Evaluating Coral Farming Strategies in Mauritius: A Comparative Study of Nursery Types, Biodiversity and Environmental Conditions at Pointe Aux Feuilles and Flic-en-Flac
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Benthic Survey and Environmental Parameters
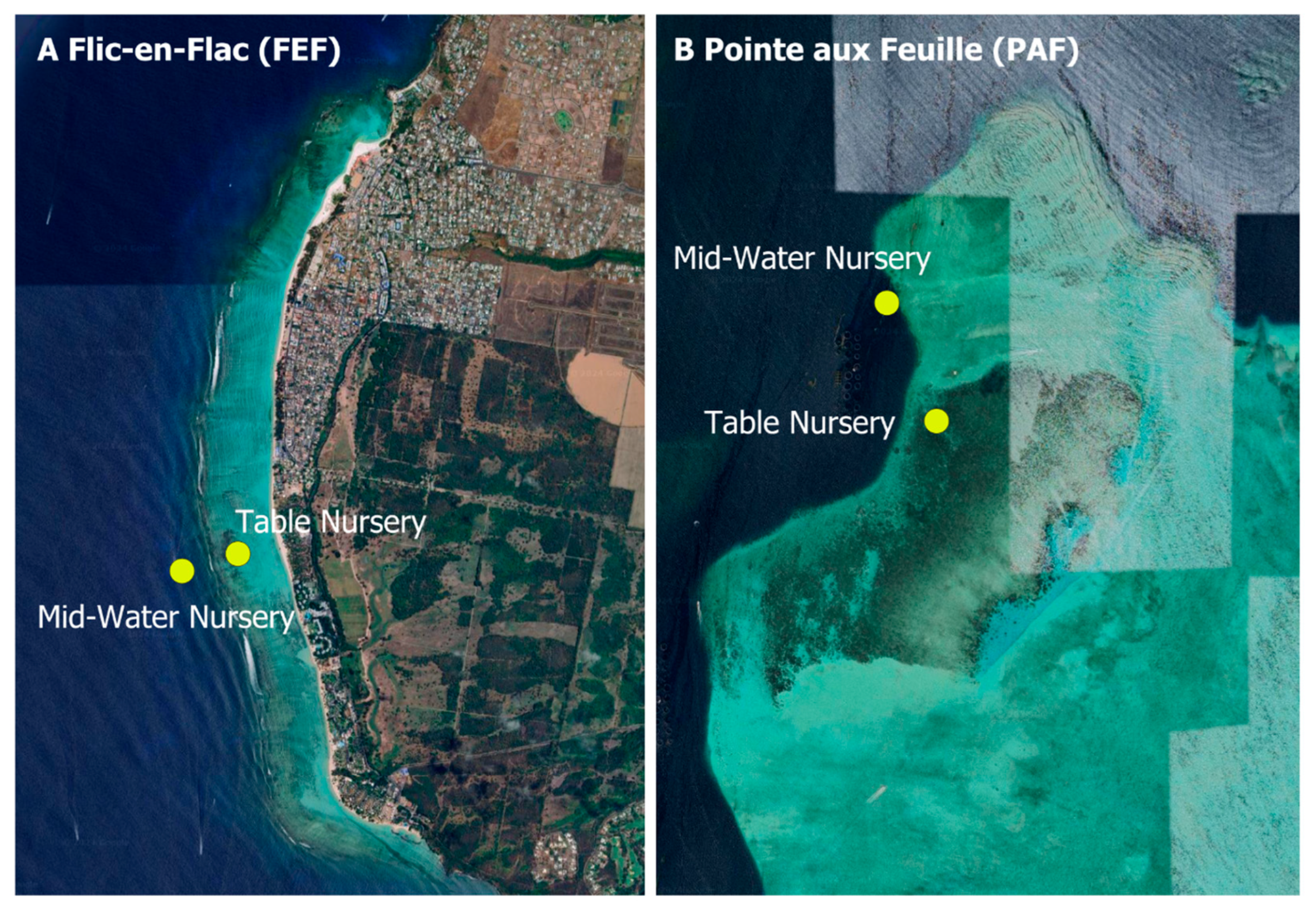
2.3. Nursery Designs, Coral Placement, Installation and Monitoring
2.4. Statistical Analysis
3. Results
3.1. Site Characteristics
3.1.1. Environmental Parameters
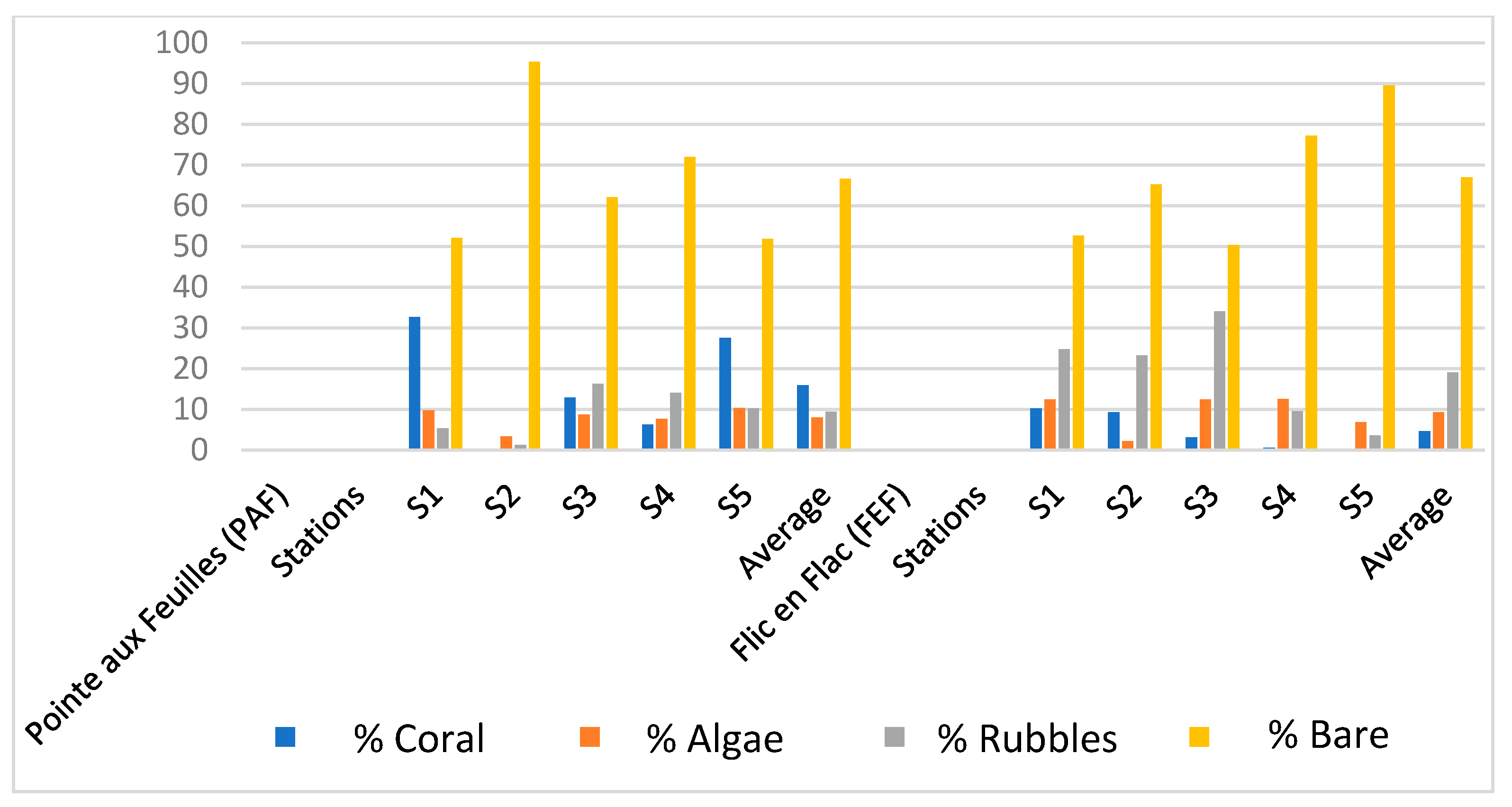
3.1.2. Percentages of Coral, Algal, Rubble, and Sand Coverage
3.1.3. Coral Biodiversity
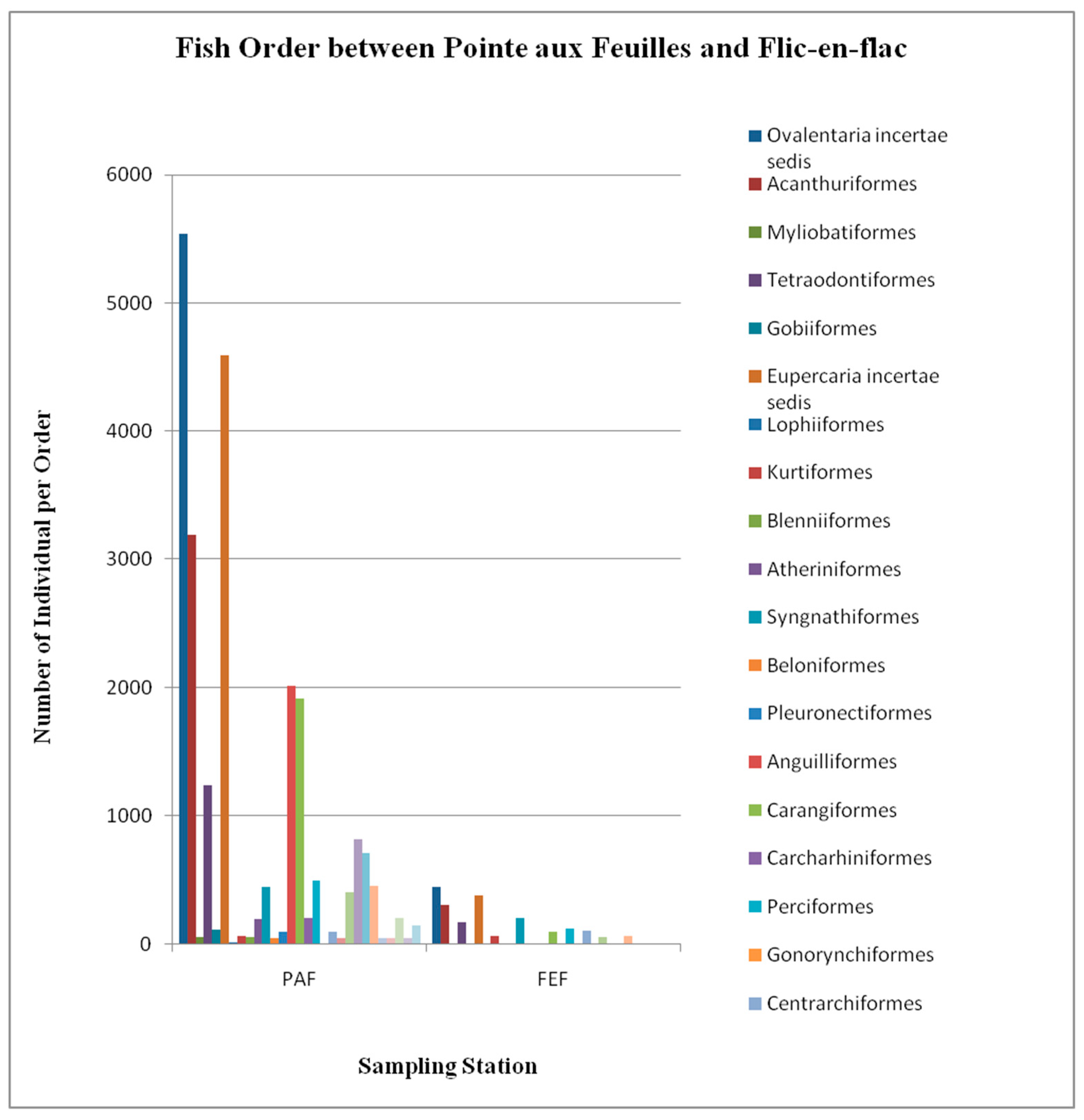
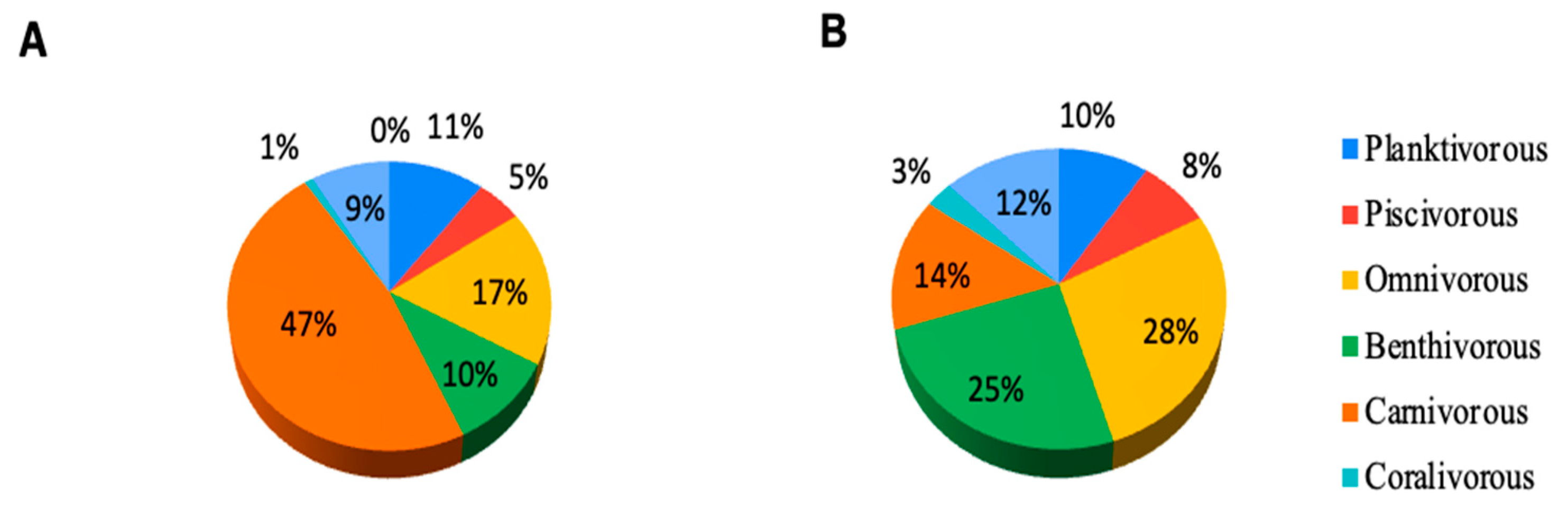
3.1.4. Fish Species and Feeding Habits
3.1.5. Fish and Coral Diversity Indices
3.2. Coral Fragments Survival
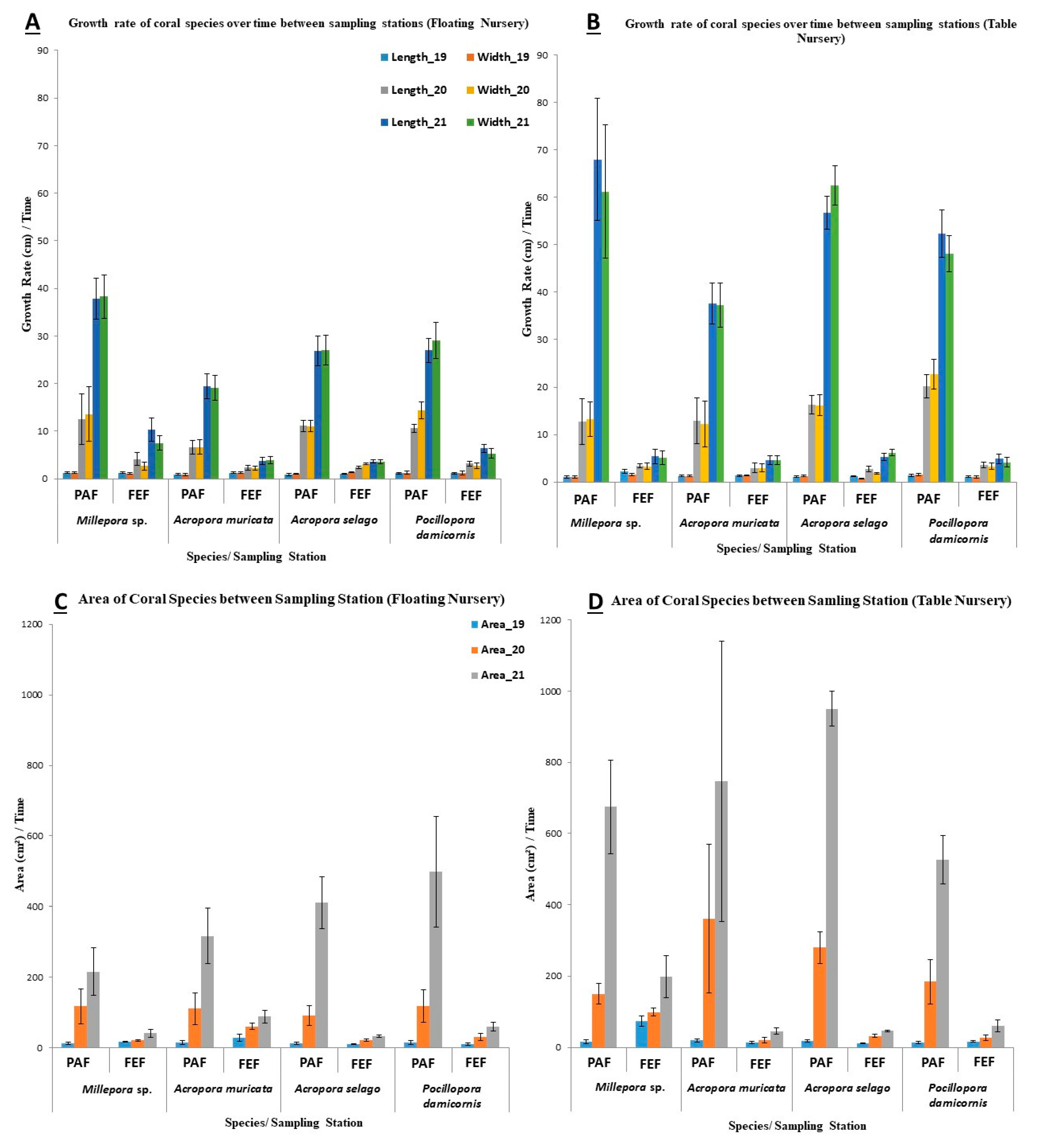
3.3. Coral Growth
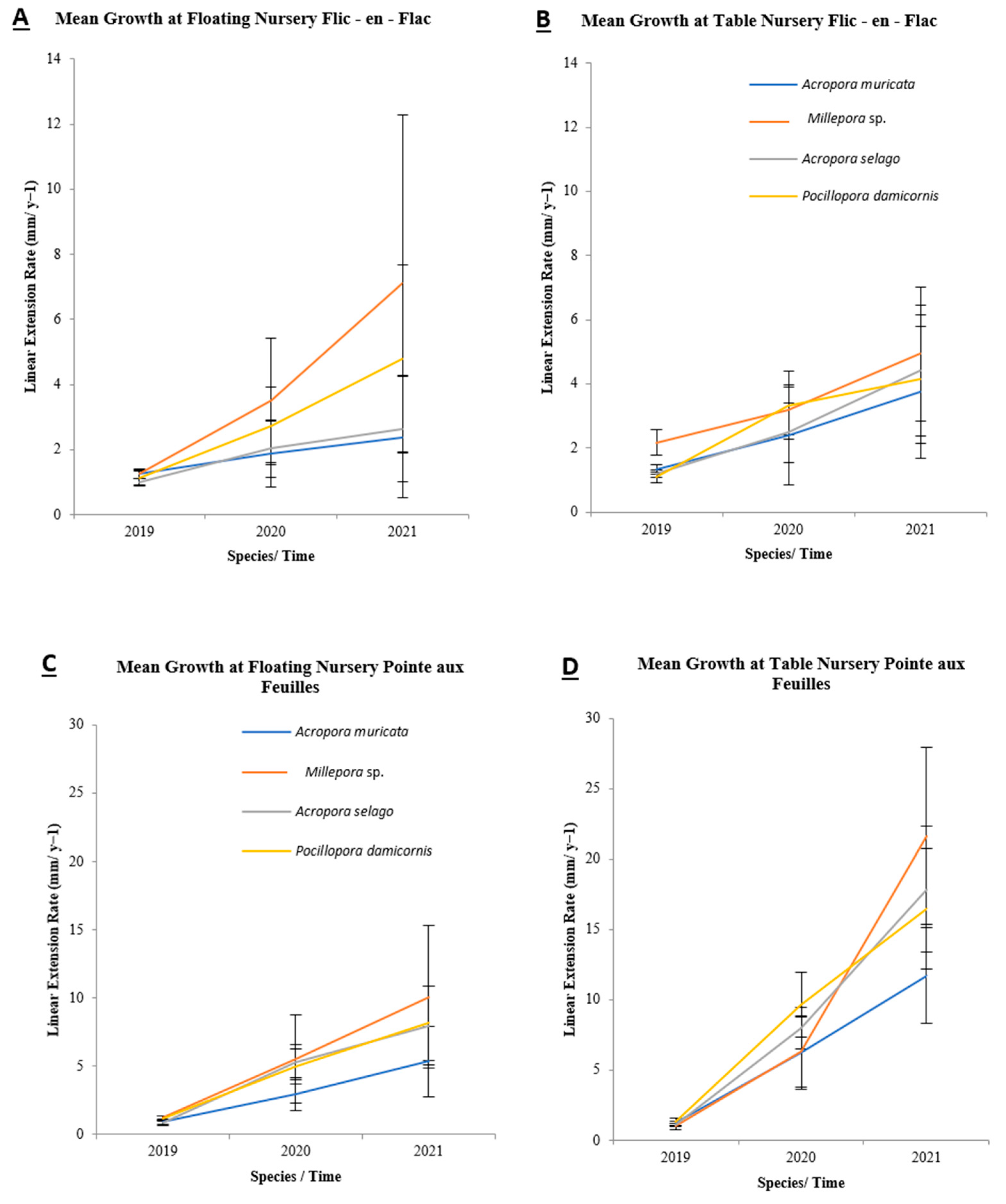
3.4. Linear Extension Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hein, M.; Banaszack, A.; Dallison, T.; Deri, W.; Grimsditch, G.; Jacob, F.; Loder, J.; McLeod, I.; Mead, D.; Moore, T.; et al. Mapping the Global Funding Landscape for Coral Reef Restoration. 2021, pp. 1–23. Available online: https://researchonline.jcu.edu.au/70966/ (accessed on 12 March 2025).
- UNDP. Coral reefs and their importance for the island economies. UNDP Mauritius & Seychelles Blog, 6 June 2021. Available online: https://www.mu.undp.org (accessed on 13 April 2022).
- Bindoff, N.L.; Cheung, W.W.; Kairo, J.G.; Arstegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Chapter 5: Changing ocean, marine ecosystems, and dependent communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Rinkevich, B. Management of coral reefs: We have gone wrong when neglecting active reef restoration. Mar. Pollut. Bull. 2008, 56, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.F.; Côté, I.M.; Toth, L.T. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Annu. Rev. Mar. Sci. 2019, 11, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Kleypas, J.A.; Allemand, D.; Anthony, K.; Baker, A.C.; Beck, M.; Hale, L.Z.; Hilme, N.; Hoegh-Guldberg, O.; Hughes, T.; Kaufman, L.; et al. Designing a blueprint for coral reef survival. Biol. Conserv. 2021, 257, 109107. [Google Scholar] [CrossRef]
- Vardi, T.; Hoot, W.C.; Levy, J.; Shaver, E.; Winters, R.S.; Banasza, A.T.; Baums, I.B.; Chamberland, V.F.; Cook, N.; Gulko, D.; et al. Six priorities to advance the science and practice of coral reef restoration worldwide. Restor. Ecol. 2021, 29, e13498. [Google Scholar] [CrossRef]
- Harris, D.L.; Rovere, A.; Casella, E.; Power, H.; Canavesio, R.; Collin, A.; Pomeroy, A.; Webster, J.M.; Parravicini, V. Coral reef structural complexity provides important coastal protection from waves under rising sea levels. Sci. Adv. 2018, 4, eaao4350. [Google Scholar] [CrossRef]
- Masselink, G.; Beetham, E.; Kench, P. Coral reef islands can accrete vertically in response to sea level rise. Sci. Adv. 2020, 6, eaay3656. [Google Scholar] [CrossRef]
- Majewska, J. The Ocean Economy in SIDS—Environmental Taxation. Ekon. XXI Wieku 2024, 27. Available online: https://journals.ue.wroc.pl/e21/article/view/1332 (accessed on 14 November 2024). [CrossRef]
- Golomb, D.; Shashar, N.; Rinkevich, B. Coral carpets- a novel ecological engineering tool aimed at constructing coral communities on soft sand bottoms. Ecol. Eng. 2020, 145, 105743. [Google Scholar] [CrossRef]
- Bozec, Y.M.; Yakob, L.; Bejarano, S.; Mumby, P.J. Reciprocal facilitation and non-linearity maintain habitat engineering on coral reefs. Oikos 2013, 122, 428–440. [Google Scholar] [CrossRef]
- Rinkevich, B. Rebuilding coral reefs: Does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sustain. 2014, 7, 28–36. [Google Scholar] [CrossRef]
- Rinkevich, B. Climate change and active reef restoration ways of constructing the ‘reefs of tomorrow’. J. Mar. Sci. Eng. 2015, 3, 111–127. [Google Scholar] [CrossRef]
- Rinkevich, B. The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. J. Mar. Sci. Eng. 2019, 7, 201. [Google Scholar] [CrossRef]
- Rinkevich, B. The coral gardening concept and the use of underwater nurseries; lessons learned from silvics and silviculture. In Coral Reef Restoration Handbook; Precht, W.F., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 291–301. [Google Scholar]
- Frias-Torres, S.; Goehlich, H.; Reveret, C.; Montoya-Maya, P.H. Reef fishes recruited at midwater coral nurseries consume biofouling and reduce cleaning time in Seychelles, Indian Ocean. Afr. J. Mar. Sci. 2015, 37, 421–426. [Google Scholar] [CrossRef]
- Dale, C.; Antoine, A.; Strona, G.; Bell, M.; Shah, N.; Saponari, L. Enhancing coral restoration practices in Seychelles: Benefits and limitations of fishing lines and rope as coral stocking methods. Restor. Ecol. 2024, 32, e14252. [Google Scholar] [CrossRef]
- Shaish, L.; Levi, G.; Katzir, G.; Rinkevich, B. Coral reef restoration (Bolinao, the Philippines) in the face of frequent natural catastrophes. Restor. Ecol. 2010, 18, 285–299. [Google Scholar] [CrossRef]
- Hamzah, S.N.; Nursinar, S. The success of coral rehabilitation through transplantation using spider modules (case study: Botutonuo marine area, Bone Bolango Regency). Aquac. Aquar. Conserv. Legis. 2021, 14, 3023–3031. [Google Scholar]
- Huawei. IUCN and Huawei Call for Greater Technology Adoption to Protect Nature. 6 June 2022. Available online: https://www.prnewswire.com/news-releases/iucn-and-huawei-call-for-greater-technology-adoption-to-protect-nature-301562325.html (accessed on 13 April 2024).
- Chineah, V.; Chooramun, V.; Nallee, M.; Rai, Y.B.; Pillay, R.M.; Jayabalan, N.; Terashima, H.; Terai, A. Status of the marine environment of the flic en Flac lagoon, Mauritius. In Proceedings of the Fifth Annual Meeting of Agricultural Scientists, April 2002; p. 219. Available online: https://www.researchgate.net/publication/237109340_STATUS_OF_THE_MARINE_ENVIRONMENT_OF_THE_FLIC_EN_FLAC_LAGOON_MAURITIUS (accessed on 12 March 2025).
- Leujak, W.; Ormond, R.F.G. Comparative accuracy and efficiency of six coral community survey methods. J. Exp. Mar. Biol. Ecol. 2007, 351, 168–187. [Google Scholar] [CrossRef]
- Nazurally, N.; Rinkevich, B. A Questionnaire-based Consideration of Coral Farming for Coastal Socio-economic Development in Mauritius. WIOMSA 2013, 12, 47–56. [Google Scholar]
- Elliott, J.A.; Patterson, M.R.; Staub, C.G.; Koonjul, M.; Elliott, S.M. Decline in coral cover and flattening of the reefs around Mauritius (1998–2010). PeerJ 2018, 6, e6014. [Google Scholar] [CrossRef]
- Pillay, R.M.; Gian, S.B.; Bhoyroo, V.; Curpen, S. Adapting Coral Culture to Climate Change: The Mauritian Experience. WIOMSA 2011, 10, 155–167. [Google Scholar]
- Tebbett, S.B.; Bennett, S.; Bellwood, D.R. A functional perspective on the meaning of the term ‘herbivore’: Patterns versus processes in coral reef fishes. Coral Reefs 2024, 43, 219–232. [Google Scholar] [CrossRef]
- Smith, H.A.; Brown, D.A.; Arjunwadkar, C.V.; Fulton, S.E.; Whitman, T.; Hermanto, B.; Mastroianni, E.; Mattocks, N.; Smith, A.K.; Harrison, P.L.; et al. Removal of macroalgae from degraded reefs enhances coral recruitment. Restor. Ecol. 2022, 30, e13624. [Google Scholar] [CrossRef]
- Williams, S.D.; Patterson, M.R. Resistance and robustness of the global coral–symbiont network. Ecology 2020, 101, e02990. [Google Scholar] [CrossRef]
- Marshall, P.A.; Baird, A.H. Bleaching of corals on the Great Barrier Reef: Differential susceptibilities among taxa. Coral Reefs 2000, 19, 155–163. [Google Scholar] [CrossRef]
- Schopmeyer, S.A.; Lirman, D.; Bartels, E.; Gilliam, D.S.; Goergen, E.A.; Griffin, S.P.; Johnson, M.E.; Lustic, C.; Maxwell, K.; Walter, C.S. Regional restoration benchmarks for Acropora cervicornis. Coral Reefs 2017, 36, 1047–1057. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, W.; Chen, R.; Rinkevich, B.; Shafir, S.; Xia, J.; Zhu, M.; Chen, R.; Wang, A.; Li, X. Framed Reef Modules: A new and cost-effective tool for coral restoration. Restor. Ecol. 2024, 32, e13997. [Google Scholar] [CrossRef]
- Dehnert, I.; Saponari, L.; Galli, P.; Montano, S. Comparing different farming habitats for mid-water rope nurseries to advance coral restoration efforts in the Maldives. PeerJ 2022, 10, e12874. [Google Scholar] [CrossRef]
- Howlett, L.; Camp, E.F.; Edmondson, J.; Henderson, N.; Suggett, D.J. Coral growth, survivorship and return-on-effort within nurseries at high-value sites on the Great Barrier Reef. PLoS ONE 2021, 16, e0244961. [Google Scholar] [CrossRef]
- Edwards, A.J. (Ed.) Reef Rehabilitation Manual; Coral Reef Targeted Research & Capacity Building for Management Program: St Lucia, Australia, 2010; p. 166. [Google Scholar]
- Highsmith, R.C. Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser. Oldendorf. 1982, 7, 207–226. [Google Scholar] [CrossRef]
- Kotb, M.M.A. Coral translocation and farming as mitigation and conservation measures for coastal development in the Red Sea: Aqaba case study, Jordan. Environ. Earth Sci. 2016, 75, 439. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Yap, H.T.; Montaño, M.N.E. Intensive fish farming in the Philippines is detrimental to the reef-building coral Pocillopora damicornis. Mar. Ecol. Prog. Ser. 2006, 316, 165–174. [Google Scholar] [CrossRef]
- Mumby, P.J.; Dahlgren, C.P.; Harborne, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science 2006, 311, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Knoester, E.G.; Murk, A.J.; Osinga, R. Benefits of herbivorous fish outweigh costs of corallivory in coral nurseries placed close to a Kenyan patch reef. Mar. Ecol. Prog. Ser. 2019, 611, 143–155. [Google Scholar] [CrossRef]
- Quimpo, T.J.; Cabaitan, P.C.; Hoey, A.S. Detachment of Porites cylindrica nubbins by herbivorous fishes. Restor. Ecol. 2020, 28, 418–426. [Google Scholar] [CrossRef]
- O’Donnell, K.E.; Lohr, K.E.; Bartels, E.; Patterson, J.T. Evaluation of staghorn coral (Acropora cervicornis, Lamarck 1816) production techniques in an ocean-based nursery with consideration of coral genotype. J. Exp. Mar. Biol. Ecol. 2017, 487, 53–58. [Google Scholar] [CrossRef]
- Migliaccio, O. Optimizing Coral Farming: A Comparative Analysis of Nursery Designs for Acropora aspera, Acropora muricata, and Montipora digitata in Anantara Lagoon, Maldives. Int. J. Mar. Sci. 2024, 14, 295–305. [Google Scholar] [CrossRef]
- Forsman, Z.H.; Kimokeo, B.K.; Bird, C.E.; Hunter, C.L.; Toonen, R.J. Coral farming: Effects of light, water motion and artificial foods. J. Mar. Biol. Assoc. UK 2012, 92, 721–729. [Google Scholar] [CrossRef]
- Doropoulos, C.; Gómez-Lemos, L.A.; Salee, K.; McLaughlin, M.J.; Tebben, J.; Van Koningsveld, M.; Feng, M.; Babcock, R.C. Limitations to coral recovery along an environmental stress gradient. Ecol. Appl. 2022, 32, e2558. [Google Scholar] [CrossRef]
- Edori, O.S. Physical and Chemical Characteristics of Water from Ede Onyima Creek, Okarki-Engenni, Rivers State, Nigeria. Chem. Res. J. 2020, 5, 144–154. [Google Scholar]
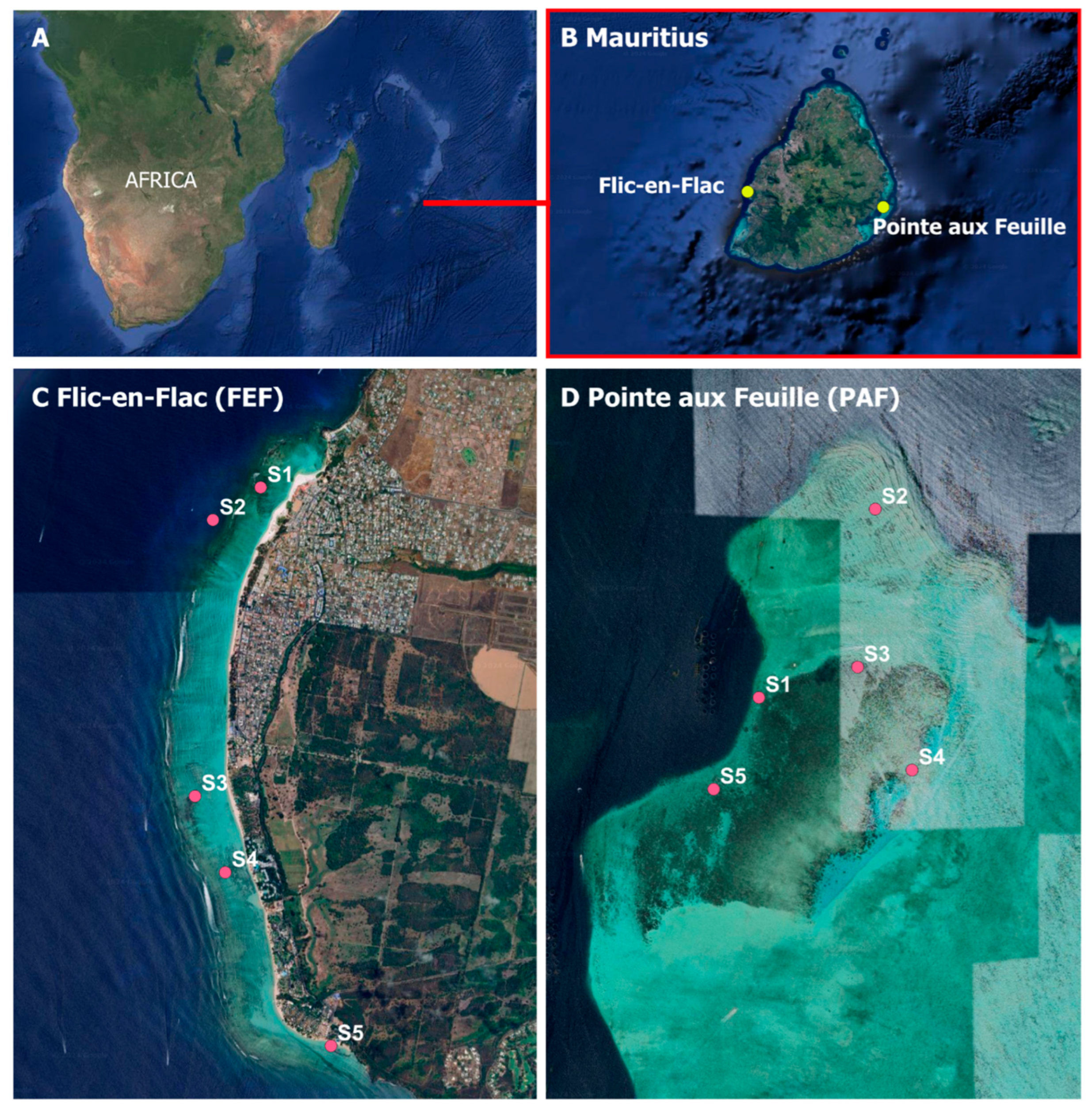

| Site | Station | Coordinates |
|---|---|---|
| Flic-en-Flac (FEF) | S1 | −20.275581° S; 57.3656333° E |
| S2 | −20.2780099° S; 57.3618442° E | |
| S3 | −20.298619° S; 57.360422° E | |
| S4 | −20.3042957° S; 57.3628205° E | |
| S5 | −20.317180° S; 57.371214° E | |
| Pointe aux Feuille (PAF) | S1 | −20.338919° S; 57.792509° E |
| S2 | −20.331829° S; 57.797158° E | |
| S3 | −20.337773° S; 57.796454° E | |
| S4 | −20.341642° S; 57.798628° E | |
| S5 | −20.342360° S; 57.790703° E |
| Parameter | df | Kruskal–Wallis Chi-Square | p Value |
|---|---|---|---|
| Temperature | 3 | 4.107 | ns |
| Salinity | 3 | 26.845 | ** |
| DO | 3 | 13.743 | ** |
| TSS | 3 | 15.461 | ** |
| pH | 3 | 28.483 | ** |
| Nitrate | 3 | 40.952 | ** |
| Phosphate | 3 | 38.921 | ** |
| Phytoplankton Density | 3 | 44.787 | ** |
| Parameters/Site | PAF | FEF | ||
|---|---|---|---|---|
| TN | FN | TN | FN | |
| Temperature (°C) | 27.24 ± 2.35 23.20–30.26 | 26.49 ± 2.37 22.91–29.55 | 27.81 ± 2.40 23.83–30.76 | 27.43 ± 2.28 23.91–30.15 |
| Salinity (%) | 35.94 ± 0.71 35.44–36.20 | 36.01 ± 0.21 35.56–36.33 | 35.30 ± 0.67 34.67–36.56 | 35.21 ± 0.33 34.67–36.10 |
| Dissolved Oxygen/DO (mg/L) | 5.81 ± 0.71 5.05–7.05 | 6.16 ± 0.54 5.59–7.05 | 6.42 ± 0.52 5.30–7.05 | 6.86 ± 0.72 6.13–8.10 |
| Total Suspended Solid/TSS (mg/L) | 12.01 ± 1.84 10.00–14.44 | 11.13 ± 2.37 4.56–14.44 | 10.37 ± 1.01 8.90–12.50 | 9.87 ± 1.10 8.60–12.50 |
| pH | 7.93 ± 0.03 7.89–7.98 | 8.10 ± 0.01 8.08–8.13 | 7.87 ± 0.32 7.00–8.10 | 7.92 ± 0.09 7.70–8.10 |
| Nitrate (mg/L) | 1.99 ± 0.25 1.47–2.25 | 1.19 ± 0.59 0.47–1.77 | 3.28 ± 0.51 2.36–3.80 | 3.06 ± 0.99 0.00–3.95 |
| Phosphate (mg/L) | 0.04 ± 0.02 0.02–0.08 | 0.04 ± 0.01 0.02–0.05 | 0.08 ± 0.01 0.06 ± 0.10 | 0.07 ± 0.00 0.06–0.07 |
| Phytoplankton Density | 70.67 × 105 ± 14.52 × 105 35.8 × 105–93.6 × 105 | 70.33 × 105 ± 21.27 × 105 4.69 × 105–91.6 × 105 | 6.52 × 103 ± 2.15 × 103 1.36 × 103–8.9 × 103 | 5.88 × 103 ± 1.55 × 103 3.47 × 103–7.8 × 103 |
| Family | PAF | FEF | Mean | Standard Deviation | Total | Percentage |
|---|---|---|---|---|---|---|
| Acroporidae | 1700 | 146 | 923 | 1098.8 | 1846 | 31.76% |
| Agariciidae | 300 | 8 | 154 | 206.5 | 308 | 5.30% |
| Antipathidae | 50 | 0 | 25 | 35.4 | 50 | 0.86% |
| Astrocoeniidae | 50 | 6 | 28 | 31.1 | 56 | 0.96% |
| Coscinaraeidae | 50 | 0 | 25 | 35.4 | 50 | 0.86% |
| Dendrophylliidae | 50 | 2 | 26 | 33.9 | 52 | 0.89% |
| Euphylliidae | 50 | 6 | 28 | 31.1 | 56 | 0.96% |
| Faviidae | 500 | 21 | 260.5 | 338.7 | 521 | 8.96% |
| Fungiidae | 350 | 18 | 184 | 234.8 | 368 | 6.33% |
| Helioporacea | 50 | 0 | 25 | 35.4 | 50 | 0.86% |
| Leptastreidae | 100 | 11 | 55.5 | 62.9 | 111 | 1.91% |
| Lobophylliidae | 100 | 5 | 52.5 | 67.2 | 105 | 1.81% |
| Merulinidae | 600 | 28 | 314 | 404.5 | 628 | 10.80% |
| Milleporidae | 50 | 0 | 25 | 35.4 | 50 | 0.86% |
| Montastraeidae | 50 | 6 | 28 | 31.1 | 56 | 0.96% |
| Plerogyridae | 50 | 0 | 25 | 35.4 | 50 | 0.86% |
| Plesiastreidae | 50 | 6 | 28 | 31.1 | 56 | 0.96% |
| Pocilloporidae | 450 | 30 | 240 | 297.0 | 480 | 8.26% |
| Poritidae | 800 | 18 | 409 | 553.0 | 818 | 14.07% |
| Psammocoridae | 0 | 2 | 1 | 1.4 | 2 | 0.03% |
| Scleractinia incertae sedis | 50 | 0 | 25 | 35.34 | 50 | 0.86% |
| Stylasteridae | 50 | 0 | 25 | 35.4 | 50 | 0.86% |
| Total | 5500 | 313 | 2906.5 | 3670.6 | 5813 | 100.00% |
| Sampling Station | S | N | D | J’ | H’ | Lamba’ |
|---|---|---|---|---|---|---|
| Corals | ||||||
| PAF | 110 | 5500 | 12.66 | 1 | 4.7 | 0.9911 |
| FEF | 59 | 313 | 10.09 | 0.9936 | 4.1 | 0.9855 |
| Fishes | ||||||
| PAF | 218 | 23,304 | 21.58 | 0.8599 | 4.6 | 0.9859 |
| FEF | 104 | 2056 | 13.5 | 0.8 | 3.8 | 0.9657 |
| Site–Nursery Type | Initial no. of Fragments | Detached | Dead | Detachment (%) | Survival (%) | |
|---|---|---|---|---|---|---|
| Millepora sp. | PAF-T | 4500 | 15 | 10 | 0.33 | 99.8 |
| PAF-F | 1500 | 20 | 15 | 1.3 | 99 | |
| FEF-T | 4500 | 280 | 250 | 6.2 | 94.4 | |
| FEF-F | 1500 | 200 | 150 | 13 | 90 | |
| Acropora muricata | PAF-T | 4500 | 20 | 15 | 0.4 | 99.7 |
| PAF-F | 1500 | 25 | 18 | 1.7 | 98.8 | |
| FEF-T | 4500 | 320 | 300 | 7.1 | 93 | |
| FEF-F | 1500 | 190 | 120 | 12.7 | 92 | |
| Acropora selago | PAF-T | 4500 | 23 | 15 | 0.5 | 99.7 |
| PAF-F | 1500 | 25 | 20 | 1.7 | 98.7 | |
| FEF-T | 4500 | 300 | 320 | 6.7 | 92.9 | |
| FEF-F | 1500 | 120 | 100 | 8 | 93 | |
| Pocillopora damicornis | PAF-T | 4500 | 10 | 10 | 0.2 | 99.8 |
| PAF-F | 1500 | 10 | 15 | 0.7 | 99 | |
| FEF-T | 4500 | 220 | 200 | 4.9 | 95.6 | |
| FEF-F | 1500 | 160 | 180 | 10.7 | 88 |
| Species/Site | Nursery Type | Linear Extension Rate (mm y−1) |
|---|---|---|
| Acropora muricata | ||
| PAF | TN | 6.41 ± 2.05 |
| FN | 3.08 ± 1.32 | |
| FEF | TN | 2.49 ± 1.27 |
| FN | 1.84 ± 1.00 | |
| Millepora sp. | ||
| PAF | TN | 9.66 ± 3.01 |
| FN | 5.61 ± 2.87 | |
| FEF | TN | 3.44 ± 1.08 |
| FN | 3.96 ± 2.41 | |
| Acropora selago | ||
| PAF | TN | 8.99 ± 2.03 |
| FN | 4.71 ± 1.47 | |
| FEF | TN | 2.70 ± 1.03 |
| FN | 1.89 ± 0.88 | |
| Pocillopora damicornis | ||
| PAF | TN | 9.17 ± 2.29 |
| FN | 4.77 ± 1.42 | |
| FEF | TN | 2.86 ± 1.07 |
| FN | 2.94 ± 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazurally, N.; Pomeroy, A.W.M.; Lowe, R.J.; Narayanan, I.; Rinkevich, B. Evaluating Coral Farming Strategies in Mauritius: A Comparative Study of Nursery Types, Biodiversity and Environmental Conditions at Pointe Aux Feuilles and Flic-en-Flac. J. Mar. Sci. Eng. 2025, 13, 1268. https://doi.org/10.3390/jmse13071268
Nazurally N, Pomeroy AWM, Lowe RJ, Narayanan I, Rinkevich B. Evaluating Coral Farming Strategies in Mauritius: A Comparative Study of Nursery Types, Biodiversity and Environmental Conditions at Pointe Aux Feuilles and Flic-en-Flac. Journal of Marine Science and Engineering. 2025; 13(7):1268. https://doi.org/10.3390/jmse13071268
Chicago/Turabian StyleNazurally, Nadeem, Andrew W. M. Pomeroy, Ryan J. Lowe, Inesh Narayanan, and Baruch Rinkevich. 2025. "Evaluating Coral Farming Strategies in Mauritius: A Comparative Study of Nursery Types, Biodiversity and Environmental Conditions at Pointe Aux Feuilles and Flic-en-Flac" Journal of Marine Science and Engineering 13, no. 7: 1268. https://doi.org/10.3390/jmse13071268
APA StyleNazurally, N., Pomeroy, A. W. M., Lowe, R. J., Narayanan, I., & Rinkevich, B. (2025). Evaluating Coral Farming Strategies in Mauritius: A Comparative Study of Nursery Types, Biodiversity and Environmental Conditions at Pointe Aux Feuilles and Flic-en-Flac. Journal of Marine Science and Engineering, 13(7), 1268. https://doi.org/10.3390/jmse13071268







