Climate Variability and Fish Community Dynamics: Impacts of La Niña Events on the Continental Shelf of the Northern South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
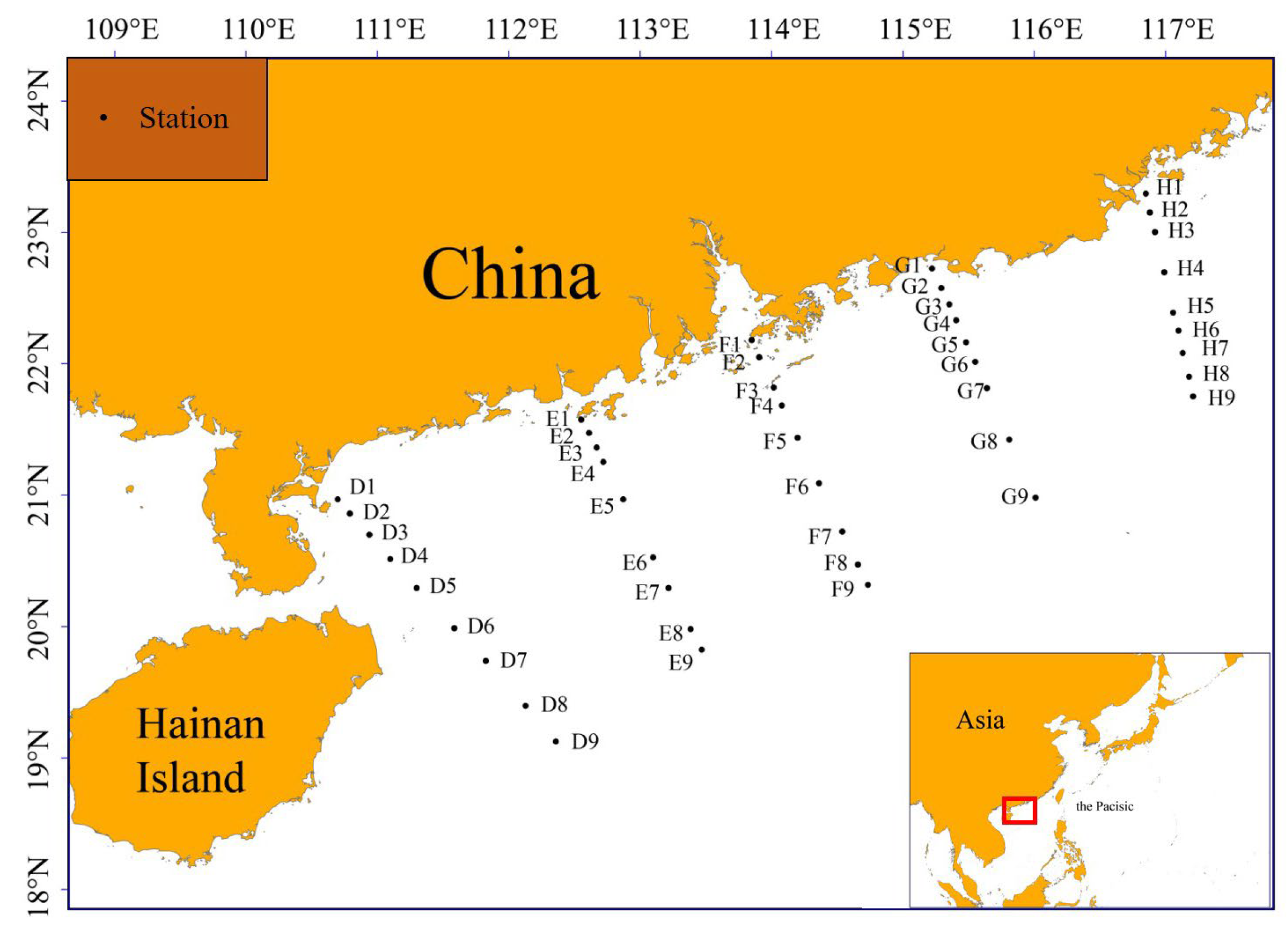
2.1.1. Survey Data
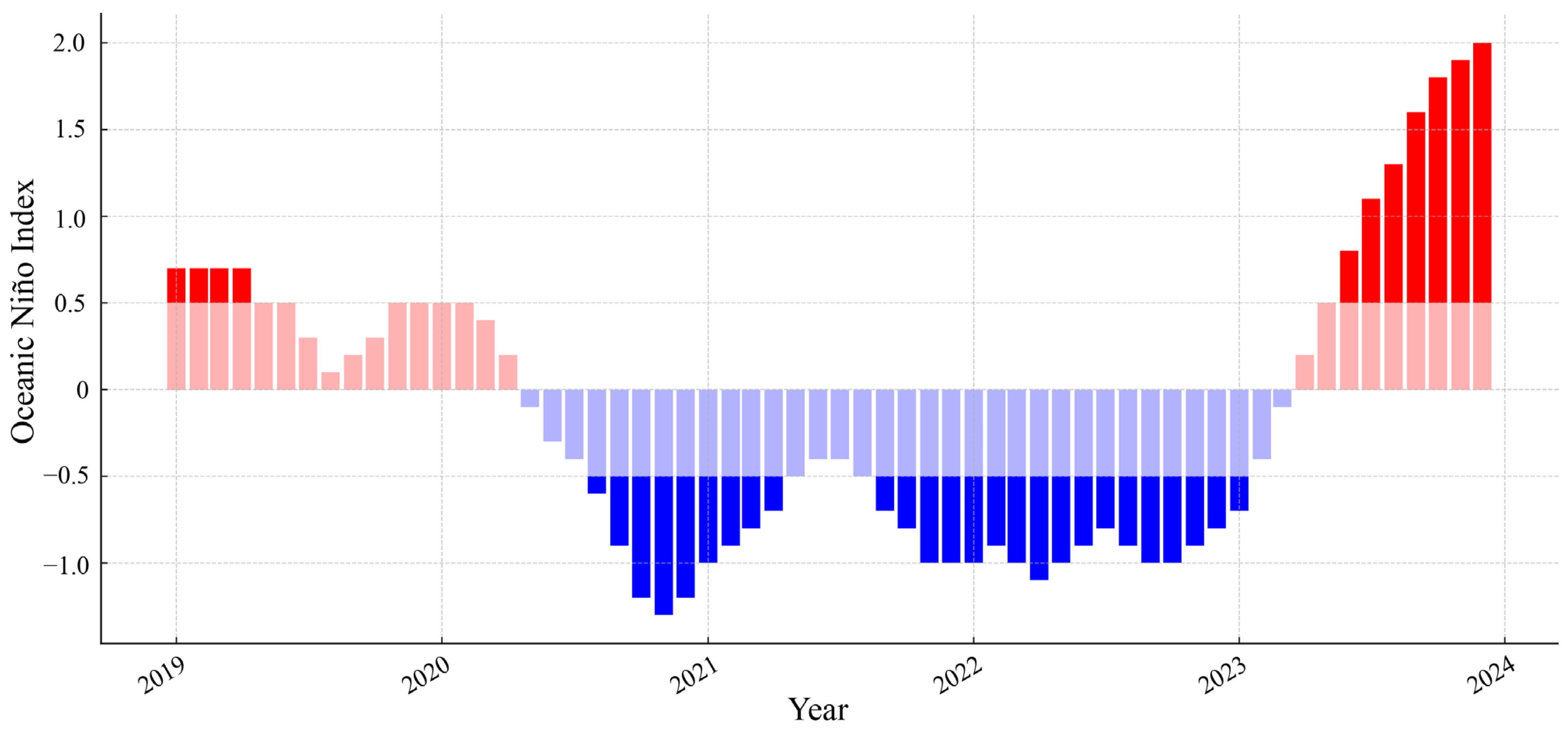
2.1.2. Oceanic Niño Index (ONI)
2.2. Data Analyses
2.2.1. Dominant Fish Species
2.2.2. Fish Diversity
2.3. Fish Community Composition
2.4. Species Distribution Models (SDMs)
2.4.1. Screening of Environmental Data
2.4.2. Ensemble Modeling
3. Results
3.1. Fish Community Composition and Dominant Species
3.2. Fish Diversity and Community Structure
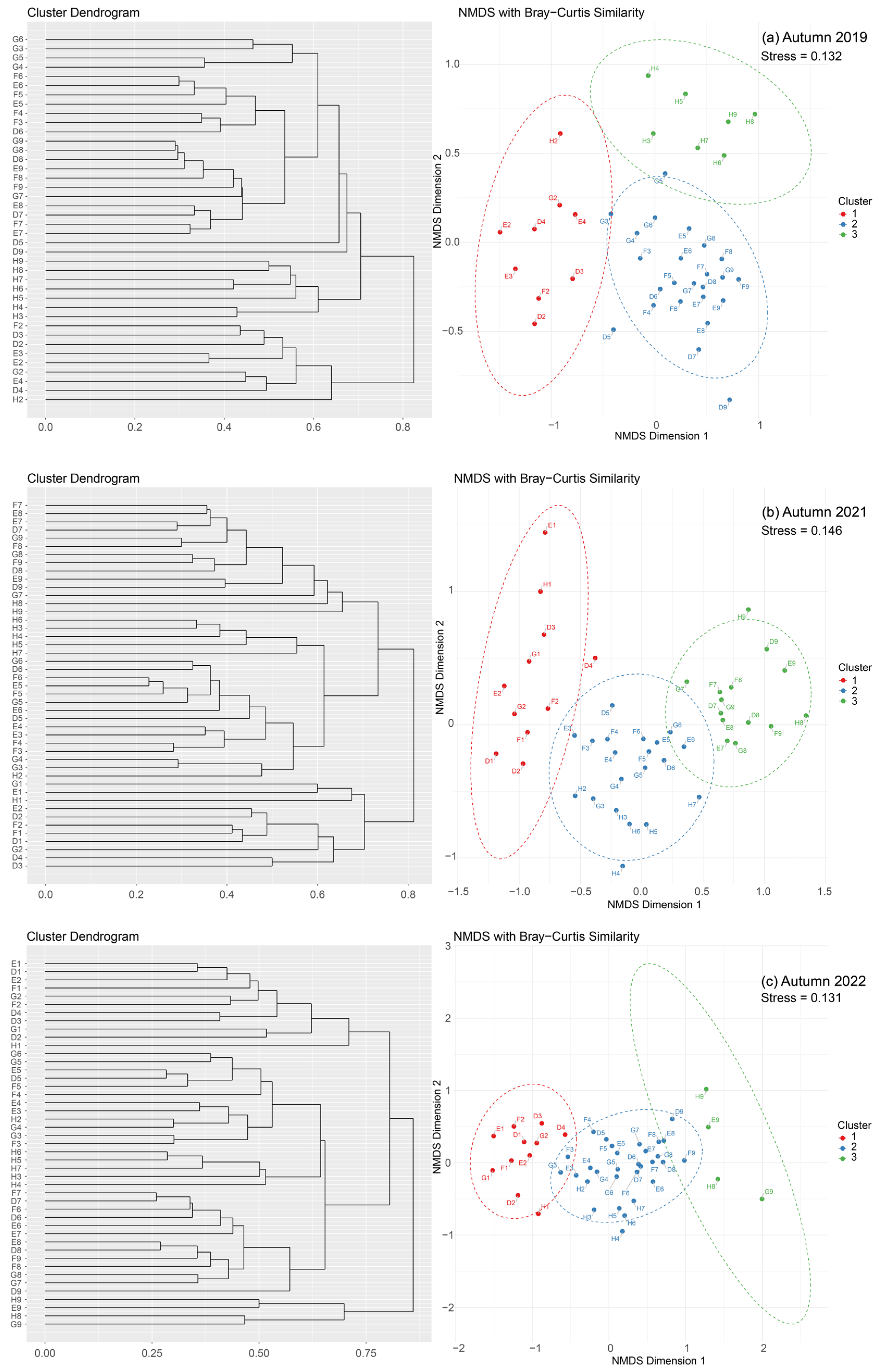
3.3. Distributions of Fish Assemblages
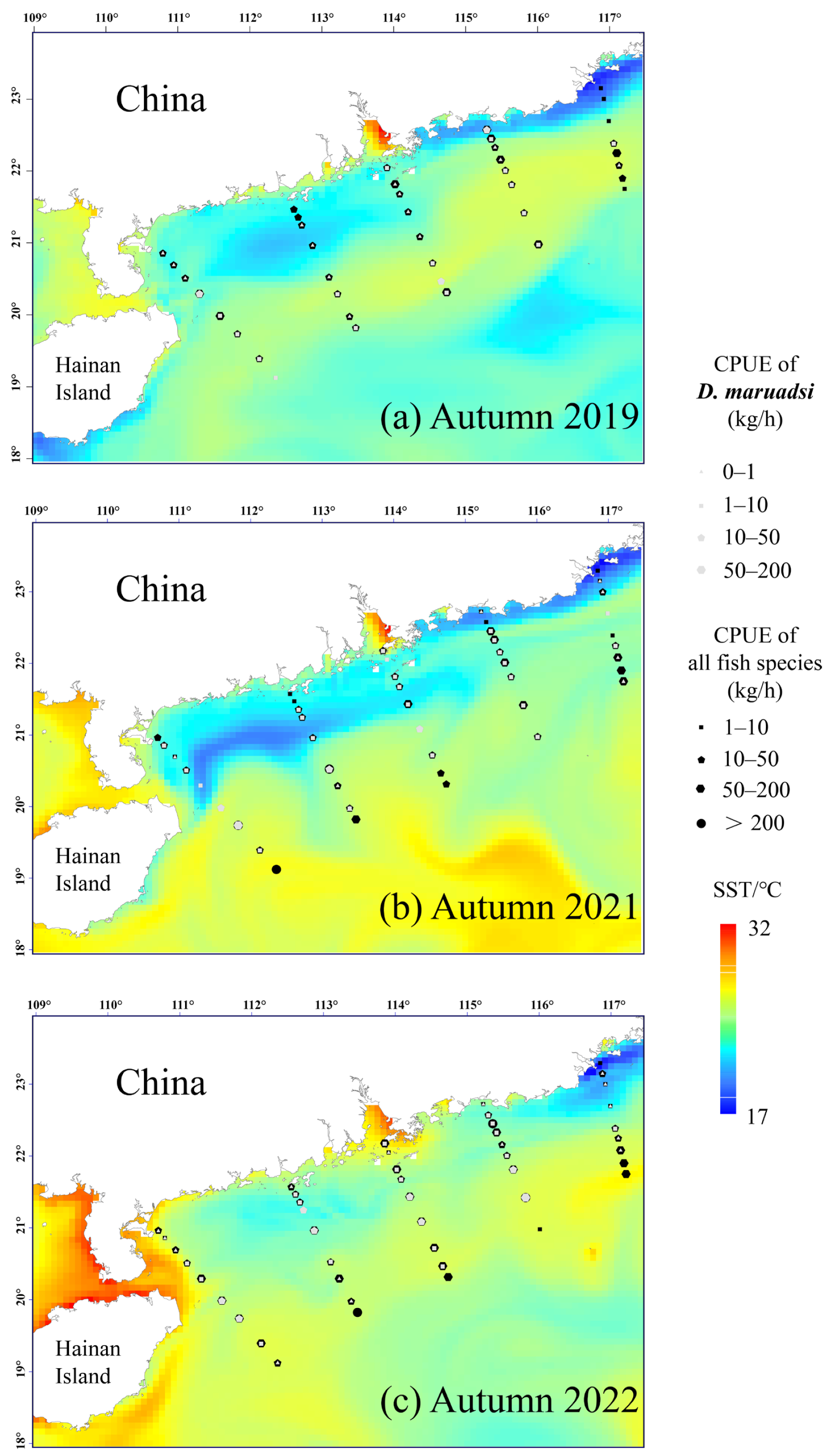
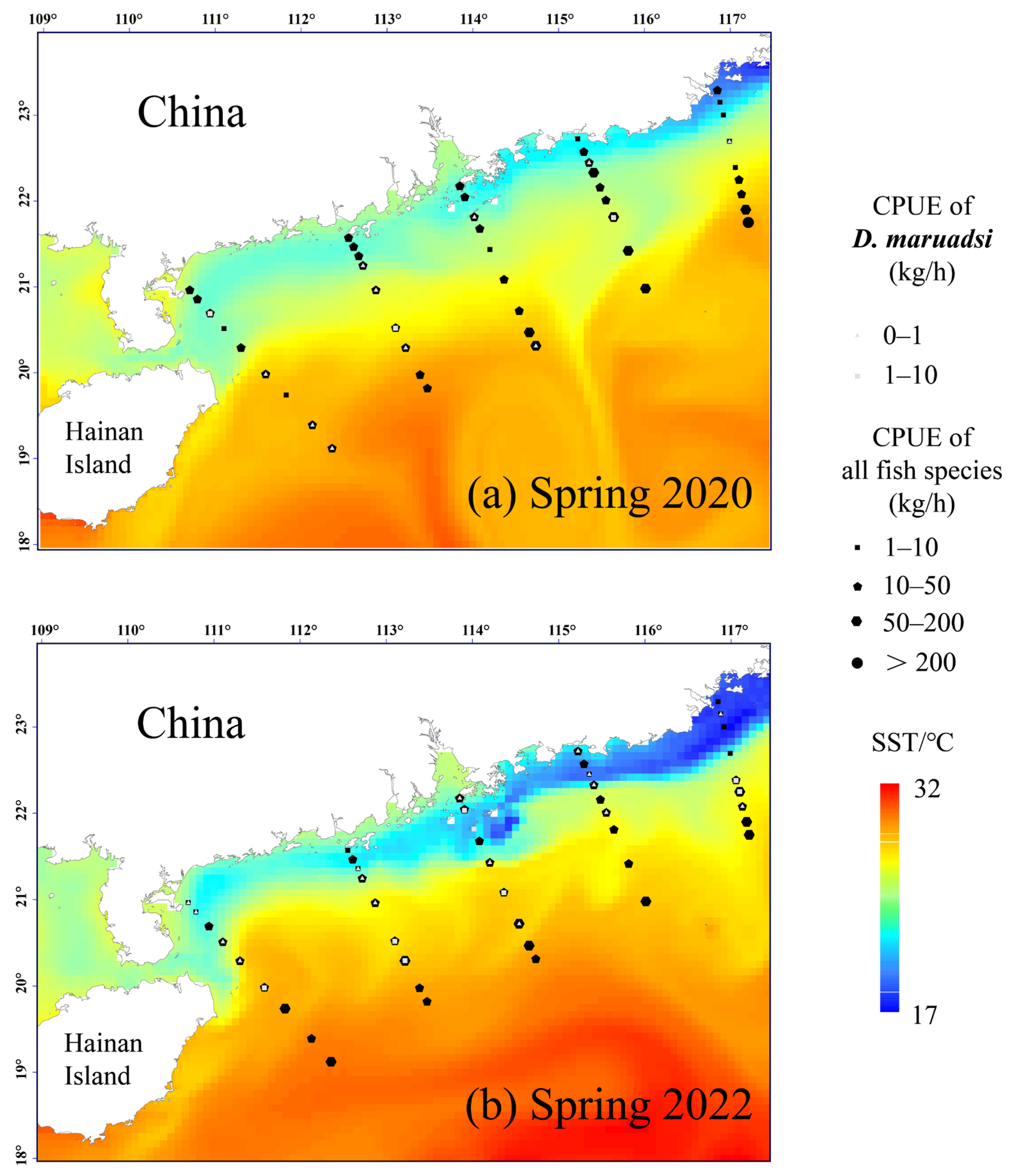
3.4. Phased Variations in the Fish Density Distribution and Habitat Preferences of D. maruadsi
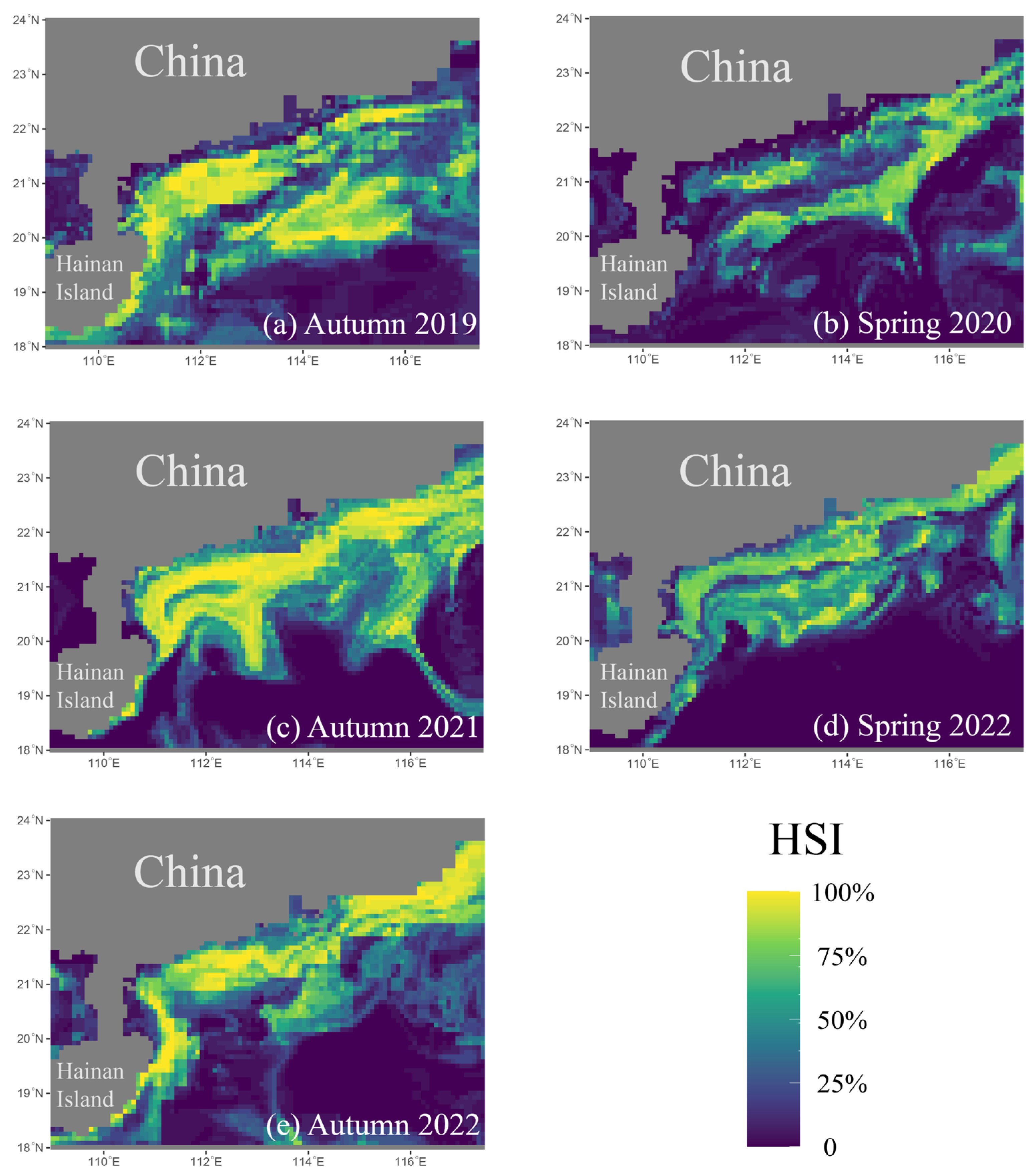
3.5. SDM Analysis
4. Discussion
4.1. Fish Species Composition and the Dominant Species
4.2. Impacts of La Niña on the Fish Community Structure and Seasonal Recovery Dynamics
4.3. Phased Changes in the Fish Distributional Density (CPUE) and Environmental Responses
4.4. SDMs for D. maruadsi
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, A.M.; Vieira, J.; Winemiller, K. Dynamics of the shallow-water fish assemblage of the Patos Lagoon estuary (Brazil) during cold and warm ENSO episodes. J. Fish Biol. 2001, 59, 1218–1238. [Google Scholar] [CrossRef]
- Jaureguizar, A.J.; De Wysiecki, A.M.; Camiolo, M.D.; Clara, M.L. Inter-annual fluctuation in the population structure of an estuarine fish: Influence of environmental drivers. J. Marine Syst. 2021, 218, 103526. [Google Scholar] [CrossRef]
- Boyce, D.G.; Tittensor, D.P.; Garilao, C.; Henson, S.; Kaschner, K.; Kesner-Reyes, K.; Pigot, A.; Reyes, R.B., Jr.; Reygondeau, G.; Schleit, K.E. A climate risk index for marine life. Nat. Clim. Change 2022, 12, 854–862. [Google Scholar] [CrossRef]
- Daly, J.; Knott, C.; Keogh, P.; Singh, G.G. Changing climates in a blue economy: Assessing the climate-responsiveness of Canadian fisheries and oceans policy. Mar. Policy 2021, 131, 104623. [Google Scholar] [CrossRef]
- Barber, R.T.; Chávez, F.P. Ocean variability in relation to living resources during the 1982–83 El Niño. Nature 1986, 319, 279–285. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Yu, W.; Chen, X. Differences in habitat pattern response to various ENSO events in Trachurus murphyi and Dosidicus gigas located outside the exclusive economic zones of Chile. J. Fish. Sci. China 2021, 28, 1195–1207. [Google Scholar]
- Brierley, A.S.; Kingsford, M.J. Impacts of Climate Change on Marine Organisms and Ecosystems. Curr. Biol. 2009, 19, R602–R614. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Allison, E.H.; Bassett, H.R. Climate change in the oceans: Human impacts and responses. Science 2015, 350, 778–782. [Google Scholar] [CrossRef]
- Bigg, G.R.; Jickells, T.D.; Liss, P.S.; Osborn, T.J. The role of the oceans in climate. Int. J. Climatol. 2003, 23, 1127–1159. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Gao, T.-X.; Meng, W.; Jiang, Y.-L. Genome-wide population structure and genetic diversity of Japanese whiting (Sillago japonica) inferred from genotyping-by-sequencing (GBS): Implications for fisheries management. Fish. Res. 2020, 225, 105501. [Google Scholar] [CrossRef]
- Bolin, J.A.; Schoeman, D.S.; Evans, K.J.; Cummins, S.F.; Scales, K.L. Achieving sustainable and climate-resilient fisheries requires marine ecosystem forecasts to include fish condition. Fish Fish. 2021, 22, 1067–1084. [Google Scholar] [CrossRef]
- Philander, S.G.H. El Nino southern oscillation phenomena. Nature 1983, 302, 295–301. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Sun, M.; Li, J.; Zhou, X.; Chen, Z.; Zhang, K. Impacts of strong ENSO events on fish communities in an overexploited ecosystem in the South China Sea. Biology 2023, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, G.F.; Alves, J.C.; Alves, D.C.; Agostinho, A.A.; Gomes, L.C. The response of fish functional diversity to the El Niño Southern Oscillation (ENSO) in a Neotropical floodplain. Hydrobiologia 2021, 848, 1207–1218. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertrand, A.; Hobday, A.; Kiyofuji, H.; McClatchie, S.; Menkes, C.; Pilling, G.; Polovina, J.; Tommasi, D. El Niño Southern Oscillation in a Changing Climate. Hydrobiologia 2020, 2, 429–451. [Google Scholar] [CrossRef]
- Hu, W.; Du, J.; Su, S.; Tan, H.; Yang, W.; Ding, L.; Dong, P.; Yu, W.; Zheng, X.; Chen, B. Effects of climate change in the seas of China: Predicted changes in the distribution of fish species and diversity. Ecol. Indic. 2022, 134, 108489. [Google Scholar] [CrossRef]
- Kang, B.; Pecl, G.T.; Lin, L.; Sun, P.; Zhang, P.; Li, Y.; Zhao, L.; Peng, X.; Yan, Y.; Shen, C. Climate change impacts on China’s marine ecosystems. Rev. Fish Biol. Fish. 2021, 31, 599–629. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, P.; Panhwar, S.K.; Li, J.; Yan, L.; Chen, Z.; Zhang, K. The initial assessment of an important pelagic fish, Mackerel Scad, in the South China Sea using data-poor length-based methods. Mar. Coast. Fish. 2023, 15, e210258. [Google Scholar] [CrossRef]
- Xiong, P.; Cai, Y.; Jiang, P.; Xu, Y.; Sun, M.; Fan, J.; Chen, Z. Impact of climate change on the distribution of Trachurus japonicus in the northern South China Sea. Ecol. Indic. 2024, 160, 111758. [Google Scholar] [CrossRef]
- Zhang, K.; Li, M.; Li, J.; Sun, M.; Xu, Y.; Cai, Y.; Chen, Z.; Qiu, Y. Climate-induced small pelagic fish blooms in an overexploited marine ecosystem of the South China Sea. Ecol. Indic. 2022, 145, 109598. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Sun, M.; Fan, J.; Li, J.; Zhang, K.; Chen, Z. Variation in fish community structure in Beibu Gulf before and after La Niña event. South China Fish. Sci. 2023, 19, 1–11. [Google Scholar]
- Tkachenko, K.S.; Hoang, D.T.; Dang, H.N. Ecological status of coral reefs in the Spratly Islands, South China Sea (East Sea) and its relation to thermal anomalies. Estuar. Coast. Shelf Sci. 2020, 238, 106722. [Google Scholar] [CrossRef]
- Cai, R.; Guo, H.; Fu, D.; Yan, X.; Tan, H. Response and adaptation to climate change in the South China Sea and Coral Sea. In Climate Change Adaptation in Pacific Countries: Fostering Resilience and Improving the Quality of Life; Leal Filho, W., Ed.; Springer: Cham, Switzerland, 2017; pp. 163–176. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhou, Z.; Zhang, Y.; Chen, Z.; Deng, W.; Shi, R. The mass coral bleaching event of inshore corals form South China Sea witnessed in 2020: Insight into the causes, process and consequence. Coral Reefs 2022, 41, 1351–1364. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Lafond, V.; Griess, V.C. Species distribution models (SDM): Applications, benefits and challenges in invasive species management. CABI Rev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Santini, L.; Benítez-López, A.; Maiorano, L.; Čengić, M.; Huijbregts, M.A. Assessing the reliability of species distribution projections in climate change research. Divers. Distrib. 2021, 27, 1035–1050. [Google Scholar] [CrossRef]
- Grenouillet, G.; Buisson, L.; Casajus, N.; Lek, S. Ensemble modelling of species distribution: The effects of geographical and environmental ranges. Ecography 2011, 34, 9–17. [Google Scholar] [CrossRef]
- Robinson, L.; Elith, J.; Hobday, A.; Pearson, R.; Kendall, B.; Possingham, H.; Richardson, A. Pushing the limits in marine species distribution modelling: Lessons from the land present challenges and opportunities. Glob. Ecol. Biogeogr. 2011, 20, 789–802. [Google Scholar] [CrossRef]
- Brodie, S. Integrating dynamic subsurface habitat metrics into species distribution models. Front. Mar. Sci 2018, 5, 219. [Google Scholar] [CrossRef]
- Fromentin, J.-M.; Stemmann, L.; Demarcq, H.; Estournel, C.; Saraux, C. Concomitant changes in the environment and small pelagic fish community of the Gulf of Lions. Prog. Oceanogr. 2020, 186, 102375. [Google Scholar] [CrossRef]
- Pincinato, R.B.M.; Asche, F.; Oglend, A. Climate change and small pelagic fish price volatility. Clim. Change 2020, 161, 591–599. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Pennino, M.G.; Bellido, J.M.; Colmenero, A.I.; Giráldez, A.; Hidalgo, M.; Gabriel Ramírez, J.; Steenbeek, J.; Torres, P.; Cousido-Rocha, M. Changes in life history traits of small pelagic fish in the Western Mediterranean Sea. Front. Mar. Sci. 2021, 8, 570354. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Fish Bulletin 152. Food Habits of Albacore, Bluefin Tuna and Bonito in Californian Waters; Scripps Digital Collection: San Diego, CA, USA, 1971; pp. 11–105. [Google Scholar]
- Gu, D.E.; Yu, F.D.; Hu, Y.C.; Wang, J.W.; Xu, M.; Mu, X.D.; Yang, Y.X.; Luo, D.; Wei, H.; Shen, Z.X.; et al. The Species Composition and Distribution Patterns of Non-Native Fishes in the Main Rivers of South China. Sustainability 2020, 12, 4566. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Hill, T.C.J.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar] [CrossRef]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Wilhm, J.L. Use of biomass units in Shannon’s formula. Ecology 1968, 49, 153–156. [Google Scholar] [CrossRef]
- Lima, A.R.; Baltazar-Soares, M.; Garrido, S.; Riveiro, I.; Carrera, P.; Piecho-Santos, A.M.; Peck, M.A.; Silva, G. Forecasting shifts in habitat suitability across the distribution range of a temperate small pelagic fish under different scenarios of climate change. Sci. Total Environ. 2022, 804, 150167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shan, X.; Gorfine, H.; Dai, F.; Wu, Q.; Yang, T.; Shi, Y.; Jin, X. Ensemble projections of fish distribution in response to climate changes in the Yellow and Bohai Seas, China. Ecol. Indic. 2023, 146, 109759. [Google Scholar] [CrossRef]
- Fujiwara, M.; Martinez-Andrade, F.; Wells, R.D.; Fisher, M.; Pawluk, M.; Livernois, M.C. Climate-related factors cause changes in the diversity of fish and invertebrates in subtropical coast of the Gulf of Mexico. Commun. Biol. 2019, 2, 403. [Google Scholar] [CrossRef]
- Yang, W.; Hu, W.; Chen, B.; Tan, H.; Su, S.; Ding, L.; Dong, P.; Yu, W.; Du, J. Impact of climate change on potential habitat distribution of Sciaenidae in the coastal waters of China. Acta Oceanol. Sin. 2023, 42, 59–71. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Z.; Feng, X.; Yu, W. Climate-related changes in seasonal habitat pattern of Sthenoteuthis oualaniensis in the South China Sea. Ecosyst. Health Sustain. 2021, 7, 1926338. [Google Scholar] [CrossRef]
- Arabameri, A.; Saha, S.; Roy, J.; Chen, W.; Blaschke, T.; Tien Bui, D. Landslide susceptibility evaluation and management using different machine learning methods in the Gallicash River Watershed, Iran. Remote. Sens. 2020, 12, 475. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Fan, W.-Y.; Wang, Z.-G. The predictive performance and stability of six species distribution models. PLoS ONE 2014, 9, e112764. [Google Scholar] [CrossRef]
- Hipfner, J.M.; Galbraith, M.; Bertram, D.F.; Green, D.J. Basin-scale oceanographic processes, zooplankton community structure, and diet and reproduction of a sentinel North Pacific seabird over a 22-year period. Prog. Oceanogr. 2020, 182, 102290. [Google Scholar] [CrossRef]
- Hixon, M. Tropical and temperate reef fishes: Comparison of community structures. In The Ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Academic Press: London, UK, 1991; pp. 509–563. [Google Scholar] [CrossRef]
- Jin, X.; Tang, Q. Changes in fish species diversity and dominant species composition in the Yellow Sea. Fish. Res. 1996, 26, 337–352. [Google Scholar] [CrossRef]
- Sugimoto, T.; Kimura, S.; Tadokoro, K. Impact of El Niño events and climate regime shift on living resources in the western North Pacific. Prog. Oceanogr. 2001, 49, 113–127. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Yi, Q.; Gao, G.; Chen, Y. Impacts of climatic and marine environmental variations on the spatial distribution of Ommastrephes bartramii in the Northwest Pacific Ocean. Acta Oceanol. Sin. 2016, 35, 108–116. [Google Scholar] [CrossRef]
- Lonhart, S.I.; Jeppesen, R.; Beas-Luna, R.; Crooks, J.A.; Lorda, J. Shifts in the distribution and abundance of coastal marine species along the eastern Pacific Ocean during marine heatwaves from 2013 to 2018. Mar. Biodivers. Rec. 2019, 12, 13. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, S.-Z.; Zhou, S.-H.; Zhao, L.; Zhu, J.-Y.; Lukwambe, B.; Nicholaus, R.; Li, C.-H.; Zheng, Z.-M. Structure and functional diversity of surface bacterioplankton communities in an overwintering habitat for large yellow croaker, Pseudosciaena crocea, of the Southern East China Sea. Front. Mar. Sci. 2020, 7, 472. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Xu, Y.; Jiang, Y.e.; Chen, Z. Long-term variations in fish community structure under multiple stressors in a semi-closed marine ecosystem in the South China Sea. Sci. Total Environ. 2020, 745, 140892. [Google Scholar] [CrossRef]
- Huang, M.; Chen, Y.; Zhou, W.; Wei, F. Assessing the response of marine fish communities to climate change and fishing. Conserv. Biol. 2024, 38, e14291. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Q.; Liu, C.; Xian, W. Seasonal and spatial variations in fish assemblage in the Yangtze estuary and adjacent waters and their relationship with environmental factors. J. Mar. Sci. Eng. 2022, 10, 1679. [Google Scholar] [CrossRef]
- Hsiao, P.-Y.; Lan, K.-W.; Lee, W.-H.; Liang, T.-Y.; Liao, C.-H.; Su, N.-J. Impacts of El Niño–Southern Oscillation (ENSO) Events on Trophodynamic Structure and Function in Taiwan Bank Marine Ecosystem. Diversity 2024, 16, 572. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Zuo, T.; Shan, X.; Jin, X.; Sun, J.; Yuan, W.; Pakhomov, E.A. Seasonal changes in zooplankton community structure and distribution pattern in the Yellow Sea, China. Front. Mar. Sci. 2020, 7, 391. [Google Scholar] [CrossRef]
- Wernberg, T.; Smale, D.A.; Tuya, F.; Thomsen, M.S.; Langlois, T.J.; De Bettignies, T.; Bennett, S.; Rousseaux, C.S. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Chaikin, S.; Dubiner, S.; Belmaker, J. Cold-water species deepen to escape warm water temperatures. Glob. Ecol. Biogeogr. 2022, 31, 75–88. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Bogan, M.T.; Bonada, N.; Rios-Touma, B.; Lytle, D.A. Seasonality and predictability shape temporal species diversity. Ecology 2017, 98, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Caballero, C.; Borges-Souza, J.; Ferse, S. Rocky reef fish community composition remains stable throughout seasons and El Niño/La Niña events in the southern Gulf of California. J. Sea Res. 2019, 146, 55–62. [Google Scholar] [CrossRef]
- Moore, J.W.; Schindler, D.E. Getting ahead of climate change for ecological adaptation and resilience. Science 2022, 376, 1421–1426. [Google Scholar] [CrossRef]
- Santiago, J.M.; Muñoz-Mas, R.; Solana-Gutiérrez, J.; Garcia de Jalon, D.; Alonso, C.; Martínez-Capel, F.; Pórtoles, J.; Monjo, R.; Ribalaygua, J. Waning habitats due to climate change: The effects of changes in streamflow and temperature at the rear edge of the distribution of a cold-water fish. Hydrol. Earth Syst. Sci. 2017, 21, 4073–4101. [Google Scholar] [CrossRef]
- Paes, E.T.; Moraes, L.d.S. A new hypothesis on the influence of the El Niño/La Niña upon the biological productivity, ecology and fisheries of the Southern Brazilian Bight. Pan-Am. J. Aquat. Sci. 2007, 2, 94–102. [Google Scholar]
- Daufresne, M.; Boet, P. Climate change impacts on structure and diversity of fish communities in rivers. Glob. Change Biol. 2007, 13, 2467–2478. [Google Scholar] [CrossRef]
- Sims, D.W.; Wearmouth, V.J.; Genner, M.J.; Southward, A.J.; Hawkins, S.J. Low-temperature-driven early spawning migration of a temperate marine fish. J. Anim. Ecol. 2004, 73, 333–341. [Google Scholar] [CrossRef]
- Badjeck, M.-C.; Allison, E.H.; Halls, A.S.; Dulvy, N.K. Impacts of climate variability and change on fishery-based livelihoods. Mar. Policy 2010, 34, 375–383. [Google Scholar] [CrossRef]
- Takasuka, A. Biological mechanisms underlying climate impacts on population dynamics of small pelagic fish. In Fish Population Dynamics, Monitoring, and Management: Sustainable Fisheries in the Eternal Ocean; Tsukamoto, K., Kawamura, T., Takeuchi, T., Beard, T.D., Jr., Kaiser, M.J., Eds.; Springer: Tokyo, Japan, 2018; pp. 19–50. [Google Scholar] [CrossRef]
- Ottersen, G.; Kim, S.; Huse, G.; Polovina, J.J.; Stenseth, N.C. Major pathways by which climate may force marine fish populations. J. Mar. Syst. 2010, 79, 343–360. [Google Scholar] [CrossRef]
- Peck, M.A.; Reglero, P.; Takahashi, M.; Catalán, I.A. Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog. Oceanogr. 2013, 116, 220–245. [Google Scholar] [CrossRef]
- Freitas, C.; Olsen, E.M.; Knutsen, H.; Albretsen, J.; Moland, E. Temperature-associated habitat selection in a cold-water marine fish. J. Anim. Ecol. 2016, 85, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, E.; Noyon, M.; McQuaid, C.D. Hierarchical effects of biogeography and upwelling shape the dietary signatures of benthic filter feeders. Mar. Ecol. Prog. Ser. 2016, 543, 37–54. [Google Scholar] [CrossRef][Green Version]
- Goldenberg, S.U.; Taucher, J.; Fernandez-Mendez, M.; Ludwig, A.; Arístegui, J.; Baumann, M.; Ortiz, J.; Stuhr, A.; Riebesell, U. Nutrient composition (Si: N) as driver of plankton communities during artificial upwelling. Front. Mar. Sci. 2022, 9, 1015188. [Google Scholar] [CrossRef]
- Lorenzen, K.; Camp, E.V. Density-dependence in the life history of fishes: When is a fish recruited? Fish Res. 2019, 217, 5–10. [Google Scholar] [CrossRef]
- Kangur, K.; Kangur, P.; Ginter, K.; Orru, K.; Haldna, M.; Möls, T.; Kangur, A. Long-term effects of extreme weather events and eutrophication on the fish community of shallow Lake Peipsi (Estonia/Russia). J. Limnol. 2013, 72, 376–387. [Google Scholar] [CrossRef]
- McPhaden, M.J. El Niño and La Niña: Causes and global consequences. In Encyclopedia of Global Environmental Change; MacCracken, M.C., Perry, J.S., Eds.; John Wiley & Sons: Chichester, UK, 2002; Volume 1, pp. 353–370. Available online: https://tao.ndbc.noaa.gov/proj_overview/pubs/ElNinoLaNina.pdf (accessed on 13 November 2024).
- Stock, C.A.; Pegion, K.; Vecchi, G.A.; Alexander, M.A.; Tommasi, D.; Bond, N.A.; Fratantoni, P.S.; Gudgel, R.G.; Kristiansen, T.; O’Brien, T.D. Seasonal sea surface temperature anomaly prediction for coastal ecosystems. Prog. Oceanogr. 2015, 137, 219–236. [Google Scholar] [CrossRef]
- Bertignac, M.; Lehodey, P.; Hampton, J. A spatial population dynamics simulation model of tropical tunas using a habitat index based on environmental parameters. Fish. Oceanogr. 1998, 7, 326–334. [Google Scholar] [CrossRef]
- Rijnsdorp, A.D.; Peck, M.A.; Engelhard, G.H.; Möllmann, C.; Pinnegar, J.K. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 2009, 66, 1570–1583. [Google Scholar] [CrossRef]
- Overland, J.E.; Alheit, J.; Bakun, A.; Hurrell, J.W.; Mackas, D.L.; Miller, A.J. Climate controls on marine ecosystems and fish populations. J. Mar. Syst. 2010, 79, 305–315. [Google Scholar] [CrossRef]
- Petitgas, P.; Rijnsdorp, A.D.; Dickey-Collas, M.; Engelhard, G.H.; Peck, M.A.; Pinnegar, J.K.; Drinkwater, K.; Huret, M.; Nash, R.D. Impacts of climate change on the complex life cycles of fish. Fish. Oceanogr. 2013, 22, 121–139. [Google Scholar] [CrossRef]
- Jorge-Romero, G.; Celentano, E.; Lercari, D.; Ortega, L.; Licandro, J.A.; Defeo, O. Long-term and multilevel impact assessment of the 2015–2016 El Niño on a sandy beach of the southwestern Atlantic. Sci. Total Environ. 2021, 775, 145689. [Google Scholar] [CrossRef]


| Variable | Description | Unit | Spatial Resolution |
|---|---|---|---|
| SSS | Seawater salinity | PSU | 0.083° × 0.083° |
| SST | Sea surface temperature | °C | 0.083° × 0.083° |
| Chl-a | Sea surface chlorophyll-a concentration | mg/m3 | 0.25° × 0.25° |
| pH | Seawater pH, reported at the total scale | 0.083° × 0.083° | |
| DO | Dissolved oxygen | mg/m3 | 0.083° × 0.083° |
| MLD | Mixed-layer depth | m | 0.083° × 0.083° |
| Sampling Date | Species | IRI |
|---|---|---|
| Autumn 2019 | Saurida tumbil | 919.1 |
| Decapterus maruadsi | 790.0 | |
| Upeneus bensasi | 738.8 | |
| Spring 2020 | Saurida undosquamis | 869.6 |
| Champsodon atridorsalis | 768.8 | |
| Upeneus bensasi | 677.5 | |
| Saurida tumbil | 613.2 | |
| Trichiurus lepturus | 502.1 | |
| Autumn 2021 | Decapterus maruadsi | 1795.9 |
| Evynnis cardinalis | 883.7 | |
| Synagrops japonicus | 679.5 | |
| Upeneus bensasi | 666.9 | |
| Champsodon atridorsalis | 664.8 | |
| Spring 2022 | Upeneus bensasi | 762.8 |
| Thamnaconus hypargyreus | 589.5 | |
| Trichiurus lepturus | 514.1 | |
| Autumn 2022 | Decapterus maruadsi | 1320.2 |
| Champsodon atridorsalis | 600.0 |
| Sampling Date | Margalef’s Richness Index (D’) | Pielou’s Evenness Index (J’) | Shannon–Wiener Diversity Index (H’) | Simpson Dominance Index (λ) |
|---|---|---|---|---|
| Autumn 2019 | 21.30 | 0.4745 | 2.595 | 0.7681 |
| Spring 2020 | 21.04 | 0.4749 | 2.597 | 0.7653 |
| Autumn 2021 | 23.18 | 0.4357 | 2.429 | 0.7548 |
| Spring 2022 | 20.69 | 0.4396 | 2.406 | 0.7559 |
| Autumn 2022 | 22.73 | 0.4602 | 2.573 | 0.7594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, J.; Zhang, J.; Chen, Z.; Zhang, K. Climate Variability and Fish Community Dynamics: Impacts of La Niña Events on the Continental Shelf of the Northern South China Sea. J. Mar. Sci. Eng. 2025, 13, 474. https://doi.org/10.3390/jmse13030474
Liu Z, Li J, Zhang J, Chen Z, Zhang K. Climate Variability and Fish Community Dynamics: Impacts of La Niña Events on the Continental Shelf of the Northern South China Sea. Journal of Marine Science and Engineering. 2025; 13(3):474. https://doi.org/10.3390/jmse13030474
Chicago/Turabian StyleLiu, Zikai, Jiajun Li, Junyi Zhang, Zuozhi Chen, and Kui Zhang. 2025. "Climate Variability and Fish Community Dynamics: Impacts of La Niña Events on the Continental Shelf of the Northern South China Sea" Journal of Marine Science and Engineering 13, no. 3: 474. https://doi.org/10.3390/jmse13030474
APA StyleLiu, Z., Li, J., Zhang, J., Chen, Z., & Zhang, K. (2025). Climate Variability and Fish Community Dynamics: Impacts of La Niña Events on the Continental Shelf of the Northern South China Sea. Journal of Marine Science and Engineering, 13(3), 474. https://doi.org/10.3390/jmse13030474







