Sound Production Characteristics of the Chorus Produced by Small Yellow Croaker (Larimichthys polyactis) in Coastal Cage Aquaculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Biological Fish Sound
2.2. Analysis of Acoustic Data
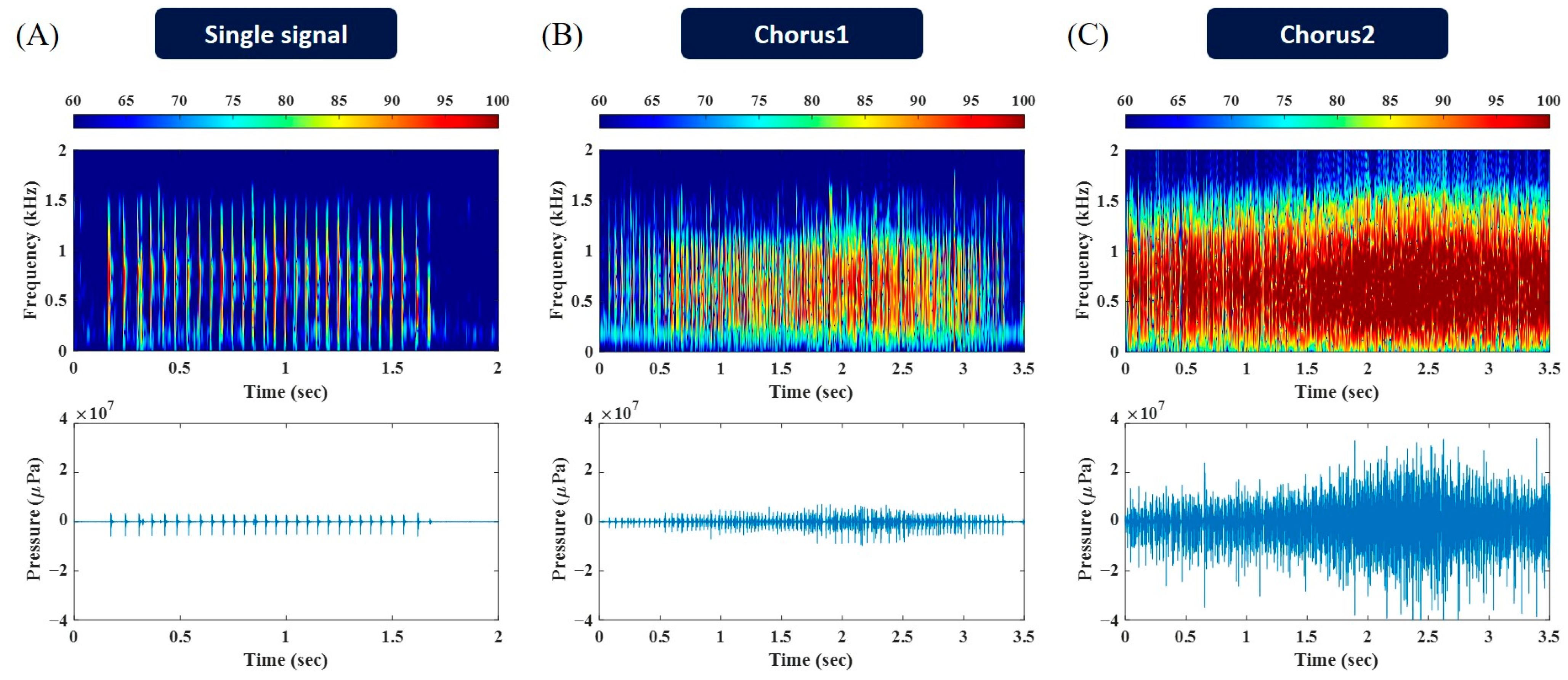
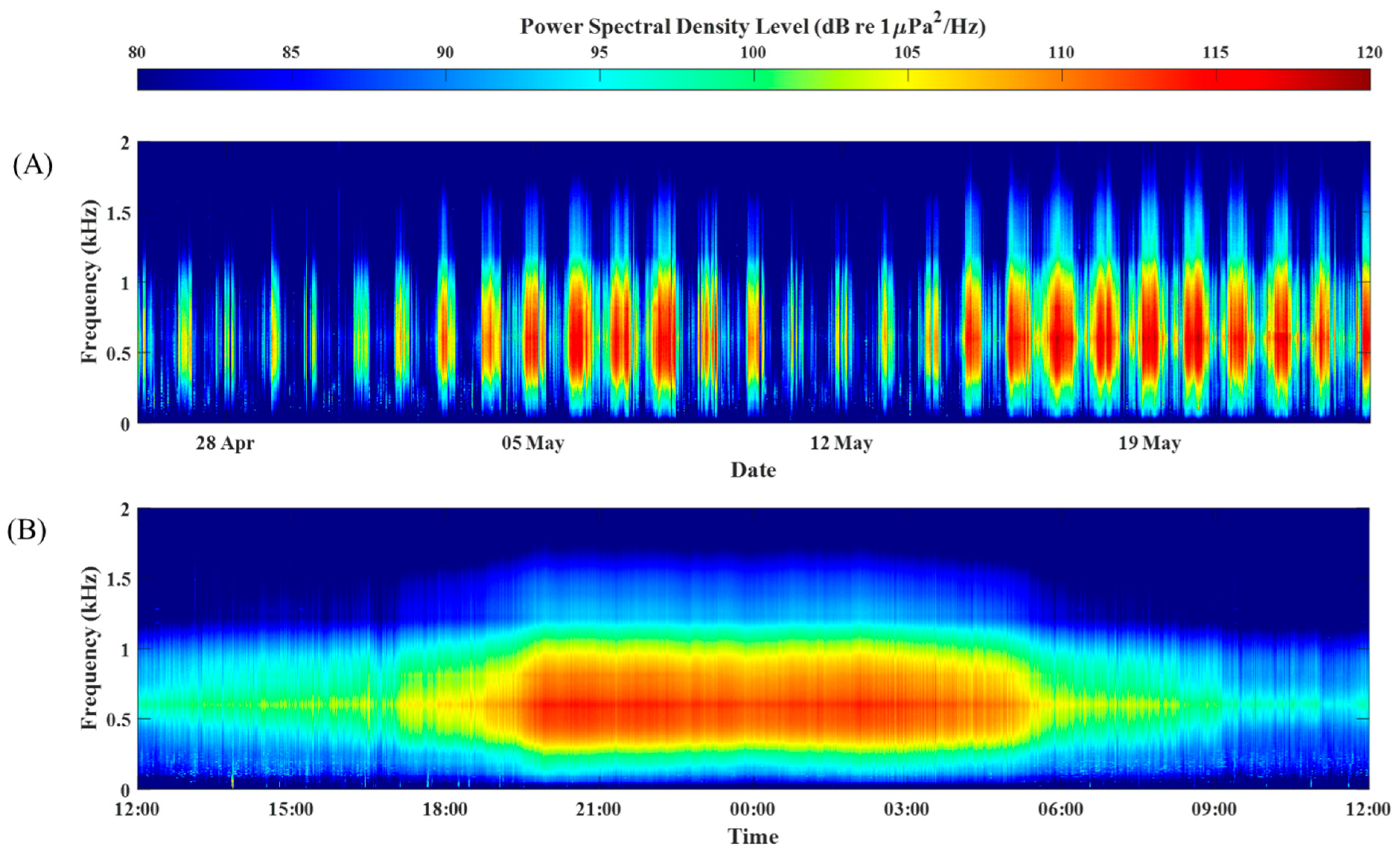
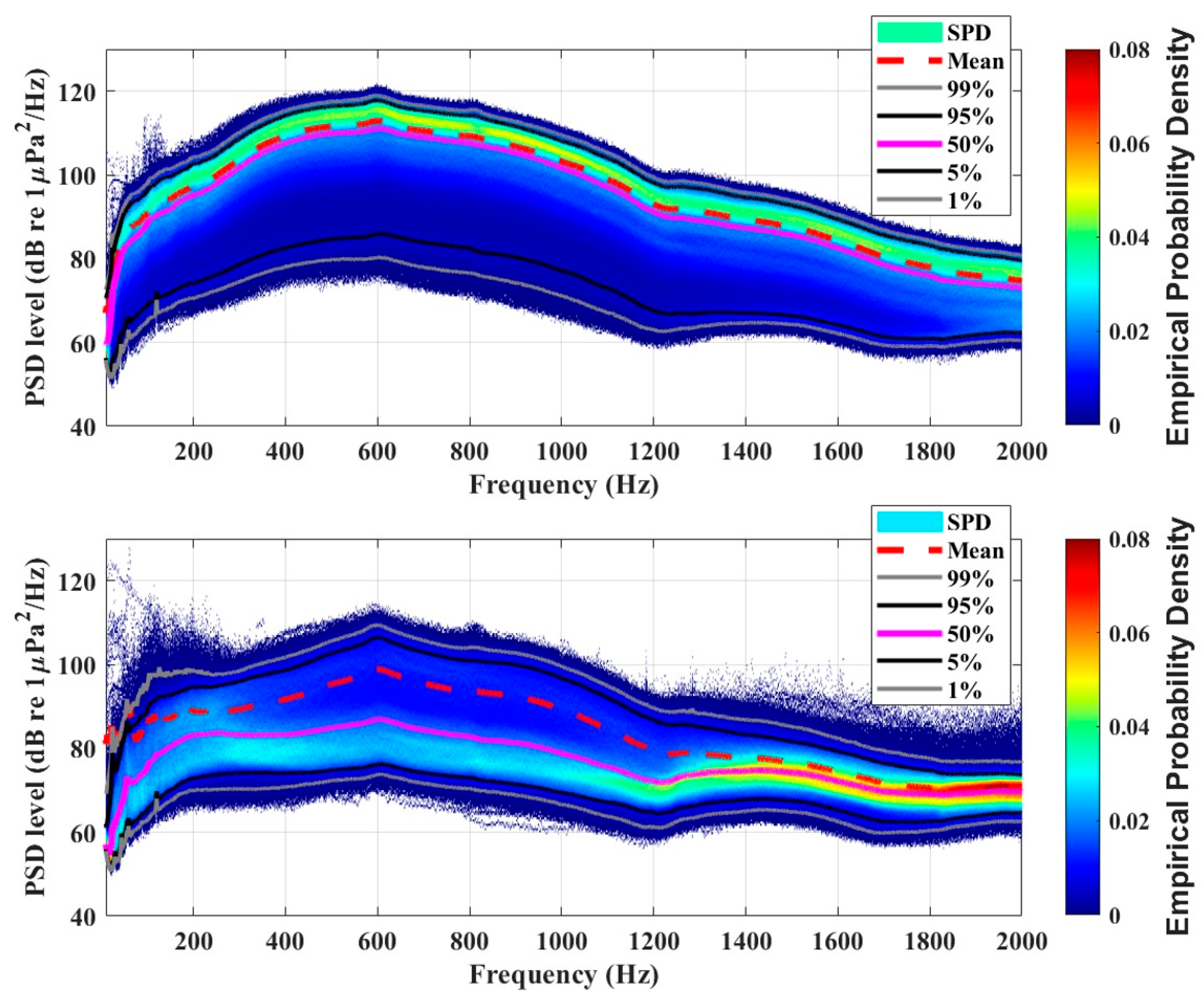
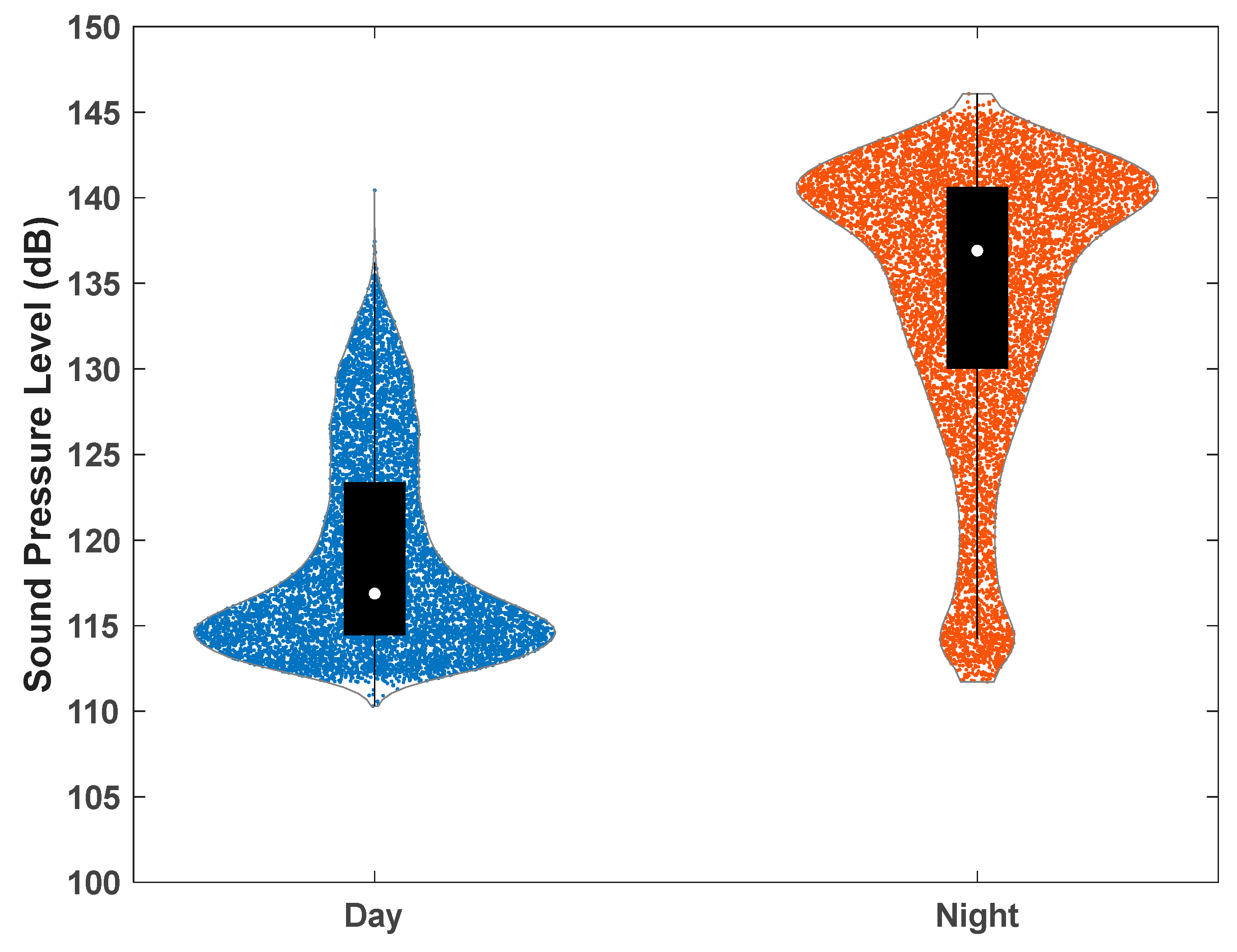
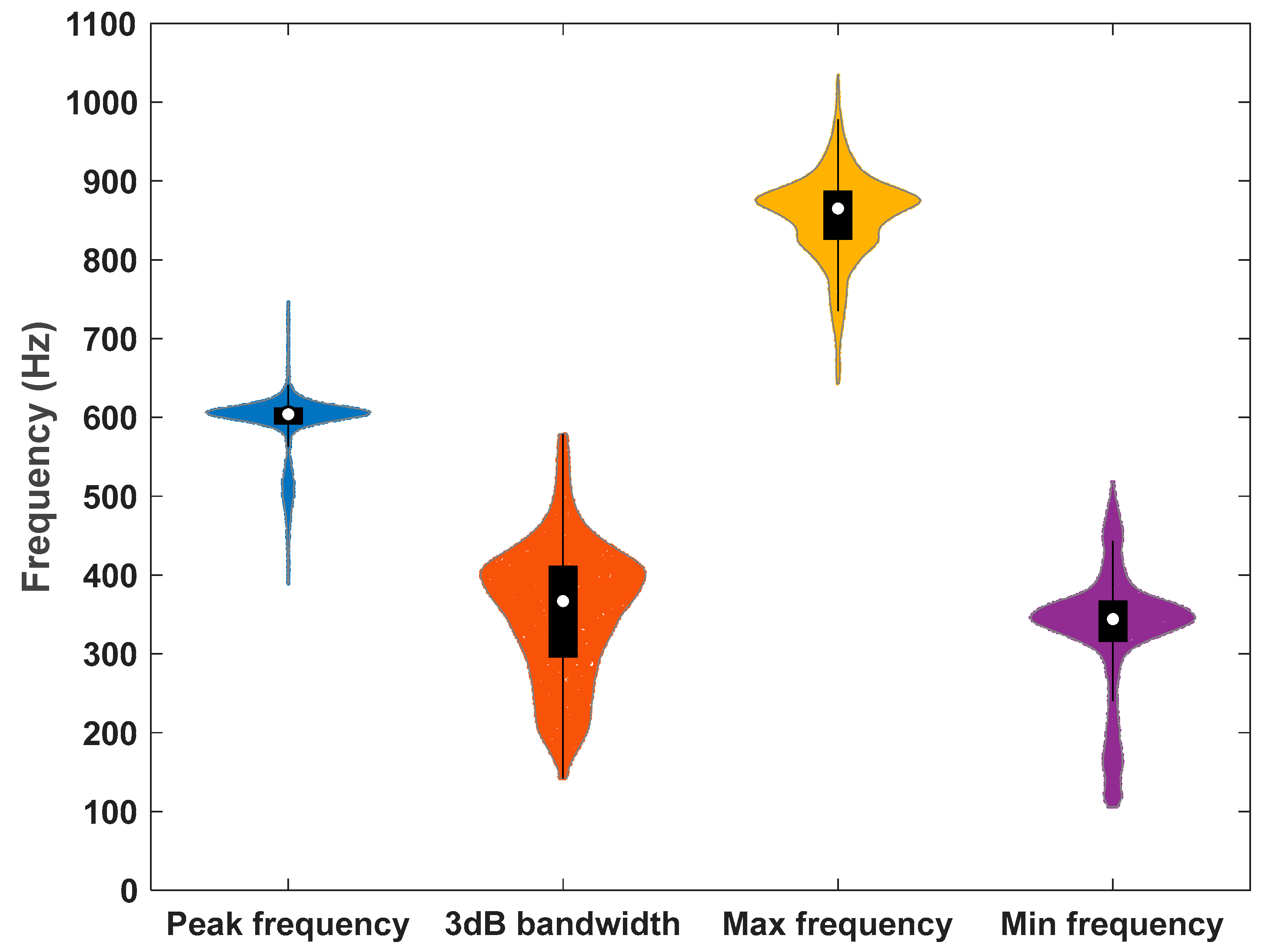
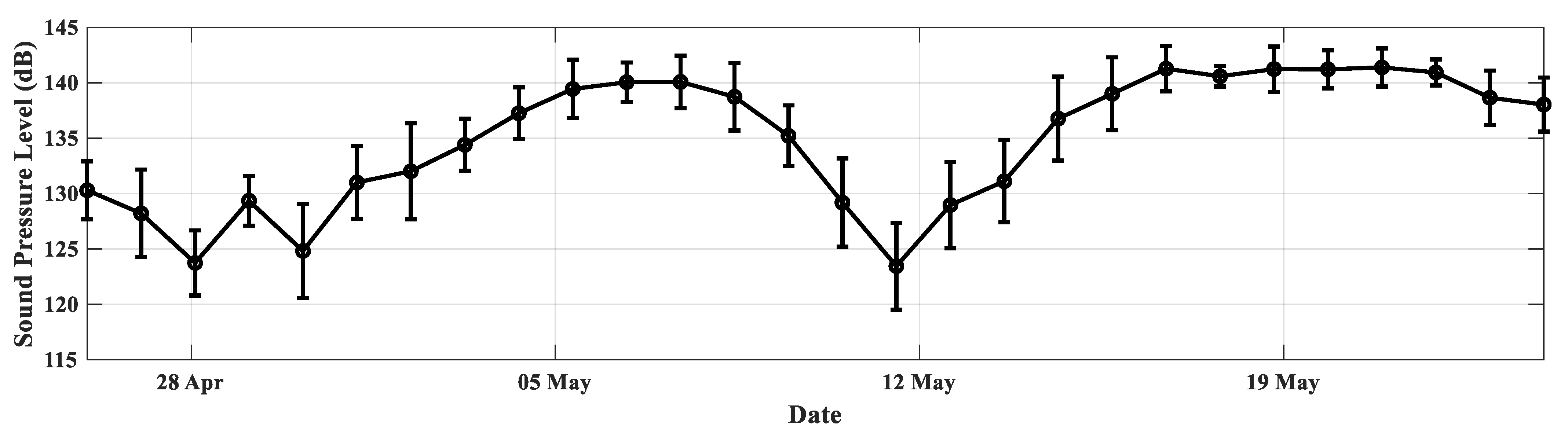
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindseth, A.V.; Lobel, P.S. Underwater soundscape monitoring and fish bioacoustics: A review. Fishes 2018, 3, 36. [Google Scholar] [CrossRef]
- Havlik, M.N.; Predragovic, M.; Duarte, C.M. State of play in marine soundscape assessments. Front. Mar. Sci. 2022, 9, 919418. [Google Scholar] [CrossRef]
- Parsons, M.J.; Lin, T.H.; Mooney, T.A.; Erbe, C.; Juanes, F.; Lammers, M.; Di Iorio, L. Sounding the call for a global library of underwater biological sounds. Front. Ecol. Evol. 2022, 10, 810156. [Google Scholar] [CrossRef]
- Chapuis, L.; Williams, B.; Gordon, T.A.; Simpson, S.D. Low-cost action cameras offer potential for widespread acoustic monitoring of marine ecosystems. Ecol. Indic. 2021, 129, 107957. [Google Scholar] [CrossRef]
- Wall, C.C.; Haver, S.M.; Hatch, L.T.; Miksis-Olds, J.; Bochenek, R.; Dziak, R.P.; Gedamke, J. The next wave of passive acoustic data management: How centralized access can enhance science. Front. Mar. Sci. 2021, 8, 703682. [Google Scholar] [CrossRef]
- Bermant, P.C.; Bronstein, M.M.; Wood, R.J.; Gero, S.; Gruber, D.F. Deep machine learning techniques for the detection and classification of sperm whale bioacoustics. Sci. Rep. 2019, 9, 12588. [Google Scholar] [CrossRef] [PubMed]
- Shiu, Y.; Palmer, K.J.; Roch, M.A.; Fleishman, E.; Liu, X.; Nosal, E.-M.; Helble, T.; Cholewiak, D.; Gillespie, D.; Klinck, H. Deep neural networks for automated detection of marine mammal species. Sci. Rep. 2020, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Martin, B.; Kowarski, K.; Gaudet, B.; Matwin, S. Marine mammal species classification using convolutional neural networks and a novel acoustic representation. arXiv 2019, arXiv:1907.13188. [Google Scholar]
- Roch, M.A.; Soldevilla, M.S.; Hoenigman, R.; Wiggins, S.M.; Hildebrand, J.A. Comparison of machine learning techniques for the classification of echolocation clicks from three species of odontocetes. Can. Acoust. 2008, 36, 41–47. [Google Scholar]
- Usman, A.M.; Ogundile, O.O.; Versfeld, D.J. Review of automatic detection and classification techniques for cetacean vocalization. IEEE Access 2020, 8, 105181–105206. [Google Scholar] [CrossRef]
- Staaterman, E.; Rice, A.N.; Mann, D.A.; Paris, C.B. Soundscapes from a tropical Eastern Pacific reef and a Caribbean Sea reef. Coral Reefs 2013, 32, 553–557. [Google Scholar] [CrossRef]
- Lillis, A.; Eggleston, D.; Bohnenstiehl, D. Estuarine soundscapes: Distinct acoustic characteristics of oyster reefs compared to soft-bottom habitats. Mar. Ecol. Prog. Ser. 2014, 505, 1–17. [Google Scholar] [CrossRef]
- Harris, S.; Shears, N.; Radford, C. Ecoacoustic indices as proxies for biodiversity on temperate reefs. Methods Ecol. Evol. 2016, 7, 713–724. [Google Scholar] [CrossRef]
- Bolgan, M.; Amorim, M.C.P.; Fonseca, P.J.; Di Iorio, L.; Parmentier, E. Acoustic complexity of vocal fish communities: A field and controlled validation. Sci. Rep. 2018, 8, 10559. [Google Scholar] [CrossRef] [PubMed]
- Miksis-Olds, J.L.; Martin, B.; Tyack, P.L. Exploring the ocean through soundscapes. Acoust. Today 2018, 14, 26–34. [Google Scholar]
- Carriço, R.; Silva, M.A.; Vieira, M.; Afonso, P.; Menezes, G.M.; Fonseca, P.J.; Amorim, M.C.P. The use of soundscapes to monitor fish communities: Meaningful graphical representations differ with acoustic environment. Acoustics 2020, 2, 382–398. [Google Scholar] [CrossRef]
- La Manna, G.; Picciulin, M.; Crobu, A.; Perretti, F.; Ronchetti, F.; Manghi, M.; Ceccherelli, G. Marine soundscape and fish biophony of a Mediterranean marine protected area. PeerJ 2021, 9, e12551. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.N.; Fay, R.R.; Platt, C.; Sand, O. Sound detection mechanisms and capabilities of teleost fishes. In Sensory Processing in Aquatic Environments; Collin, S.P., Marshall, N.J., Eds.; Springer: New York, NY, USA, 2003; pp. 1–53. [Google Scholar]
- Cato, D.H.; Noad, M.J.; McCauley, R.D. Passive acoustics as a key to the study of marine animals. In Sounds in the Sea: From Ocean Acoustics to Acoustical Oceanography; Medwin, H., Ed.; Cambridge University Press: Cambridge, UK, 2005; pp. 411–429. [Google Scholar]
- McWilliam, J.; Hawkins, A. A comparison of inshore marine soundscapes. J. Exp. Mar. Biol. Ecol. 2013, 446, 166–176. [Google Scholar] [CrossRef]
- Kaatz, I.M. Multiple sound producing mechanisms in teleost fishes and hypotheses regarding their behavioural significance. Bioacoustics 2002, 12, 230–233. [Google Scholar] [CrossRef]
- Vermeij, M.J.A.; Marhaver, K.L.; Huijbers, C.M.; Nagelkerken, I.; Simpson, S.D. Coral larvae move toward reef sounds. PLoS ONE 2010, 5, e10660. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, L.; Bshary, R. Signalling by the cleaner shrimp Periclimenes longicarpus. Anim. Behav. 2010, 79, 645–647. [Google Scholar] [CrossRef]
- Rountree, R.A.; Gilmore, G.A.; Goudey, C.A.; Hawkins, A.D.; Luczkovich, J.J.; Mann, D.A. Listening to fish: Applications of passive acoustics to fisheries science. Fisheries 2006, 31, 433–446. [Google Scholar] [CrossRef]
- Jin, X.S.; Zhao, X.Y.; Meng, T.X.; Cui, Y. The Biological Resources and Their Habitat Environment in the Bohai and Yellow Sea; Science Press: Beijing, China, 2005; p. 405. [Google Scholar]
- Li, Z.; Shan, X.; Jin, X.; Dai, F. Long-term variations in body length and age at maturity of the small yellow croaker (Larimichthys polyactis Bleeker, 1877) in the Bohai Sea and the Yellow Sea, China. Fish. Res. 2011, 110, 67–74. [Google Scholar] [CrossRef]
- Lin, L.S.; Liu, Z.L.; Jiang, Y.Z.; Huang, W.; Gao, T.X. Current status of small yellow croaker resources in the southern Yellow Sea and the East China Sea. Chin. J. Oceanol. Limnol. 2011, 29, 547–555. [Google Scholar] [CrossRef]
- Song, X.; Hu, F.; Xu, M.; Zhang, Y.; Jin, Y.; Gao, X.; Liu, Z.; Ling, J.; Li, S.; Cheng, J. Spatiotemporal distribution and dispersal pattern of early life stages of the small yellow croaker (Larimichthys polyactis) in the southern Yellow Sea. Diversity 2024, 16, 521. [Google Scholar] [CrossRef]
- Han, Q.; Shan, X.; Jin, X.; Gorfine, H.; Chen, Y.; Su, C. Changes in distribution patterns for Larimichthys polyactis in response to multiple pressures in the Bohai Sea over the past four decades. Front. Mar. Sci. 2022, 9, 941045. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, Y.; Yang, L.; Yuan, X.; Yan, L.; Zhang, Y.; Zhang, H.; Xu, M.; Song, X.; Tang, J.; et al. Improving prediction for potential spawning areas from a two-step perspective: A comparison of multi-model approaches for sparse egg distribution. J. Sea Res. 2024, 197, 102460. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Hui, J.; Su, Y.; Fu, W.; Zhang, S.; Yan, L.; Yan, K.; Huang, H.; Zhang, Y. Vocalization behavior of Chinese Bahaba (Bahaba taipingensis) during the reproduction season. J. Mar. Sci. Eng. 2023, 11, 712. [Google Scholar] [CrossRef]
- Merchant, N.D.; Fristrup, K.M.; Johnson, M.P.; Tyack, P.L.; Witt, M.J.; Blondel, P.; Parks, S.E. Measuring acoustic habitats. Methods Ecol. Evol. 2015, 6, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wilford, D.C.; Miksis-Olds, J.L.; Martin, S.B.; Howard, D.R.; Lowell, K.; Lyons, A.P.; Smith, M.J. Quantitative soundscape analysis to understand multidimensional features. Front. Mar. Sci. 2021, 8, 672336. [Google Scholar] [CrossRef]
- Helberg, G.A.; Anichini, M.; Kolarevic, J.; Sæther, B.S.; Noble, C. Soundscape characteristics of RAS tanks holding Atlantic salmon (Salmo salar) during feeding and feed withdrawal. Aquaculture 2024, 593, 741325. [Google Scholar] [CrossRef]
- Hawkins, L.A.; Parsons, M.J.G.; McCauley, R.D.; Parnum, I.M.; Erbe, C. Passive acoustic monitoring of fish choruses: A review to inform the development of a monitoring and management tool. Rev. Fish. Biol. Fish. 2025, 35, 847–874. [Google Scholar] [CrossRef]
- Stratoudakis, Y.; Vieira, M.; Marques, J.P.; Amorim, M.C.P.; Fonseca, P.J.; Quintella, B.R. Long-term passive acoustic monitoring to support adaptive management in a sciaenid fishery (Tagus Estuary, Portugal). Rev. Fish. Biol. Fish. 2024, 34, 491–510. [Google Scholar] [CrossRef]
- Welch, P.D. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- McCauley, R. Fish choruses from the Kimberley, seasonal and lunar links as determined by long term sea noise monitoring. In Proceedings of the Acoustics 2012, Fremantle, Australia, 21–23 November 2012. [Google Scholar]
- McCauley, R.D.; Cato, D.H. Patterns of fish calling in a nearshore environment in the Great Barrier Reef. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, Y.G.; Cho, S.; Kim, S.; Kim, M.; Kang, D. Acoustic characteristics of spawning biological sounds of brown croaker (Miichthys miiuy). Fishes 2024, 9, 251. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Xu, X.M.; Zhang, X.H.; Huang, L.F.; Xiao, F.G.; He, Y.W. Vocalization behavior differs across reproductive stages in cultured large yellow croaker Larimichthys crocea (Perciformes: Sciaenidae). Aquaculture 2022, 556, 738267. [Google Scholar] [CrossRef]
- Tellechea, J.S.; Bouvier, D.; Norbis, W. Spawning sounds in whitemouth croaker (Sciaenidae): Seasonal and daily cycles. Bioacoustics 2011, 20, 159–168. [Google Scholar] [CrossRef]
- McWilliam, J.N.; McCauley, R.D.; Erbe, C.; Parsons, M.J. Patterns of biophonic periodicity on coral reefs in the Great Barrier Reef. Sci. Rep. 2017, 7, 17459. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.J.G. An Investigation into Active and Passive Acoustic Techniques to Study Aggregating Fish Species. Ph.D. Thesis, Curtin University, Perth, Australia, 2009. [Google Scholar]
- Locascio, J.V.; Mann, D.A. Diel and seasonal timing of sound production by black drum (Pogonias cromis). Fish. Bull. 2011, 109, 327–338. [Google Scholar]
- Parsons, M.J.G.; Salgado-Kent, C.P.; Marley, S.A.; Gavrilov, A.N.; McCauley, R.D. Characterizing diversity and variation in fish choruses in Darwin Harbour. ICES J. Mar. Sci. 2016, 73, 2058–2074. [Google Scholar] [CrossRef]
- Siddagangaiah, S.; Chen, C.-F.; Hu, W.-C.; Danovaro, R.; Pieretti, N. Silent winters and rock-and-roll summers: The long-term effects of changing oceans on marine fish vocalization. Ecol. Indic. 2021, 125, 107456. [Google Scholar] [CrossRef]
- Hawkins, L.A.; Erbe, C.; Becker, A.; Browne, C.E.; McCordic, J.; McWiliam, J.; McCauley, R.D. The Australian fish chorus catalogue (2005–2023). Front. Remote Sens. 2024, 5, 1473168. [Google Scholar] [CrossRef]
- Monczak, A.; Mueller, C.; Miller, M.E.; Ji, Y.; Borgianini, S.A.; Montie, E.W. Sound patterns of snapping shrimp, fish, and dolphins in an estuarine soundscape of the southeastern USA. Mar. Ecol. Prog. Ser. 2019, 609, 49–68. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.G.; Kim, H.; Cho, S.; Kim, S.; Jung, Y.-H.; Kang, D. Sound Production Characteristics of the Chorus Produced by Small Yellow Croaker (Larimichthys polyactis) in Coastal Cage Aquaculture. J. Mar. Sci. Eng. 2025, 13, 1380. https://doi.org/10.3390/jmse13071380
Yoon YG, Kim H, Cho S, Kim S, Jung Y-H, Kang D. Sound Production Characteristics of the Chorus Produced by Small Yellow Croaker (Larimichthys polyactis) in Coastal Cage Aquaculture. Journal of Marine Science and Engineering. 2025; 13(7):1380. https://doi.org/10.3390/jmse13071380
Chicago/Turabian StyleYoon, Young Geul, Hansoo Kim, Sungho Cho, Sunhyo Kim, Yun-Hwan Jung, and Donhyug Kang. 2025. "Sound Production Characteristics of the Chorus Produced by Small Yellow Croaker (Larimichthys polyactis) in Coastal Cage Aquaculture" Journal of Marine Science and Engineering 13, no. 7: 1380. https://doi.org/10.3390/jmse13071380
APA StyleYoon, Y. G., Kim, H., Cho, S., Kim, S., Jung, Y.-H., & Kang, D. (2025). Sound Production Characteristics of the Chorus Produced by Small Yellow Croaker (Larimichthys polyactis) in Coastal Cage Aquaculture. Journal of Marine Science and Engineering, 13(7), 1380. https://doi.org/10.3390/jmse13071380







