Abstract
Climate change and anthropogenic stressors are accelerating coral reef degradation, prompting urgent restoration strategies. This study evaluates the performance of two coral nursery types, floating mid-water nurseries (FNs) and bottom-attached table nurseries (TNs), at two contrasting reef environments in Mauritius: the degraded, high sedimentation site of Flic-en-Flac (FEF) and the more pristine Pointe aux Feuilles (PAF). Coral fragments from Millepora sp., Acropora muricata, Acropora selago, and Pocillopora damicornis were monitored over three years for survivorship, growth, and linear extension rate (LER). Survivorship exceeded 88% in all cases, with Millepora sp. in PAF–TN achieving the highest rate (99.8%) and P. damicornis in FEF–FN the lowest (88%). Growth was greatest at PAF–TN, where Millepora sp. reached a mean length of 27.25 cm and LER of 9.66 mm y−1. In contrast, the same species in FEF–TN averaged only 3.64 cm in length and 3.44 mm y−1 in LER. Environmental conditions including higher turbidity, nitrate, and phosphate at FEF, and higher phytoplankton density at PAF significantly influenced coral performance. We propose a site-specific nursery selection framework, including FNs for high-sediment areas and TNs for protected and biodiverse sites, to support more effective coral farming outcomes in island restoration programs.
1. Introduction
Coral reefs, among the most productive and diverse marine ecosystems, support the livelihood of coastal communities at the global level, with an estimated value of USD 2.7 trillion per year of ecosystem services [1,2]. However, the continuous degradation of coral reefs [3] and the inefficacy of traditional management measures [4,5], have highlighted the urgent need for alternative approaches, such as active reef restoration measures in order to rehabilitate and restore coral reef ecosystems at the local, regional, and global scales [6,7]. The projections for future coral reef calamity, primarily due to climate change drivers like ocean warming and rising sea levels, are further intensified in the case of Small Island Developing States (SIDSs) [3]. In these cases, coral reefs provide important coastal protection in response to rising seas [8,9] and are major economic sources for states [10].
The discipline of coral reef restoration integrates ecological engineering approaches, tools, and strategies that are adapted to the needs of specific sites [11,12] and principally includes the use of ecosystem engineering species [12]. The goals in employing ecological engineering approaches are to establish or enhance a foundation ecosystem within which a more diverse ecosystem with improved ecosystem services is established. Most of these actions are performed under the conceptual umbrella of “marine silviculture”, commonly referred to as “gardening of coral reefs”, one of the most effective approaches applied globally [13,14,15]. The “gardening of coral reefs” approach uses a two-phase methodology for reef restoration: (1) the aquaculture of stocks of coral colonies in underwater coral nurseries until corals reach suitable size, and then, (2) the outplanting of farmed coral colonies from underwater nurseries to targeted locations on damaged reefs [16]. One of the novelties that “gardening of coral reefs” has offered is the use of various types of mid-water coral nursery that enable a cheap and fast methodology of farming large numbers of colonies from branching, massive, and encrusting coral species, with high survivorship and growth rates and even improved reproductive efforts [17,18]. These mid-water floating nurseries can also be lowered down during major storms and rough sea events (as suggested; [19]). In contrast, coral colonies in shallow reef restoration sites are nursed in a structure fixed to the bottom nursery bed [20]. Such shallow nurseries, usually established within lagoonal waters, are cheaper and easier to operate without expensive diving requirements, but they are prone to impacts of high sedimentation, seasonally increased seawater temperatures, and rough sea conditions [20] if they are not maintained on a regular basis. Both methods were applied in this study at two different locations and their performance was assessed.
Most of the coastline of the Republic of Mauritius is encircled by coral reefs. The average width of these reefs is 150–200 m, which results in an enclosed lagoon area of ~243 km2. The coastal zone consists of near shore wetlands and mangroves, lagoon coral, fringing coral reef, and related marine life. As a Small Island Developing State (SIDS), Mauritius depends largely on its coral reef resources for both tourism and its fisheries industry. Tourism alone accounted for about 12% of Mauritius’ gross domestic product (GDP) in 2019 before the COVID-19 era, which demonstrates the value of coral reefs as an economic asset. Yet, over recent years, episodes of bleaching have been observed in Mauritius, along with degradation due to a range of other pressures such as land-derived pollution and flash floods. Although there is limited scientific data or detailed monitoring to quantify the relative impacts of each of these pressures.
To sustain its coral reefs, various coral farming initiatives have been implemented in Mauritius, with support from organizations including Mitsui O.S.K. Lines (MOL), IUCN T4N programmes, GEF/SGP, the United Nations Development Programme (UNDP), and various private sectors initiatives. For example, the EcoMode Society, an NGO, led coral restoration efforts to mitigate the adverse effects of the MV Wakashio oil spill in 2020, in a project funded by MOL. In collaboration with Huawei and the International Union for Conservation of Nature (IUCN) through the Tech4Nature project, advanced underwater monitoring systems have also been deployed to survey coral nurseries [21]. Coral farming has also recently been prioritized by the Mauritian government, which has partnered with the UNDP to fund restoration projects around the island.
With the health of coral reefs being important not only in Mauritius but also globally, and considerable funding being used for this purpose, there is a critical need to compare the cultivation success of floating mid-water nurseries with that of shallow nurseries attached to the bottom table, as each offer attractive but different ecological and practical scalability benefits.
In this study, both nursery types were installed in two Mauritian locations. Specimens of four branching coral species (Millepora sp., Acropora muricata, Acropora selago, Pocillopora damicornis) were cultivated using both nursery types at both sites. The objective was to examine species-specific, site-specific, and cultivation-specific differences to inform future restoration designs. Given this critical need, this study aims to fill the knowledge gap by evaluating and comparing the performance of both nursery types across different reef environments in Mauritius.
To address these objectives, this study was conducted at two contrasting lagoonal reef sites, where four branching coral species were cultivated using both floating mid-water and bottom-attached table nurseries. Environmental conditions and coral performance metrics including growth, survivorship, and biodiversity were monitored over a three-year period. The findings were analyzed comparatively across nursery types and sites to assess cultivation success and site-specific suitability. The results can inform practical restoration strategies. This study concludes with recommendations for optimizing coral farming approaches in island reef systems.
2. Materials and Methods
2.1. Study Sites
Coral cultivation was performed in two Mauritian locations that were substantially different but represented the range of reef types and environmental exposure observed in many SIDSs.
(a) Flic-en-Flac (FEF; 20°18.002′ S, 57°21.642′ E; Figure 1) is a section of coastline approximately 7 km long located on the west coast of Mauritius. The shallow lagoon at Flic-en-Flac (0.5 to 2.0 m in depth) and its shoreline are shielded by a fringing reef approximately 500 to 600 m offshore. This area features a 4.5-kilometer stretch of sandy beach, making it a popular destination for both domestic and international tourists, with numerous resorts and hotels. The southern section of the beach has been facing severe erosion [22].
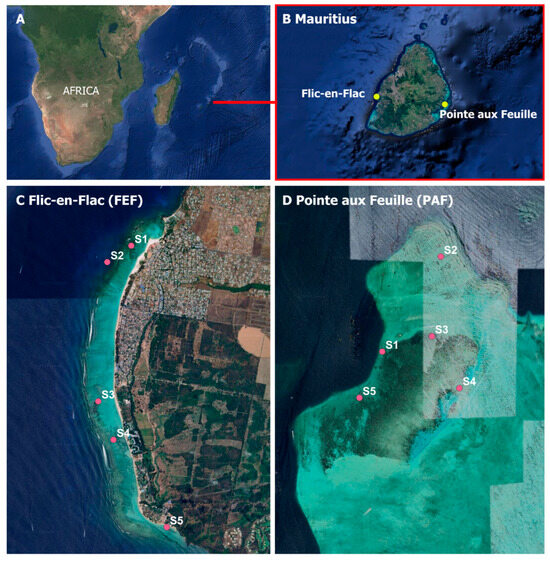
Figure 1.
(A) Regional map showing the location of Mauritius in the Indian Ocean relative to the African continent. (B) Zoomed-in map of Mauritius indicating the two study sites: Flic-en-Flac (FEF) on the west coast and Pointe aux Feuilles (PAF) on the east coast. (C,D) Detailed layout of the five monitoring stations at each site, based on GPS coordinates collected during field surveys. All map panels were generated using Google Earth Pro, and final figure assembly was completed in Microsoft PowerPoint.
(b) Pointe aux Feuilles (PAF: 20°20.361′ S, 57°47.583′ E; Figure 1) is situated on the east coast of Mauritius and is populated by coastal communities primarily engaged in fishing and seafood vending. An established fish farm has been operational here since 2002, producing around 4500 tons of finfish annually. Covering an area of 20 km2, PAF is more sheltered from high waves, strong currents, and intensive development compared with Flic-en-Flac. This area boasts high marine biodiversity, attracting both tourists and locals.
Sites were selected based on contrasting environmental characteristics. PAF exhibits high biodiversity, limited tourism, and nutrient enrichment from fish farming, while FEF is under anthropogenic stress including tourism, erosion, and pollution. These differences allowed comparative assessment of coral farming under varying environmental stressors.
2.2. Benthic Survey and Environmental Parameters
Before installing the coral nurseries, the hard substrate benthic cover (excluding sandy patches) and substrate composition at each site were assessed using the line-intercept transect (LIT) method [23]. The lagoons at FEF and PAF were each divided into five stations [Figure 1C,D, Table 1] and surveyed. At each station, three 20 m transects were laid randomly, in parallel, by snorkelers, using flexible reel fiberglass measuring tapes, which enabled the tapes to follow the seabed relief. An observer swam along the transect line measuring the benthic community and substrate coverage to the nearest centimeter, recording live coral (genus level), dead coral, algae (genus level), rubble, and algal turf.

Table 1.
GPS coordinates of the ten lagoonal monitoring stations surveyed at FEF and PAF. Five stations were selected per site based on accessibility, habitat coverage, and proximity to nursery locations. Coordinates were collected using handheld GPS units during field deployment.
In addition to the substrate composition, environmental parameters were recorded quarterly at the sites in each nursery over the three-year study period. Water samples were collected in triplicate per station and aggregated at the site level for analysis. Parameters measured included temperature
(°C), salinity (%), dissolved oxygen/DO (mg/L), total suspended solids/TSS (mg/L), pH, nitrate (mg/L), phosphate (mg/L) and phytoplankton density on a quarterly basis over the 3-year study.
2.3. Nursery Designs, Coral Placement, Installation and Monitoring
Two types of nurseries were established at both sites (Figure 2): twenty-seven type 1 nurseries, which were bottom-fixed table nurseries, and nine type 3 mid-water floating nurseries. Each of the nurseries attached to the bottom table were 2 m in length × 2 m in width and 50 cm in height, made of galvanized iron bars, and secured in place by both heavy concrete blocks and U-shaped metal bars. The water depth where the table nurseries were placed was 1.5 m to 2 m, depending on the prevailing tide. The mid-water nurseries were fixed at 17 m depth and suspended at an elevation of 10 m below the surface using 12 mm rope wrapped twice onto the bottom and the floating PVC pipes. They were built with 50 mm PVC pipes that were connected to form a 3 m square shape (Figure 3). This design allowed the structure to be lowered in case of cyclonic conditions.
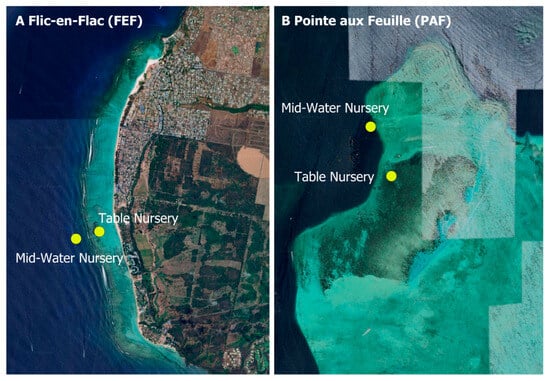
Figure 2.
(A) Location of mid-water and table nurseries at Flic-en-Flac, overlaid on the lagoon topography. (B) Corresponding nursery placements at Pointe aux Feuilles. Locations were identified via GPS and mapped in Google Earth Pro, with figures compiled using PowerPoint.

Figure 3.
(A–C) Construction and underwater deployment of table nurseries made of galvanized iron bars. (D–F) Placement of spider frames supporting coral fragments. (G,H) Deployment and buoying of mid-water nurseries using PVC pipes and ropes. (I–K) Coral fragments attached to mid-water nurseries with evidence of fish recruitment and tunicate colonization. Photographs were taken during field operations between 2019 and 2021 and reflect real-time nursery management practices.
Corals of opportunity (Millepora sp., Acropora muricata, Acropora selago, and Pocillopora damicornis) were collected from each site, fragmented into 2–2.5 cm pieces, and then placed back at the same site. A total of 4500 fragments of each coral species were placed on the table nurseries using a 6 mm opened rope to secure the coral fragments, while 1500 fragments of each species were placed on the floating nurseries using plastic pins (glued with cyanoacrylate) and also by directly fixing the corals to 0.5 mm square-shaped plastic grids.
The sites were monitored for coral growth rates, survivorship, and biodiversity in the nurseries twice per week during the first month. Thereafter, monitoring was conducted three times per month using snorkeling and scuba diving techniques. For comparison, the natural reef sites surrounding the nurseries were also monitored for diversity of corals and fish. The study period spanned from 2019 to 2021, except during the COVID-19 lockdown periods that lasted from 19 March 2020 till 31 May 2020 and March 2021 till 30 April 2021. To ensure maximum coral survivorship and create a stress-free environment for growth, macroalgae and other corallivores were regularly removed (Figure 3I). During these monitoring sessions, the fish species at each nursery were observed and recorded. Fish species were categorized as omnivorous, benthivorous, carnivorous, herbivorous, planktivorous, piscivorous, or coralivorous. Additionally, any damage to the nurseries was recorded, along with its cause and the mitigation strategies applied. This information provided valuable insights for improving nursery maintenance and informing future coral restoration efforts. The damage was mostly to the floating type nurseries, where the ropes had to be replaced more frequently (at least every 6 months) because of wear and tear. A total of five times, we lowered the floating nursery due to high waves. No damage occurred to the PVC pipes during the study period.
2.4. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics version 23.0. Normality of data was tested using the Shapiro–Wilk test. Since most environmental variables were not normally distributed (p < 0.05), non-parametric tests such as the Kruskal–Wallis test were used to evaluate differences among sites and nursery types. ANOVA and Tukey’s HSD post-hoc tests were applied where assumptions of normality and homogeneity of variance were met, particularly for comparison of coral abundance and growth. Statistical significance was set at p < 0.05. The benthic community structure and coral substrate coverage at each station were calculated based on the mean percentage cover of each benthic category within the three transects. Finally, the overall mean coral cover for Flic-en-Flac (FEF) and Pointe aux Feuilles (PAF) was calculated by averaging the values from the five stations at each site.
3. Results
3.1. Site Characteristics
3.1.1. Environmental Parameters
Water quality parameters are critical for the survival of corals and fish in an environment. During the study period, the water quality parameters were found to not be normally distributed (Shapiro–Wilk, p < 0.05). Analysis via Kruskal–Wallis testing showed the presence of significant differences (p < 0.05) in all water quality parameters between the different sampling sites, except for temperature (Table 2). Although statistical significance (p < 0.05) was found for several water parameters, the overall ecological differences across nursery types and sites were minor. These results, while statistically notable, may not reflect strong biological variation.

Table 2.
Results of Kruskal–Wallis non-parametric tests comparing environmental parameters (temperature, salinity, dissolved oxygen, total suspended solids, pH, nitrate, phosphate, and phytoplankton density) across the four nursery environments: FEF–TN, FEF–FN, PAF–TN, and PAF–FN. Data were collected quarterly over a 3-year period (2019–2021).
The table nursery at PAF recorded the highest mean TSS (12.01 ± 1.84 mg/L) and phytoplankton density (70.67 × 105 ± 14.52 × 105) but also the lowest mean DO levels (5.81 ± 0.71 mg/L), whereas the table nursery at FEF showed the highest mean temperature (27.81 ± 2.40 °C), nitrate (3.28 ± 0.51 mg/L), and phosphate values (0.08 ± 0.01 mg/L) with lowest mean pH (7.87 ± 0.32). The mid-water nursery at PAF observed the highest mean salinity (36.01 ± 0.21%) and pH values (8.10 ± 0.01) and the lowest temperature (26.49 ± 2.37 °C), nitrate (1.19 ± 0.59 mg/L) and phosphate (0.04 ± 0.01 mg/L) ranges. Results are presented as site-level averages for each nursery type (TN and FN), derived from multiple stations within each site. A summary of all values is shown in Table 3.

Table 3.
Site-level summary of water quality parameters for table nurseries (TN) and floating nurseries (FN) at Pointe aux Feuilles (PAF) and Flic-en-Flac (FEF), based on data from multiple stations per site. Values are reported as mean ± standard deviation, with minimum and maximum ranges from the 2019–2021 monitoring period.
3.1.2. Percentages of Coral, Algal, Rubble, and Sand Coverage
At both FEF and PAF sites, sand constituted the dominant benthic substrate, with coverage reaching up to 95.3%. Coral cover was notably higher at PAF, ranging from 6.3% to 32.7%, in contrast to FEF, where it varied between 0% and 10.2%. At PAF, coral cover also exceeded the percentage of rubble and algal cover. Algal coverage was relatively similar at both sites, ranging from 2.2% to 12.4%. In contrast, rubble cover was more pronounced at FEF, with values reaching up to 24.8%.
3.1.3. Coral Biodiversity
Local coral populations (at the family level) at PAF and FEF (Figure 4; Table 4) included Acroporidae (1700 and 146 at PAF and FEF, respectively), Poritidae (800 and 18 at PAF and FEF, respectively), Merulinidae (600 and 28 at PAF and FEF, respectively), Faviidae (500 and 21 at PAF and FEF, respectively), and Pocilloporidae (450 and 30 at PAF and FEF, respectively) as the most common corals. The combined coverage of corals at each of the two sites was 5813. ANOVA revealed a significant difference in coral abundance between the two sites within each locality, considering the variations across the five stations (F = 2.145, p = 0.001 < 0.05). Although diversity was assessed per station, comparisons were made between sites using station means as replicates. Mean values are presented separately for PAF and FEF to allow site-specific comparisons (Table 4).
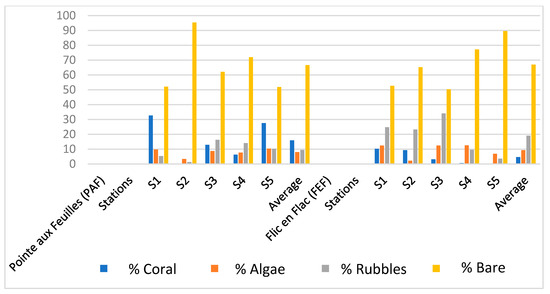
Figure 4.
Chart showing the mean percentage cover of live coral, algae, rubble, and sand at Pointe aux Feuilles (PAF) and Flic-en-Flac (FEF). Data were collected using the line-intercept transect (LIT) method, with three 20 m transects per station, across five stations per site. Percent cover values were averaged across all transects and stations, and comparisons were made at the site level.

Table 4.
Number of coral individuals recorded per family at Pointe aux Feuilles (PAF) and Flic-en-Flac (FEF), with corresponding mean values, standard deviations, total counts, and percentage representation for each family. Data were collected during benthic surveys using the line-intercept transect (LIT) method across five stations per site.
3.1.4. Fish Species and Feeding Habits
The number of fish species at both sites varied substantially between PAP and FEF (Figure 5), along with the feeding habits of the prevalent species (Figure 6). At FEF, omnivorous (27.9%) fish were more common, followed by benthivorous (25%), carnivorous (14.4%), herbivorous (12.5%), planktivorous (9.6%), piscivorous (7.7%), and lastly, coralivorous (2.9%). However, at PAF, carnivorous fish represented nearly half of the population (47.03%), followed by omnivorous (17.4%), planktivorous (10.5%), and benthivorous (10.01%), and the remainder were herbivorous, piscivorous, or coralivorous.
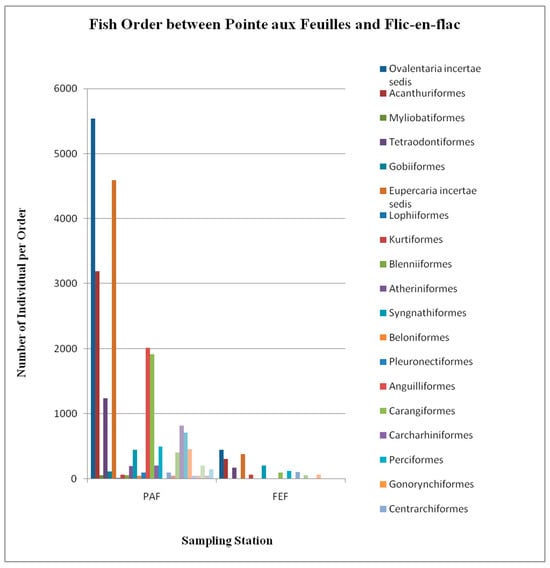
Figure 5.
Number of individual fish observed per family at Pointe aux Feuilles (PAF) and Flic-en-Flac (FEF) during the study period.
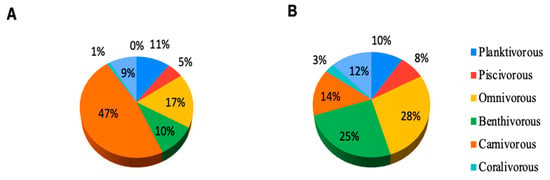
Figure 6.
Feeding group composition of fish species at (A) Pointe aux Feuilles and (B) Flic-en-Flac. Fish were classified into trophic categories (e.g., herbivorous, planktivorous, carnivorous) based on published ecological data and field observations across both sites.
3.1.5. Fish and Coral Diversity Indices
Considering diversity holistically (Table 5), the diversity differed substantially between the two sites. PAF had almost double the number of species of corals (110) and fishes (218) compared with FEF and a higher overall number of individual corals (5500) and fish (23,304). The Shannon–Weiner diversity index also showed higher values for both at PAF compared with FEF (4.7 and 4.63, respectively). However, Simpson’s diversity index showed values closer to 1 for corals (0.9911) and fish (0.9859) at PAF, while values at FEF (0.9855 and 0.9657) were also very close to 1. A value closer to 1 indicates high diversity and even distribution of species. Both PAF and FEF exhibited high Simpson’s index values, indicating an even distribution of species at both locations. While PAF had a greater number of species and individuals, the similarity in Simpson’s index values suggests that species dominance was not strongly skewed in either site, meaning no single species was overwhelmingly dominant. Although PAF maintained slightly higher diversity and evenness than FEF, the latter also demonstrated considerable biodiversity. Overall, despite differences in species richness and abundance, both sites support a high and fairly balanced level of biodiversity.

Table 5.
Diversity indices for corals and fish communities at Pointe aux Feuilles (PAF) and Flic-en-Flac (FEF). Indices include species richness (S), total individuals (N), Margalef index (D), Pielou’s evenness (J’), Shannon–Weiner diversity (H’), and Simpson’s diversity (λ’). Data were compiled from field surveys conducted at five stations per site between 2019 and 2021.
3.2. Coral Fragments Survival
After three years, >98% inclusive survival was recorded for the coral fragments of the four branching species at the PAF site, compared with ~88% at the FEF site (Table 6). The detachment rates during this period were higher at FEF, ranging between 4.9% to 13% (highest for Millepora sp. in the mid-water nursery; 13%), while detachment rates at PAF ranged between 0.2% to 1.7% (highest for Acropora muricata and Acropora selago in the mid-water nursery; 1.7%). Species detachment rates were consistently higher for Millepora sp. at both sites. Millepora sp. generally had higher survival rates (above 90%) and A. muricata had a higher percentage of detachment (above 0.4%). Detachment rates for all species at PAF remained relatively stable but increased towards the end as the corals grew larger. In contrast, at FEF, the detachment of coral fragments was observed earlier during the study period. No significant differences were observed in the survival and detachment rates between the coral species at the two sites.

Table 6.
Survival and detachment rates of each coral species across the two nursery types (table and floating) at both sites. Coral fragments were monitored monthly over three years (2019–2021). Survival (%) and detachment (%) were calculated from initial fragment numbers, with values presented per site and nursery type.
3.3. Coral Growth
The growth rates of coral in all nursery types are summarized in Figure 7. At PAF, all four coral species demonstrated significantly greater increases in length, width, and surface area in both nurseries compared with the measurements recorded at FEF over the study period. For instance, at PAF, Millepora sp. had the highest mean values for its length (27.25 ± 25.78 cm), width (25.16 ± 24.82 cm), and surface area (280.26 ± 248.52 cm2) in the table nursery, whereas at FEF, the same species recorded much lower mean values for length (3.64 ± 1.63 cm) and width (3.32 ± 1.78 cm) in the table nursery. Regarding surface area, Millepora sp. exhibited the lowest mean value (26.68 ± 13.19 cm2) in the mid-water nursery at FEF. The highest recorded values for Millepora sp. were 68.01 cm (length), 61.23 cm (width), and 675.12 cm2 (area).
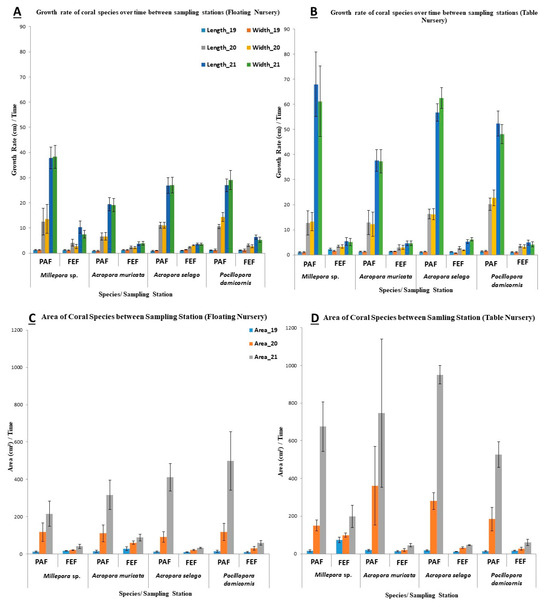
Figure 7.
Growth of the 4 coral species (Millepora sp., Acropora muricata, Acropora selago, and Pocillopora damicornis) at both nursery types (floating mid-water nurseries and bottom-attached table nurseries) from 2019 to 2021. (A) Mean length and width in floating nurseries; (B) mean length and width in table nurseries; (C) surface area in floating nurseries; (D) surface area in table nurseries. Coral growth was measured quarterly using calipers and photographic image analysis. Values represent mean ± standard deviation from three replicate nursery frames per species per site.
At PAF, Millepora sp. exhibited greater growth in area in the table nursery (280.26 ± 248.52 cm2) compared with the mid-water nursery (115.65 ± 101.30 cm2). Conversely, at FEF, Millepora sp. exhibited better growth in length and width in the mid-water nursery (5.22 ± 4.63 cm and 3.75 ± 3.35 cm, respectively) than in the table nursery (3.64 ± 1.63 cm and 3.32 ± 1.78 cm, respectively).
Within the individual sites, Acropora muricata at PAF showed better growth in the table nursery (376.14 ± 364.33 cm2), while at FEF, the species attained a larger area in the mid-water nursery (59.45 ± 30.11 cm2) compared with the table nursery (26.26 ± 16.91 cm2). This trend was consistent for other species as well. At PAF, Acropora selago achieved the highest mean values for length (24.71 ± 18.73 cm), width (26.64 ± 21.93 cm), and area (415.93 ± 381.18 cm2) in the table nursery, while the lowest mean values were observed at FEF in the table nursery.
For Pocillopora damicornis, the best growth was observed at the table nursery of PAF with a length of 24.60 ± 15.75 cm, width of 24.11 ± 23.37 cm, and area 241.46 ± 160.20 cm2.
Overall, the table nursery at PAF recorded the highest mean and maximum values for the length, width, and area of all species. Among the coral species, Millepora sp. exhibited the greatest mean length (27.25 ± 25.78 cm), while Acropora selago had the greatest mean width (26.64 ± 21.93 cm).
3.4. Linear Extension Rate
The mean linear extension rate (LER) of each coral species across different sites and nursery types differed (Table 7). Among all observations, Millepora sp. at PAF in the table nursery exhibited the highest mean LER (9.66 ± 3.01 mm y−1), whereas Acropora muricata in the mid-water nursery at FEF had the lowest mean LER (1.84 ± 1.00 mm y−1). Site-wise comparisons revealed that PAF consistently had the highest mean LER values for all studied species (Figure 8). For example, Pocillopora damicornis achieved a mean LER of 9.17 ± 2.29 mm y−1 in table nurseries at PAF, compared with 2.86 ± 1.07 mm y−1 at FEF.

Table 7.
Mean linear extension rates (LER, mm y−1) for each coral species across nursery types and study sites. Coral length measurements were taken quarterly over the 3-year period and used to calculate LER.
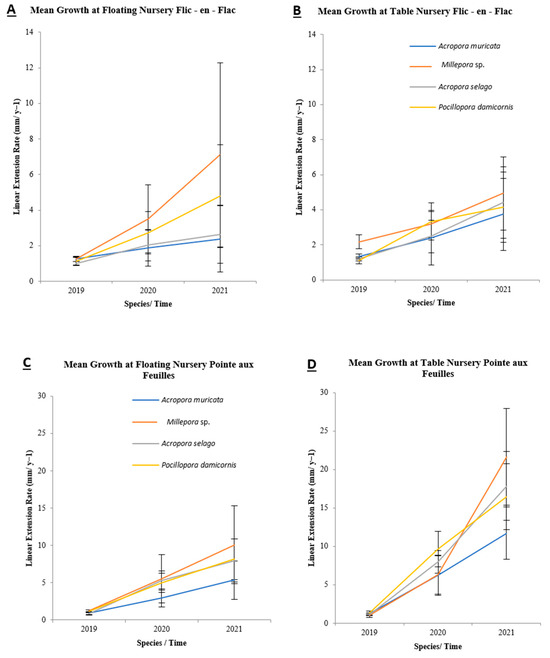
Figure 8.
Mean LER (LER, mm y−1) of coral species grown in (A,B) Flic-en-Flac and (C,D) Pointe aux Feuilles, comparing floating and table nursery types. LER was calculated from coral length differences over time, measured quarterly. Data are presented as mean ± SD per species.
Statistical analysis (analysis of variance) indicated significant differences in mean LER among species, sites, and nursery types (p < 0.05). However, these differences were statistically significant only for data collected during 2020 and 2021 (p = 0.00). Further analysis using the Tukey HSD test identified specific differences between sites and nursery types. In all cases, significant differences were observed, with p-values less than 0.05, highlighting the influence of both site and nursery types on the linear extension rate of coral growth.
4. Discussion
Mauritius, a small developing island nation, is heavily dependent on its coral reefs, which play a critical role in supporting marine biodiversity by providing habitats for species such as crustaceans and fish. These reefs also underpin key economic sectors, including fisheries and tourism, by sustaining fish stocks and protecting beaches and marine attractions [24]. However, over the years, anthropogenic pressures, including infrastructure development and urbanization, have led to significant coral cover loss on Mauritian reefs [25]. Consequently, coral restoration has become a pressing priority for Mauritius, driving increased research into coral farming techniques [24,26]. Selecting an appropriate restoration method tailored to Mauritius’ unique marine environment is crucial to ensuring successful restoration efforts.
This study compared the growth performance of four coral species between a healthy lagoon (Pointe aux Feuilles) and a lagoon heavily impacted by severe soil erosion and tourism pressure (Flic-en-Flac). Additionally, it evaluated the efficacy of two nursery types, table and mid-water, at both sites to identify the most suitable method for rearing coral fragments. Results indicated that Flic-en-Flac’s lagoon exhibited relatively low coral cover (<10%) and a high proportion of dead corals. Coral cover was higher at PAF compared with FEF, whereas algal cover was comparatively lower at PAF. This pattern may indicate effective algal regulation by herbivorous fish at PAF, potentially mitigating coral mortality [27]. Similarly, Smith et al. (2022) [28] demonstrated enhanced coral growth following the manual removal of macroalgae, further indicating the importance of algal control in promoting coral health. The genus Acropora had the highest percentage of dead corals (17.4%), whereas Porites showed significantly lower mortality (0.03%). These findings align with previous studies highlighting that some coral taxa are more resilient to environmental stress than others [29]. Fast-growing, branching taxa such as Acropora spp. are generally more vulnerable to bleaching events compared with slow-growing, massive taxa like Porites spp. [30].
Survivorship is a key indicator of success in coral farming. Across both sites and nursery types, the survival rates of all four coral species remained consistently high (88–99.8%) throughout the study period. Similar outcomes have been reported in other regions; for instance, in the Caribbean, Acropora cervicornis exhibited survival rates of 85–96% after 12 months in an in-situ nursery setting [31]. In the Red Sea, coral survivors reached 85–95% (species specific) after one year in a mid-water floating nursery. At the Great Barrier Reef, survival rates for Acropora spp. exceeded 80%, although they were slightly lower than those observed in the present study. In China, Liu et al. (2024) [32] reported coral survivorship that ranged up to 86–90% after 400 days, depending on the species of interest. In the Maldives, corals survivorship was above 90% for all species, with 89% for Acropora spp. [33]. In Singapore, following 380 days of coral farming, Acropora spp. had the highest survivorship at 31–83%, much lower than in the current study. Variations in survival rates may result from species-specific or site-specific factors [34]. Among the species studied, Acropora muricata exhibited the highest detachment rates, consistent with findings from previous studies, highlighting that branching coral species are more prone to detachment due to adhesive failures. Despite the high survivorship rates, lower survivorship was generally recorded at Flic-en-Flac (FEF) compared with Pointe aux Feuilles (PAF), coupled with higher detachment rates at FEF. This could be attributed to increased sedimentation at FEF, probably due to nearby infrastructure, as well as more frequent human interaction given its popularity as a tourist site.
While the present study monitored key parameters such as total suspended solids (TSS), nitrate, and phosphate, which are indicative of sedimentation and nutrient enrichment, a more direct quantification of anthropogenic stressors including continuous sedimentation rate monitoring, land-based nutrient source mapping, and water turbidity sensors would further confirm the causal links between human activities and coral nursery performance. Future studies should consider deploying in situ sensors and collaborating with environmental authorities to integrate long-term datasets for improved interpretation of anthropogenic impact, particularly at high-pressure sites such as FEF.
Coral growth is a critical metric for evaluating the success of coral farming [35]. Larger corals are less susceptible to mortality risks once they reach a certain size [36]. Species-wise, Millepora sp. recorded the highest mean length, while Acropora selago achieved the greatest width and coral cover. These differences are likely to have been due to variations in the morphological and physiological characteristics of each species. Conversely, Kotb (2016) [37] found no statistical differences in growth rates among the species he used, including those used in this study (Acropora spp., Millepora spp., and P. damicornis). Notably, similar to our results, P. damicornis exhibited higher growth rates than Acropora spp. in Kotb’s study. This implies that planning with consideration to the coral species to be used and selection of site is essential before coral farming.
Selecting an appropriate location for a coral nursery is crucial to ensure optimal growth of coral fragments. This study compared two regions, the highly human-impacted lagoonal area of Flic-en-Flac (FEF), and the more pristine but nutrient enriched area of Pointe aux Feuilles (PAF), located near a fish farm and less influenced by tourism. According to Tukey’s test, coral growth was significantly different between the two regions, with lower growth observed at FEF compared with PAF. This disparity may be attributed to sedimentation from infrastructure and increased turbidity at FEF. However, this finding contrasts with the results of Villanueva et al. (2006) [38], who reported that coral growth, specifically that of P. damicornis, could be adversely affected by fish farm effluent. Additionally, the higher phytoplankton density observed at PAF may have supported growth of coral fragments at that site.
The three-year monitoring period encompassed multiple seasonal cycles, allowing observations of fluctuations in temperature, salinity, and nutrient availability across monsoonal and dry seasons. While our quarterly sampling schedule captured broad seasonal trends, more granular insights into specific bleaching season impacts would benefit from monthly or continuous environmental monitoring. Temperature peaks observed in summer at both sites coincided with lower dissolved oxygen and elevated nutrient levels, potentially influencing coral performance metrics.
Data gaps caused by COVID-19 lockdowns (March–May 2020, March–April 2021) resulted in reduced temporal resolution for some parameters. Although these gaps are unlikely to have altered the overall trends identified, they may have masked short-term responses to acute stress events such as heatwaves. Future studies may benefit from applying imputation techniques or leveraging satellite data and autonomous loggers to bridge such gaps.
Differences in fish diversity between the sites may also have played a role. Herbivorous fish, such as parrotfish, are essential for maintaining healthy corals by grazing algae, which helps keep the reef and nursery settings clean [39,40]. However, accidental grazing by these fish can result in coral fragment detachment [17,41].
This study assessed the effectiveness of two nursery types for coral farming, considering site-specific conditions. At PAF, coral growth rates were higher in table nurseries compared with mid-water nurseries. Conversely, at FEF, mid-water nurseries yielded higher coral growth than table nurseries, further emphasizing the importance of environmental factors and nursery design [42,43]. The literature further reveals that mid-water nurseries, which swing in open blue waters and are moved by the water currents, can reduce the accumulation of sediment on coral fragments, leading to enhanced growth. This mobility helps prevent sediment from settling on corals, a common issue in stationary table nurseries, especially in areas with high sedimentation [16,17]. Studies also revealed that inserting bottom-attached nurseries at least 30 cm from the substrate increased resilience against impacts caused by macroalgae, turf algae, and sedimentation rates [32], with similar implications for recruited herbivores and corallivores. In this study, FEF was host to a higher presence of coralivores and herbivores, which may have disproportionately impacted the table nurseries, leading to better growth in the mid-water nurseries. Additionally, the high sedimentation at FEF is likely to have affected table nurseries more severely as sediments accumulated on stationary structures, whereas mid-water nurseries were less impacted.
This study primarily focused on ecological and environmental metrics (e.g., survivorship, growth, water quality parameters) to evaluate nursery performance. However, integrating molecular and physiological assessments such as profiling Symbiodiniaceae communities, measuring oxidative stress markers, or analyzing gene expression could yield critical mechanistic insights into coral stress tolerance and acclimatization capacity. These approaches were beyond the scope of the present study due to logistical and budgetary constraints but represent a valuable direction for future research. Such data could further clarify the physiological underpinnings behind species- or site-specific performance differences observed in coral nurseries.
Environmental parameters significantly influence coral growth rates [43,44,45]. In this study, total suspended solids (TSS) were lower around the mid-water nurseries at both sites (PAF and FEF). While coral growth was greater at PAF, this site had lower dissolved oxygen (DO) levels and higher TSS values compared to FEF. The healthier coral ecosystem and associated biodiversity at PAF may have contributed to accumulation of organic matter, including waste generation, thereby increasing TSS. The decomposition of such organic matter, along with the activity of faecal bacteria and other organisms, consumes oxygen, resulting in lower DO levels at PAF [46]. In contrast, the continuous water flow around mid-water nurseries enhances oxygen circulation, leading to higher DO levels near coral tissues. This efficient oxygen supply supports coral fragment growth, as observed at FEF, where mid-water nurseries performed better in terms of growth compared with table nurseries.
High survivorship rates were obtained for all species at both sites and on both nursery types; however, table nurseries at PAF emerged as a more favorable option for optimum coral growth. In contrast, the high levels of infrastructure development and sedimentation at FEF hindered growth, particularly in table nurseries. Therefore, while both sites are viable for coral farming, selecting nursery types and locations must account for local environmental conditions to maximize success.
Based on the observed differences in performance, we propose a site-specific nursery design framework. In high-sedimentation, high-tourism zones such as FEF, mid-water floating nurseries are more suitable due to reduced sedimentation on coral fragments and lower detachment rates. Conversely, in more sheltered environments like PAF, table nurseries promote better coral growth and are easier to maintain. Selection should therefore consider site-level environmental stressors, hydrodynamics, and the intensity of human activity. Future restoration efforts should incorporate preliminary site assessments (e.g., TSS levels, DO, fish community structure) when deciding on nursery types. Moreover, incorporating cost-effectiveness metrics such as material durability, maintenance frequency, and ease of outplanting will help refine nursery design choices and optimize resource allocation.
5. Conclusions
This study demonstrates the potential of coral farming as a viable method for coral restoration in Mauritius, highlighting the importance of site-specific nursery types and species selection. The high survivorship rates observed across all coral species at both Pointe aux Feuilles (PAF) and Flic-en-Flac (FEF) emphasize the resilience of corals within artificial nursery settings, despite contrasting environmental conditions. PAF, with its healthier ecosystem and higher phytoplankton density, proved to be more conducive to coral growth, particularly in table nurseries. Conversely, the high sedimentation and human pressures at FEF impacted growth, making mid-water nurseries more effective in this region.
The findings underscore the critical role of local environmental factors such as water quality, sedimentation, and fish diversity on the success of coral farming efforts. Future coral restoration projects in Mauritius and similar regions should prioritize careful site selection, incorporating both environmental and infrastructural considerations to ensure optimal growth conditions for coral species. Additionally, future research should explore the long-term success of coral farming in these environments, including post-restoration monitoring to assess the persistence of restoration outcomes and the recovery of surrounding ecosystems.
Further studies should investigate the interaction between coral growth and various environmental stressors, such as sedimentation, nutrient availability, and climate change impacts, to refine restoration methodologies. Additionally, exploring the use of innovative nursery structures, such as integrated multi-trophic aquaculture systems, could enhance coral farming success by providing additional ecosystem services while promoting conservation of biodiversity.
In conclusion, while both floating nurseries and table nurseries are efficient methods for coral farming, their efficiency may vary depending on the site selected. Continued research and adaptation of restoration strategies to local conditions will be essential to ensuring the long-term sustainability of coral reefs in Mauritius and beyond.
This study suggests a practical decision-making framework for coral nursery placement based on local environmental factors. Mid-water nurseries are recommended for dynamic, high-sediment environments like FEF, while table nurseries are better suited for calm, nutrient-rich, and biodiverse areas like PAF. Incorporating cost-benefit analyses and environmental diagnostics in site-selection protocols will be critical to scaling-up effective coral restoration in island nations.
Author Contributions
N.N.: Conceptualization, methodology, formal analysis, investigation, resources, writing—original draft writing, project administration. A.W.M.P.: Conceptualization, methodology, formal analysis, investigation, review. R.J.L.: Conceptualization, methodology, validation, investigation, review. I.N.: Review and editing. B.R.: Conceptualization, methodology, validation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) declare financial support was received for the research. This research work was funded by the Higher Education Commission (TEC/11/4/13/8).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors wish to thank the Ministry of Blue Economy, Marine Resources, Fisheries and Shipping of the Republic of Mauritius for granting the research authorization (Permit Ref: F/20/2) necessary for conducting coral reef surveys and restoration activities. We are also grateful to the various local communities, including fishermen, who helped in with maintenance. We sincerely thank all contributors, colleagues, and anonymous reviewers for their valuable input, which has helped improve the quality of this manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hein, M.; Banaszack, A.; Dallison, T.; Deri, W.; Grimsditch, G.; Jacob, F.; Loder, J.; McLeod, I.; Mead, D.; Moore, T.; et al. Mapping the Global Funding Landscape for Coral Reef Restoration. 2021, pp. 1–23. Available online: https://researchonline.jcu.edu.au/70966/ (accessed on 12 March 2025).
- UNDP. Coral reefs and their importance for the island economies. UNDP Mauritius & Seychelles Blog, 6 June 2021. Available online: https://www.mu.undp.org (accessed on 13 April 2022).
- Bindoff, N.L.; Cheung, W.W.; Kairo, J.G.; Arstegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Chapter 5: Changing ocean, marine ecosystems, and dependent communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Rinkevich, B. Management of coral reefs: We have gone wrong when neglecting active reef restoration. Mar. Pollut. Bull. 2008, 56, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.F.; Côté, I.M.; Toth, L.T. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Annu. Rev. Mar. Sci. 2019, 11, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Kleypas, J.A.; Allemand, D.; Anthony, K.; Baker, A.C.; Beck, M.; Hale, L.Z.; Hilme, N.; Hoegh-Guldberg, O.; Hughes, T.; Kaufman, L.; et al. Designing a blueprint for coral reef survival. Biol. Conserv. 2021, 257, 109107. [Google Scholar] [CrossRef]
- Vardi, T.; Hoot, W.C.; Levy, J.; Shaver, E.; Winters, R.S.; Banasza, A.T.; Baums, I.B.; Chamberland, V.F.; Cook, N.; Gulko, D.; et al. Six priorities to advance the science and practice of coral reef restoration worldwide. Restor. Ecol. 2021, 29, e13498. [Google Scholar] [CrossRef]
- Harris, D.L.; Rovere, A.; Casella, E.; Power, H.; Canavesio, R.; Collin, A.; Pomeroy, A.; Webster, J.M.; Parravicini, V. Coral reef structural complexity provides important coastal protection from waves under rising sea levels. Sci. Adv. 2018, 4, eaao4350. [Google Scholar] [CrossRef]
- Masselink, G.; Beetham, E.; Kench, P. Coral reef islands can accrete vertically in response to sea level rise. Sci. Adv. 2020, 6, eaay3656. [Google Scholar] [CrossRef]
- Majewska, J. The Ocean Economy in SIDS—Environmental Taxation. Ekon. XXI Wieku 2024, 27. Available online: https://journals.ue.wroc.pl/e21/article/view/1332 (accessed on 14 November 2024). [CrossRef]
- Golomb, D.; Shashar, N.; Rinkevich, B. Coral carpets- a novel ecological engineering tool aimed at constructing coral communities on soft sand bottoms. Ecol. Eng. 2020, 145, 105743. [Google Scholar] [CrossRef]
- Bozec, Y.M.; Yakob, L.; Bejarano, S.; Mumby, P.J. Reciprocal facilitation and non-linearity maintain habitat engineering on coral reefs. Oikos 2013, 122, 428–440. [Google Scholar] [CrossRef]
- Rinkevich, B. Rebuilding coral reefs: Does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sustain. 2014, 7, 28–36. [Google Scholar] [CrossRef]
- Rinkevich, B. Climate change and active reef restoration ways of constructing the ‘reefs of tomorrow’. J. Mar. Sci. Eng. 2015, 3, 111–127. [Google Scholar] [CrossRef]
- Rinkevich, B. The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. J. Mar. Sci. Eng. 2019, 7, 201. [Google Scholar] [CrossRef]
- Rinkevich, B. The coral gardening concept and the use of underwater nurseries; lessons learned from silvics and silviculture. In Coral Reef Restoration Handbook; Precht, W.F., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 291–301. [Google Scholar]
- Frias-Torres, S.; Goehlich, H.; Reveret, C.; Montoya-Maya, P.H. Reef fishes recruited at midwater coral nurseries consume biofouling and reduce cleaning time in Seychelles, Indian Ocean. Afr. J. Mar. Sci. 2015, 37, 421–426. [Google Scholar] [CrossRef]
- Dale, C.; Antoine, A.; Strona, G.; Bell, M.; Shah, N.; Saponari, L. Enhancing coral restoration practices in Seychelles: Benefits and limitations of fishing lines and rope as coral stocking methods. Restor. Ecol. 2024, 32, e14252. [Google Scholar] [CrossRef]
- Shaish, L.; Levi, G.; Katzir, G.; Rinkevich, B. Coral reef restoration (Bolinao, the Philippines) in the face of frequent natural catastrophes. Restor. Ecol. 2010, 18, 285–299. [Google Scholar] [CrossRef]
- Hamzah, S.N.; Nursinar, S. The success of coral rehabilitation through transplantation using spider modules (case study: Botutonuo marine area, Bone Bolango Regency). Aquac. Aquar. Conserv. Legis. 2021, 14, 3023–3031. [Google Scholar]
- Huawei. IUCN and Huawei Call for Greater Technology Adoption to Protect Nature. 6 June 2022. Available online: https://www.prnewswire.com/news-releases/iucn-and-huawei-call-for-greater-technology-adoption-to-protect-nature-301562325.html (accessed on 13 April 2024).
- Chineah, V.; Chooramun, V.; Nallee, M.; Rai, Y.B.; Pillay, R.M.; Jayabalan, N.; Terashima, H.; Terai, A. Status of the marine environment of the flic en Flac lagoon, Mauritius. In Proceedings of the Fifth Annual Meeting of Agricultural Scientists, April 2002; p. 219. Available online: https://www.researchgate.net/publication/237109340_STATUS_OF_THE_MARINE_ENVIRONMENT_OF_THE_FLIC_EN_FLAC_LAGOON_MAURITIUS (accessed on 12 March 2025).
- Leujak, W.; Ormond, R.F.G. Comparative accuracy and efficiency of six coral community survey methods. J. Exp. Mar. Biol. Ecol. 2007, 351, 168–187. [Google Scholar] [CrossRef]
- Nazurally, N.; Rinkevich, B. A Questionnaire-based Consideration of Coral Farming for Coastal Socio-economic Development in Mauritius. WIOMSA 2013, 12, 47–56. [Google Scholar]
- Elliott, J.A.; Patterson, M.R.; Staub, C.G.; Koonjul, M.; Elliott, S.M. Decline in coral cover and flattening of the reefs around Mauritius (1998–2010). PeerJ 2018, 6, e6014. [Google Scholar] [CrossRef]
- Pillay, R.M.; Gian, S.B.; Bhoyroo, V.; Curpen, S. Adapting Coral Culture to Climate Change: The Mauritian Experience. WIOMSA 2011, 10, 155–167. [Google Scholar]
- Tebbett, S.B.; Bennett, S.; Bellwood, D.R. A functional perspective on the meaning of the term ‘herbivore’: Patterns versus processes in coral reef fishes. Coral Reefs 2024, 43, 219–232. [Google Scholar] [CrossRef]
- Smith, H.A.; Brown, D.A.; Arjunwadkar, C.V.; Fulton, S.E.; Whitman, T.; Hermanto, B.; Mastroianni, E.; Mattocks, N.; Smith, A.K.; Harrison, P.L.; et al. Removal of macroalgae from degraded reefs enhances coral recruitment. Restor. Ecol. 2022, 30, e13624. [Google Scholar] [CrossRef]
- Williams, S.D.; Patterson, M.R. Resistance and robustness of the global coral–symbiont network. Ecology 2020, 101, e02990. [Google Scholar] [CrossRef]
- Marshall, P.A.; Baird, A.H. Bleaching of corals on the Great Barrier Reef: Differential susceptibilities among taxa. Coral Reefs 2000, 19, 155–163. [Google Scholar] [CrossRef]
- Schopmeyer, S.A.; Lirman, D.; Bartels, E.; Gilliam, D.S.; Goergen, E.A.; Griffin, S.P.; Johnson, M.E.; Lustic, C.; Maxwell, K.; Walter, C.S. Regional restoration benchmarks for Acropora cervicornis. Coral Reefs 2017, 36, 1047–1057. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, W.; Chen, R.; Rinkevich, B.; Shafir, S.; Xia, J.; Zhu, M.; Chen, R.; Wang, A.; Li, X. Framed Reef Modules: A new and cost-effective tool for coral restoration. Restor. Ecol. 2024, 32, e13997. [Google Scholar] [CrossRef]
- Dehnert, I.; Saponari, L.; Galli, P.; Montano, S. Comparing different farming habitats for mid-water rope nurseries to advance coral restoration efforts in the Maldives. PeerJ 2022, 10, e12874. [Google Scholar] [CrossRef]
- Howlett, L.; Camp, E.F.; Edmondson, J.; Henderson, N.; Suggett, D.J. Coral growth, survivorship and return-on-effort within nurseries at high-value sites on the Great Barrier Reef. PLoS ONE 2021, 16, e0244961. [Google Scholar] [CrossRef]
- Edwards, A.J. (Ed.) Reef Rehabilitation Manual; Coral Reef Targeted Research & Capacity Building for Management Program: St Lucia, Australia, 2010; p. 166. [Google Scholar]
- Highsmith, R.C. Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser. Oldendorf. 1982, 7, 207–226. [Google Scholar] [CrossRef]
- Kotb, M.M.A. Coral translocation and farming as mitigation and conservation measures for coastal development in the Red Sea: Aqaba case study, Jordan. Environ. Earth Sci. 2016, 75, 439. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Yap, H.T.; Montaño, M.N.E. Intensive fish farming in the Philippines is detrimental to the reef-building coral Pocillopora damicornis. Mar. Ecol. Prog. Ser. 2006, 316, 165–174. [Google Scholar] [CrossRef]
- Mumby, P.J.; Dahlgren, C.P.; Harborne, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science 2006, 311, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Knoester, E.G.; Murk, A.J.; Osinga, R. Benefits of herbivorous fish outweigh costs of corallivory in coral nurseries placed close to a Kenyan patch reef. Mar. Ecol. Prog. Ser. 2019, 611, 143–155. [Google Scholar] [CrossRef]
- Quimpo, T.J.; Cabaitan, P.C.; Hoey, A.S. Detachment of Porites cylindrica nubbins by herbivorous fishes. Restor. Ecol. 2020, 28, 418–426. [Google Scholar] [CrossRef]
- O’Donnell, K.E.; Lohr, K.E.; Bartels, E.; Patterson, J.T. Evaluation of staghorn coral (Acropora cervicornis, Lamarck 1816) production techniques in an ocean-based nursery with consideration of coral genotype. J. Exp. Mar. Biol. Ecol. 2017, 487, 53–58. [Google Scholar] [CrossRef]
- Migliaccio, O. Optimizing Coral Farming: A Comparative Analysis of Nursery Designs for Acropora aspera, Acropora muricata, and Montipora digitata in Anantara Lagoon, Maldives. Int. J. Mar. Sci. 2024, 14, 295–305. [Google Scholar] [CrossRef]
- Forsman, Z.H.; Kimokeo, B.K.; Bird, C.E.; Hunter, C.L.; Toonen, R.J. Coral farming: Effects of light, water motion and artificial foods. J. Mar. Biol. Assoc. UK 2012, 92, 721–729. [Google Scholar] [CrossRef]
- Doropoulos, C.; Gómez-Lemos, L.A.; Salee, K.; McLaughlin, M.J.; Tebben, J.; Van Koningsveld, M.; Feng, M.; Babcock, R.C. Limitations to coral recovery along an environmental stress gradient. Ecol. Appl. 2022, 32, e2558. [Google Scholar] [CrossRef]
- Edori, O.S. Physical and Chemical Characteristics of Water from Ede Onyima Creek, Okarki-Engenni, Rivers State, Nigeria. Chem. Res. J. 2020, 5, 144–154. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
