The Discovery and Delimitation of a New Cryptic Species of Spirinia (Nematoda: Desmodoridae) Using SSU and LSU rDNA Divergence †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Morphological Analysis
2.2. DNA Extraction and Amplification
2.3. Phylogenetic Analysis
3. Results
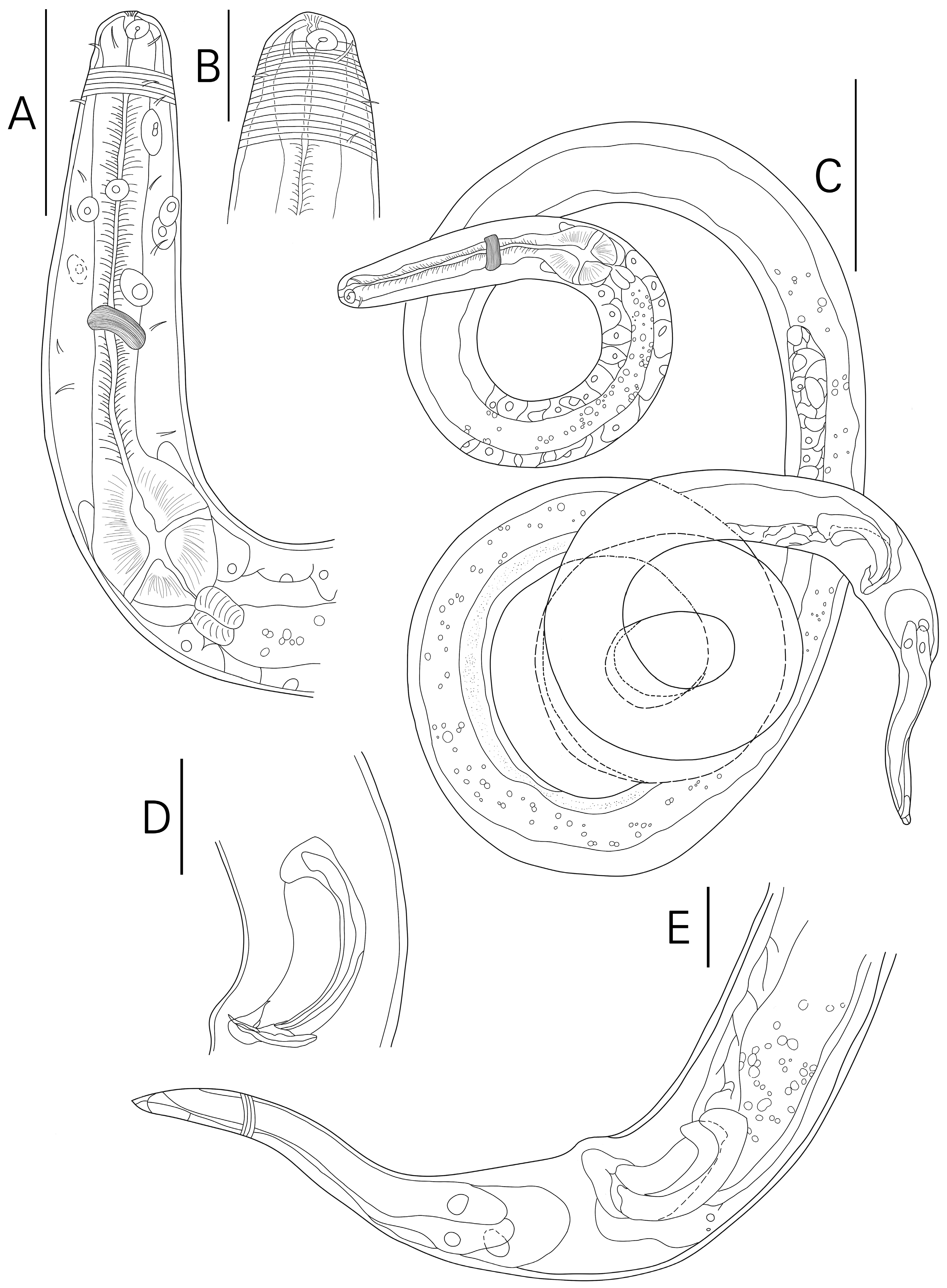
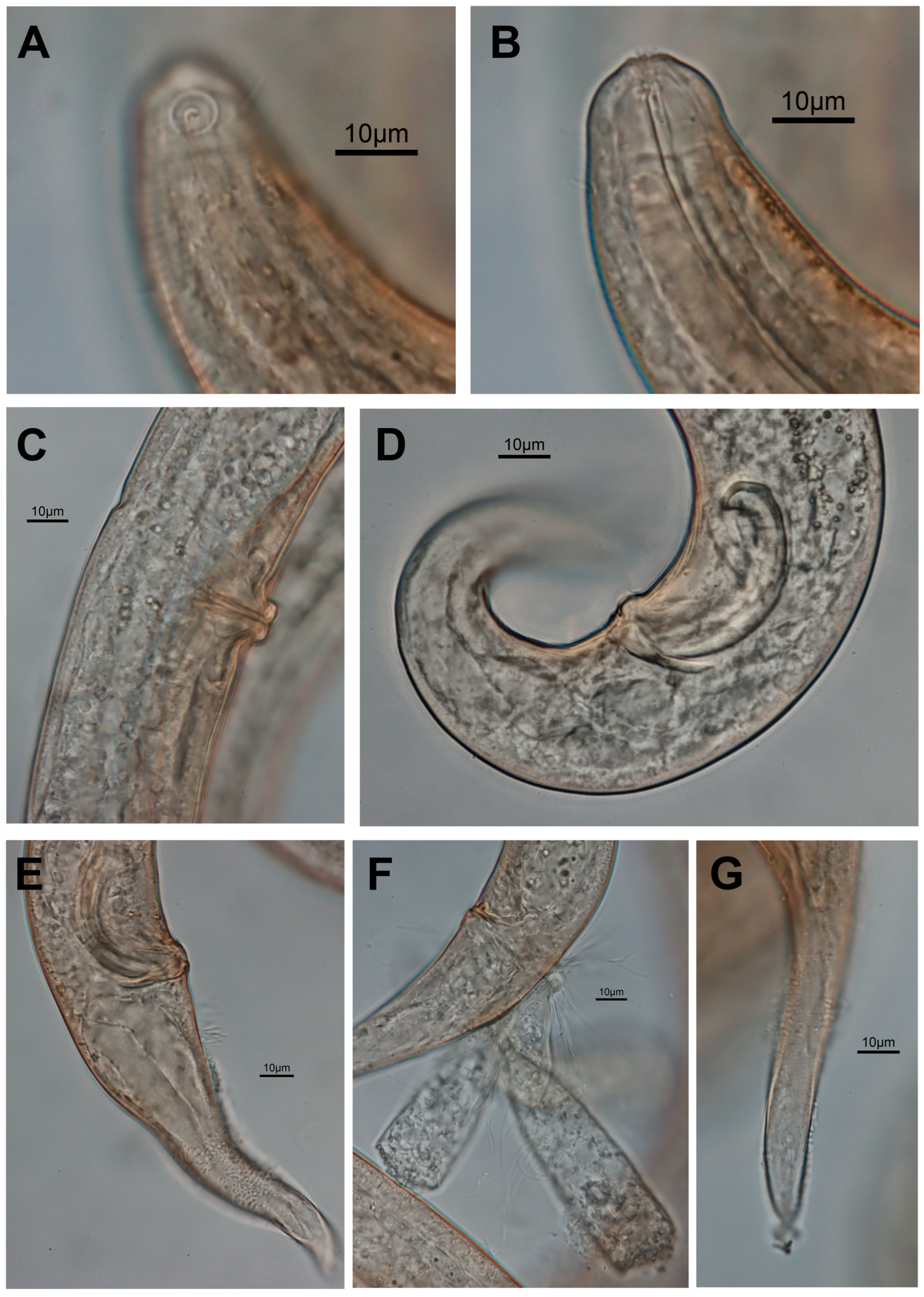
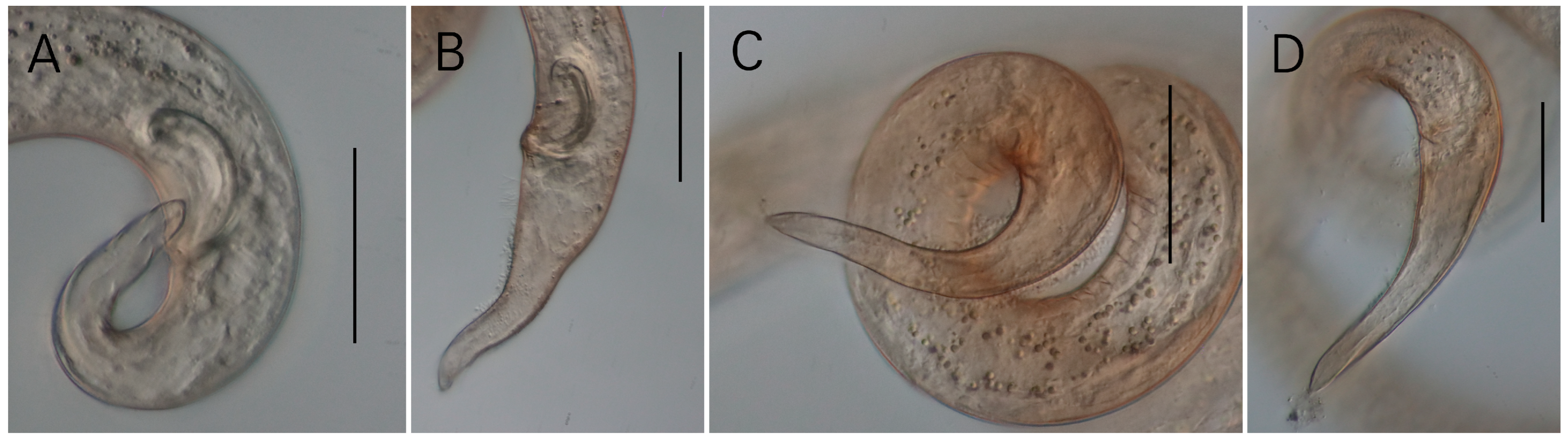
3.1. Morphological Analysis
- Systematics
- Diagnosis (updated from Leduc & Zhao 2019)
- List of valid species
- Spirinia antipodea Leduc, 2019 (Leduc & Zhao 2019: 91–105, Figures 1–3; New Zealand, Pauatahanui Inlet, North Island gravel sand, intertidal zone) [7].
- Spirinia gerlachi (Luc & De Coninck, 1959) Gerlach, 1963 (Luc & De Coninck 1959: 123–125, Figures 26–28; France, Roscoff Region, Coarse sand in a tidal pool behind Large gravel with Lithothamnium and Enteromorpha) [23].
- Spirinia gnaigeri Ott, 1977 (Ott 1977: 134–137, Figures 40–45; Bermuda, intertidal sand flat in Tuckers Town Cove) [24].
- Spirinia guanabarensis (Maria, Esteves, Smol, Vanreusel & Decraemer, 2009) Leduc & Verschelde, 2015 (Maria et al., 2009: 21–36, Figures 1 and 2; Brazil, Guanabara Bay, Bica Beach) [25].
- Spirinia hopperi Coles, 1987 (Coles 1987: 79–101, Figures 1c, 3c, 7b and 9b; England, Wembury Bay, South Devon) [2].
- Spirinia inaurita (Wieser & Hopper, 1967) Leduc & Verschelde, 2015 (Wieser & Hopper 1967: 273–274, Figure 37a–f; United States, Florida, Key Biscayne, bear cut area, flat around high-waterlevel, fine to medium sand and debris) [26].
- Spirinia laevioides Gerlach, 1963 (Gerlach 1963: 69–70, Tafel 2d–g; Maldives, Fadiffolu Atoll, Island shore of Dirudi) [5].
- Spirinia laevis (Bastian, 1865) Gerlach, 1963 (Bastian 1865: 160, Plate XIII Figures 204–206; United Kingdom, tidepools of Falmouth, Cornwall) [27].
- Spirinia okemwai (Muthumbi, Verschelde & Vincx, 1995) Leduc & Verschelde, 2015 (Muthumbi, Verschelde & Vincx 1995: 182–186, Figures 1A–H and 2A–G; Kenya, intertidal sediments of the Ceriops mangrove in Gazi Bay) [28].
- Spirinia parasitifera (Bastian, 1865) Gerlach, 1963 (Bastian 1865: 159, Plate XIII. Figures 201–203; England, Falmouth, amongst sand and small stones from tidepools) [27]
- Spirinia parma (Ott, 1972) Leduc & Verschelde, 2015 (Ott 1972: 481–483, Figures 85–96; United States, Wilmington, North Carolina, Wrightsville Beach) [29].
- Spirinia schneideri (Villot, 1875) Gerlach, 1963 (Villot 1875: 463–4644, Pl. XI, Figure 11a–c; France, Roscoff, Brittany) [30].
- Spirinia sophia Da Silva, De Castro, Da Fonseca Cavalcanti & Da Fonseca-Genevois, 2009 (Da Silva, De Castro, Da Fonseca Cavalcanti & Da Fonseca-Genevois 2009: 39–44, Figures 5–7; Brazil, Campos Basin, off the coast of Rio de Janeiro) [31].
- Spirinia verecunda Leduc & Verschelde, 2015 (Leduc & Verschelde 2015: 10–15, Figures 1–3; New Zealand, Chatham Rise) [32].
- Spirinia koreana Son & Jeong, 2025 sp. nov. (Son & Jeong, 2025: Republic of Korea, Yeongjongdo Island, sandy intertidal zone) (This study).
- Description
- Locality: collected from a sandy subtidal zone of Sunnyeobawi Beach (37.439457, 126.378285), Jung-gu, Incheon, Republic of Korea.
- Materials examined: Holotype (NIBRIV0000927233), allotype (NIBRIV0000927234), ten paratype males and eleven paratype females (NIBRIV0000927235–NIBRIV0000927240; multiple specimens on one slide, voucher number respective to slide) deposited to National Institute of Biological Resources (Republic of Korea). All specimens were collected on 28 March 2025.
- Description:
- Diagnosis and relationships:
3.2. Molecular Analysis
4. Discussion
4.1. Morphological and Molecular Differentiation of Spirinia koreana
4.2. Ambiguous Distinction Between Spirinia and Perspiria
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L | Total body length (µm) |
| a | Body length divided by maximum body diameter |
| b | Body length divided by pharynx length |
| c | Body length divided by tail length |
| c’ | Tail length divided by anal body diameter |
| cbd | Corresponding body diameter |
| spic | Spicule length as an arc |
References
- Louati, H.; Said, O.B.; Soltani, A.; Cravo-Laureau, C.; Duran, R.; Aissa, P.; Mahmoudi, E.; Pringault, O. Responses of a Free-Living Benthic Marine Nematode Community to Bioremediation of a Pah Mixture. Environ. Sci. Pollut. Res. Int. 2015, 22, 15307–15318. [Google Scholar] [CrossRef] [PubMed]
- Coles, J.W. Observations on the Marine Nematode Genus Spirinia Gerlach, 1963 (Desmodoridae: Spiriniinae) with Descriptions of Two New Species. Bull. Br. Mus. (Nat. Hist.) Zool. 1987, 53, 79–101. [Google Scholar]
- Venekey, V.; Fonseca-Genevois, V.G.; Santos, P.J.P. Biodiversity of Free-Living Marine Nematodes on the Coast of Brazil: A Review. Zootaxa 2010, 2568, 39–66. [Google Scholar] [CrossRef]
- Armenteros, M.; Ruiz-Abierno, A.; Decraemer, W. Revision of Desmodorinae and Spiriniinae (Nematoda: Desmodoridae) with Redescription of Eight Known Species. Eur. J. Taxon. 2014, 96, 1–32. [Google Scholar] [CrossRef]
- Gerlach, S.A. Freilebende Meeresnematoden Von Den Malediven Ii. Kiel. Meeresforsch 1963, 19, 67–103. [Google Scholar]
- Hourston, M.; Potter, I.C.; Warwick, R.M.; Valesini, F.J.; Clarke, K.R. Spatial and Seasonal Variations in the Ecological Characteristics of the Free-Living Nematode Assemblages in a Large Microtidal Estuary. Estuar. Coast. Shelf Sci. 2009, 82, 309–322. [Google Scholar] [CrossRef]
- Leduc, D.; Zhao, Z.Q. Morphological and Molecular Characterisation of Spirinia antipodea Leduc N. Sp.(Nematoda: Desmodoridae), a Cryptic Species Related to S. parasitifera, from the Coast of New Zealand. Nematology 2019, 21, 91–105. [Google Scholar] [CrossRef]
- Hopper, D. Drawing and Measuring Nematodes. In Laboratory Methods for Work with Plant and Soil Nematodes; Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationery Office: London, UK, 1970. [Google Scholar]
- Meeker, N.D.; Hutchinson, S.A.; Ho, L.; Trede, N.S. Method for Isolation of Pcr-Ready Genomic DNA from Zebrafish Tissues. Biotechniques 2007, 43, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Derycke, S.; Vanaverbeke, J.; Rigaux, A.; Backeljau, T.; Moens, T. Exploring the Use of Cytochrome Oxidase C Subunit 1 (Coi) for DNA Barcoding of Free-Living Marine Nematodes. PLoS ONE 2010, 5, e13716. [Google Scholar] [CrossRef]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-Wide Analysis of Ssu Rdna Reveals Deep Phylogenetic Relationships among Nematodes and Accelerated Evolution toward Crown Clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef]
- De Ley, P.; De Ley, I.T.; Morris, K.; Abebe, E.; Mundo-Ocampo, M.; Yoder, M.; Heras, J.; Waumann, D.; Rocha-Olivares, A.; Burr, A.H.J.; et al. An Integrated Approach to Fast and Informative Morphological Vouchering of Nematodes for Applications in Molecular Barcoding. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2005, 360, 1945–1958. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. Mega11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Clustal-W—Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Bell, T.G.; Kramvis, A. Fragment Merger: An Online Tool to Merge Overlapping Long Sequence Fragments. Viruses 2013, 5, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide-Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. Iq-Tree 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. Modelfinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. Jmodeltest 2: More Models, New Heuristics and High-Performance Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. Mrbayes: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A. Figtree, Version 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Luc, M.; De Coninck, L.A. Nématodes Libres Marins De La Région De Roscoff. Arch. De Zool. Expérimentale Et Générale 1959, 98, 103–165. [Google Scholar]
- Ott, J.A. New Freeliving Marine Nematodes from the West Atlantic I. Four New Species from Bermuda with a Discussion of the Genera Cytolaimium and Rhabdocoma Cobb, 1920. Zool. Anz. 1977, 198, 120–138. [Google Scholar]
- Maria, T.F.; Esteves, A.M.; Smol, N.; Vanreusel, A.; Decraemer, W. Chromaspirina guanabarensis Sp. N. (Nematoda: Desmodoridae) and a New Illustrated Dichotomous Key to Chromaspirina Species. Zootaxa 2009, 2092, 21–36. [Google Scholar] [CrossRef]
- Wieser, W.; Hopper, B. Marine Nematodes of the East Coast of North America. I. Florida. Bull. Mus. Comp. Zool. Harv. Coll. 1967, 135, 239–344. [Google Scholar]
- Bastian, H.C. Monograph of the Anguillulidae, or Free Nematoids, Marine, Land, and Freshwater; with Descriptions of 100 New Species. Trans. Linn. Soc. Lond. 1865, XXV, 73–184. [Google Scholar] [CrossRef]
- Muthumbi, A.; Verschelde, D.; Vincx, M. New Desmodoridae (Nematoda: Desmodoroidea): Three New Species from Ceriops Mangrove Sediments (Kenya) and One Related New Species from the North Sea. Cah. De Biol. Mar. 1995, 36, 181–195. [Google Scholar]
- Ott, J.A. Twelve New Species of Nematodes from an Intertidal Sandflat in North Carolina. Int. Rev. Der Gesamten Hydrobiol. Und Hydrogr. 1972, 57, 463–496. [Google Scholar] [CrossRef]
- Villot, A. Recherches Sur Les Helminthes Libres Ou Parasites Des Cotes De La Bretagne. Arch. De Zool. Expérimentale Et Générale 1875, 4, 451–482. [Google Scholar]
- Da Silva, M.C.; De Castro, F.J.V.; Cavalcanti, M.D.; Da Fonsêca-Genevois, V. Spirinia lara Sp. N. and Spirinia Sophia Sp. N. (Nematoda, Desmodoridae) from the Brazilian Continental Margin (Campos Basin, Rio De Janeiro). Zootaxa 2009, 2081, 31–45. [Google Scholar] [CrossRef]
- Leduc, D.; Verschelde, D. New Spirinia and Stygodesmodora Species (Nematoda, Spiriniinae) from the Southwest Pacific, and Revision of the Related Genera Spirinia, Chromaspirina and Perspiria. Eur. J. Taxon. 2015, 118, 1–25. [Google Scholar] [CrossRef]
- Platt, H.; Warwick, R. Free-Living Marine Nematodes; Part 2: British Chromadorids; Linnean Society of London: London, UK, 1988. [Google Scholar]
- Vincx, M.; Gourbault, N. Desmodoridae from the Bay of Morlay (Brittany) and the Southern Bight of the North Sea. Cah. De Biol. Mar. 1989, 30, 103–114. [Google Scholar]
- Baldrighi, E.; Dovgal, I.; Zeppilli, D.; Abibulaeva, A.; Michelet, C.; Michaud, E.; Franzo, A.; Grassi, E.; Cesaroni, L.; Guidi, L.; et al. The Cost for Biodiversity: Records of Ciliate-Nematode Epibiosis with the Description of Three New Suctorian Species. Diversity 2020, 12, 224. [Google Scholar] [CrossRef]
- Armenteros, M.; Rojas-Corzo, A.; Ruiz-Abierno, A.; Derycke, S.; Backeljau, T.; Decraemer, W. Systematics and DNA Barcoding of Free-Living Marine Nematodes with Emphasis on Tropical Desmodorids Using Nuclear Ssu Rdna and Mitochondrial Coi Sequences. Nematology 2014, 16, 979–989. [Google Scholar] [CrossRef]
- Bhadury, P.; Austen, M.C.; Bilton, D.T.; Lambshead, P.J.D.; Rogers, A.D.; Smerdon, G.R. Development and Evaluation of a DNA-Barcoding Approach for the Rapid Identification of Nematodes. Mar. Ecol. Prog. Ser. 2006, 320, 1–9. [Google Scholar] [CrossRef]
- Pereira, T.J.; Fonseca, G.; Mundo-Ocampo, M.; Guilherme, B.C.; Rocha-Olivares, A. Diversity of Free-Living Marine Nematodes (Enoplida) from Baja California Assessed by Integrative Taxonomy. Mar. Biol. 2010, 157, 1665–1678. [Google Scholar] [CrossRef]
- Gerlach, S.A. Über Einige Nematoden Aus Der Familie Der Desmodoriden. Zool. Anz. 1950, 145, 178–198. [Google Scholar]



| Target Gene | Primer (Direction) | Sequence 5′-3′ | Amplification Condition | Reference |
|---|---|---|---|---|
| mtCOI | JB3 (f) | TTTTTTGGGCATCCTGAGGTTTAT | 94 °C for 5 min, 5 cycles of 94 °C for 30 s, 54 °C for 30 s, and 72 °C for 30 s, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s, followed by final step of 72 °C for 10 min | Derycke et al., 2010 [10] |
| JB5 (r) | AGCACCTAAACTTAAAACATAATGAAAATG | |||
| 18S | 988F (f) | CTCAAAGATTAAGCCATGC | 94 °C for 5 min, 5 cycles of 94 °C for 30 s, 45 °C for 30 s, and 72 °C for 70 s, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 30 s, and extension at 72 °C for 70 s, followed by final step of 72 °C for 5 min | Holterman et al., 2006 [11] |
| 1912R (r) | TTTACGGTCAGAACTAGGG | |||
| 1813F (f) | CTGCGTGAGAGGTGAAAT | |||
| 2646R (r) | GCTACCTTGTTACGACTTTT | |||
| 28S | D2A (f) | ACAAGTACCGTGAGGGAAAGTTG | 95 °C for 5 min followed by 37 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 1 min, and extension at 72 °C for 1 min 30 s min, followed by final step at 72 °C for 5 min | De Ley et al., 2005 [12] |
| D3B (r) | TCGGAAGGAACCAGCTACTA |
| Character | Male | Female | ||
|---|---|---|---|---|
| Holotype | Male (n = 11) Mean ± Sd (Range) | Allotype | Female (n = 12) Mean ± Sd (Range) | |
| L | 2100 | 2052 ± 98.4 (1833–2180) | 1990 | 2105 ± 113.7 (1858–2282) |
| a (L/gbd) | 48.8 | 48 ± 3.2 (43.6–54.5) | 44.2 | 48.9 ± 3.1 (45.3–53.4) |
| b (L/pharynx length) | 13.9 | 15 ± 1.2 (12.7–17) | 15.3 | 15.5 ± 0.8 (13.9–17.1) |
| c (L/tail length) | 15.8 | 17 ± 2.2 (12.5–22) | 17 | 16.9 ± 1.4 (15.1–19.8) |
| c’ (tail length/anal body diameter) | 3.4 | 3 ± 0.3 (2.7–4) | 3.7 | 3.7 ± 0.2 (3.4–4.2) |
| Cephalic setae length | 5.6 | 6 ± 0.5 (5–6.8) | 5.6 | 5.5 ± 0.5 (4.4–6.4) |
| Cephalic setae cbd | 17 | 16 ± 1.3 (13–18) | 15 | 16.1 ± 0.9 (14.6–17.5) |
| Amphid (at center) cbd | 16 | 15 ± 1.7 (12–18) | 14 | 15.8 ± 0.8 (14–17.4) |
| Amphid height | 4.7 | 5 ± 0.5 (3.9–5.9) | 4.1 | 5 ± 0.5 (4.1–6) |
| Amphid diameter | 6.1 | 5 ± 0.5 (4.3–6.1) | 5.4 | 5.5 ± 0.6 (4.8–7) |
| Amphid diameter divided by cbd (%) | 38.1 | 35 ± 4.8 (28.3–42.3) | 38.6 | 34.9 ± 4.3 (29.8–46.7) |
| Amphid (at center) distance to anterior | 4.9 | 6 ± 1.5 (4–9.7) | 5 | 6.5 ± 1.7 (4.2–9.7) |
| Nerve ring distance to anterior | 75 | 76 ± 7.8 (65–93) | 77 | 80.2 ± 9.7 (64–94) |
| Nerve ring cbd | 32 | 31 ± 0.7 (30–32) | 31 | 31.9 ± 2.3 (27–34) |
| Pharynx length | 151 | 134 ± 8.4 (122–151) | 130 | 135.5 ± 7.2 (120–149) |
| Pharyngeal bulb width | 30 | 29 ± 0.9 (28–30) | 27 | 29.8 ± 2.2 (27–36) |
| Pharynx cbd | 37 | 36 ± 0.9 (34–37) | 35 | 35.9 ± 3.7 (30–46) |
| Max. body diameter | 43 | 43 ± 2.2 (40–47) | 45 | 43.2 ± 1.2 (41–45) |
| Spicule length (as arc) | 64 | 55 ± 4.1 (49–64) | n/a | n/a |
| Gubernaculum length (as arc) | 22 | 22 ± 1.8 (18–24) | n/a | n/a |
| Anal body diameter | 39 | 36 ± 2.8 (30–41) | 32 | 33.6 ± 2.1 (30–37) |
| Tail length (µm) | 133 | 120 ± 12.8 (95–147) | 117 | 124.7 ± 10.2 (103–140) |
| Vulva distance to anterior | n/a | n/a | 949 | 1026.5 ± 52.1 (911–1119) |
| Vulva body diameter | n/a | n/a | 47 | 45 ± 1.8 (41–48) |
| Vulva distance to anterior as percentage of total body length | n/a | n/a | 48% | 0.5 ± 0 (0.5–0.5) |
| Species | Source | Region | L | a | b | c | c’ | Spic (µm) | V |
|---|---|---|---|---|---|---|---|---|---|
| S. gerlachi | [23] | France | 1494 | 37.5 | 11 | 10.4 | ~4 | 37 | – |
| S. gnaigeri | [24] | Bermuda | 860–913 | 20–23 | 9–10 | 9–11 | 2.8–3.5 | 50–55 | 47–50 |
| S. guanabarensis | [25] | Brazil | 2110–2579 | 37–48 | 11–14 | 17–22 | 2.6–3.3 | 58–70 | 55–59 |
| S. hopperi | [2] | England | 4100–5100 | 48–67 | 20–22 | 22–30 | 2.2–3 | 110–130 | 39–44 |
| S. inaurita | [26] | USA | 1180–1350 | 38–40 (calc) | 11–12 | 13–14 | 3.9 | 27–28 | 49 |
| S. laevioides | [5] | Maldives | 1348–1367 | 27–32 | 13.5–14 | 11.8–13.9 | 3–3.3 | 42–52 | 43 |
| S. laevis | [27] | UK | ~3600 | ~40 | ~10 | ~25 | – | ~70 | 40 |
| S. okemwai | [28] | Kenya | 966–1231 | 23–27 | 6.6–8.5 | 14.5–17.4 | 1.8–2.4 | 41–52 | 55 |
| S. parma | [29] | USA | 1276–1608 | 41.1–56.1 | 11.1–12.9 | 14.7–19.2 | 3.3–3.9 | 2631 | 58 |
| S. schneideri | [23] | France | 3424– 3919 | 26–32.5 | 17.6–18.6 | 27.6–48.4 | 1 | 119–140 | 40.2–48 |
| S. sophia | [31] | Brazil | 1945–2180 | 108–112 | 17–21 | 23–24 | 4–5 | 26–27 | 55–57 |
| S. verecunda | [32] | New Zealand | 757 | 22 | 8.00 | 15 | 2 | 38 | 51–54 |
| S. parasitifera | [2,4,5,27,33,34] | Global | 1600–3796 | 28–59 | 12–26 | 14–29 | 2–4 | 45–100 | 46–53 |
| S. antipodea | [7] | New Zealand | 2182–2564 | 44–59 | 17–19 | 15–19 | 3.7–4.7 | 52–59 | 49–51 |
| S. koreana sp. nov. | Present study | Republic of Korea | 1833–2282 | 44–54 | 13–17 | 12–22 | 2.7–4.2 | 49–64 | 47–52 |
| # | Species Name | Isolate Number | GenBank Accession Number | ||
|---|---|---|---|---|---|
| mtCOI | 18S | 28S | |||
| JB3 | 988F, 1813F | D2A | |||
| /JB5 | /1912R, 2646R | /D3B | |||
| (~360 bp) | (~1600 bp) | (~750 bp) | |||
| 1 | Spirinia koreana sp. nov. | 1A | PV637276 | PV628730 | PV630260 |
| 2 | Spirinia koreana sp. nov. | 1N | PV637277 | PV628731 | PV630261 |
| 3 | Spirinia koreana sp. nov. | 1O | PV637278 | PV628732 | PV630262 |
| 4 | Spirinia koreana sp. nov. | 1P | PV637279 | PV628733 | PV630263 |
| Species | Source/ Reference | Amphid Coverage (of Annulation) | Tail Morphology | ||
|---|---|---|---|---|---|
| Textual Description | Figure Depiction | Textual Description | Figure Depiction | ||
| S. gerlachi | Luc & De Coninck 1959 [23] | not mentioned | posterior only (Figure 27) | long conico-attenuated | conical/long conico-attenuated (Figure 28) |
| S. gnaigeri | Ott 1977 [24] | not mentioned | posterior only (Figure 43) | conical | conical/long conico-attenuated (Figure 44) |
| S. guanabarensis | Maria et al., 2009 [25] | largely surrounded | mostly, but not entirely (Figures 1C and 2A,B) | conical | conical/conico-attenuated (Figures 1D,E and 2F,G) |
| S. hopperi | Coles 1987 [2] | not mentioned cuticle described as without striations entirely | not depicted (Figure 1c), but with SEM showing fine striation at anterior end of amphid (Figure 9B) | tail long; fairly long in both sexes | conical (Figure 3C) |
| S. inaurita | Wieser & Hopper 1967 [26] | not mentioned | not depicted (Figure 37A) | not described | conical/long conico-attenuated (Figure 37E) |
| S. laevioides | Gerlach 1963 [5] | cuticle shows annulation that reaches up to the front edge of the amphids | entire (Figure 2e) | conical | conical (Figure 2f) |
| S. laevis | Bastian 1865 [27] | not mentioned | –not depicted (Figure 204) –entire (Gerlach 1950 [39]: Figure 6A,B) | –narrowing to a point –cylindrical–conical with a rounded terminal cone (Gerlach 1950 [39]) | –conical (Figures 205 and 206) –cylindrical portion depicted (Gerlach 1950 [39]: Figure 6C) |
| S. okemwai | Muthumbi, Verschelde & Vincx 1995 [28] | not mentioned | entire (Figures 1B,G and 2A,B,D) | conical | conico-cylindrical (Figures 1E,F and 2E,G) |
| S. parma | Ott 1972 [29] | not mentioned | partially, beginning at front edge (Figures 87, 90 and 92) | conical | mostly conical but with terminal cylindrical flagellate portion depicted (Figures 88, 89 and 91) |
| S. schneideri | Luc & De Coninck 1959 [23] | not mentioned | –not depicted (Figure 31) –not depicted (Coles 1987 [2]: Figure 1b), but with SEM showing fine striation well above anterior end of amphid (Coles 1987 [2]: Figure 9A) | Tail is different in males and females. In females, tail is conical. In males, tail is subhemispherical with terminal point. | subhemispherical with terminal cylindrical point (Figures 32 and 33) conical (Coles 1987 [2]: Figure 3B) |
| S. sophia | Silva et al., 2009 [31] | amphid surrounded by transverse cuticular striation | entire (Figures 5B,D and 6B,C) | conical | conical (Figures 5E,F and 7G) |
| S. verecunda | Leduc & Verschelde 2015 [32] | annulations completely surrounding amphid | entire (Figure 1A,B) | conical | conical (Figure 1G,I,J) |
| S. parasitifera | Bastian 1865 [27]: Gerlach 1963 [5]: Coles 1987 (UK, Canada, USA) [2]: Platt & Warwick 1988 [33]: Vincx & Gourbault 1989 [34]: Armenteros et al., 2014 [4] | not mentioned (Bastian 1865 [27]; Gerlach 1963 [5]; Coles 1987 [2]; Platt & Warwick 1988 [33]; Vincx & Gourbault 1989 [34]; Armenteros et al., 2014 [4]) | –not depicted (Bastian 1865 [27]: Figure 201) –posterior only (Gerlach 1963 [5]: Tafel 1e, 1h; Armenteros et al., 2014 [4]: Figure 11A) –entire (Gerlach 1963 [5]: Tafel 1f, 1g, 1c; Coles 1987 [2]: Figure 1a; Platt & Warwick 1988 [33]: Figure 148A; Vincx & Gourbault 1989 [34]: Figure 3c; Armenteros et al., 2014 [4]: Figure 12A,B) | –tapering gradually to a point posteriorly (Bastian 1865 [27]) –conical (Platt & Warwick 1988 [33]) | –conical (Bastian 1865 [27]: Figures 202 and 203; Coles 1987 [2]: Figure 10A,C; Armenteros et al., 2014 [4]: Figure 11B) –long conico-attenuated (Coles 1987 [2]: Figures 2A and 3A; Platt & Warwick 1988 [33]: Figure 148B; Vincx & Gourbault 1989 [34]: Figure 3M,N) |
| S. antipodea | Leduc & Zhao 2019 [7] | amphid partially surrounded by cuticle annulation | posterior only (Figure 1C,D) | conical | –conical (Figure 2B,D) –conico-attenuated (Figures 2A and 3F) |
| S. koreana sp. nov. | Present study | only posterior half of amphid partially surrounded by cuticle striation | posterior only (Figures 1B and 2A) | conical/conico-attenuated | –conical (Figures 2C, 3E and 4A,B) –conico-attenuated (Figures 1E and 4C,D) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, K.; Jeong, R. The Discovery and Delimitation of a New Cryptic Species of Spirinia (Nematoda: Desmodoridae) Using SSU and LSU rDNA Divergence. J. Mar. Sci. Eng. 2025, 13, 1251. https://doi.org/10.3390/jmse13071251
Son K, Jeong R. The Discovery and Delimitation of a New Cryptic Species of Spirinia (Nematoda: Desmodoridae) Using SSU and LSU rDNA Divergence. Journal of Marine Science and Engineering. 2025; 13(7):1251. https://doi.org/10.3390/jmse13071251
Chicago/Turabian StyleSon, Kyeongmoon, and Raehyuk Jeong. 2025. "The Discovery and Delimitation of a New Cryptic Species of Spirinia (Nematoda: Desmodoridae) Using SSU and LSU rDNA Divergence" Journal of Marine Science and Engineering 13, no. 7: 1251. https://doi.org/10.3390/jmse13071251
APA StyleSon, K., & Jeong, R. (2025). The Discovery and Delimitation of a New Cryptic Species of Spirinia (Nematoda: Desmodoridae) Using SSU and LSU rDNA Divergence. Journal of Marine Science and Engineering, 13(7), 1251. https://doi.org/10.3390/jmse13071251







