Abstract
Marine fouling communities, characterized by a high abundance of suspension feeders, play a crucial role in regulating ecosystem services, particularly in improving seawater quality. While not typically prioritized in conservation due to their prevalence in degraded or artificial habitats, fouling communities are important for their ecological functions under increasing urbanization and climate change. Bryozoans are an important component of these communities, although their filtering activity is less understood compared to some other groups, such as bivalves and ascidians. In this paper, we aimed to investigate the filtration activity of two widespread fouling bryozoan species, namely Schizoporella errata and Bugula neritina in the northern Adriatic (Slovenia). We measured the clearance rates (CR) of both the species when fed with microalgae to assess their filtration capacity and determine the most suitable units for quantifying the biofiltration. B. neritina exhibited a higher average CR than S. errata. The maximum CR was 32 mL/(h·cm2) for the S. errata and 52 mL/(h·cm2) for the B. neritina. Due to the morphological and growth differences between the species, the surface area was determined to be the most appropriate unit for expressing the CR. We also examined the CR of the S. errata exposed to fluorescent microplastic beads and identified active feeding areas within the colonies. Feeding zooids in S. errata were concentrated at the terminal growth margins and elevated areas of the frontal budding, as indicated by a higher fluorescence and microsphere density. These results contribute to the existing knowledge on fouling bryozoans in coastal habitats and provide further insights into their potential role as biofilters and contributors to ecosystem functioning.
1. Introduction
The degradation of the marine environment is one of the main problems threatening the marine ecosystems. The development of the maritime infrastructure, such as marinas, harbors and mariculture facilities, is profoundly altering the ecosystem and causing changes in its functioning. The fouling community that thrives in these environments is generally considered a nuisance that causes problems in shipping and other marine industries. These artificial habitats do not act as a substitute for natural rocky habitats and serve as a hotspot for non-indigenous species [1]. On the other hand, the synanthropic fouling community also provides regulating ecosystem services, manifested primarily in the improvement of the seawater quality due to the prevalence of suspension feeders in this community [2]. Many studies have highlighted a strong controlling effect of large aggregations of suspension feeders, such as bivalves, on phytoplankton associated with shifts in its composition ([3] and references therein]). A better understanding of the ecological functions of the marine communities shaped by urban infrastructure is important to protect biodiversity in the face of growing coastal populations and anticipated global change [1].
Several invertebrate suspension feeders have so far been proposed as bioremediators of polluted and eutrophic environments due to their ability to effectively clear (toxic) microalgae and bacteria from the water column. The groups which were primarily studied for their bioremediation abilities were ascidians [4,5], sabellid polychaetes [6,7,8] and sponges [9,10,11]. Bryozoans are mostly considered unimportant in this regard, due to their small size and poor retention efficiency [12]. However, small epifaunal suspension feeders (e.g., bryozoans, hydroids, spirorbins, barnacles and amphipods) appear to be important in controlling local densities of suspended organic matter in seagrass meadows [13].
Early research on the clearance rates (CR) of bryozoans [14,15] made it clear that they are efficient suspension feeders, and some research suggests that bryozoans respond very plastically to their diet [12,16,17,18,19,20]. However, much is still unknown and only a few studies exist on the clearing capacity of bryozoan colonies and assemblages [21].
Our motivation in the study was to tackle a knowledge gap in the suspension feeding of fouling bryozoans. The aim of the study was to investigate the filtration activity of these animals, focusing on their role in biofiltration as an ecosystem service. The first objective was to estimate the clearance rates of two widespread cheilostomatid bryozoan species, Schizoporella errata (Waters, 1878) and Bugula neritina (Linnaeus, 1758). The second objective was to find the appropriate units to quantify the clearance rates in order to compare them among the bryozoan species. Finally, the third objective was to identify the most active feeding regions within the S. errata colonies and to examine the relationship between the colony morphology and these active regions.
2. Materials and Methods
2.1. Studied Species and the Study Area
Both species are common fouling species with a cosmopolitan distribution and a status of cryptogenic or questionable species, respectively, in Europe [22]. They are opportunistic species which thrive in a range of environmental conditions and their optimum conditions can vary depending on the location. Both species form heterogeneous erect calcified structures, which provide shelter and food for several other organisms [23,24,25].
S. errata is a fast-growing encrusting to erect bryozoan species, with the average lateral growth rate in favorable conditions of 0.73 cm2/day [26]. Autozooids have irregular shapes and highly variable dimensions, oriented differently in multilaminar colonies. Zooids are without spines, and avicularia are rare [27]. Due to its plastic budding abilities [28], this species can form large and heterogeneous colonies with tubular antler shaped to lobe-forming erect calcified structures [29]. Such colonies reach 40 cm in size [24], and the reef-like structures can be up to 1 m long [30] (Figure 1A). The species can be found in pleiomesohaline, euhaline and polyhaline waters of warm temperate to tropical regions [31] and does therefore exhibit a high tolerance to temperature and salinity. Due to the aforementioned characteristics, S. errata also has a high invasive potential [27].

Figure 1.
Studied species: (A) large colonies of Schizoporella errata observed in a mussel farm (photo: T. Makovec), (B) a colony of Bugula neritina (photo: B. Mavrič), (C) feces from a colony of S. errata fed with plastic microspheres, as viewed under a microscope (Photo: A. Fortič).
B. neritina is a part of a species complex, of which one has a worldwide distribution (Type S) [32]). B. neritina has an arborescent growth type: autozooids are arranged in two rows and have no spines; the entire frontal part of the zooid is membranous; no avicularia are present [33] (Figure 1B). This species’ recruitment occurs between 19 and 24 °C globally [34] and can survive at temperatures 9–30 °C (laboratory conditions, Japan) [35]. The range of salinity in the field where colonies of this species were observed to grow is 18–30 [36].
Piran Harbor, the place where the experimental colonies were collected, is located in the Gulf of Trieste, a shallow gulf at the northernmost end of the Adriatic Sea (Figure 2). The northern Adriatic Sea is known for pronounced tidal fluctuations [37] and low temperatures in winter, which average around 10 °C [38]. Although the salinity in the gulf is generally marine, it is significantly influenced by substantial freshwater inputs, particularly from the Soča river. This river supplies large quantities of detrital material originating from the hinterland. The area is also characterized by the resuspension of bottom sediments, increasing pollution and other anthropogenic pressures [39,40].
Figure 2.
A map of the study area.
2.2. Sampling of Bryozoans and Acclimatization
Bryozoan colonies of the two bryozoan species used in the experiments, S. errata and B. neritina, were collected from Piran Harbor (Figure 2) in November 2021 and transferred to flow-through aquaria at the Marine Biology Station (National Institute of Biology) in Piran, Slovenia. Prior to experiments, we confirmed that the animals ingested the selected particles (microalga Dunaliella sp. and plastic microspheres), which appeared in their feces (Figure 1C). The colonies were cut into small fragments (a few cm), a process shown to not affect feeding [41]. Epibionts were removed, and the cut ends were secured with nontoxic biopolymer glue, serving as holders for placement in experimental beakers. The prepared colonies were left in the aquarium for a few days to recover, then acclimated for 24 h in filtered seawater (0.22 µm) at 17.5 °C—which is the average annual surface temperature of the Slovenian coastal sea [42]. Approximately three hours before the experiment, the colonies were placed in the glass beakers filled with filtered seawater.
2.3. Experimental Setup
We conducted two experiments. The first aimed to assess the clearance rate (CR) of two bryozoan species, S. errata and B. neritina, and to determine the most appropriate units for quantifying their biofiltration capacity. In this experiment, the colonies were fed the microalga Dunaliella sp. (average size: 9 μm × 4 μm). The second experiment focused exclusively on S. errata colonies and aimed to identify active feeding areas within the colonies while quantifying their feeding activity using 10 μm fluorescent polystyrene microspheres (Fluoresbrite® YG, Polysciences, Harrisburg, PA, USA; density 1.05 g/cm3). Analysis showed the surface properties (surface tension and wettability in saltwater) of these plastic particles were comparable to those of microalgae (Tetraselmis, Nitzschia) [43]. The experimental setup is shown in Figure 3.
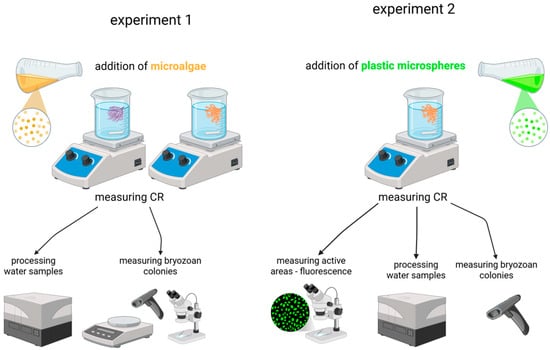
Figure 3.
The schematic representation of the experimental setup. The purple bryozoan represents B. neritina and the orange one S. errata. Created with BioRender.com.
The CR was determined by measuring the decrease in particle concentration in suspension under controlled conditions. Both experiments were conducted in 300 mL glass beakers containing filtered seawater with an initial particle concentration of around 5 × 103 particles/mL. A magnetic stirrer at a low speed ensured homogeneous suspension and prevented erroneous density measurements. Temperature, salinity and dissolved oxygen were recorded at the start and end of each experiment to ensure the conditions were optimal. During the experiments, the salinity was 37.5–38.0, the temperature was 17.2–18.0 °C and the dissolved oxygen concentration was 8.79–9.37 mg/L. The particles were added to the experimental beakers at the beginning of the experiments. We then waited 15 min for the animals to acclimatize to the food after the starvation period to see if they began with feeding activity and then took the first sample (t0), which was followed by taking samples every half hour until the end of the experiment after three hours, seven times in total. In the first experiment, there were 13 experimental beakers containing bryozoan colonies and 2 control beakers without colonies, containing only microalgae, for each species. In the second experiment, there were 11 experimental beakers with colonies and 3 control beakers containing only microalgae. The samples of microalgae/microspheres were taken in triplicate of 1 mL each and processed immediately afterwards. At the end of the experiment, bryozoan colonies were stored in FineFIX ethanol fixative (Milestone Medical, Bergamo, Italy).
2.4. Measuring the Bryozoan Colonies from Both Experiments and Measuring of the Active Feeding Areas
To measure the surface area of the colonies, two approaches were used for each bryozoan species. The B. neritina colonies from the first experiment were cut into smaller fragments, stained with crystal violet for a better contrast, washed well with distilled water and scanned (Epson Perfection 3200 Photo, Suwa, Japan). The photos were calibrated, and the area of the colonies was calculated using ImageJ 1.53t [44]. S. errata colonies from the first and second experiment were scanned with a 3D scanner (EinScan-SP, Shining 3D, Hangzhou, China) and the area of the 3D model was measured with an EinScan S program. To estimate the density of autozooids per unit area of the colony, the zooids of the B. neritina colonies were counted using a stereomicroscope. For S. errata colonies, we estimated the number of autozooids based on the average density per unit area. The colony fragments were photographed with a stereomicroscope (Olympus SZX16, Tokyo, Japan) and a digital camera (Olympus DP25, Tokyo, Japan) and analyzed in ImageJ [44]. We counted autozooids in 3–4 areas from different parts of each colony.
Later, the colonies from the first experiment were rinsed with distilled water. The S. errata colonies were then carefully broken open to remove sediment deposits and hydrozoan remains, which are frequently overgrown by the bryozoan, forming characteristic tubular structures. They were then transferred to pre-weighed evaporators and dried at 60–65 °C for at least 48 h or until the weight stabilized. After measuring the dry weight (DW) to the nearest 0.01 mg, the colonies were ashed at 500 °C for 4 h. The ash-free dry weight (AFDW) was calculated by subtracting the ash mass from the DW.
We assessed the proportion of actively feeding S. errata colonies (active surface area) and identified the most active feeding regions of microsphere-fed colonies using a stereomicroscope. The yellow-green fluorescence of the microspheres was observed with blue light excitation and a yellow filter. Previously, the microsphere-fed colonies were immersed in diluted sodium hypochlorite for 2 min to dissolve the epicuticle and visualize the fluorescence. The colonies were photographed with the colony surface perpendicular to the light path through the stereomicroscope and analyzed in ImageJ [44,45].
2.5. Processing of Water Samples
The algal density and microspheres density in the water samples from both experiments was counted using an indirect fluorescence intensity measurement method (with a Tecan Spark® (Männedorf, Switzerland) microplate reader). A calibration curve correlating the fluorescence intensity with the cell density was first created for both Dunalliela sp. and microspheres (as in [9]) and used for cell quantification in the samples. The initial density of microalgae cells in culture was checked using a Fuchs-Rosenthal counting chamber, while the initial density of microspheres was known (manufacturer’s data). Fluorescence intensity was used to measure the chlorophyll-a content in the microalgae samples and was therefore measured at an excitation wavelength of 440 ± 20 nm and an emission wavelength of 680 ± 20 nm [46]. For fluorescent microspheres, it was measured at an excitation wavelength of 441 ± 20 nm and an emission wavelength of 486 ± 20 nm (according to the manufacturer’s instructions). For the measurements, 1 mL of sample was centrifuged for 5 min at 13,000 RCF or 20,000 RCF in the case of microspheres. The supernatant was then pipetted off and the pellet of particles was resuspended using a vortex mixer and the fluorescence intensity was measured in black 96-well microtiter plates. As a negative control, filtered seawater was also measured and the measured fluorescence intensity was subtracted from the samples.
2.6. Data Analysis
To calculate the CR, we applied a logarithmic transformation to the particle density, plotted the temporal trend of particle decrease for each colony and checked the exponential decrease with a linear regression line [47]. The CR for each hour of the experiment was calculated according to Coughlan’s equation [48] and corrected with the control beaker rates, considering control suspensions and experimental suspensions were measured simultaneously. The average of three repeated particle density measurements was used for the calculations. We compared the CRs per unit area of the two species when fed with microalgae and used a t-test to check whether the differences were statistically significant (α = 0.05). For each bryozoan colony, we also determined the maximum clearance rate (CRmax), which represents the filtration capacity of the colony (as recommended by [49,50]). The CRmax was expressed per unit of total surface area (cm2), DW (g), AFDW (g) and per autozooid (zooid). For microsphere-fed colonies, the CR was also reported per estimated unit of active surface area (cm2). Data were analyzed using Microsoft Excel 2016 and R software (RStudio v. 1.4.1717; packages tidyverse, ggplot2 and stats).
3. Results
3.1. Clearance Rates and Morphological Insights in S. errata and B. neritina
In the first experiment, the algal concentrations in the beakers decreased exponentially, while the concentration in the control beakers remained relatively stable, although a slight decrease was observed in the controls measured together with B. neritina (Figure 4B). When feeding the S. errata (Figure 4A), the algae density fell to an average of 34% of the initial value, while the microalgae density in B. neritina beakers fell to an average of 16% of the initial value at the end of the experiment. The colonies in the experiment were actively feeding throughout the experiment, as evidenced by the extruding lophophores. Nevertheless, the decrease in experimental beakers with B. neritina was faster compared to S. errata.
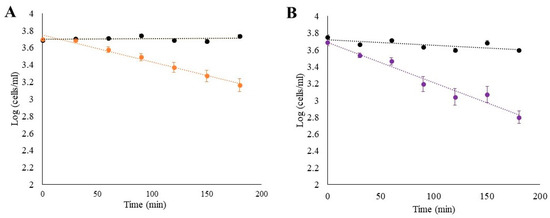
Figure 4.
A comparison of the decrease in cells of the Dunalliela sp. when two species, (A) Schizoporella errata and (B) Bugula neritina were feeding on them. The black lines represent controls, the orange line the S. errata treatment and the purple the B. neritina treatment.
The observed different decrease in the algal cell density is also due to the size of the colony fragments used in the experiments. Figure 5 shows the measurements of the colonies of S. errata and B. neritina used in the first experiment and their correlations. The colonies (or parts of colonies) of S. errata were similar in length but ranged from 3.66 to 13.13 cm2 surface area and were mostly villiform, whereas the colonies of B. neritina ranged in area from 2.37 to 7.64 cm2 and were arborescent. In S. errata, we counted an average of 575 autozooids per cm2, and the proportion of autozooids in all zooids was 85–100%. The other zooids were mostly immature zooids, in the stages of frontal or lateral zooid budding or kenozooid. The average density of B. neritina was 535 autozooids per cm2, with 100% of zooids being autozooids and the proportion of zooids in which whole polyps were present averaging 66.9%. The remainder were non-feeding zooids containing only brown bodies or zooids in the early differentiation phases. The correlation between the area and number of zooids was strong (r2 = 0.82; Figure 5C). The biomass per square centimeter of the B. neritina colonies (4.35 mg/cm2 DW, 1.59 mg/cm2 AFDW) was much lower compared to the colonies of S. errata (43.87 mg/cm2 DW, 3.98 mg/cm2 AFDW), and the values were less scattered. The proportion of AFDW averaged 9.1% of DW in S. errata and 37.2% of DW in B. neritina. The correlation between the surface area and the DW was moderate for S. errata (r2 = 0.54) and strong for B. neritina (r2 = 0.77, Figure 5A), while the correlation between the DW and AFDW was strong for S. errata (r2 = 0.98) and moderate for B. neritina (r2 = 0.45; Figure 5B).
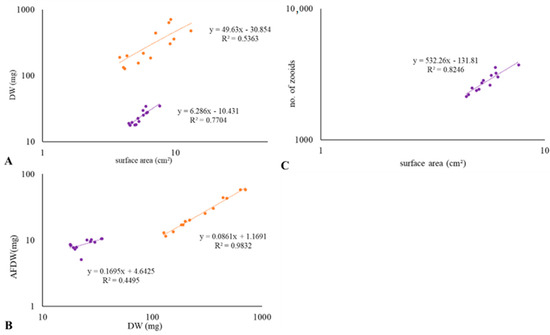
Figure 5.
Colony fragment measurements of S. errata (orange) and B. neritina (purple): (A) the correlation between the surface area and the dry weight of the colonies, (B) the correlation between the dry weight and the ash free dry weight of the colonies, (C) the correlation between the surface area and the number of zooids in colonies.
Due to the differences in weight and morphology between the species, the surface area is the most useful unit for comparing the CR. The average CR per unit area was 20.08 mL/(h·cm2) for S. errata and 33.14 mL/(h·cm2) for B. neritina (Figure 6). These differences were statistically significant (t-test; −2.5037, df = 24, p = 0.019).
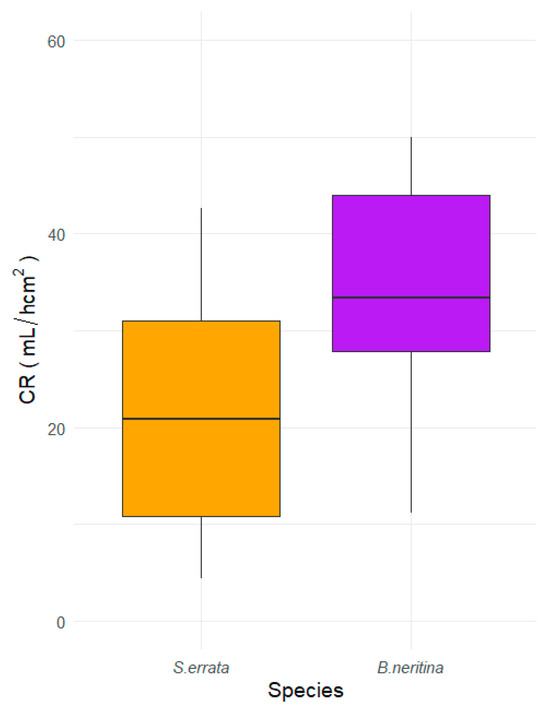
Figure 6.
Comparison of CR per surface area unit of two bryozoan species, S. errata and B. neritina, fed with Dunalliela sp.
Table 1 presents the average maximum clearance rates, which reflect the filtration capacity of the colonies for both species across different units. The data reveals significant differences in the maximum clearance rates between the colonies and species, attributed to the high plasticity of the morphologies in both species. Due to the non-comparable biomineralization and skeletal structure between the species, and in the case of S. errata, potentially also due to variations in skeletal biomass across the colonies, the use of CR per biomass unit is not an appropriate measure.

Table 1.
CRmax ± confidence interval (95%) given in different units.
The raw data for both experiments are provided in Table S1.
3.2. Microplastic Clearance Rates and Active Colony Regions in S. errata
We investigated the correlation between the CR and the surface area of the colonies (Figure 7). We found no correlation between the CR and the total surface area of the colonies (r2 = 0.14; Figure 7A); on the other hand, the correlation was more pronounced when we considered the active surface area of the colonies and not the total surface area (r2 = 0.50; Figure 7B).
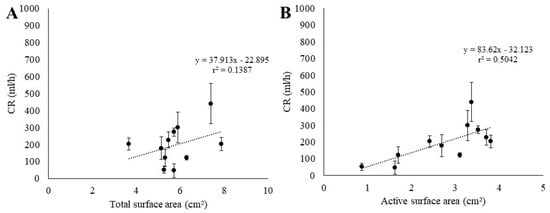
Figure 7.
Relationship between CR (average in black bullets ± SE in error bars) and estimated total surface area (A) and active surface area (B) of S. errata colonies fed with plastic beads.
Observation of S. errata colonies with fluorescence filters revealed the presence of ingested microspheres in polyps within the colony (Figure 8). In most colonies, we observed a higher fluorescence, i.e., higher density of microspheres and therefore feeding zooids, at the terminal growth edges of the colonies (Figure 8A–C) and in areas of the colony where frontal budding had occurred, i.e., at elevated parts of the colony. No colony fluoresced completely, which means that not all zooids were active everywhere in the experiment. The proportion of the colony surface actively feeding on microspheres was estimated to be 48% (16–68%) on average. Observation of the colony, which showed no feeding activity based on the absence of protruding lophophores, was supported by fluorescence analysis, revealing that only a few autozooids were feeding on the microspheres (Figure 8D). In addition, we observed some individual microspheres on the surface of the colonies, suggesting that some adhesion to the surface also occurred during the experiments.
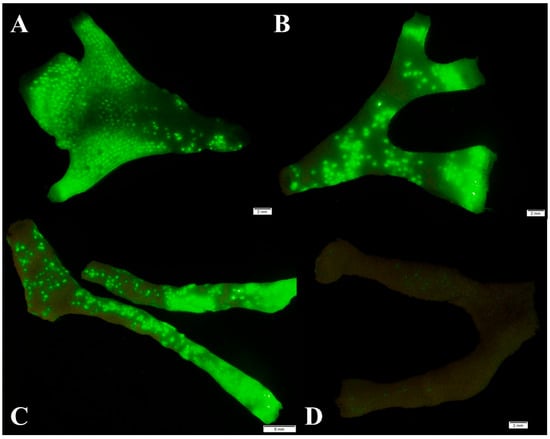
Figure 8.
Colony fragments of S. errata which were fed with fluorescent microspheres, where fluorescence of the beads in the polypides is clearly visible (A–C) and a colony fragment where the colony did not feed (D).
4. Discussion
4.1. Comparison of CR in Bryozoan and Other Species
A comparison of the CR measured in different bryozoan species is presented in Table 2. The average CRmax per unit area for the species studied here, a total CRmax of 32 mL/(h·cm2) for S. errata and 52 mL/(h·cm2) for B. neritina, closely aligns with the values reported by Lisbjerg and Petersen [21]. Additionally, we calculated the CRmax for the active portion of S. errata colonies, which was 102 mL/(h·cm2). Lisbjerg and Petersen [21] reported a CRmax of 69–115 mL/(h·cm2) for the active part of Arbocuspis bellula (Hincks, 1881) colonies at various temperatures, equivalent to 34–56 mL/(h·cm2) for the entire colony. In comparison, the CR measurements for the Membranipora membranacea (Linnaeus, 1767) at a flow velocity of 3 cm/s were at least three times higher [18], consistent with the findings of Pascoe et al. [20] for the same species.
Echevarria et al. [51] studied the CR of the B. neritina per unit biomass when exposed to a mixture of toxic and non-toxic microalgae compared to non-toxic microalgae alone. In the toxic treatment, where Karenia brevis was mixed with Rhodomonas sp., the CR was 75% lower than in the non-toxic treatment, with a rate of 13.58 L/(h·g) AFDW. These last values are about three times lower than our findings, where a CR of 35.84 L/(h·g) AFDW was recorded. The lower CR in that study may be due to the high initial algal density of approximately 2 × 104 cells/mL.
The higher CR observed in B. neritina colonies compared to S. errata colonies in our study may be attributed to the larger lophophores of B. neritina (greater diameter and more tentacles; Table 2). Additionally, in natural conditions bushy bryozoan species like B. neritina possess competitive advantages over encrusting forms, as their elevated position in the water column provides better access to food, reduces competition and predation risks and minimizes the negative effects of sedimentation.
In order to put the reported bryozoan CR into perspective, the CRs of some other suspension feeders from the Mediterranean Sea are presented. In this region, filtration activity has primarily been studied in ascidians, bivalves, sponges and some polychaetes. Most results are expressed in liters per hour per gram of dry weight (L/(h·g)). In the 1980s, Fiala-Médioni examined the filtration activity of three ascidian species in the western Mediterranean (France): Ciona intestinalis (Linnaeus, 1767), Phallusia mammillata (Cuvier, 1815), and Styela plicata (Lesueur, 1823). The CRs recorded were 4.33, 4.78 and 8.76 L/(h·g), respectively [52]. More recently, Stabili et al. (2016) investigated S. plicata from the Ionian Sea (Italy) and Polyandrocarpa zorritensis (Van Name, 1931) from the Adriatic Sea (Italy), reporting CR of 1.50 and 1.75 L/(h·g), respectively, when individuals were fed the pathogenic bacterium Vibrio alginolyticus [5].
For bivalves, the average CR of Pinna nobilis Linnaeus, 1758—one of the largest Mediterranean bivalves—ranged from 0.55 to 14.55 L/(h·g) across increasing temperatures in the northwestern Mediterranean (Spain) [53]. In Ostrea edulis Linnaeus, 1758, an average CR of 1.73 L/(h·g) was reported under high feeding conditions in Mar Menor (Spain) [54].
Among sponges, the maximum CR for Spongia (Spongia) officinalis Linnaeus, 1759 was 0.21 L/(h·g), focusing on bacterioplankton in the Ionian Sea [10]. In a separate study on four Mediterranean sponge species under varying conditions and microalgal diets, the highest CR was observed in Agelas oroides (Schmidt, 1864), reaching 0.01 L/h per gram of wet weight in the Aegean Sea (Greece) [9].
In polychaetes, the highest CR was reported for Branchiomma luctuosum (Grube, 1870) at 43.2 L/(h·g), followed by Sabella spallanzanii (Gmelin, 1791) at 12.4 L/(h·g), both fed with V. alginolyticus in the Ionian Sea [7].

Table 2.
A literature review of the clearance rates measured in the laboratory for different bryozoan species. az—autozooid, CR—clearance rate, DW—dry weight, NS—not specified.
Table 2.
A literature review of the clearance rates measured in the laboratory for different bryozoan species. az—autozooid, CR—clearance rate, DW—dry weight, NS—not specified.
| Suborder | Species | Colony Form | No. az/cm2 | CR (mL/h) | % Active Zooids During Feeding | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| per g DW (total) | per cm2 (total) | per zooid * | ||||||||
| active | total | NS | Theoretical # | |||||||
| Flustrina | Schizoporella errata | encrusting-erect | 575 a | 856 a | 32 and 49 a | 0.18 a | 0.05 and 0.09 a | 1.1–1.3 | 48 a | |
| Flustrina | Bugula neritina | erect | 535 a | 12,371 a | 521 | 0.10 a | 1.9–2.0 | |||
| Membraniporina | Arbocuspis bellula | encrusting | 1265 [21] | 9500 [21] | 34–56 [21] | 0.08 [21] | 0.42–0.50 | 49 [21] | ||
| Flustrina | Celleporella hyalina | encrusting | 0.12–0.17 [12] | 0.5 | 65 [16] | |||||
| Membraniporina | Conopeum reticulum | encrusting | 0.25–0.41 [15] | 0.42–0.46 | ||||||
| Membraniporina | Electra pilosa | encrusting-erect | 0.28 [16] | 0.20–0.39 [15] | 0.46–0.84 | 92 [16] | ||||
| Membraniporina | Membranipora membranacea | encrusting | 123 [20] | 0.36 [20] | 1.06 | |||||
| Vesicularina | Amathia verticillata | erect | 33,700 [14] | 0.37 [14] | 0.36 | |||||
* In some studies, CR is expressed per active zooids—active, in others per whole colony—total, or the information about it was not specified—NS. # Theoretical CR is based on particle velocity measurements by Strathmann [55] and species lophophore dimensions measurements from the literature [12,21,27,33,56,57,58]. CR measurements are taken from: a this study, where two figures are given for S. errata: first one was measured with microalgae and second one with plastic microspheres; [12,14,15,20,21].
4.2. Standardization of CR in Bryozoans
Physiological rates, such as CRs, are often standardized to 1 g of the DW of meat in invertebrates. However, using biomass (both DW and AFDW) as a basis for comparison between bryozoan species proved less effective in our study due to significant differences in the proportion of skeletal biomass to total biomass. This is further complicated by the challenges of isolating soft tissues for precise measurements.
The average CRmax per unit DW for B. neritina (12.37 L/(h·g)) is comparable to that of the bryozoan A. bellula (9.5 L/(h·g) for the whole colony, as reported by Lisbjerg and Petersen [21]). In contrast, the S. errata exhibited a much lower CRmax of 0.86 L/(h·g), primarily due to the deposition of its calcareous skeleton. This proportion can vary even within the same species, depending on the colony’s morphological type [24,29]. By comparison, the bryozoan Amathia verticillata (delle Chiaje, 1822) (order Ctenostomatida), which lacks a calcareous skeleton, has a CR of 33.7 L/(h·g) DW, reflecting its distinct skeletal and zooid arrangement [14].
For accurate comparisons of CR per biomass across bryozoan species and other suspension feeders, weighing only the polypide biomass of bryozoans would be ideal. However, this is very challenging in practice. According to our results, the surface area is the most appropriate unit for comparing the CR and filtration efficiency among bryozoans studied. This approach is particularly relevant for encrusting and erect or arborescent bryozoans within the order Cheilostomatida, which are common in fouling communities. However, this generalization may not apply to all morphological forms of bryozoans, particularly certain species from the orders Ctenostomatida and Cyclostomatida. For precise CR measurements and interspecies comparisons, using the zooid as the base unit is considered the most appropriate.
Strathmann [55] determined the theoretical CR for the bryozoan tentacle, utilizing observations of feeding behavior and microscopy of the lateral cilia to assess the particle movement speed. Given the varying dimensions of the lophophores, it is reasonable to infer that the filtration capabilities among different bryozoan species also vary. Nevertheless, the CRs observed in laboratory settings often deviate significantly from theoretical predictions. This discrepancy may be due to regulatory mechanisms inherent to bryozoans and the diverse experimental methodologies employed, underscoring the necessity for in situ experiments. The scientific discourse regarding whether bryozoans and other suspension feeders maintain maximal clearance rates under optimal conditions remains unresolved [20].
4.3. Active Colony Regions in S. errata and Feeding on Microplastic
In colonies fed with fluorescent microspheres, we observed that the zooids located below the growing edge and within the frontal budding zone exhibited the highest feeding activity. This pattern reflects the established zonation of feeding activity in bryozoans [59], where older zooids at the colony’s center become less active as the distance from the growing edge increases. Whether an autozooid is actively feeding primarily depends on its polypide phase. Some zooids may be completely inactive, consisting only of a brown body—a structure that forms in older zooids during unfavorable seasonal conditions [60,61]. Additionally, fully senescent zooids that no longer regenerate may also be present in the colony. Polypides can also be inactive during the differentiation phase, which occurs in newly growing regions of the colony [60]. Zonation patterns in bryozoans that exhibit frontal budding, such as S. errata, are more complex than those seen in species with only lateral zooid budding. In S. errata, budding is not restricted to the colony’s edges but occurs in multiple areas, contributing to the structural complexity of the colony. Furthermore, phenomena such as zoarial overgrowth and fusion between colonies introduce additional variability, which may explain the differences in the proportion of active zooids observed in our experiment.
Unlike laboratory-grown colonies used in previous studies [12,15,17,18], our study examined naturally occurring colonies. Natural colonies develop unevenly and adapt to diverse microhabitats, leading to variable proportions of polypides at different stages of activity. In contrast, laboratory-grown colonies exhibit more uniform growth and synchronized polypide activity cycles [17]. In S. errata, the youngest and most active zooids are typically located at the ends of tubular formations, which are usually found at the uppermost parts of the colony. In our study, we observed that at the growing edges, mostly on tubular formations, where the zooids are younger, zooid morphology was more consistent (Figure 9B), with a high proportion of mature autozooids. Clusters of active zooids were also found in the central part of erect formations, where many zooids were in earlier developmental stages. In these regions, the zooid orientation was irregular, and the size variation was greater (Figure 9C). These structural differences, along with the colony’s overall shape, which significantly impacts the CR by influencing the water mixing and refiltration rates [18], further contribute to the variations in activity levels observed across the colonies.
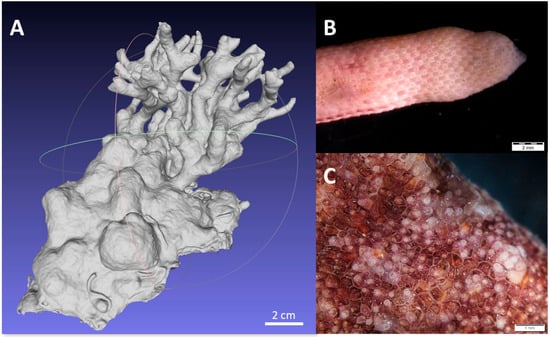
Figure 9.
A 3D model of an S. errata colony (A) and photographs showing different orientation and growth of the zooids on the top of the tubular formation (B) and in the areas of active frontal budding (C).
In experiments with four bryozoan species, Pratt [19] found that the species varied in both the proportion of feeding zooids and the proportion of actively feeding polypides. In all species studied, the average proportion of feeding zooids was below 50%, while the proportion of active polypides remained under 90%. M. membranacea exhibited the highest values for both measures. Our estimate of active colony parts, which was 48% in the S. errata, suggests that a significant portion of the colony is engaged in feeding and that the tubular formations effectively capture food particles.
Our findings also confirm that bryozoans ingest plastic particles [18,19,57,62]. However, suspension-feeding invertebrates are not suitable as potential remediation organisms for microplastic pollution because of their low capacity to retain these particles, which are mostly rapidly excreted, as confirmed also by the presence of microspheres in the feces in our study. On the other hand, mechanisms such as surface adhesion and particle encrustation are promising for the removal of these particles [63]. Bryozoans are interesting for further research in this respect. Preliminary risk assessments of the impact of ingestion of microplastics on suspension feeders suggest that bryozoans are animals at a low risk of contamination, although the paucity of data on this group makes this assessment rather unreliable at present [64].
5. Conclusions
This study shows that the two fouling bryozoan species studied are effective suspension feeders, with clearance rates (CR) comparable to those of other suspension-feeding invertebrates in the Mediterranean region. Of the two species, B. neritina had a higher CR than S. errata, probably due to the larger size of its lophophore.
Considering the morphological diversity and variable growth forms of the bryozoans, as well as the difficulty in making accurate dry weight measurements, it is more appropriate to report the CR per unit of colony surface area or per zooid rather than per gram of dry weight. This approach allows for more meaningful comparisons both within and between bryozoan species.
The study also shows that the S. errata can ingest small microplastic particles and subsequently excrete them with the feces, indicating its possible role in the microplastic cycle. Observation of feeding activity within the colonies revealed that the most active regions were associated with new growth, particularly the apical zones of tubular formations and areas with frontal budding.
These results emphasize the ecological importance of the bryozoans in coastal habitats, especially in their role as biofilters. Through their activity as suspension feeders, they contribute to the regulation of water quality and thus provide an important ecosystem service.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse13061052/s1, Table S1: Raw data for both experiments.
Author Contributions
Conceptualization, A.F., B.M. and L.L.; methodology, A.F. and B.M.; formal analysis, A.F.; investigation, A.F. and P.S.; writing—original draft preparation, A.F.; writing—review and editing, L.L., B.M. and P.S.; supervision, L.L. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Slovenian Research and Innovation Agency, grant numbers P1-0237 and Z1-60165. The APC was funded by The Slovenian Research and Innovation Agency, grant number P1-0237.
Institutional Review Board Statement
The review of the Institutional Board was not needed for this study due to the use of non-protected, marine invertebrate species. The two species examined are not protected under national legislation in Slovenia (Decree on Protected Wild Animal Species; Official Journal of the RS, nos. 46/04, 109/04, 84/05, 115/07, 32/08—odl. US, 96/08, 36/09, 102/11, 15/14, 64/16, and 62/19). Therefore, no special permits are required for their handling in the context of scientific research.
Data Availability Statement
The data supporting the findings of this study are available in Supplementary Table S1. Additional data may be obtained from the corresponding author upon reasonable request.
Acknowledgments
We thank Timotej Turk Dermastia for providing Dunaliella sp. cultures. We also acknowledge Živa Muhič and Katja Klun for their assistance in improving the fluorescence intensity measurement method. We would also like to thank the two reviewers for their valuable feedback, which significantly improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bulleri, F.; Chapman, M.G. The introduction of coastal infrastructure as a driver of change in marine environments. J. Appl. Ecol. 2010, 47, 26–35. [Google Scholar] [CrossRef]
- Layman, C.A.; Jud, Z.R.; Archer, S.K.; Riera, D. Provision of ecosystem services by human-made structures in a highly impacted estuary. Environ. Res. Lett. 2014, 9, 044009. [Google Scholar] [CrossRef]
- Cranford, P.J. Magnitude and extent of water clarification services provided by bivalve suspension feeding. In Goods and Services of Marine Bivalves; Smaal, A.C., Grant, J., Strand, Ø., Ferreira, J.G., Petersen, J.K., Eds.; Springer Open: Cham, Switzerland, 2019; pp. 119–142. [Google Scholar]
- Draughon, L.D.; Scarpa, J.; Hartmann, J.X. Are filtration rates for the rough tunicate Styela plicata independent of weight or size? J. Environ. Sci. Health A 2010, 45, 168–176. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Gravina, M.F.; Giangrande, A. Filtering activity on a pure culture of Vibrio alginolyticus by the solitary ascidian Styela plicata and the colonial ascidian Polyandrocarpa zorritensis: A potential service to improve microbiological seawater quality economically. Sci. Total Environ. 2016, 573, 11–18. [Google Scholar] [CrossRef]
- Clapin, G. The Filtration Rate, Oxygen Consumption and Biomass of the Introduced Polychaete Sabella spallanzanii Gmelin Within Cockburn Sound: Can It Control Phytoplankton Levels and Is It an Efficient Filter Feeder? Bachelor’s Thesis, Edith Cowan University, Joondalup, WA, Australia, 1996. [Google Scholar]
- Licciano, M.; Stabili, L.; Giangrande, A. Clearance rates of Sabella spallanzanii and Branchiomma luctuosum (Annelida: Polychaeta) on a pure culture of Vibrio alginolyticus. Water Res. 2005, 39, 4375–4384. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Schirosi, R.; Licciano, M.; Mola, E.; Giangrande, A. Bioremediation of bacteria in aquaculture waste using the polychaete Sabella spallanzanii. New Biotechnol. 2010, 27, 774–781. [Google Scholar] [CrossRef]
- Varamogianni-Mamatsi, D.; Anastasiou, T.I.; Vernadou, E.; Papandroulakis, N.; Kalogerakis, N.; Dailianis, T.; Mandalakis, M. A multi-species investigation of sponges’ filtering activity towards marine microalgae. Mar. Drugs 2022, 20, 24. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Marzano, C.N.; Corriero, G. Filtering activity of Spongia officinalis var. adriatica (Schmidt) (Porifera, Demospongiae) on bacterioplankton: Implications for bioremediation of polluted seawater. Water Res. 2006, 40, 3083–3090. [Google Scholar] [CrossRef]
- Milanese, M.; Chelossi, E.; Manconi, R.; Sarà, A.; Sidri, M.; Pronzato, R. The marine sponge Chondrilla nucula Schmidt, 1862 as an elective candidate for bioremediation in integrated aquaculture. Biomol. Eng. 2003, 20, 363–368. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Manríquez, P. Filter-feeding in fifteen marine ectoprocts (Bryozoa): Particle capture and water pumping. Mar. Ecol. Prog. Ser. 1997, 154, 223–239. [Google Scholar] [CrossRef]
- Lemmens, J.W.T.J.; Clapin, G.; Lavery, P.; Cary, J. Filtering capacity of seagrass meadows and other habitats of Cockburn Sound, western Australia. Mar. Ecol. Prog. Ser. 1996, 143, 187–200. [Google Scholar] [CrossRef][Green Version]
- Bullivant, J.S. The rate of feeding of the bryozoan, Zoobotryon verticillatum. N. Z. J. Mar. Freshw. Res. 1968, 2, 111–134. [Google Scholar] [CrossRef]
- Menon, N.R. Clearance rates of food suspension and food passage rates as a function of temperature in two north-sea bryozoans. Mar. Biol. 1974, 24, 65–67. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Goldson, A. Minimal scaling of the lophophore filter-pump in ectoprocts (Bryozoa) excludes physiological regulation of filtration rate to nutritional needs. Test of hypothesis. Mar. Ecol. Prog. Ser. 1997, 156, 109–120. [Google Scholar] [CrossRef]
- Lisbjerg, D.; Petersen, J.K. Feeding activity, retention efficiency, and effects of temperature and particle concentration on clearance rate in the marine bryozoan Electra crustulenta. Mar. Ecol. Prog. Ser. 2001, 215, 133–141. [Google Scholar] [CrossRef]
- Pratt, M.C. Consequences of coloniality: Influence of colony form and size on feeding success in the bryozoan Membranipora membranacea. Mar. Ecol. Prog. Ser. 2005, 303, 153–165. [Google Scholar] [CrossRef]
- Pratt, M.C. Living where the flow is right: How flow affects feeding in bryozoans. Integr. Comp. Biol. 2008, 48, 808–822. [Google Scholar] [CrossRef]
- Pascoe, P.L.; Parry, H.E.; Hawkins, A.J.S. Dynamic filter-feeding responses in fouling organisms. Aquat. Biol. 2007, 1, 177–185. [Google Scholar] [CrossRef][Green Version]
- Lisbjerg, D.; Petersen, J.K. Clearance capacity of Electra bellula (Bryozoa) in seagrass meadows of western Australia. J. Exp. Mar. Biol. Ecol. 2000, 244, 285–296. [Google Scholar] [CrossRef]
- EASIN—European Alien Species Information Network, European Commission. Available online: https://easin.jrc.ec.europa.eu/easin (accessed on 8 January 2025).
- Bryan, P.J.; Kreider, J.L.; Qian, P.Y. Settlement of the serpulid polychaete Hydroides elegans (Haswell) on the arborescent bryozoan Bugula neritina (L.): Evidence of a chemically mediated relationship. J. Exp. Mar. Biol. Ecol. 1998, 220, 171–190. [Google Scholar] [CrossRef]
- Cocito, S.; Ferdeghini, F.; Morri, C.; Bianchi, N.B. Patterns of bioconstruction in the cheilostome bryozoan Schizoporella errata: The influence of hydrodynamics and associated biota. Mar. Ecol. Prog. Ser. 2000, 192, 153–161. [Google Scholar] [CrossRef]
- Morgado, E.H.; Tanaka, M.O. The macrofauna associated with the bryozoan Schizoporella errata in southeastern Brazil. Sci. Mar. 2001, 65, 173–181. [Google Scholar] [CrossRef]
- Sokolover, N.; Ostrovsky, A.N.; Ilan, M. Schizoporella errata (Bryozoa, Cheilostomata) in the mediterranean sea: Abundance, growth rate, and reproductive strategy. Mar. Biol. Res. 2018, 14, 868–882. [Google Scholar] [CrossRef]
- Hayward, P.J.; McKinney, F.K. Northern Adriatic Bryozoa from the vicinity of Rovinj, Croatia. Bull. Am. Mus. Nat. Hist. 2002, 270, 1–139. [Google Scholar] [CrossRef]
- Banta, W.C. The body wall of cheilostome Bryozoa, V. Frontal budding in Schizoporella unicornis floridana. Mar. Biol. 1972, 14, 63–71. [Google Scholar] [CrossRef]
- Nikolić, M. Polimorfna Rast Zoarijev Ektoproktne Vrste Schizoporella violacea Canu & Bassler 1930. Ph.D. Thesis, Univerza v Ljubljani, Rovinj, Croatia, 1959. [Google Scholar]
- Zabin, C.J.; Obernolte, R.; Mackie, J.A.; Gentry, J.; Harris, L.; Geller, J. A non-native bryozoan creates novel substrate on the mudflats in San Francisco Bay. Mar. Ecol. Prog. Ser. 2010, 412, 129–139. [Google Scholar] [CrossRef]
- Winston, J.E. Distribution and Ecology of Estuarine Ectoprocts: A Critical Review. Chesap. Sci. 1977, 18, 34–57. [Google Scholar] [CrossRef]
- Fehlauer-Ale, K.H.; Mackie, J.A.; Lim-Fong, G.E.; Ale, E.; Pie, M.R.; Waeschenbach, A. cryptic species in the cosmopolitan Bugula neritina complex (Bryozoa, Cheilostomata). Zool. Scr. 2013, 43, 193–205. [Google Scholar] [CrossRef]
- Ryland, J.S.; Hayward, P.J. British Anascan Bryozoans, Synopses of the British Fauna (New Series); Academic Press Inc.: London, UK; New York, YN, USA; San Francisco, CA, USA, 1977; Volume 10. [Google Scholar]
- Lord, J.P. Impact of seawater temperature on growth and recruitment of invasive fouling species at the global scale. Mar. Ecol. 2017, 38, e12404. [Google Scholar] [CrossRef]
- Kitamura, H.; Hirayama, K. Growth of the bryozoan Bugula neritina in the sea at various water temperatures. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 1–5. [Google Scholar] [CrossRef]
- Fofonoff, P.W.; Ruiz, G.M.; Steves, B.; Simkanin, C.; Carlton, J.T. National Exotic Marine and Estuarine Species Information System. Available online: https://invasions.si.edu/nemesis/ (accessed on 30 April 2025).
- Boicourt, W.C.; Kuzmić, M.; Hopkins, T.S. The inland sea: Circulation of Chesapeake Bay and the northern Adriatic. In Ecosystems at the Land-Sea Margin: Drainage Basin to Coastal Sea; Malone, T.C., Malej, A., Harding, L.W., Jr., Smodlaka, N., Turner, R.E., Eds.; American Geophysical Union: Washington, DC, USA, 1999; pp. 81–129. [Google Scholar]
- Grilli, F.; Accoroni, S.; Acri, F.; Aubry, F.B.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and interannual trends of oceanographic parameters over 40 years in the northern Adriatic Sea in relation to nutrient loadings using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Ogorelec, B.; Mišič, M.; Faganeli, J. Marine geology of the Gulf of Trieste (northern Adriatic): Sedimentological aspects. Mar. Geol. 1991, 99, 79–92. [Google Scholar] [CrossRef]
- Turk, R. Ocena ranljivosti slovenskega obalnega pasu in njegova kategorizacija z vidika (ne)dopustnih posegov, dejavnosti in rabe. Ann. Ser. Hist. Nat. 1999, 9, 37–50. [Google Scholar]
- Okamura, B. The effects of ambient flow velocity, colony size, and upstream colonies on the feeding success of Bryozoa. I. Bugula stolonifera Ryland, an arborescent species. J. Exp. Mar. Biol. Ecol. 1984, 83, 179–193. [Google Scholar] [CrossRef]
- Mesečni Bilten ARSO, Slovenian Environment Agency. Available online: http://hmljn.arso.gov.si/o%20agenciji/knji%C5%BEnica/mese%C4%8Dni%20bilten/ (accessed on 22 January 2021).
- Rosa, M.; Ward, J.E.; Shumway, S.E.; Wikfors, G.H.; Pales-Espinosa, E.; Allam, B. Effects of particle surface properties on feeding selectivity in the eastern oyster Crassostrea virginica and the blue mussel Mytilus edulis. J. Exp. Mar. Biol. Ecol. 2013, 446, 320–327. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Bankhead, P. Analyzing Fluorescence Microscopy Images with ImageJ; Heidelberg University: Heidelberg, Germany, 2014; p. 187. [Google Scholar]
- Chamberlin, J.F. Algal Wastewater Treatment and Biofuel Production: An Assessment of Measurement Methods, and Impact of Nutrient Availability and Species Composition. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2016. [Google Scholar]
- Randløv, A.; Riisgård, H.U. Efficiency of particle retention and filtration rate in four species of ascidians. Mar. Ecol. Prog. Ser. 1979, 1, 55–59. [Google Scholar] [CrossRef]
- Coughlan, J. The estimation of filtering rate from the clearance of suspensions. Mar. Biol. 1969, 2, 356–358. [Google Scholar] [CrossRef]
- Navarro, J.M.; Widdows, J. Feeding physiology of Cerastoderma edule in response to a wide range of seston concentrations. Mar. Ecol. Prog. Ser. 1997, 152, 175–186. [Google Scholar] [CrossRef]
- Widdows, J.; Staff, F. Biological Effects of Contaminants: Measurement of Scope for Growth in Mussels; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2006; p. 40. [Google Scholar] [CrossRef]
- Echevarria, M.; Naar, J.P.; Tomas, C.; Pawlik, J.R. Effects of Karenia brevis on clearance rates and bioaccumulation of brevetoxins in benthic suspension feeding invertebrates. Aquat. Toxicol. 2012, 106, 85–94. [Google Scholar] [CrossRef]
- Fiala-Médioni, A. filter-feeding ethology of benthic invertebrates (Ascidians). IV. Pumping rate, filtration rate, filtration efficiency. Mar. Biol. 1978, 48, 243–249. [Google Scholar] [CrossRef]
- Hernandis, S.; Ibarrola, I.; Tena-Medialdea, J.; Albentosa, M.; Prado, P.; Vázquez-Luis, M.; García-March, J.R. Physiological responses of the fan mussel Pinna nobilis to temperature: Ecological and captivity implications. Mediterr. Mar. Sci. 2023, 24, 259–271. [Google Scholar] [CrossRef]
- Albentosa, M.; Akinyemi, M.I.; Vera, M.; Ibarrola, I.; Filgueira, R.; Galimany, E.; da Costa, F.; Pardo, B.G.; Vázquez-Luis, M.; Hernández, A.; et al. Recovery of eutrophized marine ecosystems using the European flat oyster, Ostrea edulis. Aquat. Conserv. 2023, 33, 645–660. [Google Scholar] [CrossRef]
- Strathmann, R. Function of lateral cilia in suspension feeding of lophophorates (Brachiopoda, Phoronida, Ectoprocta). Mar. Biol. 1973, 23, 129–136. [Google Scholar] [CrossRef]
- Winston, J.E. Bryozoans (Ectoprocta) of Indian River. Bull. Am. Mus. Nat. Hist. 1982, 173, 99–176. [Google Scholar]
- Okamura, B. Particle size and flow velocity induce an inferred switch in bryozoan suspension-feeding behavior. Biol. Bull. 1987, 173, 222–229. [Google Scholar] [CrossRef]
- Shunatova, N.N.; Ostrovsky, A.N. Individual autozooidal behaviour and feeding in marine bryozoans. Sarsia 2001, 86, 113–142. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Clarke, A. Seasonal variation in the feeding activity of four species of Antarctic bryozoan in relation to environmental factors. J. Exp. Mar. Biol. Ecol. 1994, 181, 117–133. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Clarke, A. Seasonality of polypide recycling and sexual reproduction in some erect Antarctic bryozoans. Mar. Biol. 1998, 131, 647–658. [Google Scholar] [CrossRef]
- Bock, P.E. Bryozoans (Phylum Bryozoa or Ectoprocta). In Marine Invertebrates of Southern Australia, Part I; Shepherd, S.A., Thomas, I.M., Eds.; South Australian Government: Adelaide, Australia, 1982; pp. 319–394. [Google Scholar]
- Okamura, B. Particle Size, Flow Velocity, and Suspension-Feeding by the Erect Bryozoans Bugula neritina and B. stolonifera. Mar. Biol. 1990, 53, 33–38. [Google Scholar] [CrossRef]
- Masiá, P.; Sol, D.; Ardura, A.; Laca, A.; Borrell, Y.J.; Dopico, E.; Laca, A.; Machado-Schiaffino, G.; Díaz, M.; Garcia-Vazquez, E. Bioremediation as a promising strategy for microplastics removal in wastewater treatment plants. Mar. Pollut. Bull. 2020, 156, 111252. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S. A Risk Analysis of Microplastic Consumption in Filter Feeders. Master’s Thesis, Nova Southeastern University, Fort Lauderdale-Davie, FL, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).