Abstract
The Mediterranean Sea, a semi-enclosed basin at mid-latitudes, is experiencing significant environmental changes driven by global warming. This study examines recent shifts in fish species composition within Spanish Mediterranean waters, focusing on the potential tropicalization of marine communities. Using an updated dataset derived from the Spanish marine fishes checklist, we analyzed newly recorded species across two Spanish demarcations: the Levantine-Balearic (LEBA) and the Strait of Gibraltar and Alboran Sea (ESAL). A total of 25 new records (including 23 new species) were reported, with 15 new records in LEBA and 10 new records in ESAL and also including 2 new species recorded occurring in both demarcations. To assess changes in species’ thermal preferences, we compared the mean temperature of newly recorded species with that of previously established species in each demarcation using the Mann–Whitney U test. While no significant differences were found in LEBA, a marked increase of up to 6.08 °C in thermal preference was observed in ESAL. These findings suggest that tropicalization is occurring unevenly across the Spanish Mediterranean, with the Alboran Sea and Strait of Gibraltar being particularly affected. The complex oceanography of the Alboran Sea, coupled with extreme weather events and biological invasions, may exacerbate these shifts.
1. Introduction
The Mediterranean Sea is a semi-enclosed sea at mid-latitudes, where both temperate and subtropical biota converge [1]. The Mediterranean biota origin settles in the ancient Tethys Ocean, but significant changes occurred between 5.96 and 5.33 million years ago; during the Messinian Salinity Crisis (MSC), the Mediterranean Sea underwent a partial desiccation due to the closure of the Strait of Gibraltar, leading to the extinction of 89% endemic species [2,3]. The subsequent Zanclean Flood, caused by the reopening of the Strait of Gibraltar, allowed for the recolonization of the Mediterranean by Atlantic species [4]. The MSC played a crucial role in shaping the current biogeographic and ecological characteristics of the Mediterranean’s biodiversity [3]. Currently, the Mediterranean region is known to be an important biodiversity hotspot [5] and exhibit a southeastward decreasing gradient in species richness that has been variously thought to be associated with changing temperature, salinity, productivity, or distance from the Atlantic connection at Gibraltar [3,5,6].
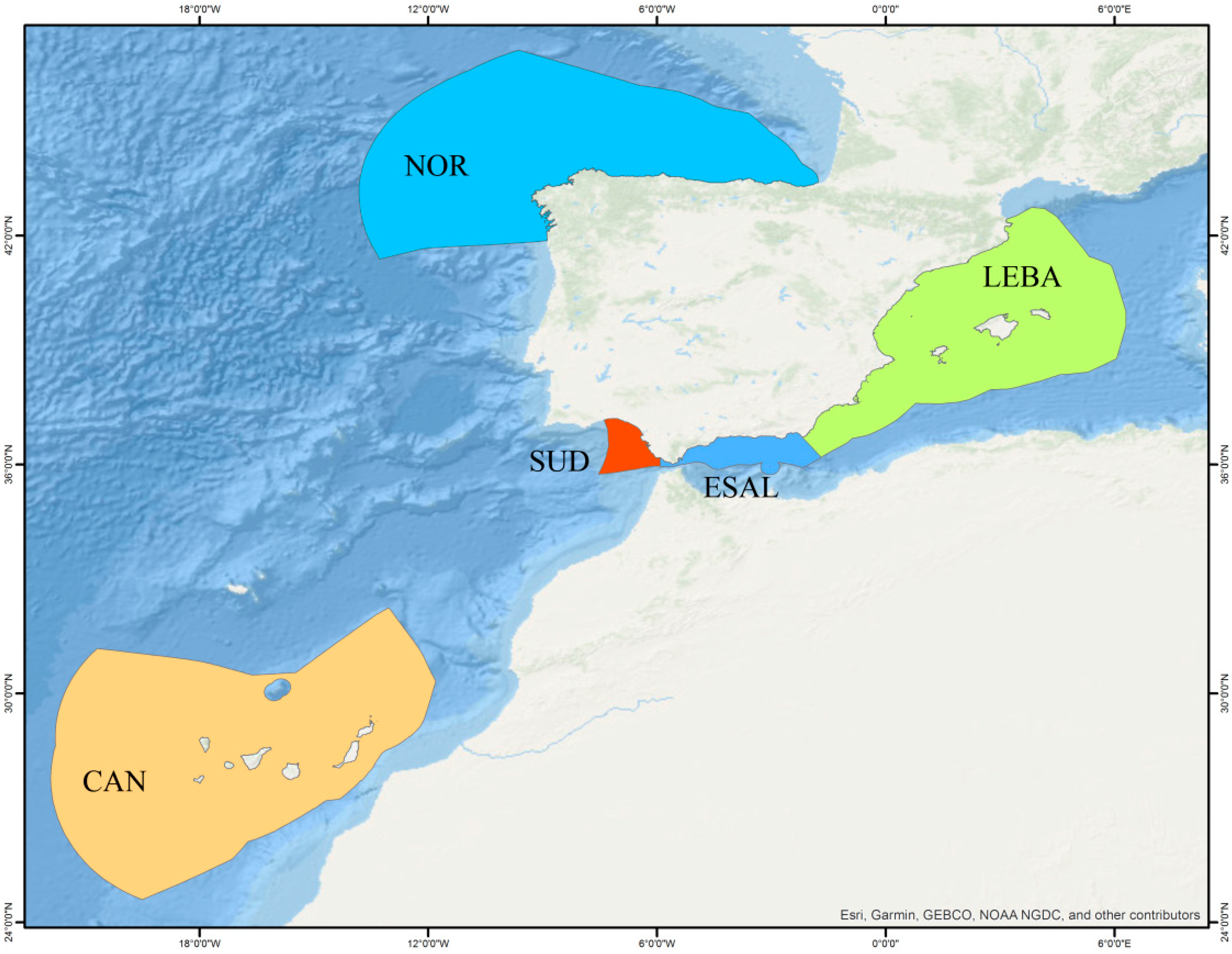
Báez et al. [7] provided an updated checklist of fish species within Spanish marine demarcations, estimating that there are a total of 1075 species in the exclusive economic zones of Spanish waters including the Atlantic and Mediterranean demarcations. The Mediterranean region comprises two demarcations: the Levantine-Balearic (LEBA, East coast of Spain and Balearic Islands) and the Strait of Gibraltar and Alboran Sea (ESAL) with 533 species (49.6% of the whole Spanish checklist) (Figure 1) [7]. Despite the high biodiversity of the region, the distribution of numerous species remains insufficiently understood, particularly in the case of small-sized organisms exhibiting cryptic behavior or inhabiting deep-sea environments.

Figure 1.
Limits of Spanish marine demarcations. Key: Mediterranean region, including ESAL (blue): Strait of Gibraltar and Alboran Sea; LEBA (green): east coast of Spain and Balearic Islands. Atlantic region includes NOR (light blue): Spanish north coast; CAN (orange): Canary Islands; SUD (red): Spanish coast of Gulf of Cádiz. Source: Instituto Español de Oceanografía (CSIC).
The planet is experiencing global warming, primarily reflected in the oceans due to climate change. Oceans act as major heat sinks of the excess heat generated by anthropogenic greenhouse gas emissions [8]. Among the world’s marine ecosystems, the Mediterranean Sea has been identified as one of the most affected regions [9], due to it being a semi-enclosed sea, having a relatively small volume, and having high sensitivity to atmospheric temperature fluctuations [9]. As a result of this warming trend, the new environment conditions of the Mediterranean Sea facilitate the migration and establishment of tropical and subtropical species, a process referred to as Mediterranean tropicalization [10,11]. This phenomenon involves a shift in biodiversity toward a more thermophilic or tropical assemblage, altering ecosystem dynamics and species interactions [11]. In this context, the primary objective of the present study is to assess the potential tropicalization of Spanish Mediterranean waters by analyzing recent shifts in fish species composition. This study aims to contextualize these findings within the broader framework of Mediterranean tropicalization and its implications for marine biodiversity conservation and management.
2. Materials and Methods
The study region of the present study encompasses all jurisdictional waters of Spain within the Mediterranean Sea, including the Strait of Gibraltar.
For the compiled new records of fish species within Mediterranean Spanish waters, we performed an exhaustive bibliographic search in the scientific literature and gray literature. We used Web search engines such as Google Scholar and also used multiple database references such as Scopus. We also consulted the Global Biodiversity Information Facility (GBIF.org).
For each new record and species, we obtained the preferred temperature estimation provided by Fishbase [12]. However, in certain cases, direct temperature data for a given species were not available. In such instances, we estimated the mean temperature by referencing ecologically similar species within the same genus (Supplementary Material, S1).
Finally, we conducted a statistical comparison of the mean temperature preferences of the fish species listed in the checklist provided by Báez et al. [7] with the mean temperature preferences of newly recorded species for the ESAL and LEBA marine demarcations obtained from the literature, as we currently lack precise information on the specific temperature ranges that the newly reported species occupy in their newly colonized areas of the Mediterranean. For this reason, our analysis is based on bibliographically reported values from the species’ native or previously known ranges.
It is important to acknowledge that these species likely tolerate a wide range of temperatures—something that is indeed reflected in the bibliographic compilation we conducted. However, due to the unavailability of empirical data on actual temperature use within the new area, we adopted a comparative approach using mean values. Specifically, we compared the mean temperatures associated with newly recorded species (based on bibliographic data) to the mean temperatures of resident species already known in the studied regions. This allowed us to conduct a viable statistical analysis, despite the limitations in available data. To assess potential differences between these groups, we applied a Mann–Whitney U test, as the assumption of normality could not be verified. This test is appropriate for continuous or ordinal data and does not require equal variances. It was selected for its robustness and sensitivity to differences in central tendency when comparing two independent samples [13].
3. Results
Of the 533 species reported for the Spanish Mediterranean exclusive economic zone (EEZ) by Báez et al. [7], 6 species were considered to have doubtful presence in the Mediterranean by Kovačić et al. [14] (including Alepisaurus ferox, Ammodytes tobianus, Buenia jeffreysii, Diogenichthys atlanticus, Remora brachyptera, and Symphodus bailloni). However, Buenia jeffreysii, Diogenichthys atlanticus, Remora brachyptera, and Symphodus bailloni were recorded from Global Biodiversity Information Facility (GBIF.org). However, in the case of Buenia jeffreysii, there is a possibility that this may be due to a misidentification. Therefore, we recommend excluding Alepisaurus ferox, Ammodytes tobianus, and Buenia jeffreysii from the Spanish checklist. Since the last update of the Spanish marine fishes checklist [7], a total of 25 new records (including 23 new species) were reported, with 15 new records in LEBA and 10 new records in ESAL and also including 2 new species recorded occurring in both demarcations (Table 1).

Table 1.
New recorded species with respect to Levantine-Balearic (LEBA) and Strait of Gibraltar and Alboran Sea (ESAL) Spanish demarcations and distribution and temperature preferences by species are provided.
Table 2 presents a comparison of the mean temperature preferences of species previously reported in each marine demarcation (LEBA and ESAL) against those of the recently recorded species within each demarcation. The Mann–Whitney U test identified significant differences between species previously reported versus recently recorded species only in the case of the ESAL demarcation (ESAL, Mann–Whitney U = 958.5; p = 0.002; N = 471), while in the case of LEBA (LEBA, Mann–Whitney U = 3557; p > 0.05; N = 510) and total species (Mann–Whitney U = 4585.5; p > 0.05; N = 550), there were no significant differences.

Table 2.
Mean temperature preferences of all species and confidence intervals for maximum and minimum temperatures with respect to Levantine-Balearic (LEBA) and Strait of Gibraltar and Alboran Sea (ESAL) Spanish demarcations and total. Keys: Maximum mean temperature preference: Max. mean T°; Minimum mean temperature preference: Minim. mean T °C.
4. Discussion
Our findings indicate that while no significant changes in temperature preferences were observed in fish communities within the LEBA demarcation, a notable shift has occurred in the ESAL demarcation. In ESAL, we recorded an increase of up to 6.08 °C in the temperature preferences of fish species, suggesting an ongoing process of tropicalization. As a result, this may accelerate the replacement of native species with thermophilic species. This warming trend aligns with previous studies indicating that the Mediterranean Sea, particularly its western basin, is experiencing rapid ecological transformations due to climate change [9,32]. The influx of thermophilic species, combined with the decline in cold-adapted species, is reshaping community structures, with potential implications for trophic interactions and ecosystem stability. However, the observed tropicalization trend is not geographically uniform; it is more pronounced in the Alboran Sea and the Strait of Gibraltar than in the LEBA demarcation. This spatial variability may be influenced by regional oceanographic conditions, connectivity to the Atlantic, and current dynamics. Specifically, the Alboran Sea is characterized by unique oceanographic processes shaped by both Atlantic inflows and Mediterranean outflows, which make it particularly susceptible to rapid ecological changes [33].
When we consider the geographical origin of newly recorded species (Table 1), the majority of new records in both the ESAL and LEBA demarcations correspond to species already present in the Mediterranean basin (20% and 32%, respectively). These are followed by Atlantic species (8% and 16%, respectively), Circumtropical species (8% and 12%), and, lastly, Indo-Pacific species—such as the lionfish, which has been documented in the ESAL demarcation. Thus, it appears that the observed pattern is not solely driven by the proximity of ESAL to the Atlantic Ocean nor by the prevailing current flow from the Atlantic into the Alboran Sea. Other underlying factors must be contributing to this dynamic.
In this context, Micheli et al. [34] identified the Alboran Sea as falling within the 20.5% of marine regions worldwide experiencing medium-high to very high cumulative human impact. Indeed, the Alboran Sea is among the busiest maritime traffic zones globally [35], and it is also one of the most densely populated coastal areas in the Mediterranean region. This pressure is intensified during the summer months due to significant increases in tourism. As a result, pollution levels in the area may adversely affect resident marine populations, reducing their survival probabilities [36].
Furthermore, the recent large-scale invasion of the non-native macroalga Rugulopteryx okamurae in the Alboran Sea introduces additional ecological disturbances. This species may alter habitat structure, reshape competitive interactions, and disrupt trophic relationships, further complicating ecosystem dynamics [37,38,39]. Future studies should investigate the extent to which such invasive species interact with climate-induced stressors and whether they are actively contributing to the ongoing restructuring of local fish communities.
In agreement with the conclusions of Bianchi and Morri [10], our findings support the idea that current changes in fish community composition are increasingly driven by anthropogenic climate change, rather than by historical geological processes. Therefore, we cannot argue that the differences in the greater tropicalization of the ESAL demarcation compared to LEBA are due to differential environmental factors, nor to the proximity of ESAL to the Atlantic, but rather to the greater anthropogenic impact together with the oceanographic complexity of the demarcation. Extreme climatic events, such as marine heatwaves, may further intensify these changes by impacting foundational species negatively while favoring the movement of other alien species [40].
The continuous new records of fish species in the Mediterranean highlights the dynamic nature of its marine biodiversity. Several studies [16,21,22,41,42,43] have reported the annual appearance of previously unrecorded species, which can be attributed, according to Bianchi and Morri [10], to three primary mechanisms: (1) the range expansions of tropical species driven by rising sea temperatures, (2) the introductions of exotic species through human-mediated pathways such as ballast water or aquaculture escapees, and (3) the identification of cryptic species that had previously gone unnoticed due to limited observations or taxonomic ambiguity. Moreover, Lenanton et al. [44] proposed another important mechanism: the sporadic occurrences of vagrant species, favored by the increase in temperature. Understanding the relative contribution of each mechanism is crucial for effective monitoring and conservation efforts in the Mediterranean, particularly in the context of ongoing climate change.
Among the 11 newly reported species from ESAL, the presence of the whale shark (Rhincodon typus) may be attributed to sporadic occurrences of vagrant species, including high-profile taxa. In contrast, the record of the lionfish (Pterois miles) may be associated with the introductions of exotic species through human-mediated pathways by lessepsian migration. Undoubtedly, the detection of Buenia massutii, Gobius incognitus, Gobius roulei, Grammonus ater, and Millerigobius macrocephalus is likely the result of the identification of a cryptic species that had previously gone unnoticed due to limited field observations or taxonomic ambiguity. Conversely, the remaining cases (Cephalopholis taeniops, Lobotes surinamensis, and Paranthias furcifer) are most plausibly explained by the ongoing process of tropicalization affecting the Mediterranean Sea. Thereby, 36.4% of new records from ESAL (including the whale shark records) could be due to the ongoing process of tropicalization.
In conclusion, our study provides evidence that Mediterranean fish communities, particularly in the ESAL region, are undergoing tropicalization as a response to climate change. The observed shift in species’ temperature preferences underscores the urgency of implementing adaptive management strategies to mitigate the ecological consequences of this transformation. Future research should integrate environmental and anthropogenic factors to refine our understanding of the mechanisms driving biodiversity shifts and to inform conservation policies tailored to the Mediterranean’s rapidly changing ecosystems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jmse13061119/s1, Table S1: Checklist species with respect to Levantine-Balearic (LEBA) and Strait of Gibraltar and Alboran Sea (ESAL) Spanish demarcations and temperature preferences by species.
Author Contributions
Conceptualization, J.C.B.; formal analysis, J.C.B.; investigation, D.T.; data curation, D.T.; writing—original draft preparation, D.T.; writing—review and editing, J.C.B.; funding acquisition, J.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
D. Torreblanca received support for the BIODIV project “Scientific and technical advice for monitoring marine biodiversity: marine protected areas and species under state jurisdiction (2022–2025)”. This project was funded by the European Union (NextGenerationEU) through the Recovery, Transformation, and Resilience Plan and promoted by the General Directorate of Biodiversity, Forests, and Desertification of the Ministry for Ecological Transition and the Demographic Challenge and the CSIC (Spanish National Council of Oceanologists), through the Spanish Institute of Oceanography (IEO). JC Báez was financially supported by the project ‘Plan complementario de I +D + i en el ‘area de Biodiversidad (PCBIO)’, funded by the European Union within the framework of the Recovery, formation and Resilience Plan—NextGenerationEU; by the Spanish Ministry of Science, Innovation and Universities; and by the Regional Government of Andalusia.
Data Availability Statement
The original contributions presented in this study are included in the article and supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely thank the three anonymous reviewers for their valuable insights and constructive feedback, which have significantly enhanced the quality of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ESAL | Strait of Gibraltar and Alboran Sea Spanish demarcation |
| LEBA | Levantine-Balearic Spanish demarcation |
References
- Bianchi, C.N.; Morri, C. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Krijgsman, W.; Hilgen, F.J.; Raffi, I.; Sierro, F.J.; Wilson, D.S. Chronology, causes and progression of the Messinian salinity crisis. Nature 1999, 400, 652–655. [Google Scholar] [CrossRef]
- Agiadi, K.; Hohmann, N.; Gliozzi, E.; Thivaiou, D.; Bosellini, F.R.; Taviani, M.; Bianucci, G.; Collareta, A.; Londeix, L.; Faranda, C.; et al. The marine biodiversity impact of the Late Miocene Mediterranean salinity crisis. Science 2024, 385, 986–991. [Google Scholar] [CrossRef]
- Garcia-Castellanos, D.; Estrada, F.; Jiménez-Munt, I.; Gorini, C.; Fernàndez, M.; Vergés, J.; De Vicente, R. Catastrophic flood of the Mediterranean after the Messinian salinity crisis. Nature 2009, 462, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Ben Rais Lasram, F.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar] [CrossRef]
- Báez, J.C.; Rodriguez-Cabello, C.; Banon, R.; Brito, A.; Falcon, J.M.; Maño, T.; Baro, J.; Macías, D.; Meléndez, M.J.; Camiñas, J.A.; et al. Updating the national checklist of marine fishes in Spanish waters: An approach to priority hotspots and lessons for conservation. Mediterr. Mar. Sci. 2019, 20, 260–270. [Google Scholar] [CrossRef]
- Venegas, R.M.; Acevedo, J.; Treml, E.A. Three decades of ocean warming impacts on marine ecosystems: A review and perspective. Deep-Sea Res. II Top. Stud. Oceanogr. 2023, 212, 105318. [Google Scholar] [CrossRef]
- Moullec, F.; Velez, L.; Verley, P.; Barrier, N.; Ulses, C.; Carbonara, P.; Esteban, A.; Follesa, C.; Gristina, M.; Jadaud, A.; et al. Capturing the big picture of Mediterranean marine biodiversity with an end-to-end model of climate and fishing impacts. Prog. Oceanogr. 2019, 178, 102179. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Global sea warming and “tropicalization” of the Mediterranean Sea: Bieogeographic and ecological aspects. Biogeograhia 2003, 24, 319–327. [Google Scholar] [CrossRef]
- Azzurro, E.; Ballerini, T.; Antoniadou, C.; Aversa, G.D.; Souissi, J.B.; Blašković, A.; Cappanera, V.; Chiappi, M.; Cinti, M.F.; Colloca, F.; et al. ClimateFish: A Collaborative Database to Track the Abundance of Selected Coastal Fish Species as Candidate Indicators of Climate Change in the Mediterranean Sea. Front. Mar. Sci. 2022, 9, 910887. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Version (10/2024). 2024. Available online: https://www.fishbase.org (accessed on 1 May 2025).
- Emerson, R.W. Mann-Whitney U test and t-test. J. Visual Impair. Blin. 2023, 117, 99–100. [Google Scholar] [CrossRef]
- Kovačić, M.; Lipej, L.; Dulčić, J.; Iglesias, S.P.; Goren, M. Evidence-based checklist of the Mediterranean Sea fishes. Zootaxa 2021, 4998, 1–115. [Google Scholar] [CrossRef] [PubMed]
- Fortič, A.; Al-Sheikh Rasheed, R.; Almajid, Z.; Badreddine, A.; Báez, J.C.; Belmonte-Gallegos, A.; Bettoso, N.; Borme, D.; Camisa, F.; Caracciolo, D.; et al. New records of introduced species in the Mediterranean Sea (April 2023). Mediterr. Mar. Sci. 2023, 24, 182–202. [Google Scholar] [CrossRef]
- Digenis, M.; Akyol, O.; Benoit, L.; Biel-Cabanelas, M.; Çamlik, Ö.; Charalampous, K.; Chatzispyrou, A.; Crocetta, F.; Cengiz Deval, M.; Di Capua, I.; et al. New records of rarely reported species in the Mediterranean Sea (March 2024). Mediterr. Mar. Sci. 2024, 25, 84–115. [Google Scholar] [CrossRef]
- Ordines, F.; Kovačić, M.; Vivas, M.; García-Ruiz, C.; Guijarro, B. Westernmost Mediterranean records of three gobiid species (Actinopterygii: Perciformes: Gobiidae). Acta Ichthyol. Piscator. 2019, 49, 275–282. [Google Scholar] [CrossRef]
- Stern, N.; Badreddine, A.; Bitar, G.; Crocetta, F.; Deidun, A.; Dragičević, B.; Dulčić, J.; Durgham, H.; Galil, B.; Galiya, M.; et al. New Mediterranean Biodiversity Records (July 2019). Mediterr. Mar. Sci. 2019, 20, 409–426. [Google Scholar] [CrossRef]
- Dragicevic, B.; Anadoli, O.; Angel, D.; Benabdi, M.; Bitar, G.; Castriota, L.; Crocetta, F.; Deidun, A.; Dulčić, J.; Edelist, D.; et al. New Mediterranean Biodiversity Records (December 2019). Mediterr. Mar. Sci. 2019, 20, 645–656. [Google Scholar] [CrossRef]
- Ordines, F.; Deudero, S.; Sinte-Vila, J.; Sbragaglia, V.; Fricke, R.; Azzurro, E. A new record of Diodon hystrix (Actinopterygii: Tetraodontiformes: Diodontidae) in the Mediterranean Sea. Acta Ichthyol. Piscator. 2018, 48, 403–407. [Google Scholar] [CrossRef]
- Bo, M.; Al Mabruk, S.A.; Balistreri, P.; Bariche, M.; Batjakas, I.E.; Betti, F.; Bilan, M.; Canese, S.; Cattaneo-Vietti, R.; Corsini-Foka, M.; et al. New records of rare species in the Mediterranean Sea (October 2020). Mediterr. Mar. Sci. 2020, 21, 608–630. [Google Scholar]
- Santin, A.; Aguilar, R.; Akyol, O.; Begburs, C.R.; Benoit, L.; Chimienti, G.; Crocetta, F.; Dalyan, C.; De La Linde Rubio, A.; Dragicevic, B.; et al. New records of rare species in the Mediterranean Sea (March 2021). Mediterr. Mar. Sci. 2021, 22, 199–217. [Google Scholar]
- Ahnelt, H.; Dorda, J. Gobioid fishes from the north eastern Atlantic and the Mediterranean: New records and rarely found species. Ann. Naturhist. Mus. Wien. 2004, 105B, 5–19. [Google Scholar]
- Kovačić, M.; Renoult, J.P.; Pillon, R.; Bilecenoglu, M.; Tiralongo, F.; Bogorodsky, S.V.; Engin, S.; Kovtun, O.; Louisy, P.; Patzner, R.A.; et al. The Delimitation of Geographic Distributions of Gobius bucchichi and Gobius incognitus (Teleostei: Gobiidae). J. Mar. Sci. Eng. 2023, 11, 516. [Google Scholar] [CrossRef]
- Kovačić, M.; Renoult, J.P.; Pillon, R.; Svensen, R.; Bogorodsky, S.V.; Engin, S.; Louisy, P. Identification of Mediterranean marine gobies (Actinopterygii: Gobiidae) of the continental shelf from photographs of in situ individuals. Zootaxa 2022, 5144, 1–103. [Google Scholar] [CrossRef]
- Kovačić, M.; Ramírez-Amaro, S.; Farriols, M.T.; Ordines, F. The Second Record of Gymnesigobius medits Kovačić., Ordines., Ramirez-Amaro & Schliewen., 2019., the Deepest Benthic Gobiiform Species., and the Additional Records of Gobius xoriguer Iglésias., Vukić & Šanda., 2021 (Actinopterygii: Gobiiformes: Gobiidae). Fishes 2023, 8, 331. [Google Scholar] [CrossRef]
- Guallart, J.; Morey, G.; Bartolí, À. New record of a sharpnose sevengill shark Heptranchias perlo (Elasmobranchii., Hexanchidae) from the Balearic Sea., western Mediterranean Sea. J. Fish Biol. 2019, 94, 526–531. [Google Scholar] [CrossRef]
- Schliewen, U.K.; Kovačić, M.; Cerwenka, A.F.; Svensen, R.; Ordines, F. Lebetus patzneri (Teleostei: Gobiidae)., a new goby species from the Balearic Islands., western Mediterranean., with first records of Lebetus guilleti (Le Danois., 1913) from this area and Norway., and with notes on its biology. Zootaxa 2019, 4706, 231–254. [Google Scholar] [CrossRef]
- Báez, J.C.; Akyol, O.; Azzurro, E.; Battaglia, P.; Belmonte-Gallegos, A.; Christidis, G.; Crocetta, F.; Dalyan, C.; Gönül, Y.; Grati, F.; et al. New records of rarely reported species in the Mediterranean Sea (March 2025). Mediterr. Mar. Sci. 2025, 26, 256–276. [Google Scholar] [CrossRef]
- Orfanidis, S.; Alvito, A.; Azzurro, E.; Badreddine, A.L.; Souissi, J.B.; Chamorro, M.; Crocetta, F.; Dalyan, C.E.; Fortic, A.; Galanti, L.; et al. New Alien Mediterranean Biodiversity Records (March 2021). Mediterr. Mar. Sci. 2021, 22, 180–198. [Google Scholar]
- Pallaoro, A.; Kovacic, M. Vanneaugobius dollfusi Brownell., 1978 a rare fish new to the Adriatic Sea. J. Fish Biol. 2000, 57, 255–257. [Google Scholar] [CrossRef]
- Schroeder, K.; Chiggiato, J.; Josey, S.A.; Borghini, M.; Aracri, S.; Sparnocchia, S. Rapid response to climate change in a marginal sea. Sci. Rep. 2017, 7, 4065. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Garrido, J.C.; Nadal, I. The Alboran Sea circulation and its biological response: A review. Front. Mar. Sci. 2022, 9, 933390. [Google Scholar] [CrossRef]
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS ONE 2013, 8, e79889. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, F.P. Assessing impacts to maritime shipping from marine chokepoint closures. Commun. Transp. Res. 2023, 3, 100083. [Google Scholar]
- Pons, M.; Stephanis, R.; de Verborgh, P.; Genovart, M. Sharp decreases in survival probabilities in the long-finned pilot whales in Strait of Gibraltar. Mar. Biol. 2022, 169, 44. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Moreira, J.; Espinosa, F.; Ros, M.; Rallis, I.; Sempere-Valverde, J.; Ostalé-Valriberas, E.; Altamirano, M.; García-Gómez, J.C.; Guerra-García, J.M. Evaluating the vulnerability of coralligenous epifauna to macroalgal invasions. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2305–2319. [Google Scholar] [CrossRef]
- Borriglione, M.; Ruitton, S.; Boyer, F.; Thibault, D.; Blanfuné, A.; Guillemain, D.; Verlaque, M.; Boudouresque, C.-F.; Thibaut, T. Impact of the invasive brown alga Rugulopteryx okamurae on the benthic communities in the Northwestern Mediterranean Sea. Estuar. Coast. Shelf Sci. 2024, 310, 109010. [Google Scholar] [CrossRef]
- Souviron-Priego, L.; Márquez, A.L.; Korbee, N.; Figueroa, F.L.; Real, R. Understanding the invasion of the macroalga Rugulopteryx okamurae (Ochrophyta) in the northern Alboran Sea through the use of biogeographic models. Sci. Total Environ. 2024, 955, 176851. [Google Scholar] [CrossRef]
- Smith, K.E.; Aubin, M.; Burrows, M.T.; Filbee-Dexter, K.; Hobday, A.J.; Holbrook, N.J.; King, N.G.; Moore, P.J.; Sen Gupta, A.; Thomsen, M.; et al. Global impacts of marine heatwaves on coastal foundation species. Nat. Commun. 2024, 15, 5052. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Akyol, O.; Al-Hosne, Z.; Rasheed, R.A.; Atac, E.; Bello, G.; Ćetković, I.; Corsini-Foka, M.; Crocetta, F.; Denitto, F.; et al. New records of rare species in the Mediterranean Sea (May 2020). Mediterr. Mar. Sci. 2020, 21, 340–359. [Google Scholar]
- Tsagarakis, K.; Darmanin, S.A.; Al Mabruk, S.; Auriemma, R.; Azzurro, E.; Badouvas, N.; Bakiu, R.; Bariche, M.; Battaglia, P.; Betti, F.; et al. New records of rare species in the Mediterranean Sea (October 2021). Mediterr. Mar. Sci. 2021, 22, 627–652. [Google Scholar] [CrossRef]
- Grech, D.; Asciutto, E.; Bakiu, R.; Battaglia, P.; Ben-Grira, C.; Camlik, O.Y.; Cappuccinelli, R.; Carmona, L.; Chebaane, S.; Crocetta, F.; et al. New records of rarely reported species in the Mediterranean Sea (July 2023). Mediterr. Mar. Sci. 2023, 24, 392–418. [Google Scholar] [CrossRef]
- Lenanton, R.C.J.; Dowling, C.E.; Smith, K.A.; Fairclough, D.V.; Jackson, G. Potential influence of a marine heatwave on range extensions of tropical fishes in the eastern Indian Ocean—Invaluable contributions from amateur observers. Reg. Stud. Mar. Sci. 2017, 13, 19–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).