Abstract
Hydrothermal vents are “oases” of biological productivity and endemicity on the seafloor. Chemosynthetic communities at deep-sea hydrothermal vents are characterized by high abundance and endemism. The distribution of species among these isolated habitats supports regional biodiversity and stability, so understanding the fundamental processes is a key target of conservation. Larval dispersal resulting from deep-ocean circulations is one of the major factors influencing the diversity and distributions of vent animals. By combining a biophysical model with biological larvae traits, we quantify potential larval dispersal of vent species via ocean circulation in the Azores Triple Junction. Here we present results from a biophysical model of larval dispersal run for the hydrothermal vent benthic mussel Bathymodiolus azoricus. Several scenarios were implemented, based on similar data sets, although changing values for one or two parameters, such as swimming behaviour and planktonic larvae duration. Results showed that larvae retention is the most common pattern from the Azores Triple Junction vent fields mussel. The Rainbow vent field is rather isolated, being the sink population of the Menez Gwen and Lucky Strike but with a very low number of larvae exchange. Results are discussed in the framework of spatial management to maintain the populations after an impact by natural or human disturbance.
1. Introduction
Connectivity, defined as the exchange of individuals among spatially separated populations, is a fundamental concept in marine conservation biology. It underpins the maintenance of genetic diversity and population resilience to environmental disturbances [1,2,3]. Dispersal is central to connectivity and is especially critical during the larval stage in many marine species [3]. For benthic and sessile organisms, early life stages such as larvae, eggs, or spores represent the primary opportunity for dispersal [3,4]. The spatial and temporal scales over which dispersal occurs determine whether populations can recover from disturbances, recolonize degraded habitats, or continue to supply larvae to dependent populations.
Larval dispersal is shaped by multiple biological and physical factors, including spawning output, larval behaviour, ocean currents, and mortality rates [2,5]. Pelagic larval duration (PLD) plays a major role in determining dispersal distance and timing of settlement [6,7,8]. Spawning dynamics, such as synchrony, seasonality, and location, further influence connectivity patterns [9]. For species with planktonic larvae, successful settlement requires suitable habitat at the time larvae become competent, making dispersal potential a central factor in biogeography and marine population dynamics.
Deep-sea hydrothermal vents, characterized by high habitat fragmentation and unique chemosynthetic communities, offer a natural laboratory for studying connectivity [10,11,12]. While some vent species disperse through stepping-stone mechanisms along mid-ocean ridges [13], others exhibit surprisingly high gene flow despite natural barriers like ridge offsets and depth gradients [14,15]. Nonetheless, empirical connectivity data in deep-sea vents are limited [13,14,15,16,17,18,19]. Increasing anthropogenic impacts—fishing, oil and gas extraction, climate change, and deep-sea mining—threaten these ecosystems, making species’ capacity to reproduce, disperse, and colonize new habitats vital for their persistence [20,21,22,23,24,25,26].
Understanding deep-sea connectivity has become a central focus of recent research, particularly at hydrothermal vents along the Mid-Atlantic Ridge (MAR). Genetic approaches such as mitochondrial DNA sequencing and population genomics have revealed both connectivity and isolation among vent species like mussels and limpets [8,27,28,29]. However, these tools often lack the resolution to identify source-sink dynamics. Biophysical models complement genetic studies by simulating larval transport based on oceanographic circulation, larval traits like PLD, and behaviour [5,9]. On the northern MAR, models using Argo float data have identified persistent dispersal corridors and barriers shaped by ridge topography and bathymetry [30], helping guide spatial conservation strategies under changing environmental conditions [31].
The Azores region hosts several hydrothermal vent fields within the MAR, including Menez Gwen, Lucky Strike, and Rainbow, which support Bathymodiolus azoricus, an endemic vent mussel and key habitat-forming species [32,33]. These sites are protected under the Azores Marine Park and the OSPAR MPA network [34,35], yet potential mining interest in mineral-rich deposits remains [36]. Despite similarities with other Bathymodiolus species found globally [37,38,39], B. azoricus has a unique biogeographic range and appears to follow an annual reproductive cycle [40,41,42,43]. While biophysical models have enhanced understanding of connectivity elsewhere [8,44,45,46], they have not yet been applied to the Azores vent systems.
Identifying larval sources and sinks is essential for spatial planning and regional conservation management strategies. In this study, we present results from a biophysical larval dispersal model developed for B. azoricus [47], a dominant benthic species inhabiting hydrothermal vent ecosystems in the Azores.
We examine patterns of ecological connectivity among hydrothermal vent fields and assess the extent to which the current marine protected area (MPA) network supports long-term population viability. Specifically, we test two hypotheses: (1) connectivity among vent fields is spatially structured and shaped by regional oceanographic processes, and (2) the existing MPA network adequately captures the key connectivity pathways necessary to maintain viable populations.
2. Materials and Methods
2.1. Study Area
The Mid-Atlantic Ridge south of the Azores hosts three hydrothermal vent fields—Menez Gwen, Lucky Strike, and Rainbow—each characterized by distinct geological settings, fluid geochemistry, and depths. This region lies near the intersection of three tectonic plates—the North American, Eurasian, and African plates—and is known as the Azores Triple Junction.
The Menez Gwen vent field is located at a depth of approximately 850 m on the volcanic segment north of Lucky Strike, near the summit of a young volcano at the base of a graben [48]. Lucky Strike, situated at 1750 m depth, features active vents surrounding a central lava lake, with the most intense activity occurring on its western side [49]. Hydrothermal discharges here include high-temperature black smokers (~324 °C), intermediate-temperature flanges rich in barite, iron, and zinc sulphides (~170 °C), and low-temperature diffuse flows depositing amorphous silica [50].
Rainbow, the deepest of the three vent fields at 2300 m, is located on the northern part of the AMAR segment. The active vent area lies at the intersection of the ridge with a non-transform fault and is hosted in an ultramafic geological setting, with only a small basaltic outcrop located about 1 km east of the vent field. The site is characterized by massive sulphide deposits and chimneys enriched in copper, zinc, cobalt, and nickel, with values notably higher than those found in basalt-hosted systems [49]. Fluids emitted at Rainbow reach temperatures of 360 °C and are acidic (pH 2.9–3.1). Compared to other MAR vent fields, Rainbow fluids have low hydrogen sulphide (H2S) content but are enriched in hydrogen (H2) and methane (CH4), with a relatively uniform chemical composition of major and trace elements [50,51,52].
The Menez Gwen vent field [53] is located approximately 89 km north of Lucky Strike, while Rainbow lies 183 km to the south of Lucky Strike and 272 km from Menez Gwen. All these three vent fields also have satellite vents: Bubbylon for Menez Gwen [54], Ewan for Lucky Strike [54] and Rainbow Pit [30] for Rainbow. The Marine Protected area includes these satellite vents.
2.2. Model Setup
2.2.1. Hydrodynamic Mode
To simulate the 3D dispersal of larvae in our study, we implemented an offline particle-tracking model that utilizes stored 3D hydrodynamic hindcast results. An offline setup was chosen to concentrate computational resources on releasing enough particles, thereby ensuring statistical robustness in the model outputs [55].
The hydrodynamic data were obtained using the MOHID Water Modelling System, developed by the Marine and Environmental Technology Research Center at Instituto Superior Técnico, University of Lisbon [56]. Larval dispersal simulations were carried out using the Biophysical Connectivity Modelling System (CMS), which integrates biological parameters such as larval duration, mortality, and reproductive periods.
A high-resolution (~6 km) 3D ocean model (MOHID) has been developed specifically for the Azores region and is currently operational. The MOHID system adopts an integrated modelling approach, accounting for both physical and biogeochemical processes across different spatial and temporal scales. The model is open-source and has been under development since 1985 at IST. It follows an object-oriented architecture, representing water bodies as modular components, and includes a fully 3D hydrodynamic core coupled with modules for water quality, atmospheric processes, discharges, oil dispersion, and point-source mixing zones [57].
The hydrodynamic model is forced by tides, atmospheric data, and open boundary conditions. The MOHID Lagrangian Transport Module is used to track the trajectories of individual particles or water masses. MOHID has been successfully applied across a wide range of environments and spatial scales (see [58]).
Hydrodynamic Model Configuration for the Azores Region
The model was implemented using a nested downscaling approach with three grid levels (Level 1 to Level 3), each with increasing horizontal resolution (see Table 1 and Figure 1 for details of each domain). The system is forced by the following:
- Tidal data from the FES2012 global tidal model;
- Atmospheric forcing from the Global Forecast System (GFS);
- Open boundary conditions (OBCs) from MERCATOR-OCEAN (PSY2V4; [58]).

Table 1.
Principal characteristics of model implementation in the Azores region.
Table 1.
Principal characteristics of model implementation in the Azores region.
| Domain Level | Bathymetry Data (Source) | Type | Dimension (dx/dy) | Spacial Resolution (dx/dy) | Time Step (seconds) | Period Simulated |
|---|---|---|---|---|---|---|
| Level 1 | EMODnet + Gebco | 2-D barotropic | 300 × 200 | 6 km × 6 km | 120 | 2011–2013 |
| Level 2 | EMODnet | 3-D baroclinic | 113 × 92 | 6 km × 6 km | 120 | 2011–2013 |
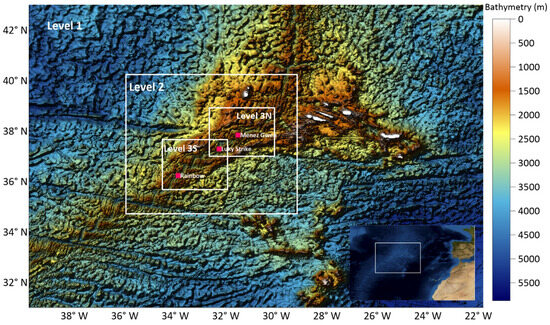
Figure 1.
Domain areas with the respective bathymetries in the Azores region model systems. Red points are the location of the tree hydrothermal vents.
The model setup follows the configuration used in the Azores operational ocean forecast system, which has been running since 2008 [59,60] and has been applied in other regions by the authors [61,62].
The Level 2 (regional) and Level 3 (north and south) domains are fully 3D baroclinic circulation models with 50 vertical layers: 43 in Cartesian coordinates and 7 sigma layers near the surface, providing a resolution down to 1 m in the upper ocean. The FES2012 tidal data set is applied to the Level 1 domain. FES2012 was developed by Noveltis, LEGOS, and the CLS Space Oceanography Division and is distributed by AVISO with support from CNES (AVISO site).
The outputs from the hydrodynamic model—including horizontal and vertical current velocities, sea surface height, temperature, and salinity—are used as input to the CMS for simulating larval dispersal in offline mode.
2.2.2. Bathymetric Data
Several sources of bathymetric data were used (summarized in Table 1) to take advantage of the best resolution on each product (Figure 1).
The GEBCO data was extracted from the General Bathymetric Chart of the Oceans (GEBCO, www.gebco.net/ accessed on 14 July 2024) while the EMODnet data was from the EMODnet Bathymetry portal (http://www.emodnet-bathymetry.eu. accessed on 14 July 2024).
Also, local multibeam bathymetry obtained by the University of the Azores (Uaz) has been used in Level 3 domain to better resolve some places in the Middle Atlantic Ridge, where all the emissions points of the present study are located. The final bathymetries and the location of the three hydrothermal vents are shown in Figure 2 (Rainbow, Lucky Strike and Menez Gwen), and Table 2 indicates their depths.
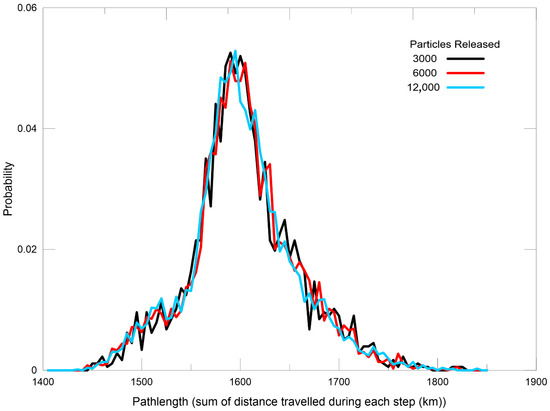
Figure 2.
Larval Travel Distance Probability and Release Magnitude: The X-axis represents the path length—defined as the cumulative distance travelled by each larva across all time steps—grouped into 100 km bins. The Y-axis shows the probability of distribution of these distances. Line colour indicates increasing numbers of larvae released during a given period, ranging from black to red and blue. A smoother curve observed beyond 6000 released larvae reflects stochastic saturation, suggesting this is the minimum number needed to capture consistent dispersal patterns. These values represent larvae released from a single site without considering mortality.
2.2.3. Validations Results
The model configuration, selected parameters, and validation results are detailed in [60]. Model validation was performed by comparing outputs from the MOHID hydrodynamic model with temperature and salinity data obtained from both remote sensing and in situ ARGO float observations. The ARGO data were collected and made freely available by the International Argo Project and the national programmes contributing to it (http://www.argo.ucsd.edu, http://argo.ucsd.edu/jcommops, accessed on 28 June 2025). ARGO is a pilot programme of the Global Ocean Observing System.
In Domain 2, the vertical thermohaline structure simulated by the hydrodynamic model showed very high agreement with ARGO float data, with Pearson correlation coefficients exceeding 0.95—most values were around 0.99 (see Supplementary Figures S1–S3). The hydrodynamic model outputs—including horizontal and vertical velocity components, sea level, temperature, salinity, and density fields—were then used as input for the CMS (Connectivity Modelling System).
The agreement between the model and observational data was strong. Figure S3 (Supplementary Materials) shows scatter diagrams representing the statistical validation results for the year 2011, focusing on the Level 2 (regional) model domain.
2.3. Biophysical Modelling System
To simulate larval dispersal and estimate population connectivity, we used Lagrangian particle-tracking approaches, which are widely applied to oceanographic transport studies, including larval movement and recruitment [3,63]. Specifically, we implemented the Connectivity Modelling System (CMS) [64,65] and integrated Behavior components adapted from the Lagrangian TRANSport (LTRANS) model [66,67]. The CMS was originally developed for modelling complex larval trajectories within three-dimensional flow fields, while LTRANS allows detailed behavioural parameterization based on turbulence, vertical migration, and other biological cues.
We modified the CMS framework by integrating behavioural modules from LTRANS to better simulate species-specific larval dynamics. These included vertical swimming and sinking velocities, which were either set as constants or defined by probabilistic functions. Behavioural cues were introduced to direct vertical movement, mimicking ontogenetic shifts or habitat preferences. To replicate natural variability in behaviour, a random component was applied to both swimming speed and orientation. This hybrid model allowed for the incorporation of key life-history traits influencing larval transport outcomes, including vertical migration and passive drift.
Larval trajectories were computed using CMS driven by hydrodynamic output from a regional ocean circulation model. This setup enabled particle tracking in a three-dimensional flow field over time, beginning from ecologically relevant release points. The model incorporated biologically realistic processes such as egg buoyancy, diel or ontogenetic vertical migration, and stochastic mortality [42,68]. Additionally, vertical orientation near the surface and bottom layers was parameterized using LTRANS-derived logic [66,67], enhancing ecological realism.
Following the principle that larval dispersal distance can be approximated by combining circulation dynamics and pelagic larval duration (PLD) [4], we used PLD-informed parameters to define the dispersal window for each simulation. PLD was modelled as a species-specific distribution, capturing variability in development time and settlement readiness. Behavioural modules allowed larvae to become competent after a threshold period and settle when suitable bottom conditions were met.
To ensure sufficient sampling for accurate dispersal probability estimates, we tested particle-release scenarios with varying sample sizes. A probabilistic saturation analysis was conducted to determine the minimum number of particles required for robust statistical outcomes, accounting for mortality [69]. Preliminary simulations were run with 1000, 3000, 6000, and 12,000 (Figure 2). Results indicated that a threshold of ≥12,000 particles was sufficient to produce consistent connectivity metrics across replicate runs.
Our analysis indicated that releasing 6000 particles is the minimum required to obtain accurate dispersal estimates. For the present study, 8000 particles were released over the one-month simulation period for each site, corresponding to the spawning season.
2.4. The Vent Mussel
Reproductive Timing of Bathymodiolus azoricus: Histological studies from May 1994 indicated a reproductive pause and recent spawning in Bathymodiolus azoricus populations at Lucky Strike and Menez Gwen [70]. Newly settled juveniles with prodissoconch II shell lengths of 472–602 µm were observed in early June [41], while a heavy larval settlement occurred at Rainbow between 28 August and 1 September, suggesting larval ages of 1–2 months [70]. Additional evidence from aquaria and field studies confirmed annual spawning in January–February, based on oocyte sizes of 70–80 µm [43,44]. These findings led to the use of two pelagic larval durations (PLDs)—90 days for early summer settlers and 240 days for those observed later in the year.
Settlement Observations and Knowledge Gaps: Newly settled mussels (~500 µm) were also observed in early June 1994, and juveniles of ~300 µm and ~500 µm were found at vent fields in July 1996 [42,45]. Sediment trap samples showed heavy settlement at Rainbow from late August to early September, with only sparse settlement at Menez Gwen during the same period [70]. Despite similar reproductive patterns across sites, no prior modelling study has assessed larval dispersal and connectivity among the Menez Gwen, Lucky Strike, and Rainbow vent fields.
Study Design and Parameter Selection: To simulate larval dispersal, species-specific parameters were input into the Connectivity Modelling System (CMS). When direct data for B. azoricus were unavailable, information from the related mussel Gigantidas childressi or coastal mytilids was used. This cross-referencing approach is supported by similarities in reproductive traits across Bathymodiolus species, cold seep mussels, and littoral mytilids [71,72,73,74,75,76]. These substitutions enabled biologically realistic simulations despite data gaps.
Larval Life Cycle and Release Conditions: Simulated larvae followed the known developmental trajectory of Mytilidae mussels. Fertilized eggs form ciliated embryos, which progress to trochophores and D-veligers (prodissoconch I shell stage) before becoming competent pediveligers [77]. This planktonic period is critical for dispersal and habitat selection. The simulations modelled four larval stages—blastula, trochophore, D-veliger, and pediveliger—with each scenario informed by ocean circulation and species biology (Table 2 and Table 3).
Behavioural Scenarios in Simulations: Three larval behaviours were modelled. In the “neutral “scenario, particles remained neutrally buoyant after 40 h, drifting passively. In “Behaviour 1,” larvae began surface-oriented swimming post-blastula, with a 55% probability of rising during the veliger stage and becoming bottom-oriented after PLD [78]. “Behaviour 2” mirrored “Behaviour 1” but introduced downward swimming 10 days before PLD. These behaviours captured stage-specific responses to environmental cues and vertical habitat preferences.
PLD and Habitat-Constrained Settlement: Both 90-day and 240-day PLDs were simulated, reflecting known variability in development rates. Settlement was defined as arrival within Marine Protected Areas (MPAs) encompassing the target vent fields. Larvae dispersing outside MPAs were not considered settled, as suitable benthic habitat is limited to hydrothermal vents, making successful recruitment outside those zones ecologically not possible (Table 2).
Connectivity Matrix and Settlement Probabilities: Model results were recorded as raw connectivity matrices, showing the number of larvae settling from each source site i to each sink site j. These values were converted into settlement probabilities using:
in which Pset(i,j) was the settlement probability representing the proportion of larvae exported from a source i to a sink j( relative to the number of larvae released from the source (Ni) [63]. A total of 6 matrices were generated in this study based on 1 organism, 10 years, and 3 vent fields.
Connectivity Interpretation and Retention Patterns: Six matrices were generated across 10 years of simulated data, incorporating three vent fields and one focal species. Diagonal elements in the matrices represent local retention—the proportion of larvae that returned and settled at their origin vent site. High retention indicates site-specific self-recruitment potential, a critical factor in designing effective conservation measures within MPAs [79].

Table 2.
Summary of larval characteristics used in the larval dispersal modelling.
Table 2.
Summary of larval characteristics used in the larval dispersal modelling.
| Biological Trait | Model Parameterization (B. azoricus) | Reference |
|---|---|---|
| Spawning time | A large proportion of the animals recovered in early February 2003 were releasing eggs and motile sperms; the same happened when animals were kept in captivity for one year February | [42,45] |
| Spawning location | Central coordinates of each vent field (Menez Gwen, Lucky Strike, Rainbow) | [33,34] |
| Spawning depth | Mean depth of each vent field | |
| Egg diameter | Mean diameter of Gigantidas childressi eggs collected in the Gulf of Mexico 69.15 ± 2.36 µm | [71] |
| Egg density | Calculated Based on Mytilus edulis | [80] |
| Blastula density | Calculated Based on Mytilus edulis | [80] |
| Trochophore density | Calculated Based on Mytilus edulis | [80] |
| D veliger density | Calculated Based on mussel (Mytilus edulis) parameters | [79,81] |
| Competent larvae density | Calculated Based on mussel (Mytilus edulis) parameters | [79,81] |
| Planktonic larval duration | Based on calculations for Gigantidas childressi from the Gulf of mexico; also from the difference between spawning time and observation of competent B. azoricus larvae. 90 and 240 days | [41,42,43,70,71] |
| Larval swimming velocity | Based on Mytilus data and temperature dependent | [80,82] |
| Larval Mortality | Based on the PLD as calculated by the model | [64] |
| Settlement habitat | Inside the designated MPA for each hydrothermal vent field, that also include the satellite hydrothermal vents | [35] |
Several scenarios were implemented, based on similar data sets, although changing values for the planktonic larvae duration and comparing swimming behaviour with neutral. Results are discussed in the framework of spatial management to maintain the populations after impacted by human activities.

Table 3.
Input parameters used for the simulation of larval dispersal.
Table 3.
Input parameters used for the simulation of larval dispersal.
| Parameter | Adopted Values |
|---|---|
| Hydrodynamic model | MOHID |
| High resolution (6 km) days | 10 years |
| Depth of emissions | bottom depth at location |
| Location (Lat, Long, water depth) | |
| Menez Gwen hydrothermal vent field | 37.850° N, 32.517, 840 m |
| Lucky Strike hydrothermal vent field | 37.283° N, 32.283º W, 1700 m |
| Rainbow hydrothermal vent field | 36.217° N, 33,900 W, 2300 m |
| Date of emission | 15 January–15 February |
| Number of larvae emitted | 8000 (31 + 1) × 250 |
| Number of development stages | 4 |
| Turbulence | yes |
3. Results
In this study, we used high-resolution particle-tracking models, incorporating species-specific and larval traits, to estimate the degree of contemporary connectivity between mussel populations at the Azores Triple Junction MAR vent fields. Our biophysical model indicated that the Rainbow vent field receives very few dispersing larvae from the northern vent fields, and this exchange only occurs under the longest PLD scenario.
3.1. Connectivity Matrices
3.1.1. The Settlement/Potential Connectivity Matrix
To evaluate the regional-scale connectivity among hydrothermal vent fields in the Azores Triple Junction, settlement data were visualized as a connectivity matrix (Figure 3), illustrating the percentage of larval particles exchanged between source and settlement locations. The diagonal of the matrix highlights self-seeding or larval retention, which was the dominant dispersal pattern across simulations. This suggests that most larvae settled near their point of origin, while exchange between different vent fields was consistently low.
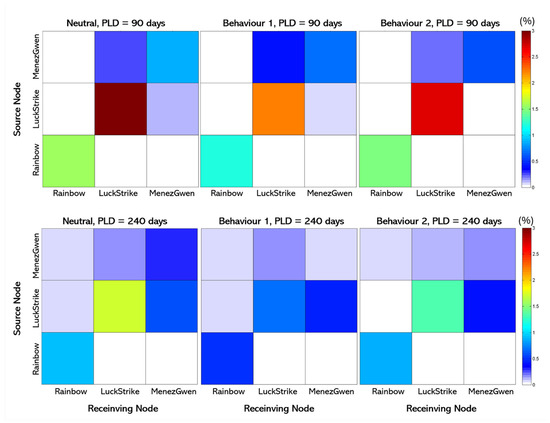
Figure 3.
Connectivity matrices for larvae dispersing as neutral, Behaviour 1, and Behaviour 2, for ten years (2008 to 2017), considering different PLDs (90, and 240 days). After the PLD, larvae would become competent and sink onto the seafloor (neutral and Behaviour 1). Behaviour 2 involves the larvae beginning to gradually swim down 10 days before the PLD age. Source locations appear as rows, and settlement locations appear as columns; the diagonal denotes larvae retention. Colour spectrum indicates the percentage (%) number of larvae successfully settling in each area. White cells indicate settlement rates < 0.05%. Spectrum goes from blues to red (3%).
The simulations indicated limited but directional larval exchange between vent fields. Menez Gwen (MG) and Lucky Strike (LS) showed mutual, though minimal, larval supply, while connectivity between LS and Rainbow (Rb) was extremely rare, occurring only in specific behavioural scenarios (e.g., Behaviour 1). Similarly, Menez Gwen provided low levels of larval input to Rainbow. These patterns persisted across a 10-year model period, implying that while the vent fields are technically connected, realized connectivity is weak and asymmetric.
Overall, Lucky Strike was largely isolated from Rainbow, with simulations showing only rare dispersal events between the two. Larval recruitment patterns revealed strong larvae retention in all vent fields (Figure 3), underscoring the importance of local retention. Dispersal from Rainbow was especially constrained; regardless of larval duration or behaviour, most Rainbow larvae failed to find suitable habitat and were lost during transit.
Dispersal outcomes were heavily influenced by planktonic larval duration (PLD) and current dynamics. For neutral buoyancy larvae, a PLD of 90 days enabled some exchange between Menez Gwen and Lucky Strike, with limited dispersal to Rainbow only appearing at 240-day PLD. Even then, Rainbow-originating larvae rarely reached other vents (Figure 4). Most larvae continued to recruit back to their natal vent, showing high retention across simulation years.
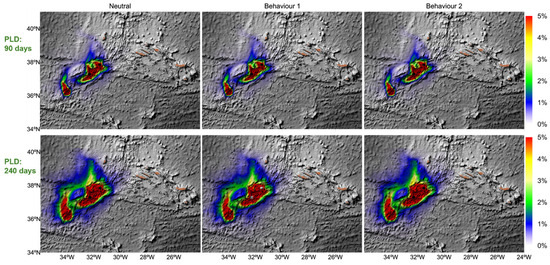
Figure 4.
Dispersal kernels for larvae on top of the bathymetric dispersing as neutral, Behaviour 1, and Behaviour 2, for ten years (2008 to 2017). After the PLD, larvae would become competent and sink onto the seafloor (neutral and Behaviour 1). Behaviour 2—the larvae begin gradually swimming down 10 days before the PLD age. Colour spectrum indicates the larvae position probability during dispersal. Colour scale goes from 0 in blues to 5% in red.
Buoyant and behaviourally swimming larvae generally travelled shorter distances than neutrally buoyant ones, due to vertical constraints. Even in longer PLD scenarios (240 days), successful settlement primarily occurred between Menez Gwen and Lucky Strike, with only occasional success at Rainbow. While swimming behaviour theoretically enhanced dispersal range, it did not significantly increase successful settlement due to the sparsity of suitable vent habitat. Thus, despite the potential for long-distance transport (Figure 5), realized connectivity remained low across the Azores vent system.
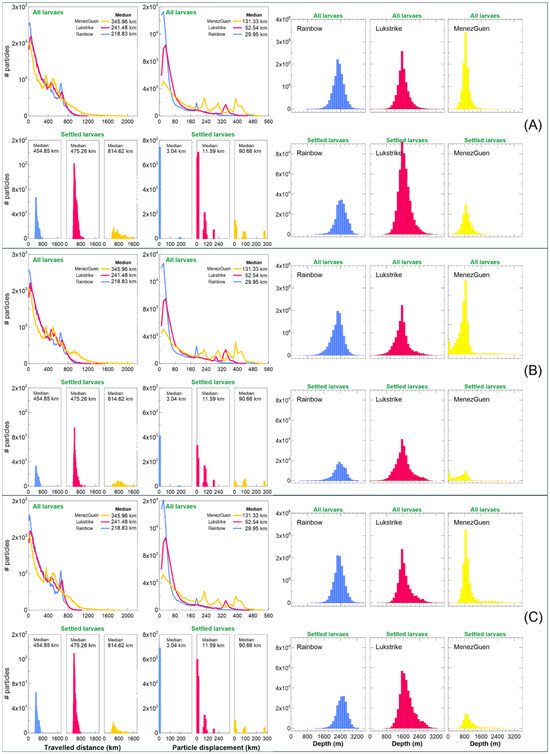
Figure 5.
Median of the estimated dispersal kernels for the travelled linear distance and Frequency distributions of the travel distance, particle displacement, and depth of the Bathymodiolus azoricus settled larvae released in February with a PLD of 240 days from 2008 to 2017. Bars show the median dispersal probability values over the 240 days from the release sites until they settled. The x-axis is in kilometres or metres (depth), and the y-axis refers to the density of the estimated dispersal kernel. (A) refers to neural scenario, (B)—Behaviour 1, and (C)—Behaviour 2 scenario.
The dispersal kernel reveals that topography significantly influences larval connectivity between vent fields, with physical barriers such as seamounts restricting movement (Figure 4); for instance, larvae from Rainbow are confined by a nearby seamount and do not reach Menez Gwen or Lucky Strike, although the reverse movement is possible. Across all modelled scenarios, larval dispersal is dominated by short-distance travel (larvae retention), with limited long-distance exchange, contributing to the observed low connectivity among vent fields (Figure 5). Moreover, larvae generally remain within specific depth layers, and only those from the shallowest site, Menez Gwen, reach the surface, likely enabling their broader dispersal due to stronger surface currents.
3.1.2. Back Tracking
Identifying the source and destination of dispersing larvae is a key challenge in connectivity studies, especially given the prevalence of local retention, and to address this, a backtracking module was applied to simulate larval trajectories over two years for different planktonic larval durations (PLDs). The results (Figure 6) reveal significant interannual variability, indicating that larval exchange between vent fields does not occur every year. Notably, only the 240-day PLD enabled connectivity between Menez Gwen and Lucky Strike, while Rainbow remained largely isolated across both years. At a 90-day PLD, backtracking failed to identify clear source locations, making it impossible to confirm connectivity between sites. Despite the occasional long-distance transport at extended PLD, local retention remained the dominant pattern, as shown by the high percentages of larvae returning to or maintaining their origin (Figure 6).
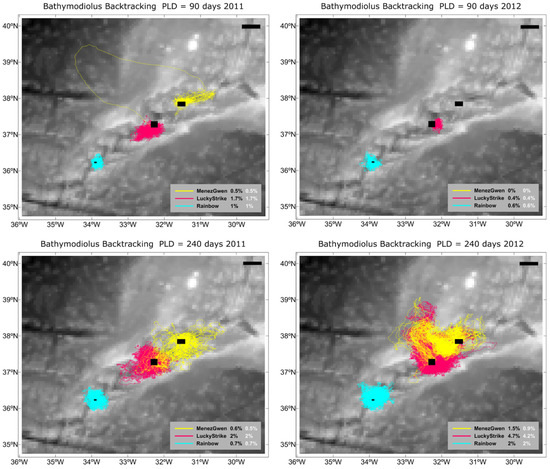
Figure 6.
Backtracking plot for two years (2011 and 2012) taking into account different PLDs (90, 240). Orange yellow colours are Menez Gwen, Red/Pink are Lucky Strike, and Blues are Rainbow. White percentages indicate larvae retention in the MPA. Black bar refers to 50 km.
4. Discussion
Deep-Sea Connectivity: A Critical Knowledge Gap
Understanding population connectivity is vital for predicting ecological responses to disturbance and informing conservation strategies. While extensive research exists on shallow-water systems (e.g., [9,83]), deep-sea environments, particularly hydrothermal vents, remain understudied, with limited data available from both larval dispersal modelling (e.g., [4,8,17,74]) and population genetics (e.g., [17]). The specialized communities in deep-sea hydrothermal vents, such as those at the Azores Triple Junction, depend heavily on species like Bathymodiolus mussels, which function as ecosystem engineers [34,35,84]. Their ability to recolonize disturbed sites is fundamental to the resilience of these ecosystems.
Dispersal Dynamics and Retention Patterns
Using high-resolution Lagrangian particle-tracking models (LTRANS and PARIS), we simulated larval dispersal under various biological and oceanographic scenarios. Across simulations, a common trend emerged: most larvae exhibited strong local retention, likely self-recruitment, and with limited dispersal between vent fields. Dispersal from Menez Gwen to Lucky Strike was the most frequent, while Rainbow remained largely isolated. Only under the longest planktonic larval duration (PLD = 240 days) and active swimming behaviour did Rainbow receive larvae from neighbouring fields (Figure 3). These findings are consistent with studies showing that larval retention is a dominant feature of marine populations, particularly when habitats are spatially constrained [5,85,86].
Increasing PLD generally enhanced connectivity, but with diminishing returns due to increased mortality risks [9,30]. While longer PLDs allow for potential long-distance dispersal, they do not guarantee settlement success, especially in environments where suitable habitat is rare. In swimming larvae scenarios, realized dispersal distances were often greater at shorter PLDs due to more efficient settlement near natal sites (Figure 4). This reflects the fact that suitable vent habitat is highly localized, and larvae drifting too far are unlikely to find appropriate cues for settlement [87,88,89].
Vertical behaviour was a major driver of dispersal outcomes. Negatively buoyant larvae generally remained near the seafloor, limiting their dispersal range, whereas larvae capable of vertical movement encountered faster currents at shallower depths [90,91,92], increasing dispersal potential between Menez Gwen and Lucky Strike. However, only larvae from Menez Gwen (at 850 m) reached surface waters, consistent with observations from other shallow-living Bathymodiolus populations [13,74,93,94]. The capacity to survive pressure changes remains uncertain, yet it may be a limiting factor for deeper populations like Rainbow.
Isolation of Rainbow Vent Field
Rainbow was consistently isolated in all dispersal scenarios. This may be attributed to its greater depth and associated weak bottom currents or the presence of stratified water masses that limit vertical mixing and larval exchange. Genetic studies on deep-sea species also support the idea that depth-related isolation can result in high population structure [89,90,91,92]. Additionally, ontogenetic changes in buoyancy—from positively buoyant early stages to negatively buoyant late stages—reduce the dispersal capability of larvae, especially near the seafloor where current velocities are low.
Anthropogenic and Climatic Threats to Connectivity
Future connectivity could be severely affected by deep-sea mining and climate change. Artificial particles, such as those from mining plumes, may interfere with larval behaviour or settlement [94]. Moreover, warming oceans may reduce PLD and increase mortality in Bathymodiolus mussels, particularly for larvae from deeper vents like Rainbow [72,93,95,96,97,98]. Incorporating natural climate variability such as the North Atlantic Oscillation (NAO) and Atlantic Multidecadal Oscillation (AMO), which operate on multi-year cycles, into hydrodynamic models would improve predictions under changing oceanographic conditions [98,99].
One limitation of this study is the 10-year time span of oceanographic data used in simulations. Interannual variability, as demonstrated in our backtracking results, suggests that rare but ecologically significant dispersal events may occur outside this window. This may lead to an underestimation of connectivity. If larval exchange is minimal but persistent, populations may remain demographically linked even if they appear isolated in short-term models. However, recolonization potential after disturbance may still be low, especially for isolated sites like Rainbow.
Conservation Implications and MPA Effectiveness
Deep-sea conservation is particularly challenging due to limited data and access. Nevertheless, spatial protection frameworks such as the Azores Marine Park, Natura 2000, and the OSPAR network encompass the three studied vent fields. Our larval dispersal simulations show that key Marine Protected Areas (PMA02, 03, 04, and 13) align with predicted larval pathways (Figure 6), suggesting that this MPA network may effectively preserve both source populations and connectivity corridors—crucial for ecosystem resilience [30].
Aligning Modelling and Genetic Evidence
Although many genetic studies suggest widespread gene flow and low population structure along the Mid-Atlantic Ridge (e.g., [14,100,101]), this apparent discrepancy with our model results is not contradictory. Genetic connectivity reflects evolutionary timescales, while biophysical models capture ecological timescales [101]. Thus, occasional long-distance dispersal events—even if rare—may be sufficient to maintain gene flow without supporting frequent demographic exchange.
5. Conclusions
This study provides the first estimate of larval dispersal and population connectivity among hydrothermal vent fields in the Azores, focusing on Bathymodiolus azoricus. Our results show that connectivity is generally low and mostly limited to exchanges between Menez Gwen and Lucky Strike, with Rainbow appearing largely isolated and potentially acting as a sink population. This pattern aligns with observed mussel densities, raising concerns about the resilience of these populations to anthropogenic disturbances. While the Azores Marine Park currently encompasses all three vent fields, including their associated ridge systems, the weak connectivity—especially with Rainbow—highlights the vulnerability of these ecosystems.
To enhance protection, particularly for the more isolated Rainbow field, conservation strategies should extend beyond spatial designation. Although the Marine Protected Areas (MPAs) are well delineated, formal regulations must be implemented to ensure effective conservation. These should include not only protection of the benthic habitat but also of the overlying water column, which is essential for larval transport. Limiting pollutants such as mineral particles from industrial activities will help preserve the “sphere of influence” that supports larval dispersal and population sustainability [102]. Such integrated management is critical to maintaining functional connectivity and supporting ecosystem resilience in the deep sea.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse13091642/s1, Figure S1: Model data (MOHID and Mercator) vs. Argo float data for 3 floats found in the Azores region, for Level 2 domain. Red dot is the Argo location.; Figure S2: Model data (MOHID and Mercator) vs. Argo float data for 3 floats found in the Azores region, for Level 2 domain. Red dot is the Argo location; Figure S3: Scatter diagrams showing the statistical results from the validations of the MOHID results and Argo float data, for the temperature (top) and salinity (bottom).
Author Contributions
Conceptualization, A.C.; methodology, A.C. and M.J.; formal analysis, M.J.; investigation, A.C. and M.J.; data curation, M.J.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and M.J.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to this work has received funding from the European Union’s Seventh Framework Programme under the grant agreement No 603418 (MIDAS). This research is part of the DEEP REST project that was funded through the 2020–2021 Biodiversa and Water JPI joint call for research projects, under the BiodivRestore ERA-NET Cofund (GA N°101003777), with the EU and the following funding organizations: Agence Nationale de la Recherche (ANR-21-BIRE-0003), France, Ministry of Agriculture, Nature and Food Quality (LNV), The Netherlands, Research Foundation—Flanders (FWO), Belgium, German Federal Ministry of Research (BMBF) through VDI/VDE-IT, Germany, Environmental Protection Agency (EPA), Ireland, Fundação para a Ciência e a Tecnologia (FCT), Portugal, Fundo Regional para a Ciência e Tecnologia (FRCT) (Refª M2.2/DEEPREST/004/2022), Portugal-Azores and State Research Agency (AEI), Spain. AC was supported by the FCT-IP Programme Stimulus of Scientific Employment (CEECIND/00101/2021). AC also received support from the Operational Programme Azores 2020, through the Fund 01-0145-FEDER-000140 “MarAZ Researchers: Consolidate a body of researchers in Marine Sciences in the Azores” of the European Union. AC and MJ also acknowledge funds through the FCT—Foundation for Science and Technology, I.P., under the project OKEANOS UIDB/05634/2023 and UIDP/05634/2023.
Data Availability Statement
The 3-D Lagrangian particle model results will be available up request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gaines, S.D. Dependence of sustainability on the configuration of marine reserves and larval dispersal distance. Ecol. Lett. 2001, 4, 144–150. [Google Scholar] [CrossRef]
- Cowen, R.K.; Gawarkiewicz, G.; Pineda, J.; Thorrold, S.R.; Werner, F.E. Population connectivity in marine systems an overview. Oceanography 2007, 20, 14–21. [Google Scholar] [CrossRef]
- Cowen, R.K.; Sponaugle, S. Larval dispersal and marine population connectivity. Ann. Rev. Mar. Sci. 2009, 1, 443–466. [Google Scholar] [CrossRef]
- Young, C.M.; He, R.; Emlet, R.B.; Li, Y.; Qian, H.; Arellano, S.M.; Van Gaest, A.; Bennett, K.C.; Wolf, M.; Smart, T.I.; et al. Dispersal of deep-sea larvae from the Intra-American Seas: Simulations of trajectories using ocean models. Integr. Comp. Biol. 2012, 52, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.P.; Srinivasan, M.; Almany, G.R. Population connectivity and conservation of marine biodiversity. Oceanography 2007, 20, 100–111. [Google Scholar] [CrossRef]
- Pineda, J. Linking larval settlement to larval transport: Assumptions, potentials, and pitfalls. Oceanogr. East. Pac. 2000, 1, 84–105. [Google Scholar]
- Strathmann, R.R. What controls the type of larval development? Summary statement for the evolution session. Bull. Mar. Sci. 1986, 39, 616–622. [Google Scholar]
- Mitarai, S.; Watanabe, H.; Nakajima, Y.; Shchepetkin, A.F.; McWilliams, J.C. Quantifying dispersal from hydrothermal vent fields in the western Pacific Ocean. Proc. Natl. Acad. Sci. USA 2016, 113, 2976–2981. [Google Scholar] [CrossRef]
- Pineda, J.; Hare, J.A.; Sponaugle, S.U. Larval transport and dispersal in the coastal ocean and consequences for population connectivity. Oceanography 2007, 20, 22–39. [Google Scholar] [CrossRef]
- Boschen-Rose, R.E.; Colaço, A. Northern Mid-Atlantic Ridge Hydrothermal Habitats: A Systematic Review of Knowledge Status for Environmental Management. Front. Mar. Sci. 2021, 8, 657358. [Google Scholar] [CrossRef]
- Gollner, S.; Colaço, A.; Gebruk, A.; Halpin, P.; Higgs, N.; Menini, E.; Mestre, N.C.; Qian, P.Y.; Sarrazin, J.; Szafranski, K.; et al. Application of scientific criteria for identifying hydrothermal ecosystems in need of protectionS. J. Mar. Policy 2021, 132, 104641. [Google Scholar] [CrossRef]
- Mullineaux, L.S.; Metaxas, A.; Beaulieu, S.E.; Bright, M.; Gollner, S.; Grupe, B.M.; Herrera, S.; Kellner, J.B.; Levin, L.A.; Mitarai, S.; et al. Exploring the ecology of deep-sea hydrothermal vents in a metacommunity framework. Front. Mar. Sci. 2018, 5, 49. [Google Scholar] [CrossRef]
- Breusing, C.; Vrijenhoek, R.C.; Reusch, T.B.H. Widespread introgression in deep-sea hydrothermal vent mussels. BMC Evol. Biol. 2017, 17, 13. [Google Scholar] [CrossRef]
- Teixeira, S.; Serrão, E.A.; Arnaud-Haond, S. Panmixia in a fragmented and unstable environment: The hydrothermal shrimp Rimicaris exoculata disperses extensively along the Mid-Atlantic Ridge. PLoS ONE 2012, 7, e38521. [Google Scholar] [CrossRef]
- Vrijenhoek, R.C. Genetic diversity and connectivity of deep-sea hydrothermal vent metapopulations. Mol. Ecol. 2010, 19, 4391–4411. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Sun, J.; Xu, Q.; Qian, P.Y. Structure and connectivity of hydrothermal vent communities along the mid-ocean ridges in the West Indian Ocean: A review. Front. Mar. Sci. 2021, 8, 744874. [Google Scholar] [CrossRef]
- Breusing, C.; Johnson, S.B.; Mitarai, S.; Beinart, R.A.; Tunnicliffe, V. Differential patterns of connectivity in Western Pacific hydrothermal vent metapopulations: A comparison of biophysical and genetic models. Evol. Appl. 2023, 16, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, T.; Fukumori, H.; Warén, A.; Kano, Y. Population connectivity of hydrothermal-vent limpets along the northern Mid-Atlantic Ridge (Gastropoda: Neritimorpha: Phenacolepadidae). J. Mar. Biol. Assoc. UK 2019, 99, 179–185. [Google Scholar] [CrossRef]
- Brunner, O.; Chen, C.; Giguère, T.; Kawagucci, S.; Tunnicliffe, V.; Watanabe, H.K.; Mitarai, S. Species assemblage networks identify regional connectivity pathways among hydrothermal vents in the Northwest Pacific. Ecol. Evol. 2022, 12, e9612. [Google Scholar] [CrossRef]
- Smith, C.R.; Levin, L.A.; Koslow, A.; Tyler, P.A.; Glover, A.G. The near future of the deep-sea floor ecosystems. In Aquatic Ecosystems: Trends and Global Prospects; Polunin, N.V.C., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 334–352. [Google Scholar]
- Ramirez-Llodra, E.; Rogers, A.D. An Ecosystem View of Anthropogenic Impacts in the Deep Ocean. In Volume 2: Marine Ecology; CRC Press: Boca Raton, FL, USA, 2025; pp. 201–228. [Google Scholar]
- Danovaro, R.; Snelgrove, P.V.; Tyler, P. Challenging the paradigms of deep-sea ecology. Trends Ecol. Evol. 2014, 29, 465–475. [Google Scholar] [CrossRef]
- Burgess, S.C.; Baskett, M.L.; Grosberg, R.K.; Morgan, S.G.; Strathmann, R.R. When is dispersal for dispersal? Unifying marine and terrestrial perspectives. Biol. Rev. 2016, 91, 867–882. [Google Scholar] [CrossRef]
- Chesson, P. Recruitment limitation: A theoretical perspective. Aust. J. Ecol. 1998, 23, 234–240. [Google Scholar] [CrossRef]
- Underwood, A.J.; Fairweather, P.G. Supply-side ecology and benthic marine assemblages. Trends Ecol. Evol. 1989, 4, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.K.; Sewell, M.A.; Byrne, M. Revisiting the larval dispersal black box in the Anthropocene. ICES J. Mar. Sci. 2018, 75, 1841–1848. [Google Scholar] [CrossRef]
- Jollivet, D.; Portanier, E.; Matabos, M. Connecting the Vents: Using Genetics to Understand Ecosystem Connectivity Along the Mid-Atlantic Ridge; iAtlantic Science Brief: Edinburgh, Scotland, 2024. [Google Scholar]
- Breusing, C.; Biastoch, A.; Drews, A.; Metaxas, A.; Jollivet, D.; Vrijenhoek, R.C.; Bayer, T.; Melzner, F.; Sayavedra, L.; Petersen, J.M.; et al. Biophysical and population genetic models predict the presence of “phantom” stepping stones connecting Mid-Atlantic Ridge vent ecosystems. Curr. Biol. 2016, 26, 2257–2267. [Google Scholar] [CrossRef]
- Yearsley, J.M.; Salmanidou, D.M.; Carlsson, J.; Burns, D.; Van Dover, C.L. Biophysical models of persistent connectivity and barriers on the northern Mid-Atlantic Ridge. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 180, 104819. [Google Scholar] [CrossRef]
- Escartin, J.; Andreani, M.; The Arc-en-Sub Science Party. Diversity and dynamics of ultramafic-hosted hydrothermal activity at mid-ocean ridges: First results from the Arc-en-Sub oceano-graphic cruise, Rainbow Massif, 36°14’N MAR. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 24–28 April 2023. EGU23-13265. [Google Scholar] [CrossRef]
- Baco, A.R.; Etter, R.J.; Ribeiro, P.A.; Von der Heyden, S.; Beerli, P.; Kinlan, B.P. A synthesis of genetic connectivity in deep-sea fauna and implications for marine reserve design. Mol. Ecol. 2016, 25, 3276–3298. [Google Scholar] [CrossRef]
- Colaço, A.; Desbruyères, D.; Comtet, T.; Alayse, A.M. Ecology of the Menez Gwen hydrothermal vent field (Mid-Atlantic Ridge/Azores Triple Junction). Cah. Biol. Mar. 1998, 39, 237–240. [Google Scholar]
- Desbruyères, D.; Biscoito, M.; Caprais, J.C.; Colaco, A.; Comtet, T.; Crassous, P.; Fouquet, Y.; Khripounoff, A.; Le Bris, N.; Olu, K.; et al. Variations in deep-sea hydrothermal vent communities on the Mid-Atlantic Ridge near the Azores plateau. Deep Sea Res. Part I 2001, 48, 1325–1346. [Google Scholar] [CrossRef]
- Decreto Legislativo Regional n.° 14/2024/A de 24 de dezembro de 2024. Segunda alteração ao Decreto Legislativo Regional n.° 28/2011/A, de 11 de novembro, alterado e republicado pelo Decreto Legislativo Regional n.° 13/2016/A, de 19 de julho, que estrutura o Parque Marinho dos Açores. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-legislativo-regional/14-2024-901147128 (accessed on 15 July 2024).
- Hennicke, J.; Blanchard, S.; Chaniotis, P.; Cornick, L.; Hauswirth, M.; Schellekens, T.; Vonk, S.; Werner, T. Report and Assessment of the Status of the OSPAR Network of Marine Protected Areas in 2021; OSPAR Commision: London, UK, 2022. [Google Scholar]
- Morato, T.; Juliano, M.; Pham, C.K.; Carreiro-Silva, M.; Martins, I.; Colaço, A. Modelling the dispersion of Seafloor Massive Sulphide mining plumes in the Mid Atlantic Ridge around the Azores. Front. Mar. Sci. 2022, 9, 910940. [Google Scholar] [CrossRef]
- Sibuet, M.; Olu, K. Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep Sea Res. 1998, 45, 517–567. [Google Scholar] [CrossRef]
- Tunnicliffe, V.; McArthur, A.G.; McHugh, D. A biogeographical perspective of the deep-sea hydrothermal vent fauna. Adv. Mar. Biol. 1998, 34, 353–442. [Google Scholar]
- Van Dover, C.L.; Humphris, S.E.; Fornari, D.; Cavanaugh, C.M.; Collier, R.; Goffredi, S.K.; Hashimoto, J.; Liley, M.D.; Reysenbach, A.L.; Shank, T.M.; et al. Biogeography and ecological setting of Indian Ocean Hydrothermal Vents. Science 2001, 294, 818–823. [Google Scholar] [CrossRef]
- Tyler, P.A.; Young, C.M. Reproduction and dispersal at vents and cold seeps. J. Mar. Biol. Assoc. UK 1999, 79, 193–208. [Google Scholar] [CrossRef]
- Comtet, T.; Desbruyères, D. Population structure and recruitment in mytilid bivalves from the Lucky Strike and Menez Gwen hydrothermal vent fields (37 degrees 17′ N and 37 degrees 50′ N on the Mid-Atlantic Ridge). Mar. Ecol. Prog. Ser. 1998, 163, 165–177. [Google Scholar] [CrossRef]
- Dixon, D.; Lowe, D.; Miller., P.; Villemin, G.; Colaço, A.; Serrão-Santos, R.; Dixon, L. Evidence for seasonal reproduction in the Atlantic vent mussel Bathymodiolus azoricus, and an apparent link to the timing of photosynthetic primary production. J. Mar. Biol. Assoc. UK 2006, 86, 1363–1371. [Google Scholar] [CrossRef]
- Colaço, A.; Martins, I.; Laranjo, M.; Pires, L.; Leal, C.; Prieto, C.; Costa, V.; Lopes, H.; Rosa, D.; Dando, P.R.; et al. Annual spawning of the hydrothermal vent mussel, Bathymodiolus azoricus, under controlled aquarium conditions at atmospheric pressure. J. Exp. Mar. Biol. Ecol. 2006, 333, 166–171. [Google Scholar] [CrossRef]
- Thomson, R.E.; Subbotina, M.M.; Anisimov, M.V. Numerical simulation of mean currents and water property anomalies at Endeavour Ridge: Hydrothermal versus topographic forcing. J. Geophys. Res. 2009, 114, 9020. [Google Scholar] [CrossRef]
- McGillicuddy, D.J.; Lavelle, J.W.; Thurnherr, A.M.; Kosnyrev, V.K.; Mullineaux, L.S. Larval dispersion along an axially symmetric mid-ocean ridge. Deep Sea Res. Part I 2010, 57, 880–892. [Google Scholar] [CrossRef][Green Version]
- Ross, R.E.; Nimmo-Smith, W.A.M.; Howell, K. Towards ‘ecological coherence’: Assessing larval dispersal within a network of existing marine protected areas. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 126, 128–138. [Google Scholar] [CrossRef]
- Cosel, R.V.; Comtet, T.; Krylova, E.M. Bathymodiolus (Bivalvia: Mytilidae) from hydrothermal vents on the Azores Triple Junction and the Logatchev hydrothermal field, Mid-Atlantic Ridge. Veliger 1999, 42, 218–248. [Google Scholar]
- Fouquet, Y.; Ondréas, H.; Charlou, J.L.; Donval, J.P.; Radford-Knoery, J.; Costa, I.; Lourenço, N.; Tivey, M.K. Atlantic lava lakes and hot vents. Nature 1995, 377, 201. [Google Scholar] [CrossRef]
- Fouquet, Y.; Charlou, J.L.; Costa, I.; Donval, J.P.; Radford-Knoery, J.; Pellé, H.; Ondréas, H.; Lourenço, N.; Ségonzac, M.; Kingston Tivey, M. A detailed study of the Lucky Strike hydrothermal vent site and discovery of a new hydrothermal site: Menez Gwen; Preliminary results of the DIVA 1 cruise (2–29 May). InterRidge News 1994, 3, 14–17. [Google Scholar]
- Charlou, J.L.; Donval, J.P.; Douville, E.; Knoery, J.; Fouquet, Y.; Bougault, H.; Jean Baptiste, P.; Stievenard, M.; German, C. High methane flux between 15ºN and the Azores Triple Junction, Mid-Atlantic Ridge. Hydrothermal and serpentinization processes. EOS 1997, 78, 46. [Google Scholar]
- Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Jean-Baptiste, P.; Holm, N. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36 14′ N, MAR). Chem. Geol. 2002, 191, 345–359. [Google Scholar] [CrossRef]
- Douville, E.; Charlou, J.L.; Oelkers, E.H.; Bienvenu, P.; Colon, C.J.; Donval, J.P.; Fouquet, Y.; Prieur, D.; Appriou, P. The rainbow vent fluids (36 14′ N, MAR): The influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids. Chem. Geol. 2002, 184, 37–48. [Google Scholar] [CrossRef]
- Marcon, Y.; Sahling, H.; Borowski, C.; dos Santos Ferreira, C.; Thal, J.; Bohrmann, G. Megafaunal distribution and assessment of total methane and sulfide consumption by mussel beds at Menez Gwen hydrothermal vent, based on geo-referenced photomosaics. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2013, 75, 93–109. [Google Scholar] [CrossRef]
- Escartin, J.; Barreyre, T.; Cannat, M.; Garcia, R.; Gracias, N.; Deschamps, A.; Salocchi, A.; Sarradin, P.M.; Ballu, V. Hydrothermal activity along the slow-spreading Lucky Strike ridge segment (Mid-Atlantic Ridge): Distribution, heatflux, and geological controls. Earth Planet. Sci. Lett. 2015, 431, 173–185. [Google Scholar] [CrossRef]
- North, E.W.; Adams, E.E.; Schlag, Z.; Sherwood, C.R.; He, R.; Hyun, K.H.; Socolofsky, S.A. Simulating oil droplet dispersal from the Deepwater Horizon spill with a Lagrangian approach. In Monitoring and Modeling the Deepwater Horizon Oil Spill: A Record-Breaking Enterprise; Liu, Y., Macfadyen, A., Ji, Z.-G., Weisberg, R.H., Eds.; AGU: Washington, DC, USA, 2011; Volume 195, pp. 217–226. [Google Scholar] [CrossRef]
- Mateus, M.; Neves, R. Ocean Modelling for Coastal Management—Case Studies With MOHID; IST Press: Lisbon, Portugal, 2013; 265p, ISBN 978-989-8481-24-5. [Google Scholar]
- Neves, R. THE MOHID CONCEPT. In Ocean Modelling for Coastal Management—Case Studies with MOHID; Mateus, M., Neves, R., Eds.; IST Press: Lisbon, Portugal, 2013; pp. 1–11. [Google Scholar]
- Drillet, Y.; Bourdallé-Badie, R.; Siefridt, L.; Le Provost, C. Meddies in the Mercator North Atlantic and Mediterranean Sea eddy-resolving model. J. Geophys. Res. Oceans 2005, 110, 03016. [Google Scholar] [CrossRef]
- Riflet, G.; Juliano, M.; Fernandes, L.; Leitão, P.C.; Neves, R. Operational Ocean forecasting of the Portuguese waters. In Mercator Ocean Quarterly Newsletter; Mercator Ocean International: Toulouse, France, 2008; pp. 20–32. [Google Scholar]
- Fernandes, L.; Montero, P.; Garcia, C.; Neves, R.; Obaton, D.; Pérez-Muñuzuri, V.; Juliano, M.F.; Ayensa, G. Building a polycentric structure for the Atlantic Arc—EASY project. In Proceedings of the 5th EuroGOOS Conference, Exeter, UK, 20–22 May 2008. [Google Scholar]
- Juliano, M.; Neves, R.; Rodrigues, P.P.G.W.; Junior, J.L.; Fernandes, R. Aplicação da Plataforma MOHID para simulação computacional de deriva oceânica de petróleo na bacia de campos—RJ. Bol. Do Obs. Ambient. Alberto Ribeiro Lamego 2012, 6, 161–172. [Google Scholar]
- Mateus, M.; Riflet, G.; Chambel, P.; Fernandes, L.; Fernandes, R.; Juliano, M.; Campuzano, F.; de Pablo, H.; Neves, R. An operational model for the West Iberian coast: Products and services. Ocean. Sci. 2012, 8, 713–732. [Google Scholar] [CrossRef]
- Werner, F.E.; Cowen, R.K.; Paris, C.B. Coupled biological and physical models: Present capabilities and necessary developments for future studies of population connectivity. Oceanography 2007, 20, 54–69. [Google Scholar] [CrossRef]
- Paris, C.B.; Helgers, J.; Van Sebille, E.; Srinivasan, A. Connectivity Modeling System: A probabilistic modeling tool for the multi-scale tracking of biotic and abiotic variability in the ocean. Environ. Model. Softw. 2013, 42, 47–54. [Google Scholar] [CrossRef]
- Paris, C.B.; Cowen, R.K.; Claro, R.; Lindeman, K.C. Larval transport pathways from Cuban snapper (Lutjanidae) spawning aggregations based on biophysical modeling. Mar. Ecol. Prog. Ser. 2005, 296, 93–106. [Google Scholar] [CrossRef]
- Laurent, C.; Querin, S.; Solidoro, C.; Canu, D.M. Modelling marine particle dynamics with LTRANS-Zlev: Implementation and validation. Environ. Model. Softw. 2020, 125, 104621. [Google Scholar] [CrossRef]
- Schlag, Z.R.; North, E.W. Lagrangian TRANSport Model (LTRANS v. 2) Users Guide; University of Maryland Center for Environmental Science (UMCES): Cambridge, MD, USA, 2012. [Google Scholar]
- Wood, S.; Paris, C.B.; Ridgwell, A.; Hendy, E.J. Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Glob. Ecol. Biogeogr. 2014, 23, 1–11. [Google Scholar] [CrossRef]
- Kough, A.S.; Paris, C.B.; Butler, M.J., IV. Larval Connectivity and the International Management of Fisheries. PLoS ONE 2013, 8, e64970. [Google Scholar] [CrossRef]
- Comtet, T.; Jollivet, D.; Khripounoff, A.; Segonzac, M.; Dixon, D.R. Molecular and morphological identification of settlement-stage vent mussel larvae, Bathymodiolus azoricus (Bivalvia: Mytilidae), preserved in situ at active vent fields on the Mid-Atlantic Ridge. Limnol. Oceanog. 2000, 45, 1655–1661. [Google Scholar]
- Arellano, S.M.; Young, C.M. Spawning, development, and the duration of larval life in a deep-sea cold-seep mussel. Biol. Bull. 2009, 216, 149–162. [Google Scholar] [CrossRef]
- Le Pennec, M.; Beninger, P.G. Aspects of the reproductive strategy of bivalves from reducing-ecosystem. Cah. Biol. Mar. 1997, 38, 132–133. [Google Scholar]
- Le Pennec, M.; Beninger, P.G. Reproductive characteristics and strategies of reducing-system bivalves. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 126, 1–16. [Google Scholar] [CrossRef]
- Portanier, E.; Nicolle, A.; Rath, W.; Monnet, L.; Le Goff, G.; Le Port, A.-S.; Daguin-Thiébaut, C.; Morrison, C.L.; Cunha, M.R.; Betters, M.; et al. Coupling large-spatial scale larval dispersal modelling with barcoding to refine the amphi-Atlantic connectivity hypothesis in deep-sea seep mussels. Front. Mar. Sci. 2023, 10, 1122124. [Google Scholar] [CrossRef]
- Eckelbarger, K.J.; Young, C.M. Ultrastructure of gametogenesis in a chemosynthetic mytilid bivalve (Bathymodiolus childressi) from a bathyal, methane seep environment (northern Gulf of Mexico). Mar. Biol. 1999, 135, 635–646. [Google Scholar] [CrossRef]
- Siegel, D.; Kinlan, B.; Gaylord, B.; Gaines, S. Lagrangian descriptions of marine larval dispersion. Mar. Ecol. Prog. Ser. 2003, 260, 83–96. [Google Scholar] [CrossRef]
- Widdows, J. Physiological ecology of mussel larvae. Aquaculture 1991, 94, 147–163. [Google Scholar] [CrossRef]
- McVeigh, D.M.; Eggleston, D.B.; Todd, A.C.; Young, C.M.; He, R. The influence of larval migration and dispersal depth on potential larval trajectories of a deep-sea bivalve. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2017, 127, 57–64. [Google Scholar] [CrossRef]
- Troost, K.; Veldhuizen, R.; Stamhuis, E.J.; Wolff, W.J. Can bivalve veligers escape from feeding currents of adult bivalves? J. Exp. Mar. Biol. Ecol. 2008, 358, 185–196. [Google Scholar] [CrossRef]
- Sprung, M. Physiological energetics of mussel larvae(Mytilus edulis). I. Shell growth and biomass. Mar. Ecol. Prog. Ser. 1984, 17, 283–293. [Google Scholar] [CrossRef]
- Fuchs, H. Mussel larval responses to turbulence are unaltered by larval age or light conditions. Limnol. Oceanogr. Fluids Environ. 2011, 1, 120–134. [Google Scholar] [CrossRef]
- Schwalb, A.N.; Ackerman, J.D. Settling velocities of juvenile Lampsilini mussels (Mollusca:Unionidae): The influence of behavior. J. N. Am. Benthol. Soc. 2011, 30, 702–709. [Google Scholar] [CrossRef]
- Bradbury, I.R.; Laurel, B.; Snelgrove, P.V.; Bentzen, P.; Campana, S.E. Global patterns in marine dispersal estimates: The influence of geography, taxonomic category and life history. Proc. R. Soc. B. 2008, 275, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, J.; Portail, M.; Legrand, E.; Cathalot, C.; Laes, A.; Lahaye, N.; Sarradin, P.M.; Husson, B. Endogenous versus exogenous factors: What matters for vent mussel communities? Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 160, 103260. [Google Scholar] [CrossRef]
- Sinclair, M. Marine Populations: An Essay on Population Regulation and Speciation; Washington Sea Grant Program: Seattle, WA, USA, 1988; p. 252. [Google Scholar]
- Sponaugle, S.; Cowen, R.K.; Shanks, A.L.; Morgan, S.G.; Leis, J.; Pineda, J.; Boehlert, G.; Kingsford, M.J.; Lindeman, K.; Grimes, C.; et al. Predicting self-recruitment in marine populations: Biophysical correlates and mechanisms. Bull. Mar. Sci. 2002, 49, 341–375. [Google Scholar]
- Metaxas, A. Behaviour in flow: Perspectives on the distribution and dispersion of meroplanktonic larvae in the water column. Can. J. Fish. Aquat. Sci. 2001, 58, 86–98. [Google Scholar] [CrossRef]
- Morgan, S.G. Behaviorally mediated larval transport in upwelling systems. Adv. Oceanogr. 2014, 2014, 364214. [Google Scholar] [CrossRef]
- Arellano, S.M.; Van Gaest, A.L.; Johnson, S.B.; Vrijenhoek, R.C.; Young, C.M. Larvae from deep-sea methane seeps disperse in surface waters. Proc. R. Soc. B 2014, 281, 20133276. [Google Scholar] [CrossRef]
- Yahagi, T.; Kayama Watanabe, H.; Kojima, S.; Kano, Y. Do larvae from deep-sea hydrothermal vents disperse in surface waters? Ecology 2017, 98, 1524–1534. [Google Scholar] [CrossRef]
- Zardus, J.D.; Etter, R.J.; Chase, M.R.; Rex, M.A.; Boyle, E.E. Bathymetric and geographic population structure in the pan-Atlantic deep-sea bivalve Deminucula atacellana (Schenck, 1939). Mol. Ecol. 2006, 15, 639–651. [Google Scholar] [CrossRef]
- Yorisue, T.; Kado, R.; Watanabe, H.; Høeg, J.T.; Inoue, K.; Kojima, S.; Chan, B.K.K. Influence of water temperature on the larval development of neoverruca sp. and Shinkailepas seepiophila—Implication for larval dispersal and settlement in the vent and seep environments. Deep Sea Res. Part I 2013, 71, 33–37. [Google Scholar] [CrossRef]
- Arellano, S.M.; Young, C.M. Temperature and salinity tolerances of embryos and larvae of the deep-sea mytilid mussel ‘Bathymodiolus’ childressi. Mar. Biol. 2011, 158, 2481–2493. [Google Scholar] [CrossRef]
- Yahagi, T.; Chen, C.; Kawagucci, S. What we know, what we can know, and what we will never know about the larval dispersal process at deep-sea chemosynthetic ecosystems. Oceanography 2019, 28, 97–125. [Google Scholar] [CrossRef]
- Teixeira, S.; Cambon-Bonavita, M.; Serrão, E.A.; Desbruyéres, D.; Arnaud-Haond, S. Recent population expansion and connectivity in the hydrothermal shrimp Rimicaris exoculata along the Mid-Atlantic Ridge. J. Biogeogr. 2011, 38, 564–574. [Google Scholar] [CrossRef]
- Teixeira, S.; Olu, K.; Decker, C.; Cunha, R.L.; Fuchs, S.; Hourdez, S.; Serrão, E.A.; Arnaud-Haond, S. High connectivity across the fragmented chemosynthetic ecosystems of the deep Atlantic Equatorial Belt: Efficient dispersal mechanisms or questionable endemism? Mol. Ecol. 2013, 22, 4663–4680. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, K.; Petersen, J.M.; Dubilier, N.; Borowski, C. Genetic connectivity between north and south Mid- Atlantic Ridge chemosynthetic bivalves and their symbionts. PLoS ONE 2012, 7, e39994. [Google Scholar] [CrossRef] [PubMed]
- Nnamchi, H.C.; Farneti, R.; Keenlyside, N.S.; Kucharski, F.; Latif, M.; Reintges, A.; Martin, T. Pan-Atlantic decadal climate oscillation linked to ocean circulation. Commun. Earth Environ. 2023, 4, 121. [Google Scholar] [CrossRef]
- Delworth, T.L.; Zeng, F.; Zhang, L.; Zhang, R.; Vecchi, G.A.; Yang, X. The central role of ocean dynamics in connecting the North Atlantic Oscillation to the extratropical component of the Atlantic multidecadal oscillation. J. Clim. 2017, 30, 3789–3805. [Google Scholar] [CrossRef]
- Kinlan, B.P.; Gaines, S.D. Propagule dispersal in marine and terrestrial environments: A community perspective. Ecology 2003, 84, 2007–2020. [Google Scholar] [CrossRef]
- Etter, R.J.; Bower, A.S. Dispersal and population connectivity in the deep North Atlantic estimated from physical transport processes. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2015, 104, 159–172. [Google Scholar] [CrossRef]
- Levin, L.A.; Baco, A.R.; Bowden, D.A.; Colaco, A.; Cordes, E.E.; Cunha, M.R.; Demopoulos, A.W.J.; Gobin, J.; Grupe, B.M.; Le, J.; et al. Hydrothermal Vents and Methane Seeps: Rethinking the Sphere of Influence. Front. Mar. Sci. 2016, 3, 72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).