Evaluation of the Effect of Floating Treatment Wetlands Planted with Sesuvium portulacastrum on the Dynamics of Dissolved Inorganic Nitrogen, CO2, and N2O in Grouper Aquaculture Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Analysis
2.2.1. Sampling Procedure
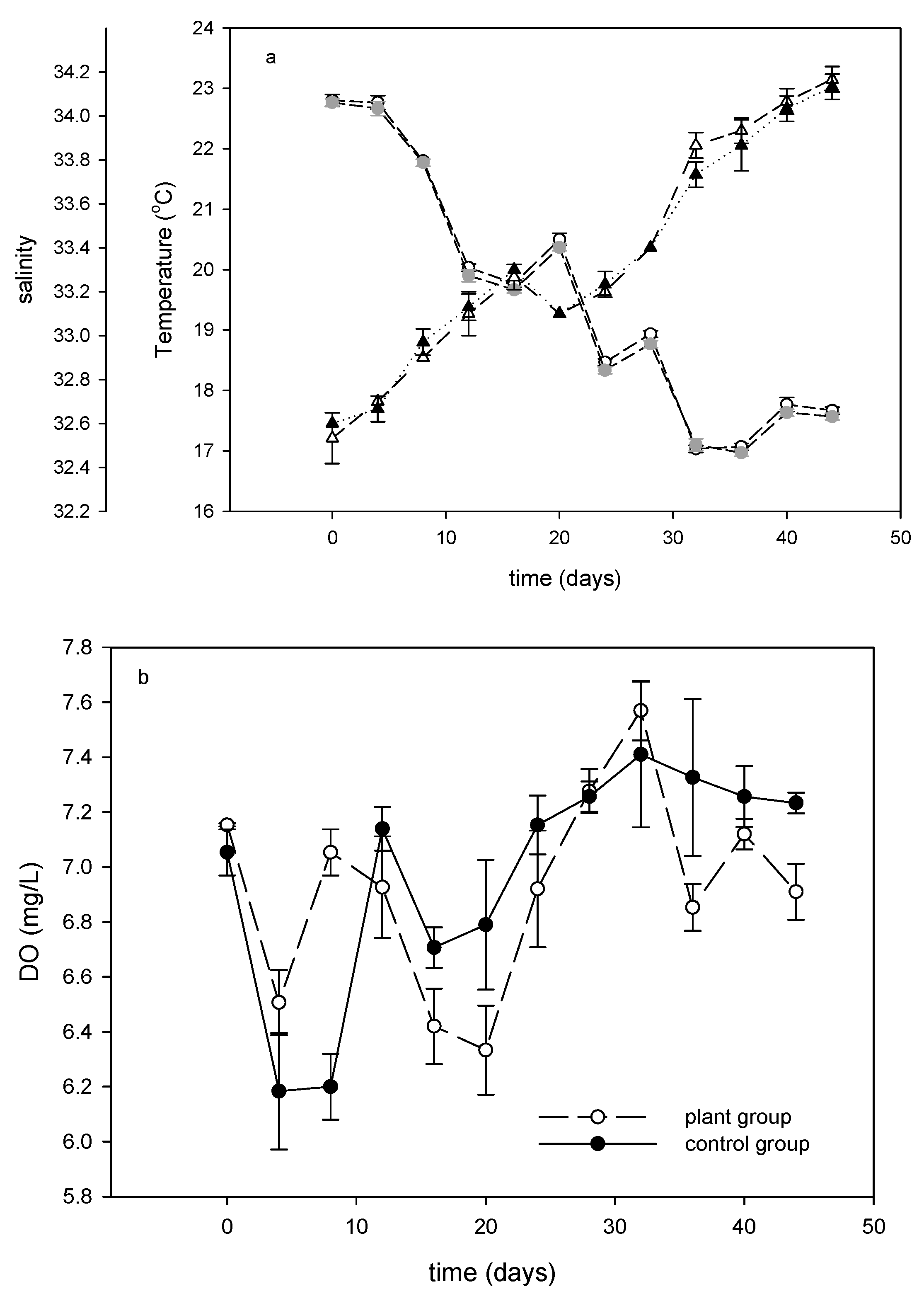
2.2.2. Temperature, Salinity, and Dissolved Oxygen Measurements
2.2.3. Concentrations of NH4+, NO2−, and NO3− in the Water Mass
2.2.4. Analysis of Nitrogen Content in Plants
2.2.5. DIC and N2O Analysis
2.2.6. Calculation of Greenhouse Emission Fluxes
3. Results and Discussion
3.1. Temperature, Salinity, and Dissolved Oxygen
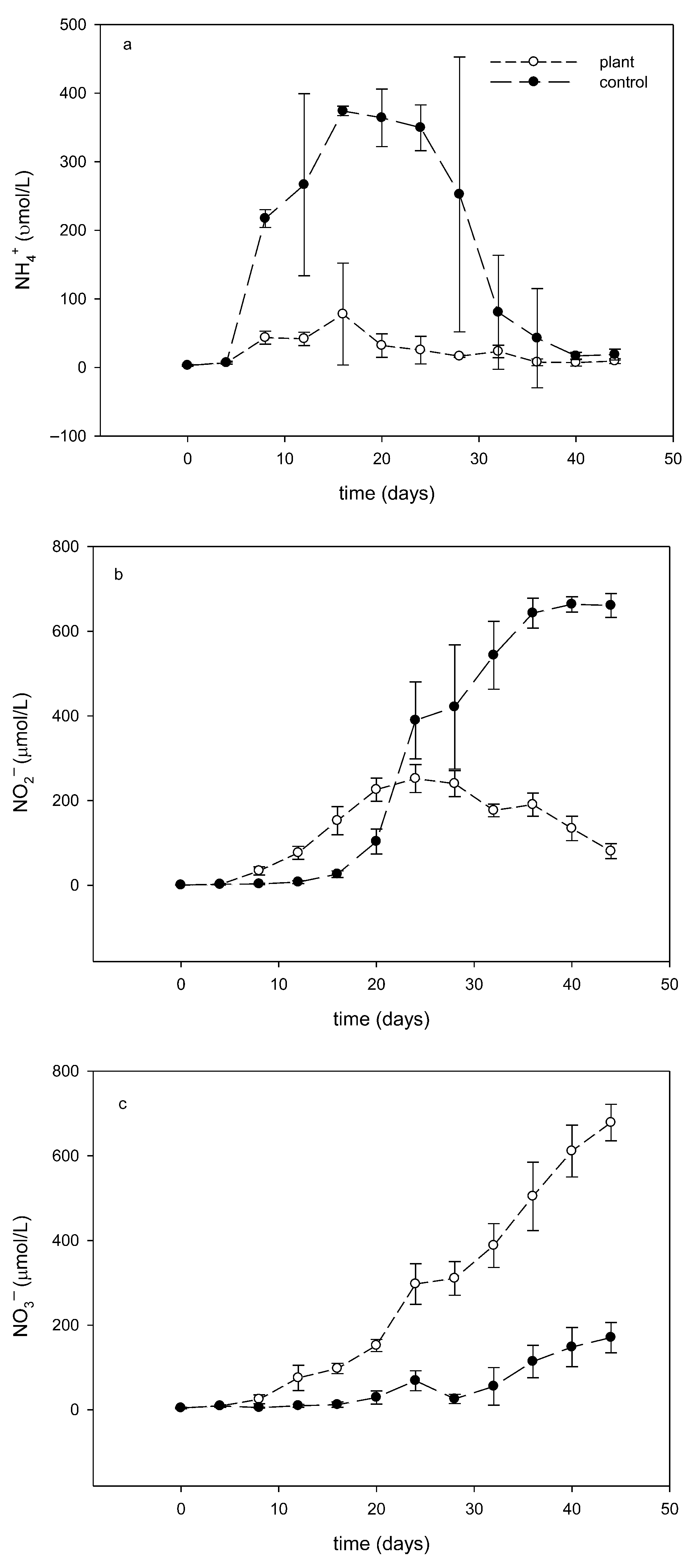
3.2. Effect of Floating Treatment Welands on Dynamics of Dissolve Inorganic Nitrogen
3.2.1. Dissolve Inorganic Nitrogen Variation During the Experiment
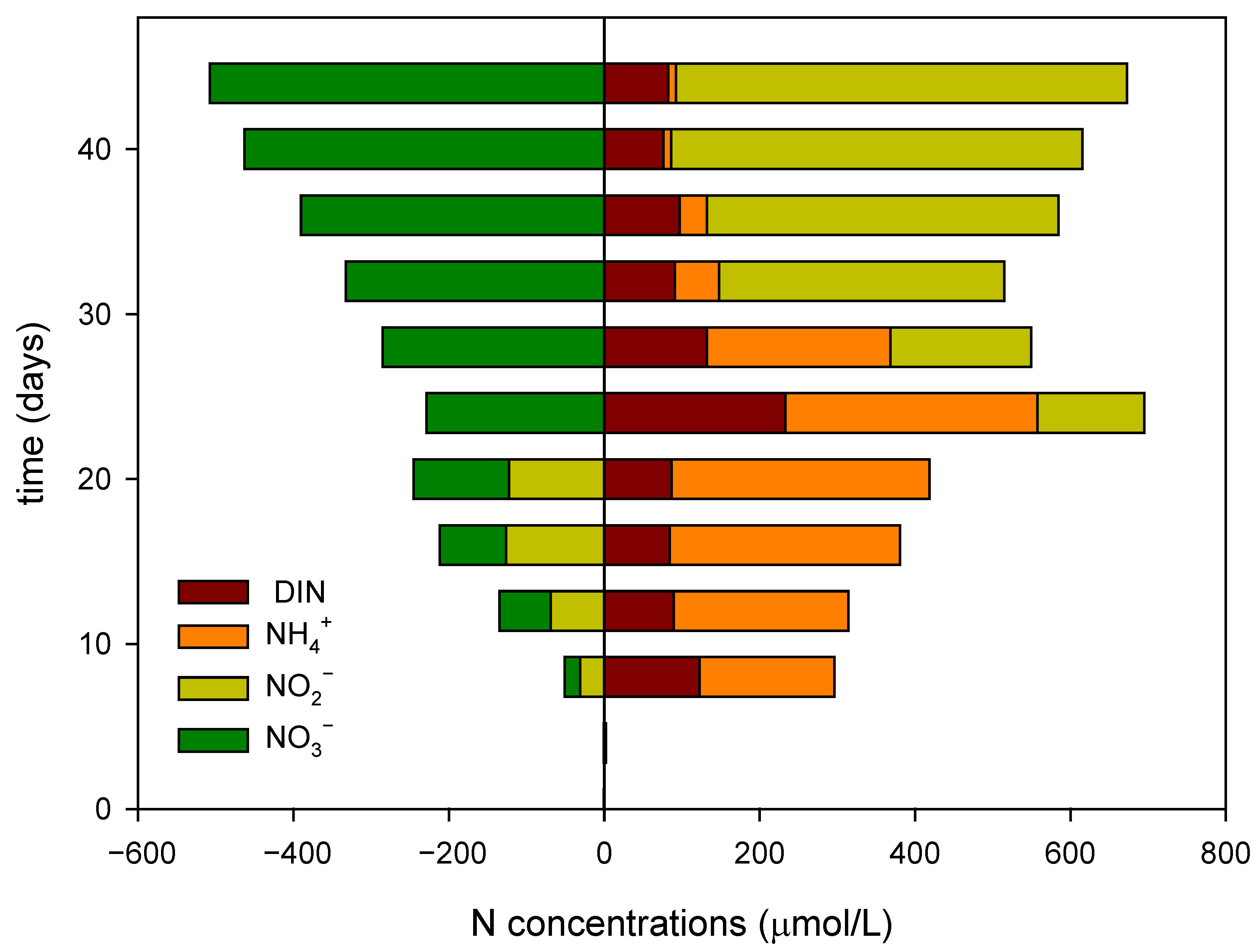
3.2.2. Differences in Dissolved Inorganic Nitrogen Dynamics Between the Plant and Control Groups
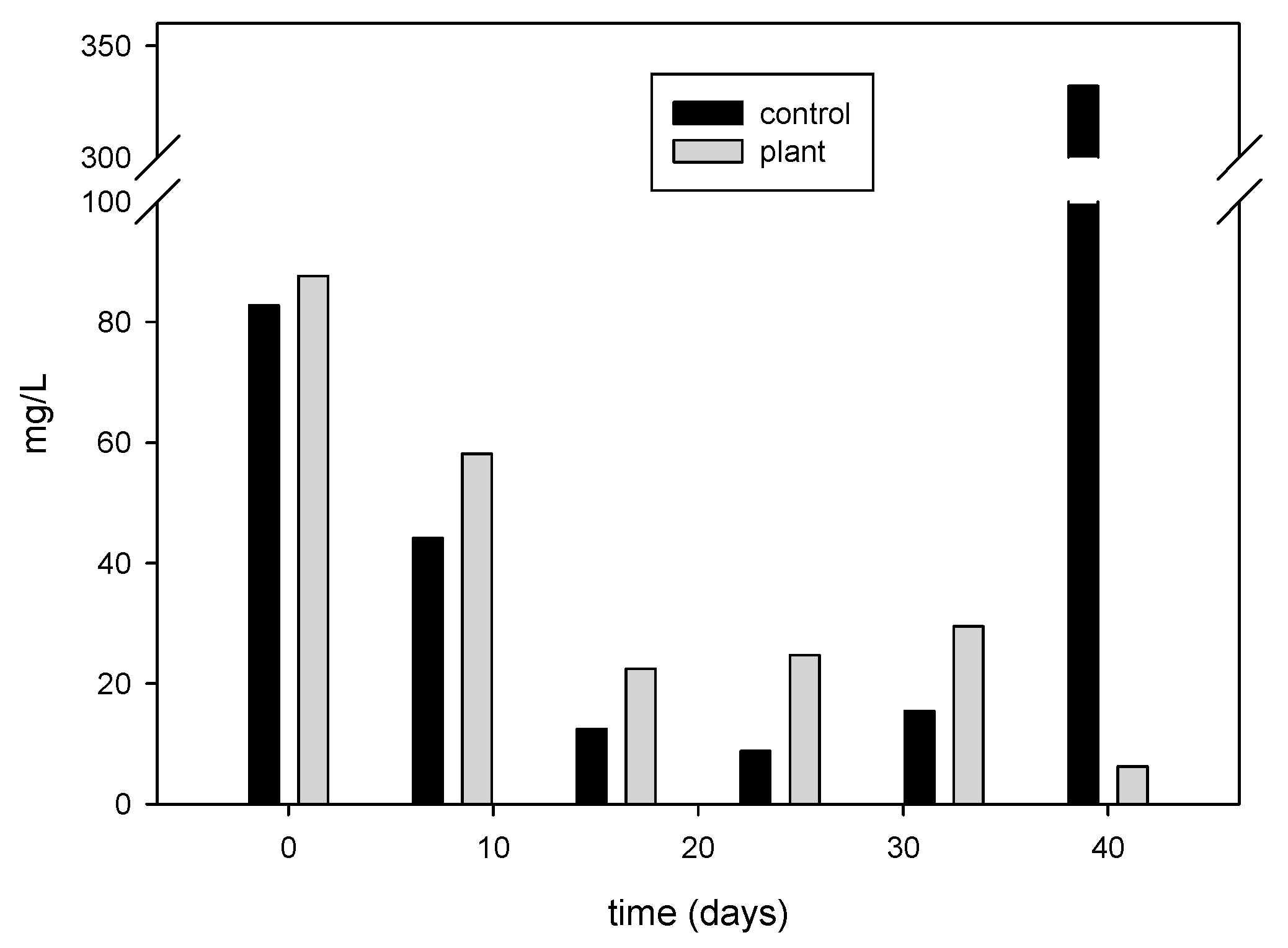
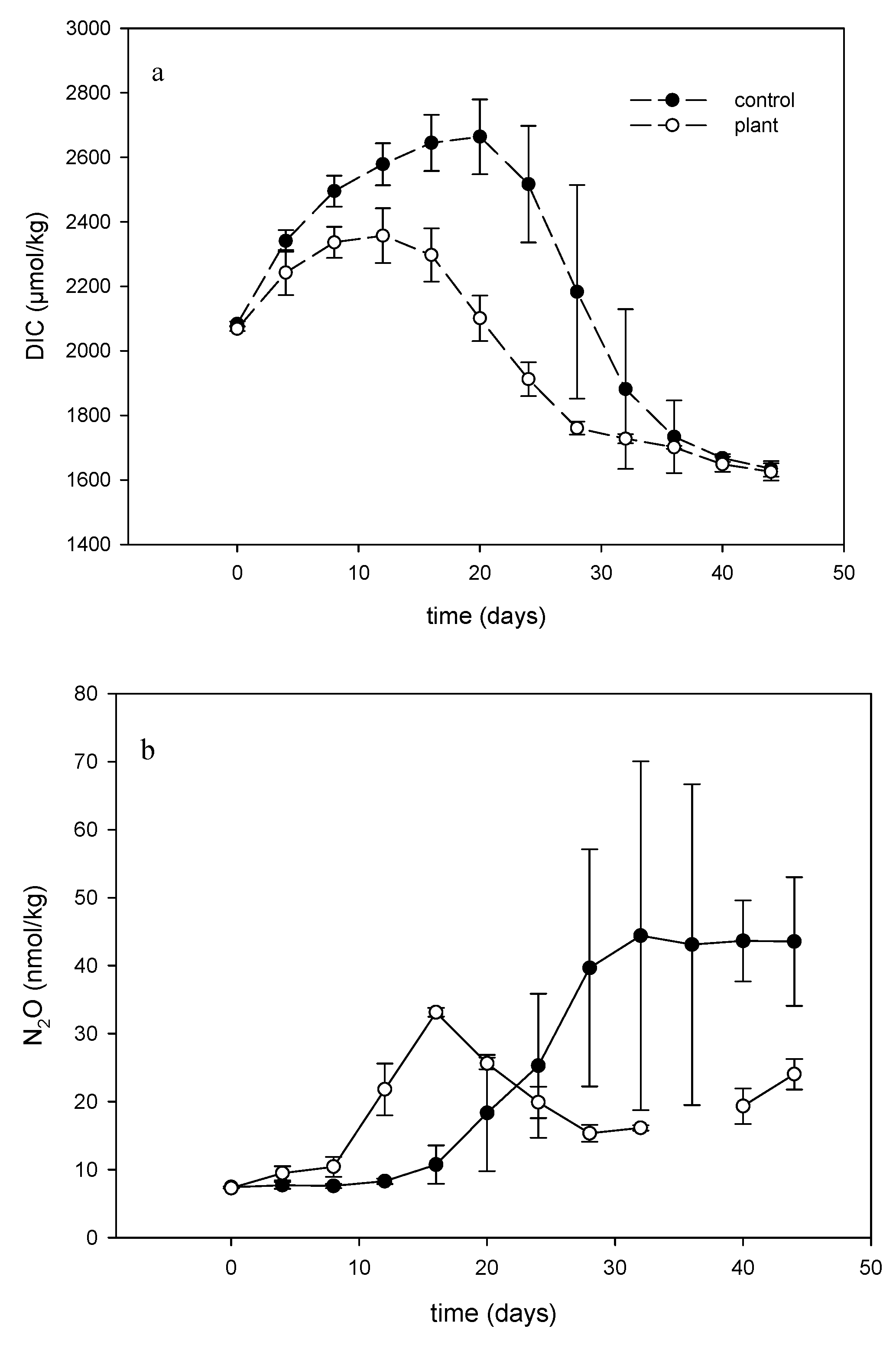
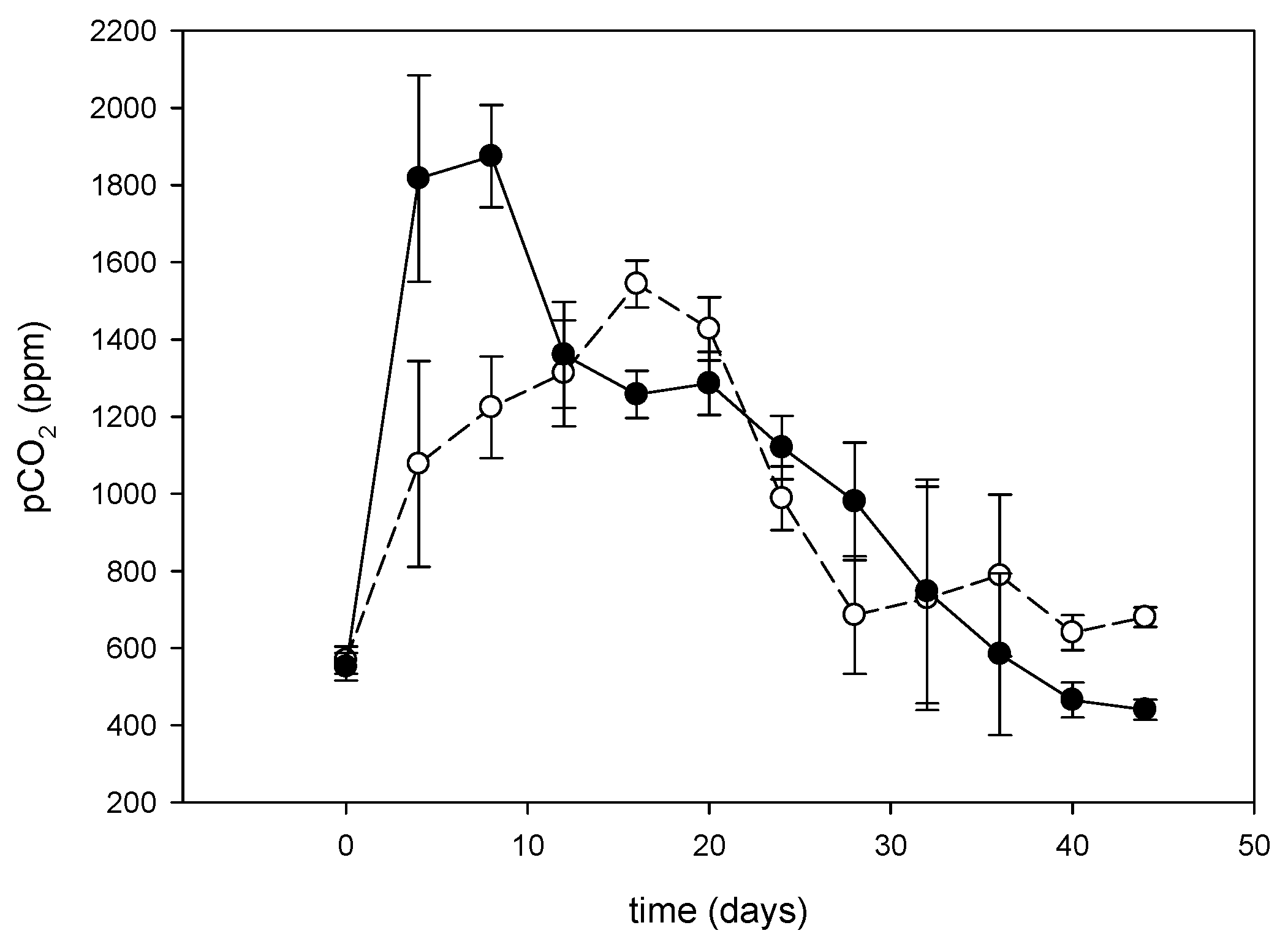
3.3. Effect of Floating Treatment Welands on Dynamics of Dissolved Inorganic Carbon and N2O in the Water
3.3.1. Dissolved Inorganic Carbon and N2O in the Water
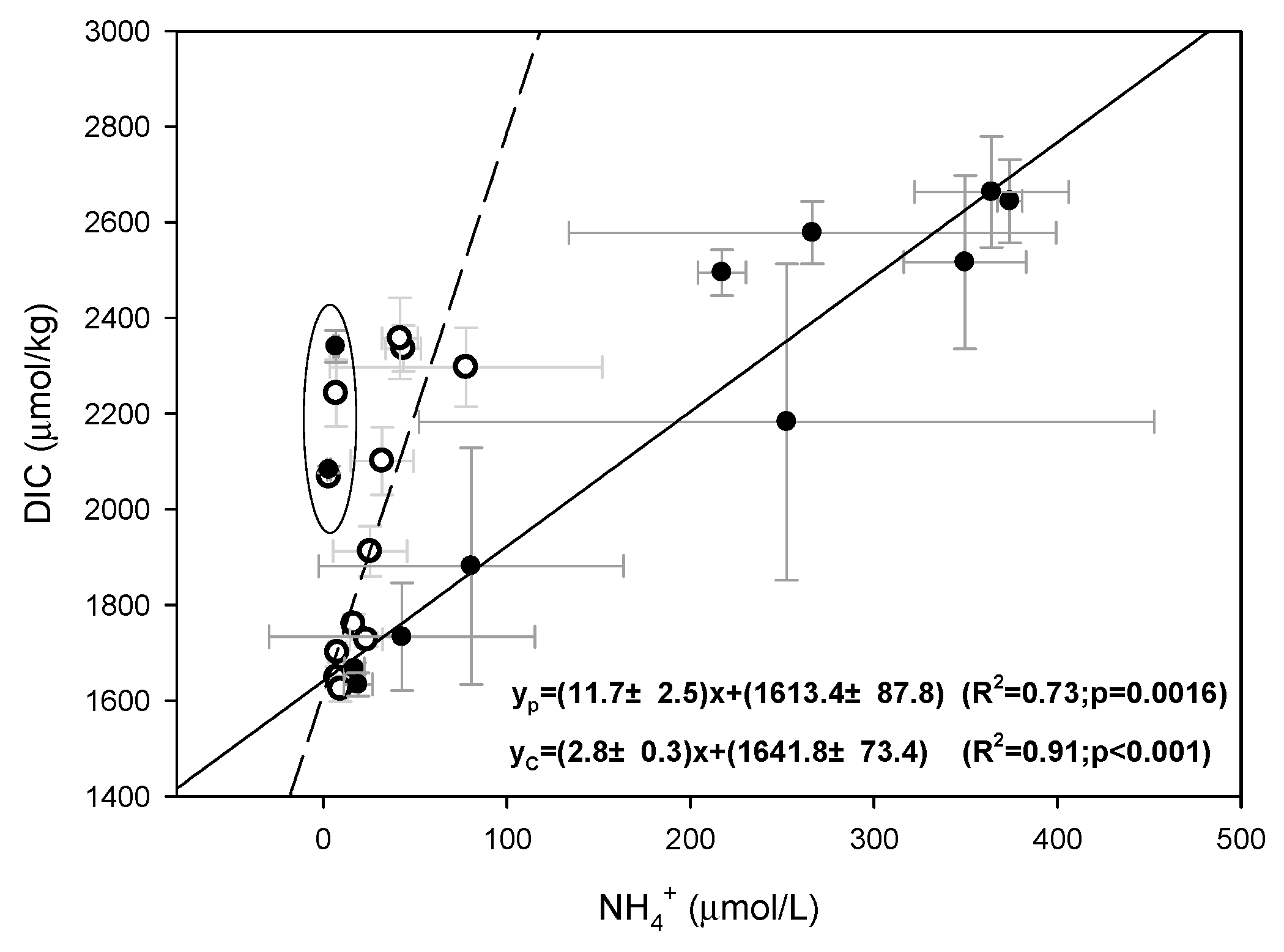
3.3.2. Response of CO2 Dynamics to Dissolved Inorganic Nitrogen Transformation
3.3.3. Dissolved Inorganic Nitrogen Transformation and N2O Dynamics
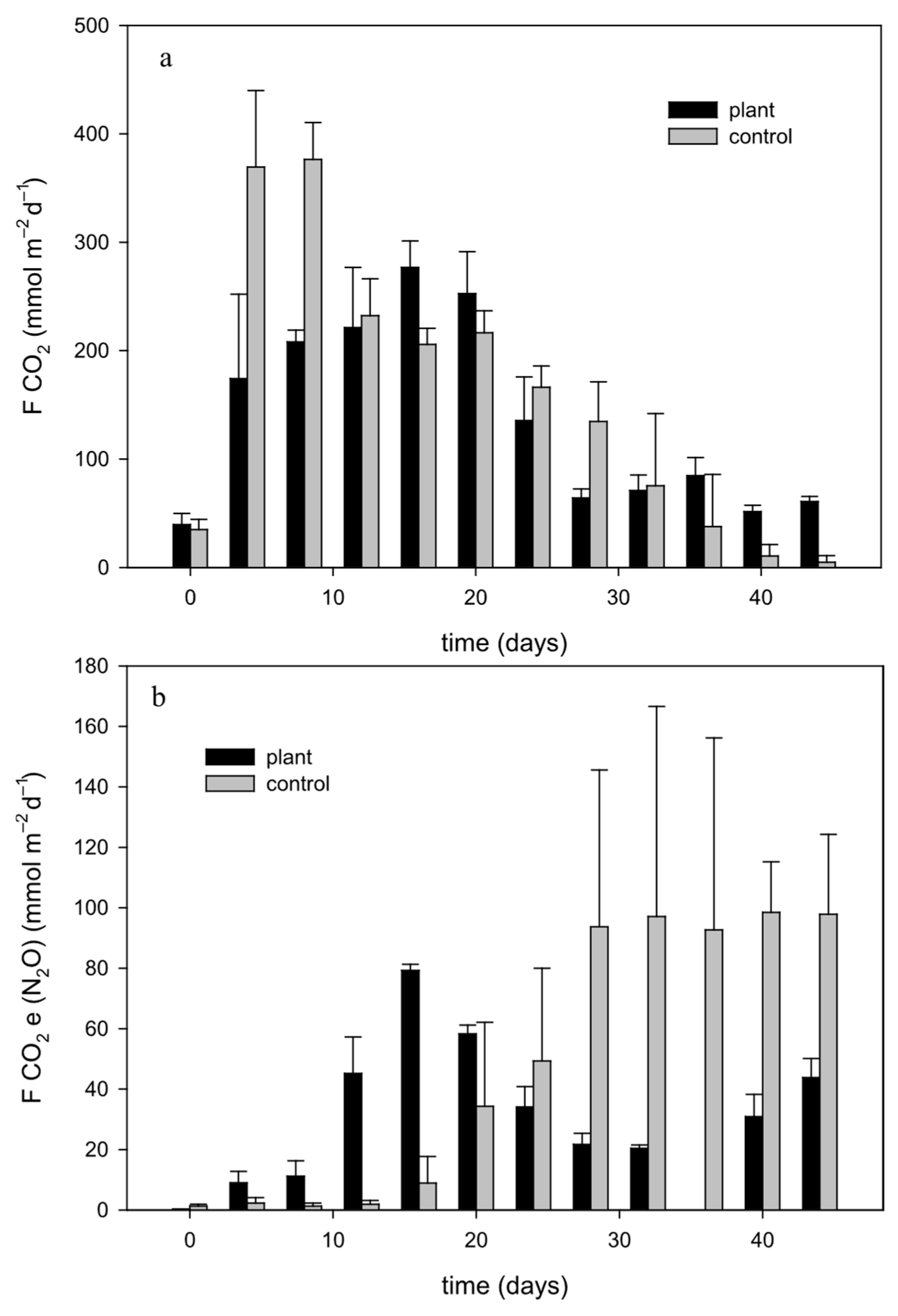
3.4. Effects of FTWs on the Potential Climate Effects of Grouper Culture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. In Brief to the State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; Food and Agricultural Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- Lothmann, R.; Sewilam, H. Potential of innovative marine aquaculture techniques to close nutrient cycles. Rev. Aquac. 2023, 15, 947–964. [Google Scholar] [CrossRef]
- Yuan, J.; Xiang, J.; Liu, D.; Kang, H.; He, T.; Kim, S.; Lin, Y.; Freeman, C.; Ding, W. Rapid growth in greenhouse gas emissions from the adoption of industrial-scale aquaculture. Nat. Clim. Change 2019, 9, 318–322. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, K.W.; Yang, P.; Yang, H.; Tong, C.; Song, C.; Tan, L.; Zhao, G.; Zhou, X.; Sun, D. Assessing carbon greenhouse gas emissions from aquaculture in China based on aquaculture system types, species, environmental conditions and management practices. Agric. Ecosyst. Environ. 2022, 338, 108110. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Hum-borg, C. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Khanal, S.K. Nitrous oxide (N2O) emission from aquaculture: A review. Environ. Sci. Technol. 2012, 46, 6470–6480. [Google Scholar] [CrossRef]
- Costa, M.H.; da Cunha, L.C.; Cox, P.M.; Eliseev, A.V.; Hensen, S.; Ishii, M.; Jaccard, S.; Koven, C.; Lohila, A.; Patra, P.K. Global carbon and other biogeochemical cycles and feedbacks. In Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 673–816. [Google Scholar]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Williams, J.; Crutzen, P. Nitrous oxide from aquaculture. Nat. Geosci. 2010, 3, 143. [Google Scholar] [CrossRef]
- Xiao, Q.; Hu, C.; Gu, X.; Zeng, Q.; Liu, Z.; Xiao, W.; Zhang, M.; Hu, Z.; Wang, W.; Luo, J. Aquaculture farm largely increase indirect nitrous oxide emission factors of lake. Agric. Ecosyst. Environ. 2023, 341, 108212. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, G.; Tong, C.; Tang, K.W.; Lai, D.Y.; Li, L.; Tang, C. Assessing nutrient budgets and environmental impacts of coastal land-based aquaculture system in southeastern China. Agric. Ecosyst. Environ. 2021, 322, 107662. [Google Scholar] [CrossRef]
- Ye, W.; Sun, H.; Li, Y.; Zhang, J.; Zhang, M.; Gao, Z.; Yan, J.; Liu, J.; Wen, J.; Yang, H. Greenhouse gas emissions from fed mollusk mariculture: A case study of a Sinonovacula constricta farming system. Agric. Ecosyst. Environ. 2022, 336, 108029. [Google Scholar] [CrossRef]
- Chen, G.; Bai, J.; Bi, C.; Wang, Y.; Cui, B. Global greenhouse gas emissions from aquaculture: A bibliometric analysis. Agric. Ecosyst. Environ. 2023, 348, 108405. [Google Scholar] [CrossRef]
- Boxman, S.E.; Nystrom, M.; Ergas, S.J.; Main, K.L.; Trotz, M.A. Evaluation of water treatment capacity, nutrient cycling, and biomass production in a marine aquaponic system. Ecol. Eng. 2018, 120, 299–310. [Google Scholar] [CrossRef]
- Chang, Y.; Cui, H.; Huang, M.; He, Y. Artificial floating islands for water quality improvement. Environ. Rev. 2017, 25, 350–357. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Yan, B.; Shutes, B.; Xing, D.; Banuelos, G.; Cheng, R.; Wang, X. Greenhouse gas emissions and wastewater treatment performance by three plant species in subsurface flow constructed wetland mesocosms. Chemosphere 2020, 239, 124795. [Google Scholar] [CrossRef]
- Jahangir, M.M.; Richards, K.G.; Healy, M.G.; Gill, L.; Müller, C.; Johnston, P.; Fenton, O. Carbon and nitrogen dynamics and greenhouse gas emissions in constructed wetlands treating wastewater: A review. Hydrol. Earth Syst. Sci. 2016, 20, 109–123. [Google Scholar] [CrossRef]
- Nie, X.; Mubashar, M.; Zhang, S.; Qin, Y.; Zhang, X. Current progress, challenges and perspectives in microalgae-based nutrient removal for aquaculture waste: A comprehensive review. J. Clean. Prod. 2020, 277, 124209. [Google Scholar] [CrossRef]
- Xie, B.; Wu, J.; Huang, L. Temporal and spatial variations of macrofouling organisms on ecological floating beds in Yundang Lagoon, China. Mar. Pollut. Bull. 2019, 148, 156–167. [Google Scholar] [CrossRef]
- Chanda, A.; Das, S.; Bhattacharyya, S.; Das, I.; Giri, S.; Mukhopadhyay, A.; Samanta, S.; Dutta, D.; Akhand, A.; Choudhury, S. CO2 fluxes from aquaculture ponds of a tropical wetland: Potential of multiple lime treatment in reduction of CO2 emission. Sci. Total Environ. 2019, 655, 1321–1333. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Sinhababu, D.P.; Roy, K.S.; Dash, P.K.; Sahu, P.K.; Dandapat, R.; Neogi, S.; Mohanty, S. Effect of fish species on methane and nitrous oxide emission in relation to soil C, N pools and enzymatic activities in rainfed shallow lowland rice-fish farming system. Agric. Ecosyst. Environ. 2013, 176, 53–62. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Yan, B.; Shutes, B.; Cheng, X.; Chen, X. Removal of nutrients in saline wastewater using constructed wetlands: Plant species, influent loads and salinity levels as influencing factors. Chemosphere 2017, 187, 52–61. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements; North Pacific Marine Science Organization: Sidney, BC, Canada, 2007. [Google Scholar]
- Zhan, L.Y.; Chen, L.Q.; Zhang, J.X.; Lin, Q. A system for the automated static headspace analysis of dissolved N2O in seawater. Int. J. Environ. Anal. Chem. 2013, 93, 828–842. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean revisited. Limnol. Oceanogr. Methods 2014, 12, 351–362. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Li, J.; Ren, J.; Cheng, X. Iron-carbon could enhance nitrogen removal in Sesuvium portulacastrum constructed wetlands for treating mariculture effluents. Bioresour. Technol. 2021, 325, 124602. [Google Scholar] [CrossRef]
- Liu, X.; Pu, X.; Luo, D.; Lu, J.; Liu, Z. Model assessment of nutrient removal via planting Sesuvium portulacastrum in floating beds in eutrophic marine waters: The case of aquaculture areas of Dongshan Bay. Acta Oceanol. Sin. 2019, 38, 91–100. [Google Scholar] [CrossRef]
- Tang, S.; Liao, Y.; Xu, Y.; Dang, Z.; Zhu, X.; Ji, G. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: A review. Bioresour. Technol. 2020, 314, 123759. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Proença, D.C.; David, F.S.; Durborow, R.M.; Moraes-Valenti, P.; Valenti, W.C. The budget of carbon in the farming of the Amazon river prawn and tambaqui fish in earthen pond monoculture and integrated multitrophic systems. Aquac. Rep. 2020, 17, 100340. [Google Scholar] [CrossRef]


| Plant Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Day | NH4+/DIN | NO2−/DIN | NO3−/DIN | NH4+/DIN | NO2−/DIN | NO3−/DIN |
| 0 | 40.8 | 5.1 | 54.1 | 39.6 | 4.5 | 55.8 |
| 4 | 40.4 | 9.6 | 50.0 | 36.4 | 13.6 | 50.0 |
| 8 | 42.7 | 33.2 | 24.0 | 96.5 | 1.3 | 2.1 |
| 12 | 21.6 | 39.4 | 38.9 | 94.1 | 2.6 | 3.3 |
| 16 | 23.8 | 46.5 | 29.7 | 90.8 | 6.3 | 2.9 |
| 20 | 7.8 | 55.1 | 37.1 | 73.4 | 20.8 | 5.8 |
| 24 | 4.4 | 43.8 | 51.7 | 43.3 | 48.2 | 8.5 |
| 28 | 2.9 | 42.3 | 54.8 | 36.1 | 60.2 | 3.6 |
| 32 | 4.0 | 30.0 | 66.0 | 11.9 | 80.0 | 8.1 |
| 36 | 1.1 | 27.1 | 71.8 | 5.3 | 80.4 | 14.3 |
| 40 | 0.9 | 17.8 | 81.3 | 2.0 | 80.1 | 17.9 |
| 44 | 1.2 | 10.4 | 88.4 | 2.2 | 77.7 | 20.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Wu, M.; Liu, J.; Ye, W.; Lin, Y.; Yang, M.; Zheng, H.; Yang, F.; Luo, D.; Zhan, L. Evaluation of the Effect of Floating Treatment Wetlands Planted with Sesuvium portulacastrum on the Dynamics of Dissolved Inorganic Nitrogen, CO2, and N2O in Grouper Aquaculture Systems. J. Mar. Sci. Eng. 2025, 13, 1342. https://doi.org/10.3390/jmse13071342
Zheng S, Wu M, Liu J, Ye W, Lin Y, Yang M, Zheng H, Yang F, Luo D, Zhan L. Evaluation of the Effect of Floating Treatment Wetlands Planted with Sesuvium portulacastrum on the Dynamics of Dissolved Inorganic Nitrogen, CO2, and N2O in Grouper Aquaculture Systems. Journal of Marine Science and Engineering. 2025; 13(7):1342. https://doi.org/10.3390/jmse13071342
Chicago/Turabian StyleZheng, Shenghua, Man Wu, Jian Liu, Wangwang Ye, Yongqing Lin, Miaofeng Yang, Huidong Zheng, Fang Yang, Donglian Luo, and Liyang Zhan. 2025. "Evaluation of the Effect of Floating Treatment Wetlands Planted with Sesuvium portulacastrum on the Dynamics of Dissolved Inorganic Nitrogen, CO2, and N2O in Grouper Aquaculture Systems" Journal of Marine Science and Engineering 13, no. 7: 1342. https://doi.org/10.3390/jmse13071342
APA StyleZheng, S., Wu, M., Liu, J., Ye, W., Lin, Y., Yang, M., Zheng, H., Yang, F., Luo, D., & Zhan, L. (2025). Evaluation of the Effect of Floating Treatment Wetlands Planted with Sesuvium portulacastrum on the Dynamics of Dissolved Inorganic Nitrogen, CO2, and N2O in Grouper Aquaculture Systems. Journal of Marine Science and Engineering, 13(7), 1342. https://doi.org/10.3390/jmse13071342






