural complexity, favor the coexistence of multiple species [54,66,67,68,69,70]. Moreover, the interactions among these structural organisms likely facilitated the establishment of multiple species by generating a more complex and heterogeneous habitat [71], along with factors such as marine geomorphology and environmental conditions typical of coastal upwelling zones, which play a key role in shaping biodiversity [72].
The macrobenthic assemblage was dominated by mollusks, arthropods, and annelids—a pattern that is consistent with reports from other marine systems [73]. The most conspicuous species exhibited specific adaptations to bathymetric and spatial conditions, reflecting a close association with habitat structure. For example, P. moerchi, Eatoniellidae, Amphipoda, and Syllis showed a preference for shallow strata, whereas O. kroeyeri clearly dominated deeper areas. These distributions appear to reflect the functional roles played by aggregations of P. chilensis and A. atra mussel beds [61,70,74,75,76]. Therefore, incorporating these habitat-forming organisms—which are highly sensitive to extraction and have considerable structural influence on benthic communities—into the PCNR management plan is a priority measure to ensure the conservation of biologically complex assemblages.
Despite its important contributions, this study has certain limitations. The sampling, although extensive for establishing a baseline, lacked temporal resolution, which may obscure significant biodiversity variations influenced by climatic variability, particularly during warm and cold ENSO phases, as well as seasonal oceanographic events. Furthermore, while geomorphology and major habitat types have been described, deeper integration with biological variables, such as grazing and predation [77,78,79], and environmental parameters (temperature, dissolved oxygen) [80,81] is considered necessary. Future research should focus on long-term monitoring to better understand the ecological processes underlying community structure in the PCNR, which is essential for the design and implementation of more effective management and conservation strategies.
Our findings highlight the ecological significance of the reserve and the urgent need to implement conservation measures that recognize the functional value of its benthic assemblages. The data on diversity, composition, and community structure provided here are essential for evaluating the effectiveness of current conservation measures, particularly given the susceptibility of these systems to climate variability associated with ENSO [82] and fishing pressure [13,83]. This is especially relevant considering that the potential loss of biodiversity within the reserve could trigger cascading effects on food web structure, alter ecosystem response thresholds to disturbances, and compromise its long-term stability [84].
5. Conclusions
Our study not only contributes to the understanding of the PCNR ecosystem but also complements previous research on coastal fauna and marine resources conducted by other institutions, providing an important baseline and strengthening the generation of regional scientific knowledge on marine biodiversity. These findings underscore the importance of continuing research within the PCNR and reinforcing conservation efforts in the context of increasing the vulnerability of these ecosystems to anthropogenic and environmental pressures.
The findings confirm the need to integrate key habitat-forming species—such as subtidal kelp forests (Lessonia trabeculata), mussel beds (Aulacomya atra), and the red sea squirt (Pyura chilensis)—into the conservation strategies of the PCNR. Their abundance, distribution, ecological role, and susceptibility to extreme climatic events make them critical components of ecosystem stability. In this context, we propose their inclusion as “umbrella species”, since their conservation would indirectly protect a variety of associated habitats and species, thereby promoting a more integrated and ecosystem-based management approach for the area.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse13081400/s1, Supplementary Material S1. Mean (X ̅) ± standard error (SE) of the percentage coverage (%) of organisms by depth stratum of the Punta de Coles Natural Reserve. Supplementary Material S2. Mean (X ̅) ± standard error (SE) of the percentage coverage (%) of organisms by sector of the Punta de Coles Natural Reserve. Supplementary Material S3. Mean density (N° ind./m 2) of megabenthic organisms bydepth stratum in the Punta Coles Natural Reserve. Supplementary Material S4. Mean density (N° ind./m 2) of megabenthic organisms by sector in the Punta Coles Natural Reserve. Supplementary Material S5. Comprehensive list of the species identified in this study.
Author Contributions
Conceptualization, S.M.-A., R.P.-V., H.H.S.G., D.E.B.C., A.W.Z.-C. and J.G.A.; Data curation, S.M.-A., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Formal analysis, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Funding acquisition, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M. and D.E.B.C.; Investigation, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Methodology, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Project Administration, S.M.-A.; Resources, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Software, S.M.-A., H.H.S.G., A.W.Z.-C. and J.G.A.; Supervision, A.W.Z.-C.; Validation, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Visualization, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Writing—original draft, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A.; Writing—review and editing, S.M.-A., R.P.-V., H.H.S.G., J.L.C.A., Y.A.M., A.T.C., D.E.B.C., A.W.Z.-C. and J.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National University of Moquegua (UNAM), through institutional grants authorized by Resolutions No. 1279-2019-UNAM and 0102-2020-UNAM. UNAM is a public higher education institution that funds research projects carried out by its academic staff. Institutional address: Prolongación Av. Ancash, Ilo, Moquegua, Peru. Phone: +51 (053) 463514.
Institutional Review Board Statement
The study of benthic biodiversity in the Punta de Coles Nature Reserve did not require ethics approval, as research related to hydrobiological resources in Peru is regulated and authorized by the Ministry of Production (PRODUCE). In this case, the corresponding permit was granted through Directorial Resolution No. 00538-2023-PRODUCE/DGPCHDI.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
The authors express their gratitude to the National University of Moquegua UNAM for the facilities provided at the Laboratorio de Biología Molecular y Biotecnología UNAM subsidiary Ilo. This work was carried out in collaboration with the Euro-Latin Network of Symbiosis for Sustainable Aquaculture (SEASOS).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Sala, E.; Giakoumi, S. No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci. 2018, 75, 1166–1168. [Google Scholar] [CrossRef]
- Sarker, S.; Rahman, M.J.; Wahab, M.A. Modeling the role of marine protected area in biodiversity conservation. J. Sea Res. 2023, 196, 102457. [Google Scholar] [CrossRef]
- Trowbridge, C.D.; Kachmarik, K.; Plowman, C.Q.; Little, C.; Stirling, P.; McAllen, R. Biodiversity of shallow subtidal, underrock invertebrates in Europe’s first marine reserve: Effects of physical factors and scientific sampling. Estuar. Coast. Shelf Sci. 2017, 187, 43–52. [Google Scholar] [CrossRef]
- Pichegru, L.; Ryan, P.; Le Bohec, C.; van der Lingen, C.; Navarro, R.; Petersen, S.; Lewis, S.; van der Westhuizen, J.; Grémillet, D. Overlap between vulnerable top predators and fisheries in the Benguela upwelling system: Implications for marine protected areas. Mar. Ecol. Prog. Ser. 2009, 391, 199–208. [Google Scholar] [CrossRef]
- Guajardo, A.; Navarrete, C. Gestión adaptativa en áreas marinas protegidas de Chile: Un método para su evaluación. Lat. Am. J. Aquat. Res. 2012, 40, 608–612. [Google Scholar] [CrossRef]
- Roura, R. El debate sobre áreas marinas protegidas en la Antártida: ¿conservación o pesca? Ecol. Polít. 2013, 46, 48–56. [Google Scholar]
- Grau Tomás, E.; García Sanabria, J. Comparative analysis of marine-protected area effectiveness in the protection of marine mammals: Lessons learned and recommendations. Front. Mar. Sci. 2022, 9, 940803. [Google Scholar] [CrossRef]
- Henriques, R.; Mann, B.; Nielsen, E.; Hui, C.; von der Heyden, S. Extending biodiversity conservation with functional and evolutionary diversity: A case study of South African sparid fishes. Afr. J. Mar. Sci. 2020, 42, 315–321. [Google Scholar] [CrossRef]
- Kirkman, S.P.; Mann, B.Q.; Sink, K.J.; Adams, R.; Livingstone, T.C.; Mann-Lang, J.B.; Pfaff, M.C.; Samaai, T.; van der Bank, M.G.; Williams, L.; et al. Evaluating the evidence for ecological effectiveness of South Africa’s marine protected areas. Afr. J. Mar. Sci. 2021, 43, 389–412. [Google Scholar] [CrossRef]
- Tarazona, J.; Arntz, W. The Peruvian coastal upwelling system. In Coastal Marine Ecosystems of Latin America, Ecological Studies; Seeliger, U., Kjerfeve, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 144, pp. 229–244. [Google Scholar]
- Thiel, M.; Macaya, E.; Acuña, E. The Humboldt current system of northern-central Chile: Oceanographic processes, ecological interactions. Oceanogr. Mar. Biol. Annu. Rev. 2007, 45, 195–344. [Google Scholar]
- Thiel, M.; Erasmo, C.; Macaya, E.A.; Wolf, E.A. El sistema de la corriente de Humboldt del norte y centro de Chile: Procesos oceanográficos, interacciones ecológicas y retroalimentación socioeconómica. Oceanogr. Y Biol. Mar. 2007, 45, 195–344. [Google Scholar]
- Tejada, A.; Baldarrago, D.; Pastor, R.; Ortiz, M. Assessing the benefits of the marine protected area of Punta Coles, southern Peru (south-east Pacific) on productivity of conspicuous benthic species using preimage population analysis. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 29–43. [Google Scholar] [CrossRef]
- Línea Base Biológica Terrestre y Marina de la Reserva Nacional Sistema de Islas, Islotes y Puntas Guaneras—Punta Coles (Ilo, Moquegua); SERNANP: Lima, Perú, 2016; p. 150.
- Libro Rojo de la Fauna Silvestre Amenazada del Perú, 1st ed.; SERFOR (Servicio Nacional Forestal y de Fauna Silvestre): Lima, Perú, 2018; pp. 1–548.
- Figueroa, J.; Roca, M.; Torres, D.; Guillermo, E.; Paredes, F.; Barraza, D. Capítulo 1: Caracterización de la fauna: Aves, mamíferos y reptiles. In Línea Base Biológica Terrestre y Marina de la Reserva Nacional Sistemas de Islas, Islotes y Puntas Guaneras—Punta Coles (Moquegua); SERNANP: Lima, Perú, 2016. [Google Scholar]
- Méndez-Ancca, S.; Pepe-Victoriano, R.; Gonzales, H.H.S.; Aguilar, J.L.C.; Meza, Y.A.; Pacho, M.A.Q.; Cáceres, A.T.; Baldarrago Centeno, D.E.; Aguilera, J.G. Discovering the Bathylithology and Bioengineering Organisms of the Punta Coles Marine Natural Reserve, Moquegua, Peru. J. Mar. Sci. Eng. 2024, 12, 2265. [Google Scholar] [CrossRef]
- Tejada Cáceres, A.; González Vargas, A.; Baldarrago, D.; Liza, C. Bancos naturales de invertebrados marinos en las regiones Moquegua y Tacna, 2016. Inf. Inst. Mar Perú 2020, 47, 261–316. [Google Scholar]
- Plan Maestro de la Reserva Nacional Sistema de Islas, Islotes y Puntas Guaneras 2016–2020; SERNANP: Lima, Perú, 2016; p. 108.
- Tejada, A.; Baldarrago, D.; Aragón, B.; Vizcarra, Y.; Villanueva, J. El chanque Concholepas concholepas en el litoral de las regiones Moquegua y Tacna, 2017. Inst. Mar Perú Inf. 2019, 46, 557–577. [Google Scholar]
- Jahncke, J.; Garcia-Godos, A.; Goya, E. La dieta del guanay Leucocarbo bougainvilli y el piquero peruano Sula variegata en la costa peruana, agosto de 1997. Inst. Mar Perú Inf. 1997, 72, 25–37. [Google Scholar]
- Tarazona, J.; Gutiérrez, D.; Paredes, C.; Indacochea, A. Overview and challenges of marine biodiversity research in Peru una revision y desafios Para la investigacion en biodiversidad marina en Peru. Gayana 2003, 67, 206–231. [Google Scholar]
- Tasso, V.; El Haddad, M.; Assadi, C.; Canales, R.; Aguirre, L.; Vélez-Zuazo, X. Macrobenthic fauna from an upwelling coastal area of Peru (Warm Temperate Southeastern Pacific province-Humboldtian ecoregion). Biodivers. Data J. 2018, 6, e28937. [Google Scholar] [CrossRef] [PubMed]
- Arntz, W.; Gallardo, V.; Gutiérrez, D.; Isla, E.; Levin, L.; Mendo, J.; Neira, C.; Rowe, G.T.; Tarazona, J.; Wolff, M. El Niño and similar perturbation effects on the benthos of the Humboldt, California and Benguela current upwelling ecosystems. Adv. Geosci. 2006, 6, 243–265. [Google Scholar] [CrossRef]
- Correa, D.; Chamorro, A.; Tam, J. Clasificación pentadal de vientos frente a la costa peruana. Rev. Investig. Fís. 2020, 23, 61–65. [Google Scholar] [CrossRef]
- Cárdenas, F.; Pastor, R.; Baldarrago, D.; Castañeda, V.; Romucho, Y. Trazas de metales en agua, sedimento y organismos bentónicos en bancos naturales de las regiones de Tacna y Moquegua. Inst. Mar Perú Inf. 2015, 42, 122–136. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Conway-Cranos, L. Geographic Patterns of Recovery in Intertidal Communities; Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO) Scientific Symposium: Corvallis, OR, USA, 2007. [Google Scholar]
- Banks, S.; Acuña, D.; Brandt, M.; Calderón, R.; Delgado, J.; Edgar, G.; Garske-García, L.; Keith, I.; Kuhn, A.; Pépolas, R.; et al. Manual de Monitoreo Submareal; Conservación Internacional Ecuador y Fundación Charles Darwin: Quito, Ecuador, 2016. [Google Scholar]
- Fauchald, K. The polychaete worms. Definitions and keys to the orders, families and genera. Science Series; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1977; p. 188. [Google Scholar]
- Rozbaczylo, N.; Bolados, J. Nereidos de Iquique, Chile. (Polychaeta, Nereidae). Boletín Mus. Nac. Hist. Nat. 1980, 37, 205–224. [Google Scholar] [CrossRef]
- De León-Gonzales, J.A.; Bastida-Zavala, J.R.; Carrerra-Parra, L.F.; Garcia-Garza, M.E.; Peña-Rivera, A.; Salazar-Vallejo, S.I.; Solis-Weiss, V. Poliquetos (Annelida: Polychaeta) de México y América Tropical; Universidad Autónoma de Nuevo León: Monterrey, Mexico, 2009; p. 737. [Google Scholar]
- Alamo, V.; Valdivieso, V. Lista Sistemática de Moluscos Marinos del Perú, 2nd ed.; Publicación Especial; Inst. Mar Callao-Perú: Callao, Peru, 1997. [Google Scholar]
- Carbajal-Enzian, P.; Santamaría, J.; Baldárrago, D. Guía Ilustrada Para el Reconocimiento de Poliplacóforos, Gasterópodos y Cefalópodos Con Valor Comercial en el Perú; Instituto del Mar del Perú (Imarpe): Lima, Peru, 2018; p. 34. [Google Scholar]
- Osorio, C. Moluscos Marinos en Chile: Especies de Importancia Económica: Guía Para su Identificación; Universidad de Chile: Santiago, Chile, 2022; p. 211. [Google Scholar]
- Garth, J.S. Brachyura of the Pacific coast of America, Oxyrhyncha. Allan Hancock Pac. Exped. 1958, 21, 1–499. [Google Scholar]
- Chirichigno, N. Lista de Crustáceos del Perú (Decapoda y Stoma-topoda) con datos de distribución geográfica. Inf. Inst. Del Mar Del Perú 1970, 35, 95. [Google Scholar]
- Moscoso, V. Catálogo de crustáceos decápodos y estomatópodos del Perú. Boletín Inst. Del Mar Del Perú 2012, 27, 8–207. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + Primer: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Oksanen, J.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; Wagner, H.; et al. Vegan: Community Ecology Package, R package version 2.3-2; 2015. Available online: https://cran.r-project.org/package=vegan (accessed on 22 January 2024).
- Wickham, H. Getting Started with ggplot2. In ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016; pp. 11–31. [Google Scholar]
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2004. Available online: https://www.R-project.org/ (accessed on 22 January 2024).
- Clarke, K.; Warwick, R. Statistical analysis and interpretation of marine Community data. I.O.C. In Draft, Manuals and Guides 22; UNESCO: Paris, France, 1990; p. 52. [Google Scholar]
- Clarke, K.; Warwick, R. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E, Ltd., Plymouth Marine Laboratory: Plymouth, UK, 1994; p. 144. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER v5: User Manual/Tutorial. PRIMER-E: Plymouth. Bull. Mar. Coast. Res. 2001, 50, 91. [Google Scholar]
- Kruskal, J.B. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Pastor, R.; Gonzáles Araujo, A.; Zavalaga Talledo, F. Comunidades bentónicas de los ecosistemas de fondos blandos y duros en el intermareal y submareal somero. Sitio piloto isla Lobos de Tierra, 2014. Inf. Inst. Mar. Perú 2017, 44, 385–408. [Google Scholar]
- Ramírez Díaz, P.; De la Cruz Galloso, J.; Castro Gálvez, J. Biodiversidad marina en isla Lobos de Tierra, Lambayeque (agosto, 2017). Inf. Inst. Mar. Perú 2020, 47, 549–565. [Google Scholar]
- Ramírez Díaz, P.; De la Cruz Galloso, J.; Torres, D. Biodiversidad en la isla Lobos de Tierra, Región Lambayeque, setiembre 2015. Inf. Inst. Mar. Perú 2019, 46, 444–461. [Google Scholar]
- Pastor, R.; Gonzáles Araujo, A.; Zavalaga Talledo, F. Comunidades bentónicas de los ecosistemas de fondos blandos y duros en el intermareal y submareal somero. Sitio piloto Islas Ballestas. Setiembre-Octubre 2013. Inf. Inst. Mar. Perú 2017, 44, 303–331. [Google Scholar]
- Betti, F.; Enrichetti, F.; Bavestrello, G.; Costa, A.; Moreni, A.; Bo, M.; Ortiz Saini, P.; Daneri, G. Hard-bottom megabenthic communities of a Chilean fjord system: Sentinels for climate change? Front. Mar. Sci. 2021, 8, 635430. [Google Scholar] [CrossRef]
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef]
- Hastings, A.; Byers, J.E.; Crooks, J.A.; Cuddington, K.; Jones, C.G.; Lambrinos, J.G.; Talley, T.S.; Wilson, W.G. Ecosystem engineering in space and time. Ecol. Lett. 2007, 10, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T.; Gribben, P.E.; Latzel, S. Native ecosystem engineer facilitates recruitment of invasive crab and native invertebrates. Biol. Invasions 2016, 18, 3163–3173. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Buschmann, A.H. Herbivore-kelp interactions in Chilean subtidal communities: A review Interacciones alga-herbívoro en comunidades submareales chilenas: Una revision. Rev. Chil. De Hist. Nat. 1997, 70, 41–52. [Google Scholar]
- Vásquez, J.A.; Donoso, G.A. Loxechinus albus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elservier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 285–296. [Google Scholar] [CrossRef]
- Rodriguez, S.; Ojeda, F. Distribution patterns of Tetrapygus niger (Echinodermata: Echinoidea) off the central Chilean coast. Mar. Ecol. Prog. Ser. 1993, 101, 157. [Google Scholar] [CrossRef]
- Rodriguez, S.R.; Ojeda, F.P. Behavioral responses of the sea urchin Tetrapygus niger to predators and food. Mar. Freshw. Behav. Phy. 1998, 31, 21–37. [Google Scholar] [CrossRef]
- Gianguzza, P.; Bonaviri, C.A. Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 275–283. [Google Scholar]
- Barahona, M.; Navarrete, S. Movement patterns of the seastar Heliaster helianthus in central Chile: Relationship with environmental conditions and prey availability. Mar. Biol. 2010, 157, 647–661. [Google Scholar] [CrossRef]
- McClintock, J.B.; Lawrence, J.B. Characteristics of foraging in the soft-bottom benthic starfish Luidia clathrata (Echinodermata: Asteroidea): Prey selectivity, switching behavior, functional responses and movement patterns. Oecologia 1985, 66, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Baldarrago, D.; Aragón, B.; Vizcarra, Y.; Tejada Cáceres, A. Estructura Bentónica en el submareal somero de Punta Coles (Ilo–Moquegua)–2017. Inst. Mar Perú Lima-Callao 2019, 46, 578–600. [Google Scholar]
- Baldarrago, D.; Aragón, B.; Vizcarra, Y.; Mamani, L.; Tejada Cáceres, A. Caracterización de la Estructura Bentónica en el submareal somero de la reserva de Punta Coles (Ilo, Región Moquegua), 2019. Inst. Mar Perú Lima-Callao 2022, 49, 275–295. [Google Scholar]
- Tokeshi, M.; Romero, L. Filling a gap: Dynamics of space occupancy on a mussel-dominated subtropical rocky shore. Mar. Ecol. Prog. Ser. 1995, 119, 167–176. [Google Scholar] [CrossRef]
- Thiel, M.; Ullrich, N. Hard rock versus soft bottom: The fauna associated with intertidal mussel beds on hard bottoms along the coast of Chile, and considerations on the functional role of mussel beds. Helgol. Mar. Res. 2002, 56, 21–30. [Google Scholar] [CrossRef]
- Roff, J.C.; Taylor, M.E.; Lauhgren, J. Geophysical approaches to the habitat classification, delineation and monitoring of marine habitats and their communities. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 77–90. [Google Scholar] [CrossRef]
- Gribben, P.E.; Wright, J.T. Invasive seaweed enhances recruitment of a native bivalve: Roles of refuge from predation and habitat choice. Mar. Ecol. Prog. Ser. 2006, 318, 177–185. [Google Scholar] [CrossRef]
- Cerda, M.; Castilla, J. Diversidad y biomasa de macroinvertebrados en matrices intermareales del tunicado Pyura praeputialis (Heller, 1878) en la Bahía de Antofagasta, Chile. Rev. Chil. Hist. Nat. 2001, 74, 841–853. [Google Scholar] [CrossRef]
- Altieri, A.H.; Silliman, B.R.; Bertness, M.D. Hierarchical organization via a facilitation cascade in intertidal cordgrass bed communities. Am. Nat. 2007, 169, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Frost, N.J.; Hawkins, S.J. Interactive effects of losing key grazers and ecosystem engineers vary with environmental context. Mar. Ecol. Prog. Ser. 2011, 430, 223–234. [Google Scholar] [CrossRef]
- Branch, G.M.; Griffiths, C.L. The Benguela ecosystem. Part V. The coastal zone. Oceanogr. Mar. Biol. Annu. Rev. 1988, 26, 395–486. [Google Scholar]
- Monteiro, S.; Champman, M.; Underwood, A. Patches of the ascidian Pyura stolonifera (Heller, 1878): Structure of habitat and associated intertidal assemblajes. Exp. Mar. Biol. Ecol. 2002, 270, 171–189. [Google Scholar] [CrossRef]
- Sepulveda, R.; Camus, P.; Moreno, C. Diversity of faunal assemblages associated with ribbed mussel beds along the South American coast: Relative roles of biogeography and bioengineering. Mar. Ecol. 2016, 37, 943–956. [Google Scholar] [CrossRef]
- Craeymeersch, J.A.; Jansen, H.M. Bivalve Assemblages as Hotspots for Biodiversity. Goods and Services of Marine Bivalves; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Orostica, M.H.; Aguilera, M.A.; Donoso, G.A.; Vásquez, J.A.; Broitman, B.R. Effect of grazing on distribution and recovery of harvested stands of Lessonia berteroana kelp in northern Chile. Mar. Ecol. Prog. Ser. 2014, 511, 71–82. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Vega, J.M.A. El Niño 1997–1998 en el norte de Chile: Efectos en la estructura y en la organización de comunidades submareales dominadas por algas pardas. In El Niño–La Niña 1997–2000. Sus Efectos en Chile; Avaria, S., Carrasco, J., Rutllant, J., Yáñez, E., Eds.; CONA: Valparaíso, Chile, 2004; pp. 119–135. [Google Scholar]
- Skein, L.; Robinson, T.B.; Alexander, M.E. Impacts of mussel invasions on the prey preference of two native predators. Behav. Ecol. 2018, 29, 353–359. [Google Scholar] [CrossRef]
- Coleman, N.; Cuff, W.; Moverley, J.; Gason, A.; Heiselrs, S. Depth, sediment type, biogeography and high species richness in shallow-water benthos. Mar. Freshw. Res. 2007, 58, 293–305. [Google Scholar] [CrossRef]
- Pacheco, A.S.; Laudien, J.; Thiel, M.; Oliva, M.; Heilmayer, O. Succession and seasonal onset of colonization in subtidal hard-bottom communities off northern Chile. Mar. Ecol. 2011, 32, 75–87. [Google Scholar] [CrossRef]
- McClanahan, T.R. Response of the coral reef benthos and herbivory to fishery closure management and the 1998 ENSO disturbance. Oecologia 2008, 155, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Manríquez, P.H.; Castilla, J.C.; Ortiz, V.; Jara, M.E. Empirical evidence for large-scale human impact on intertidal aggregations, larval supply and recruitment of Pyura praeputialis around the Bay of Antofagasta, Chile. Aust. Ecol. 2016, 41, 701–714. [Google Scholar] [CrossRef]
- Albertson, L.K.; Sklar, L.S.; Tumolo, B.B.; Cross, W.F.; Collins, S.F.; Woods, H.A. The ghosts of ecosystem engineers: Legacy effects of biogenic modifications. Funct. Ecol. 2024, 38, 52–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).