An Experimental Study on the Effects of Deflector Baffles and Circular Fish School Swimming Patterns on Flow Field Characteristics in Aquaculture Vessels
Abstract
1. Introduction
2. Experimental Methods
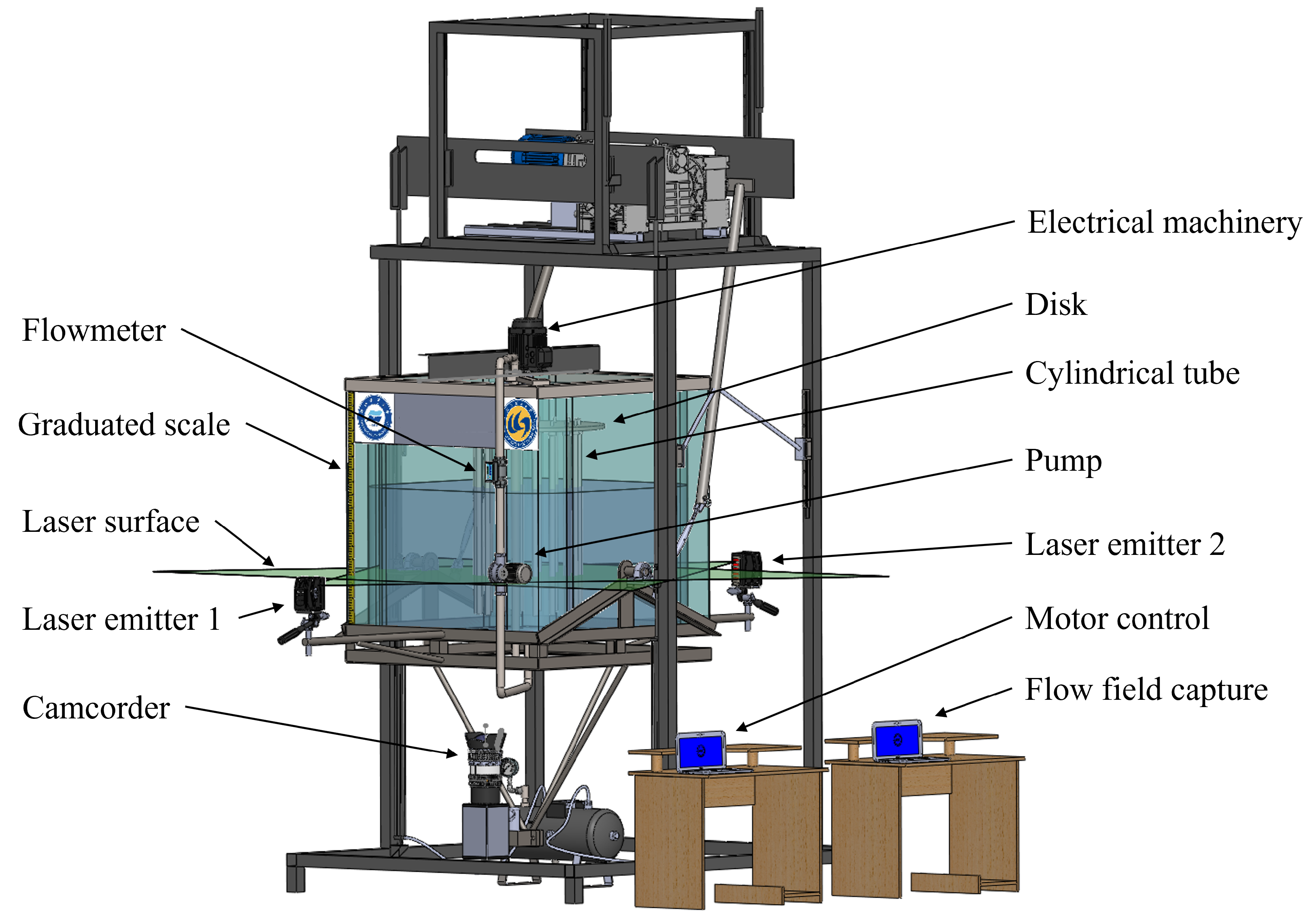
2.1. Experimental Setup
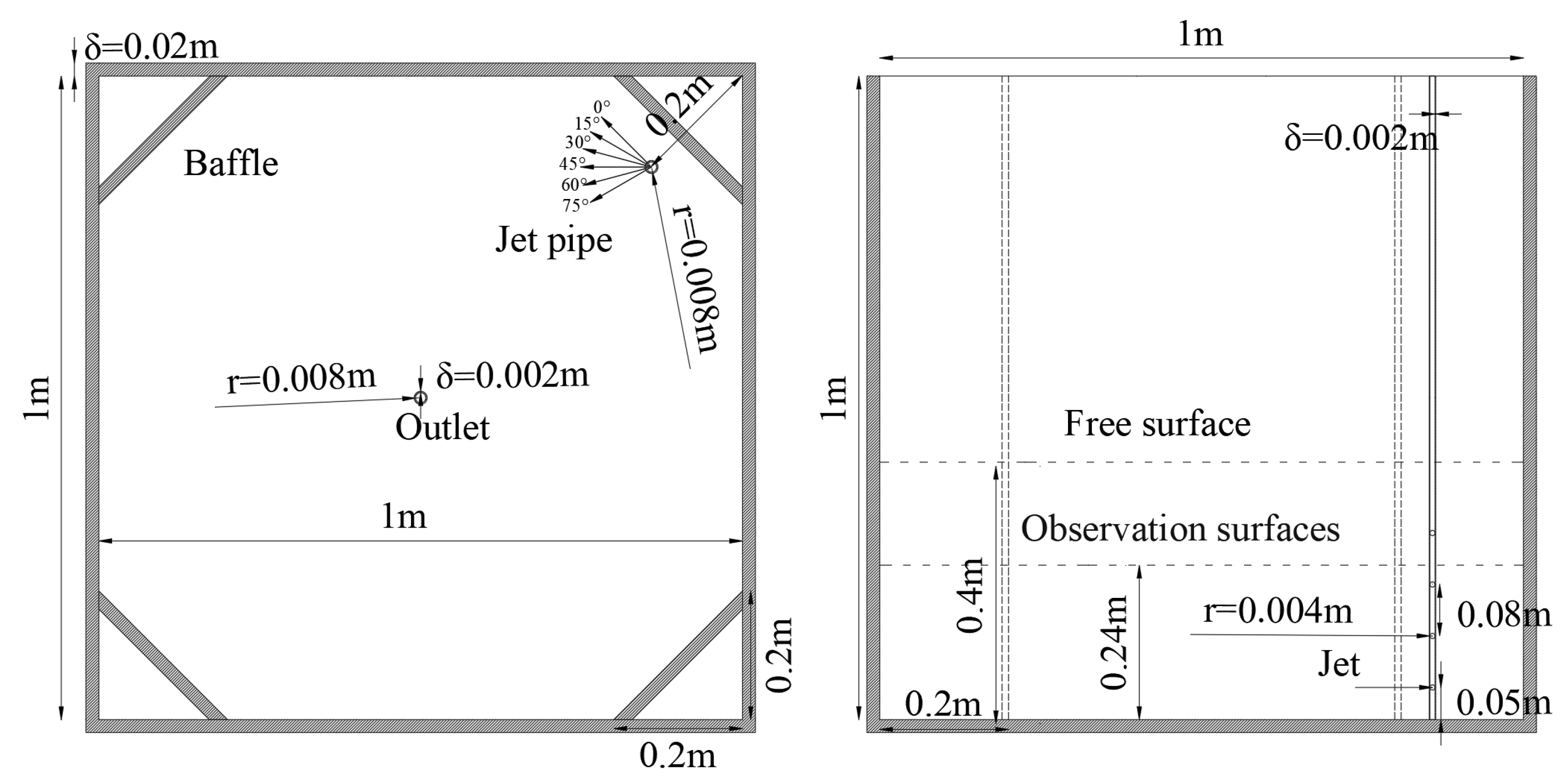
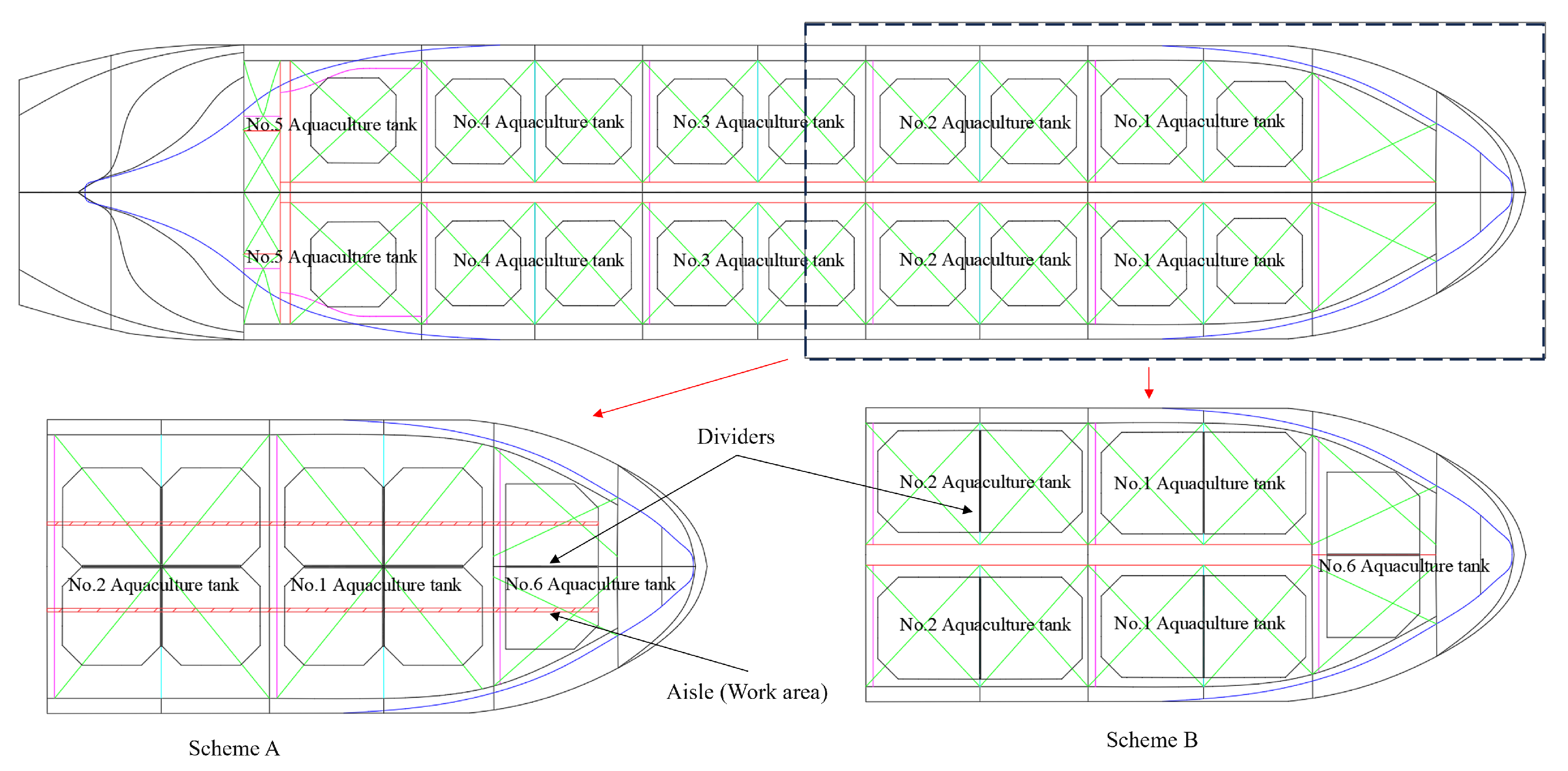
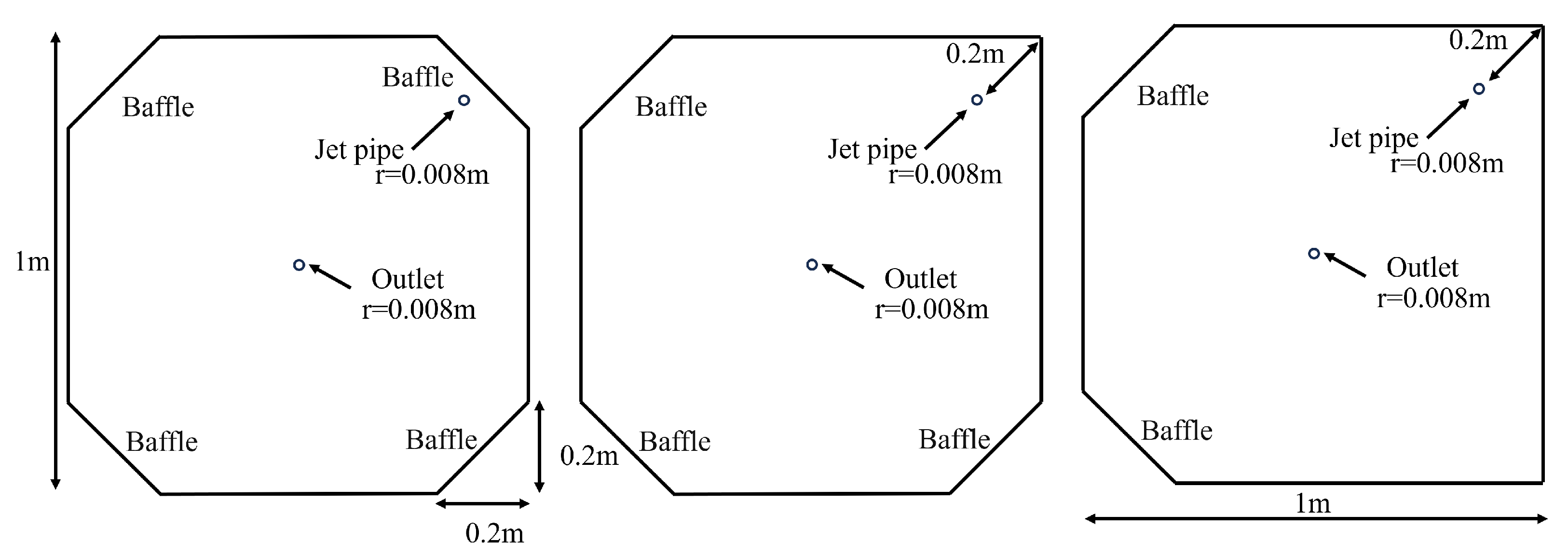
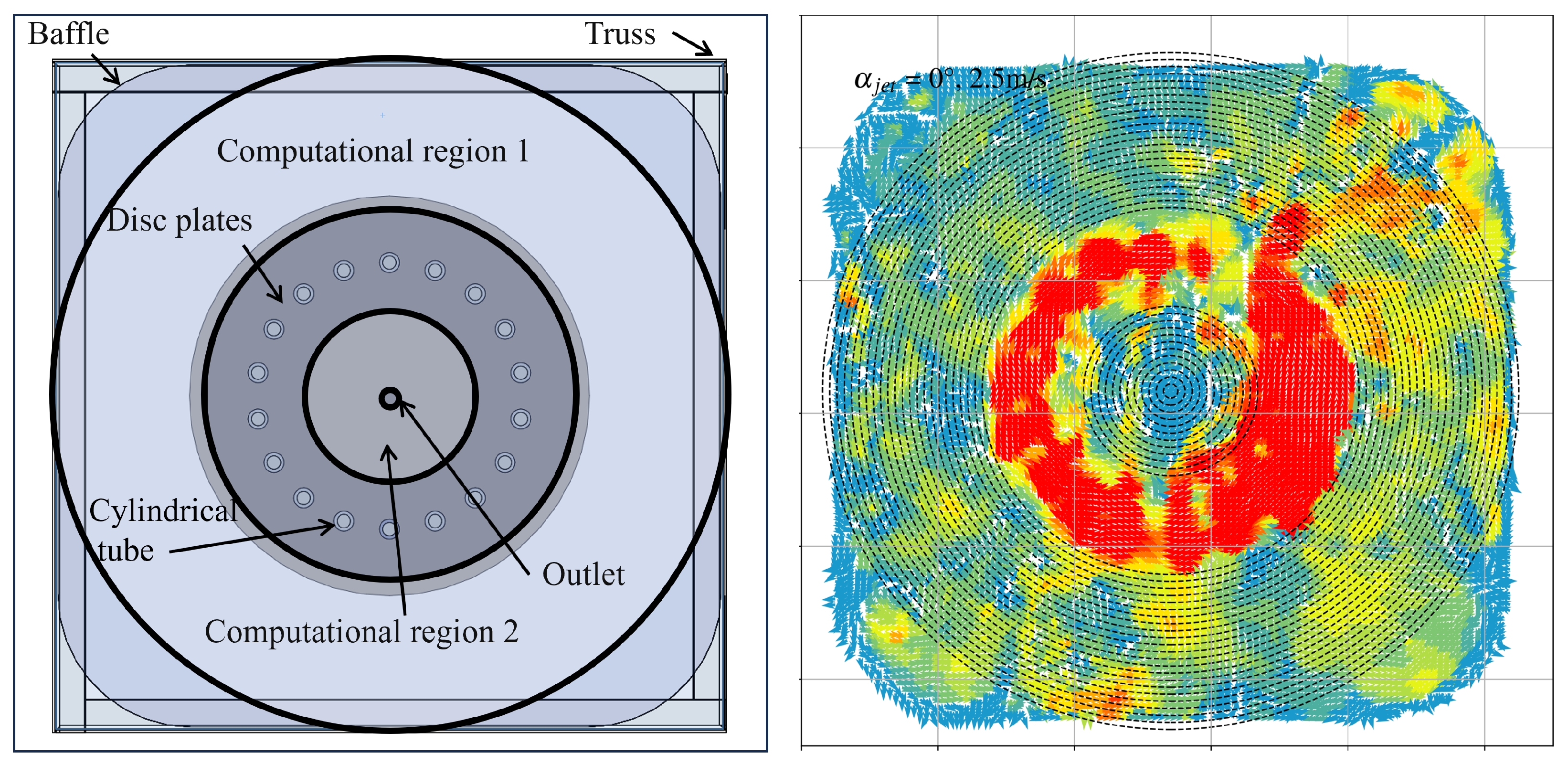
2.2. Aquaculture Tank Setup
3. Results and Discussion
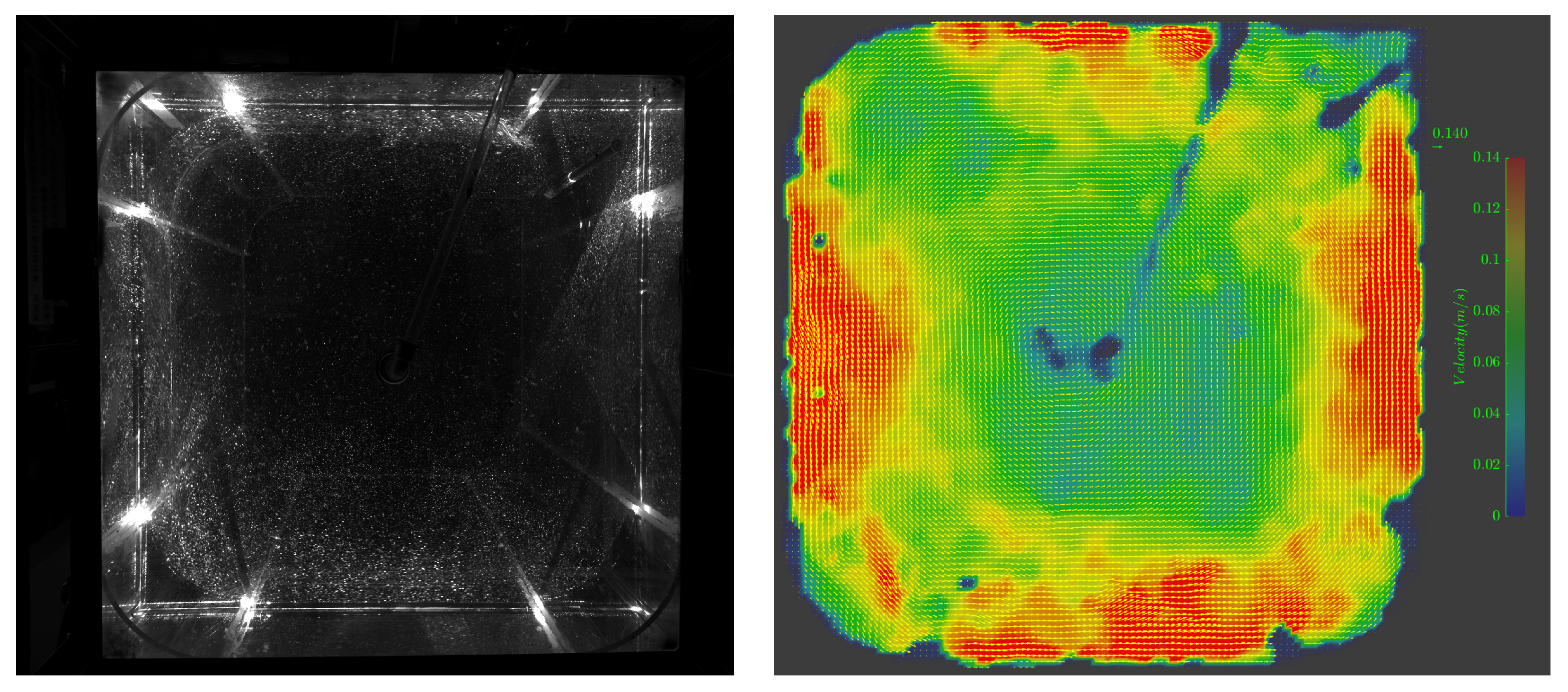
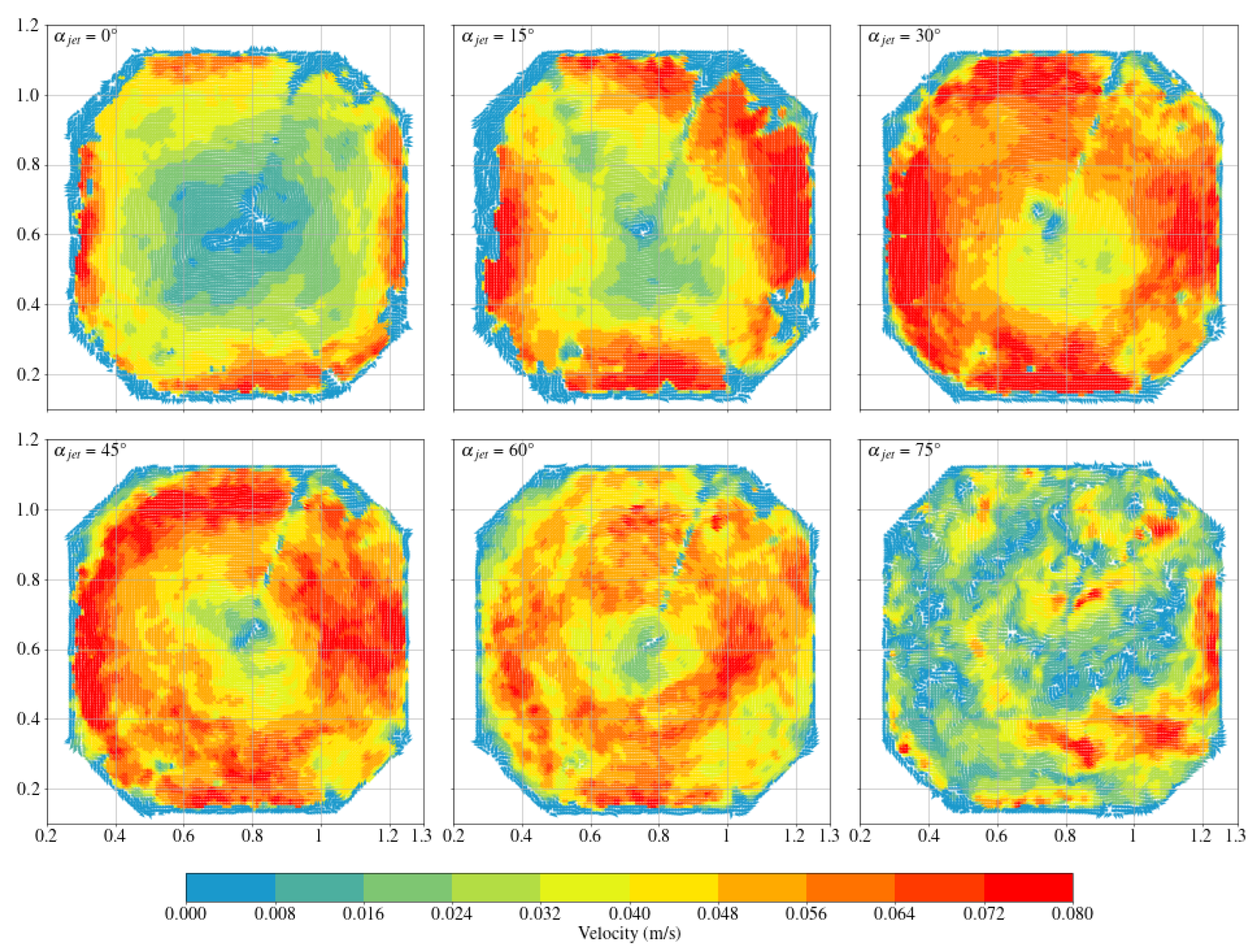
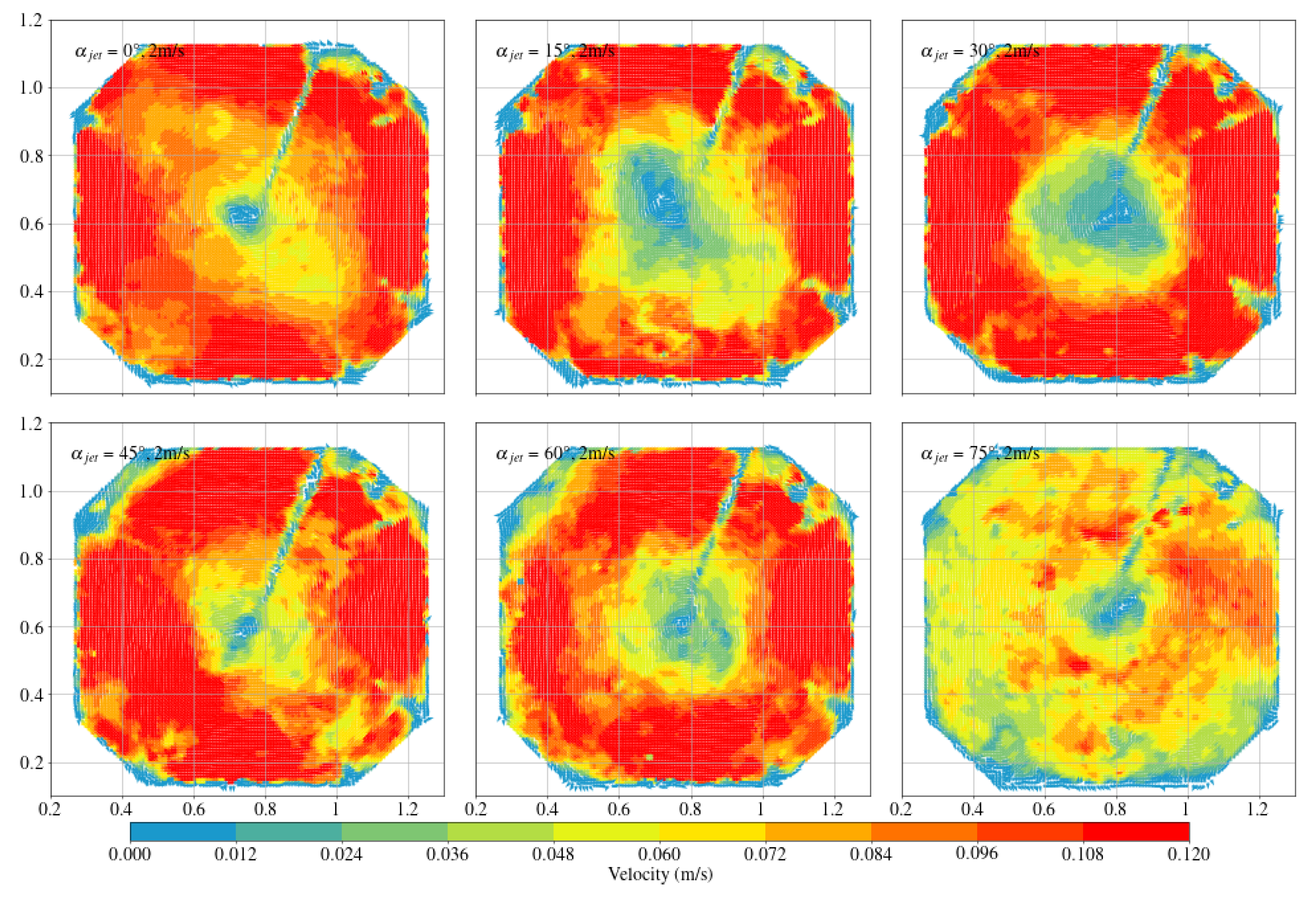
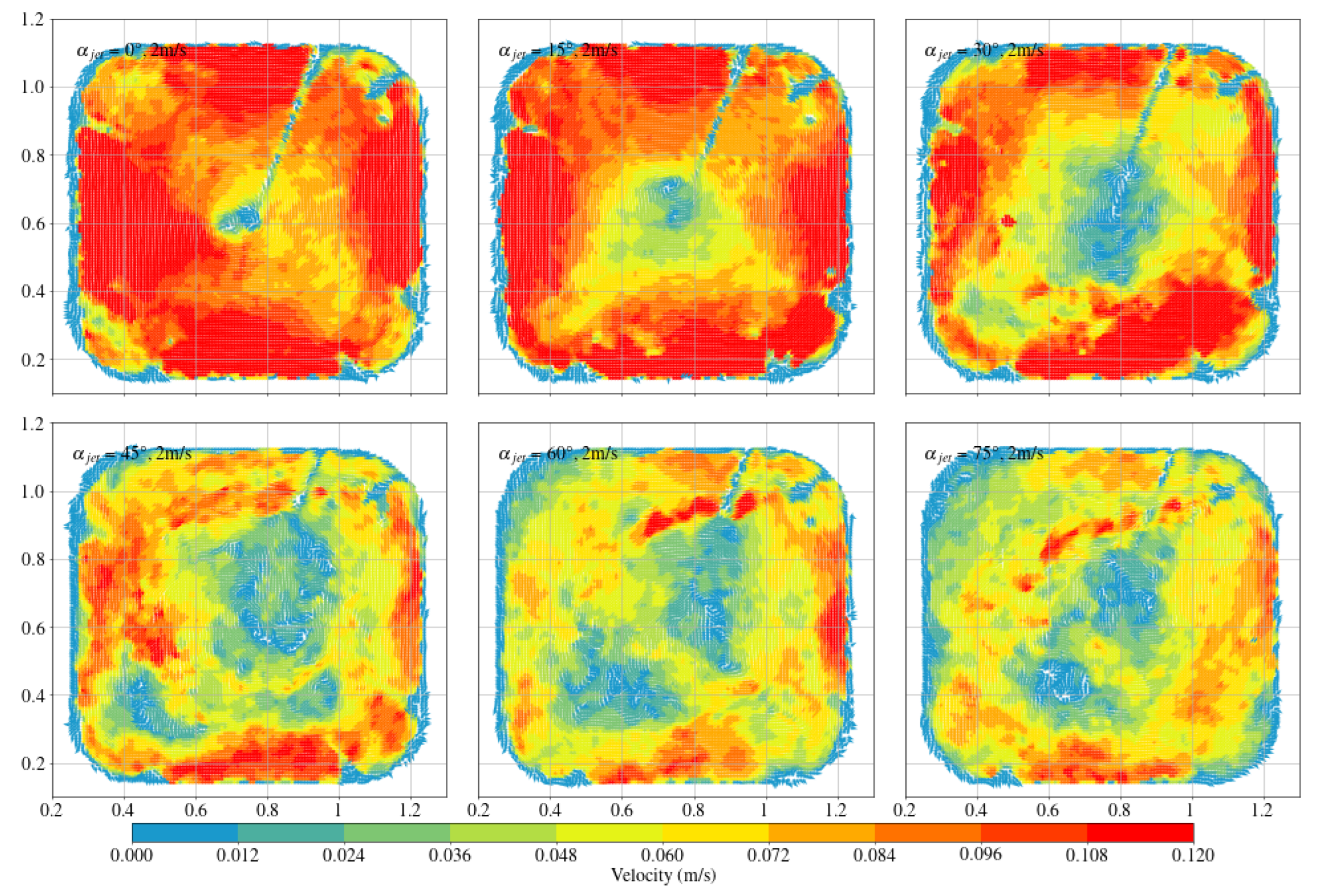
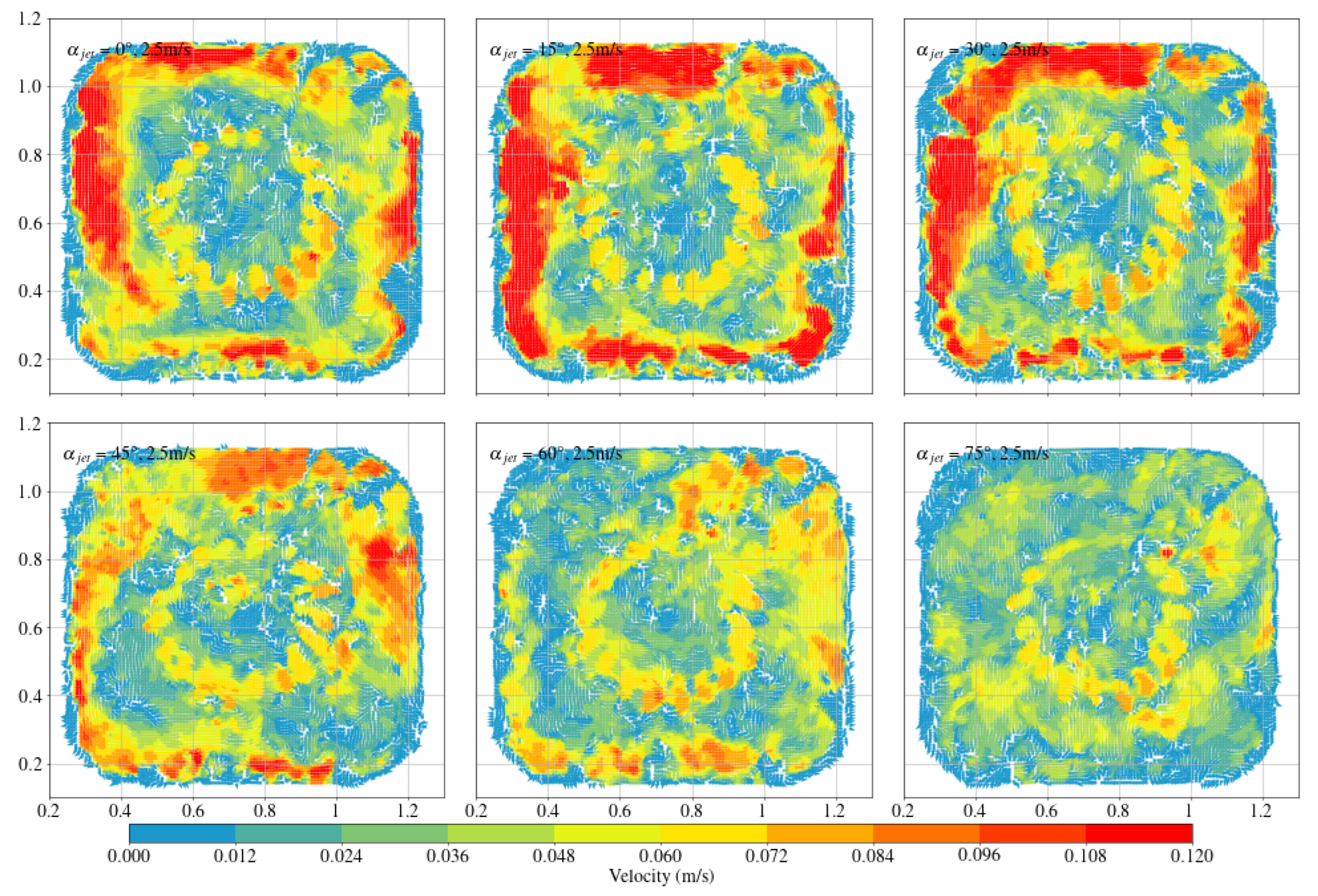
3.1. Octagonal Aquaculture Tanks
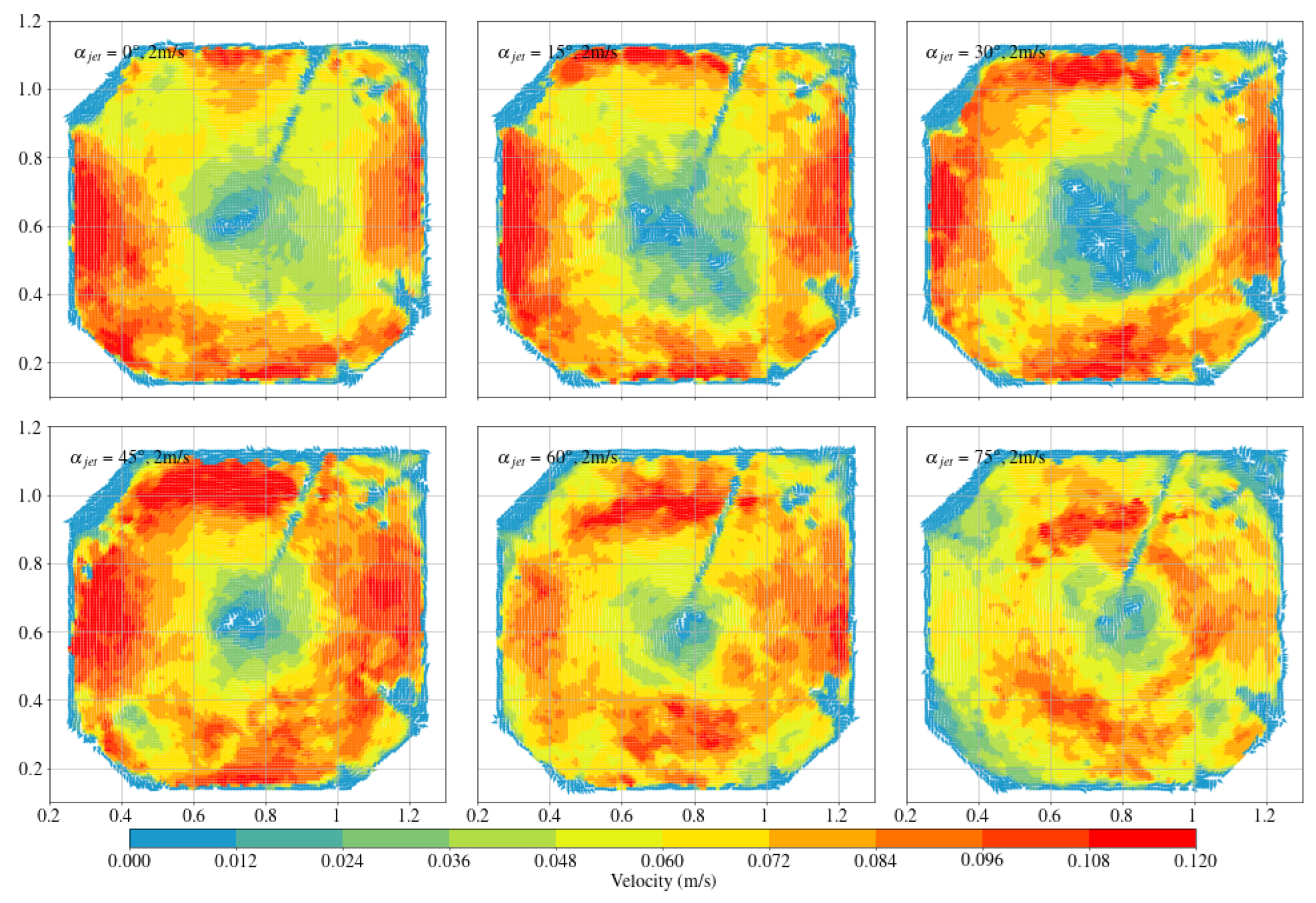
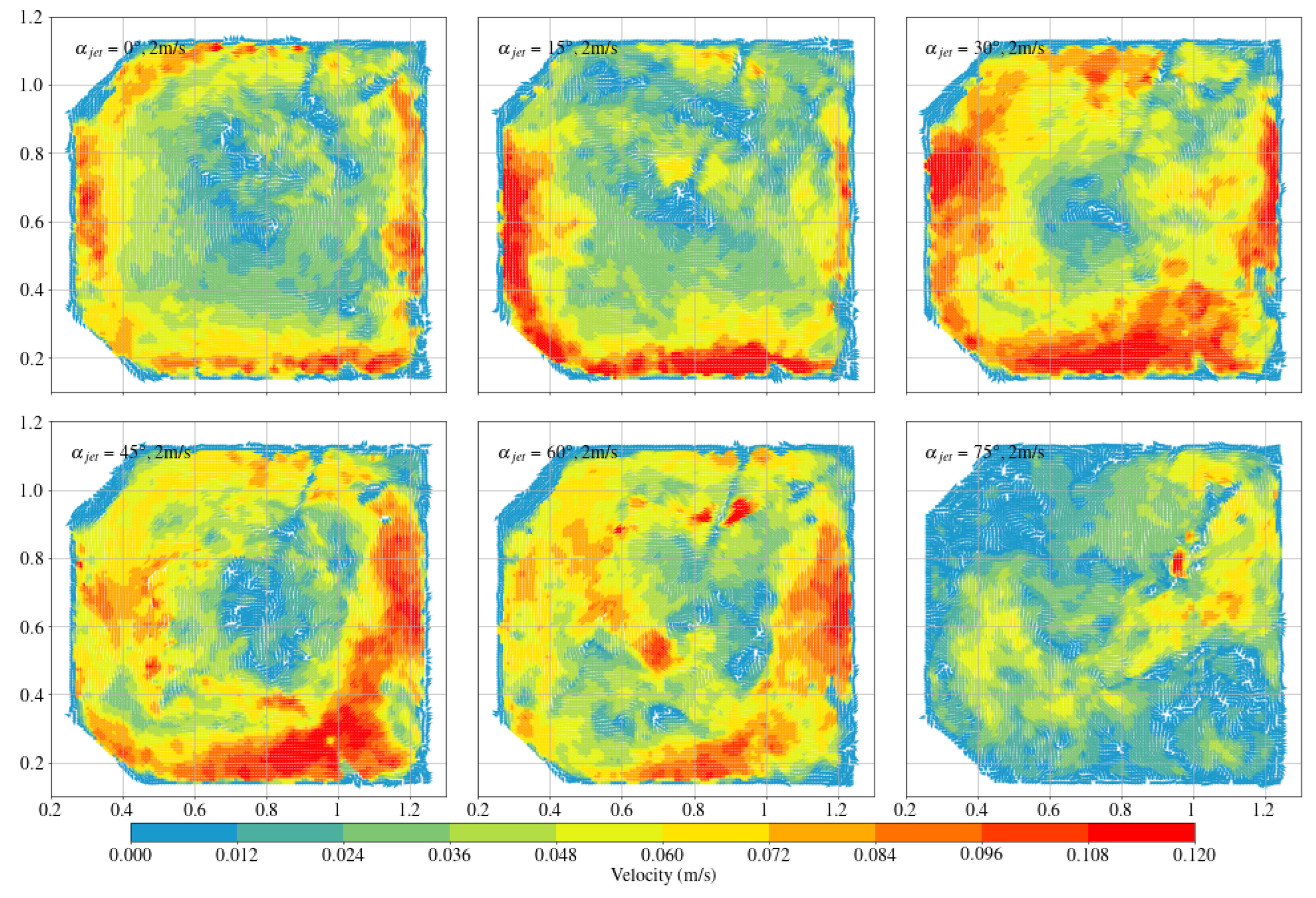
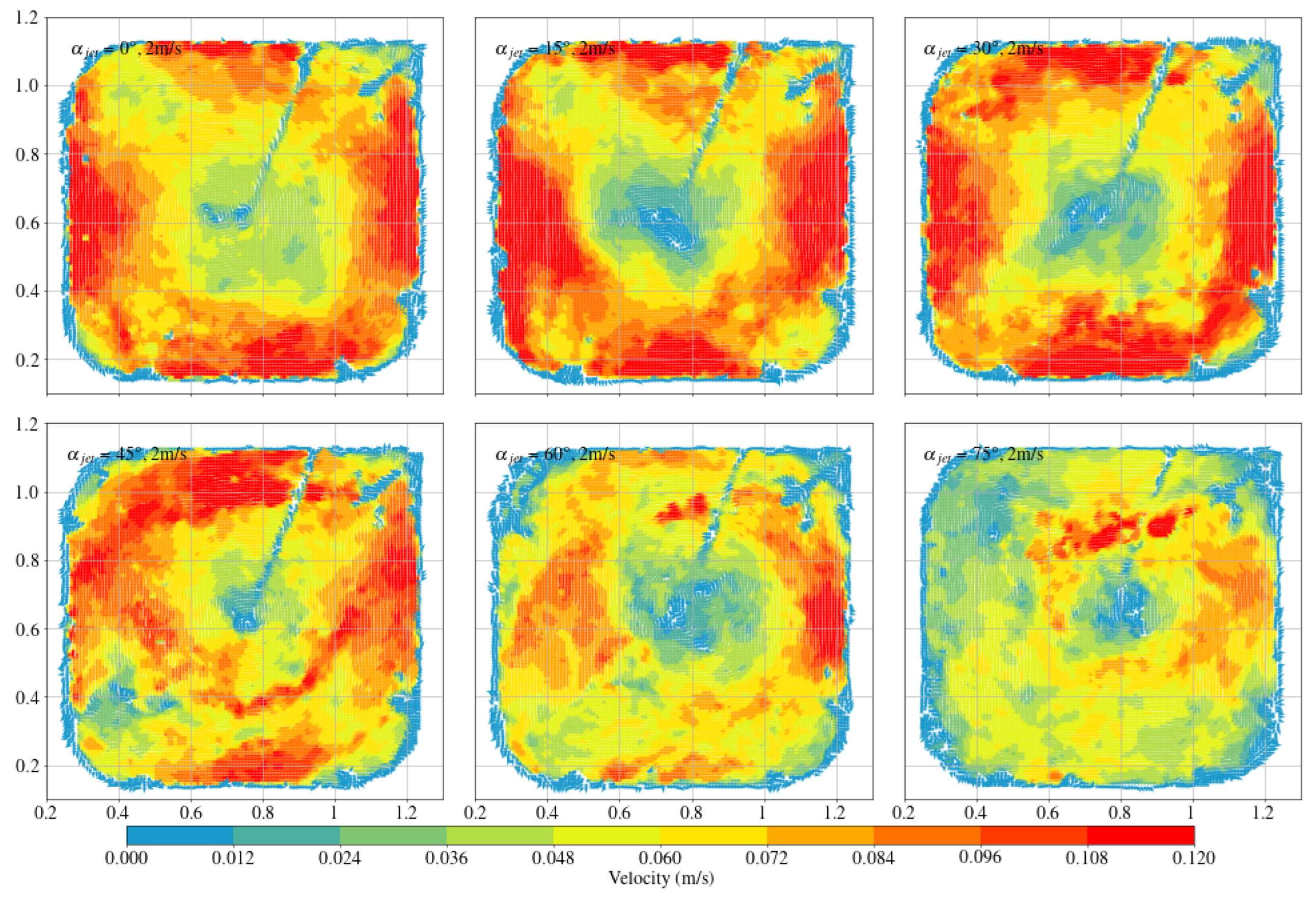
3.2. Arc-Shaped Aquaculture Tanks
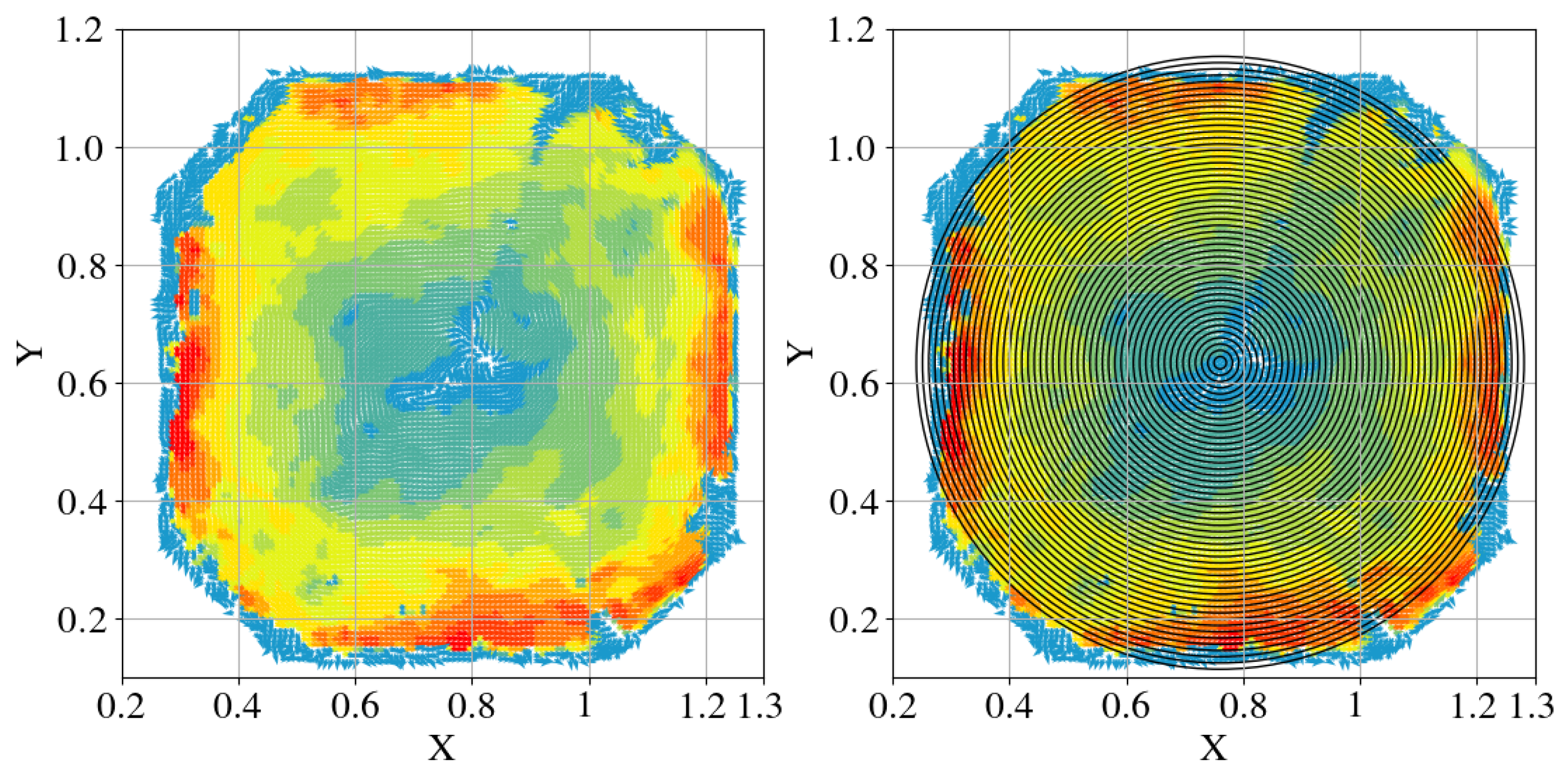
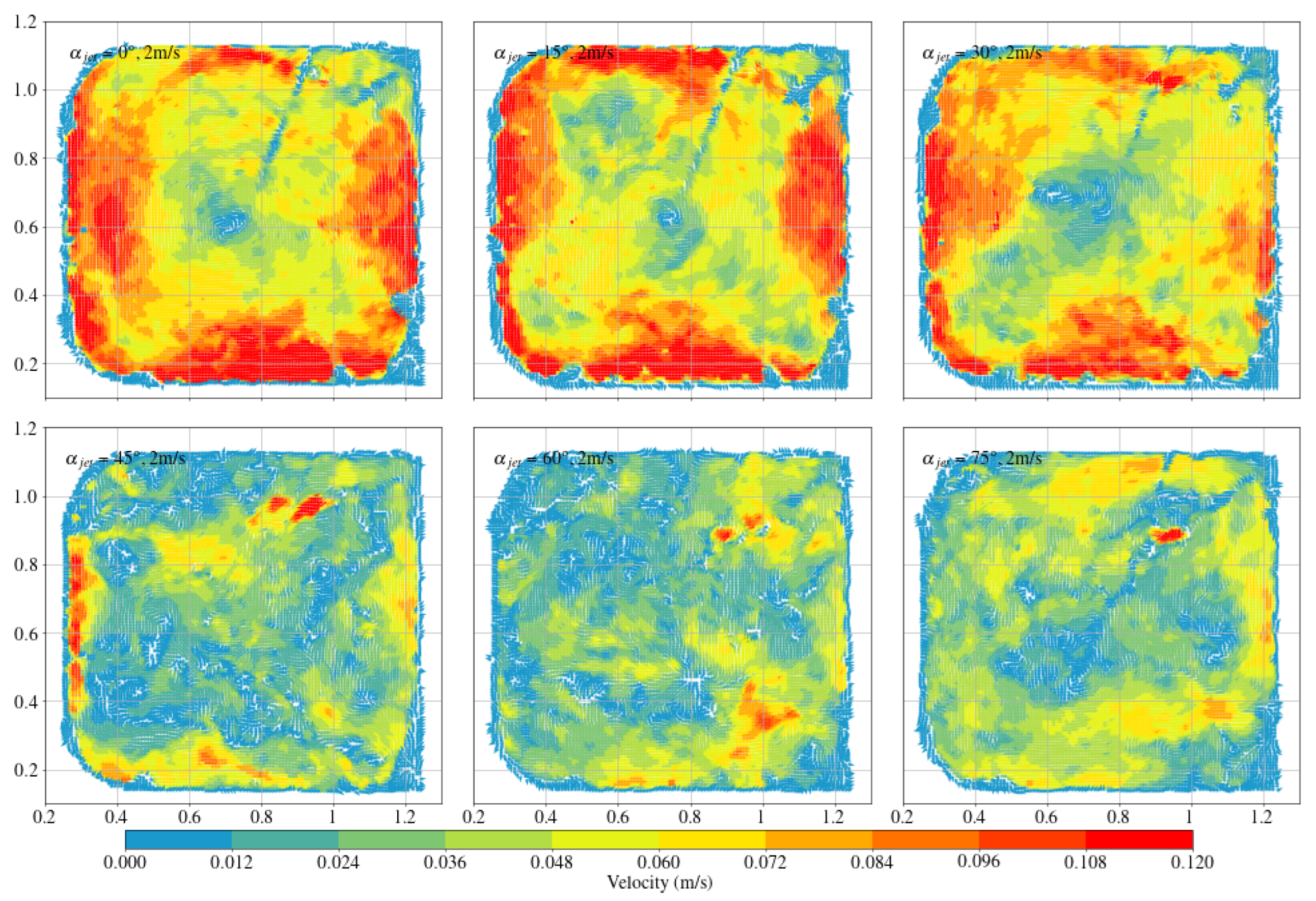
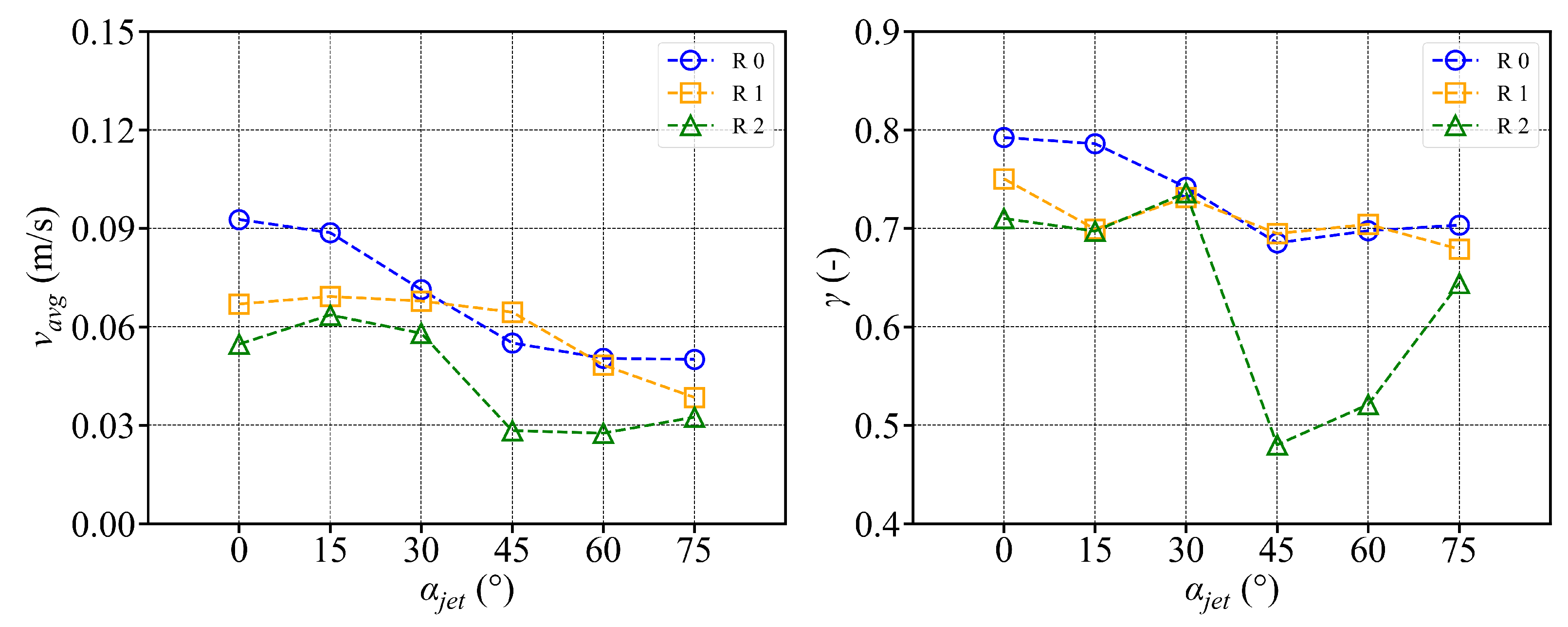
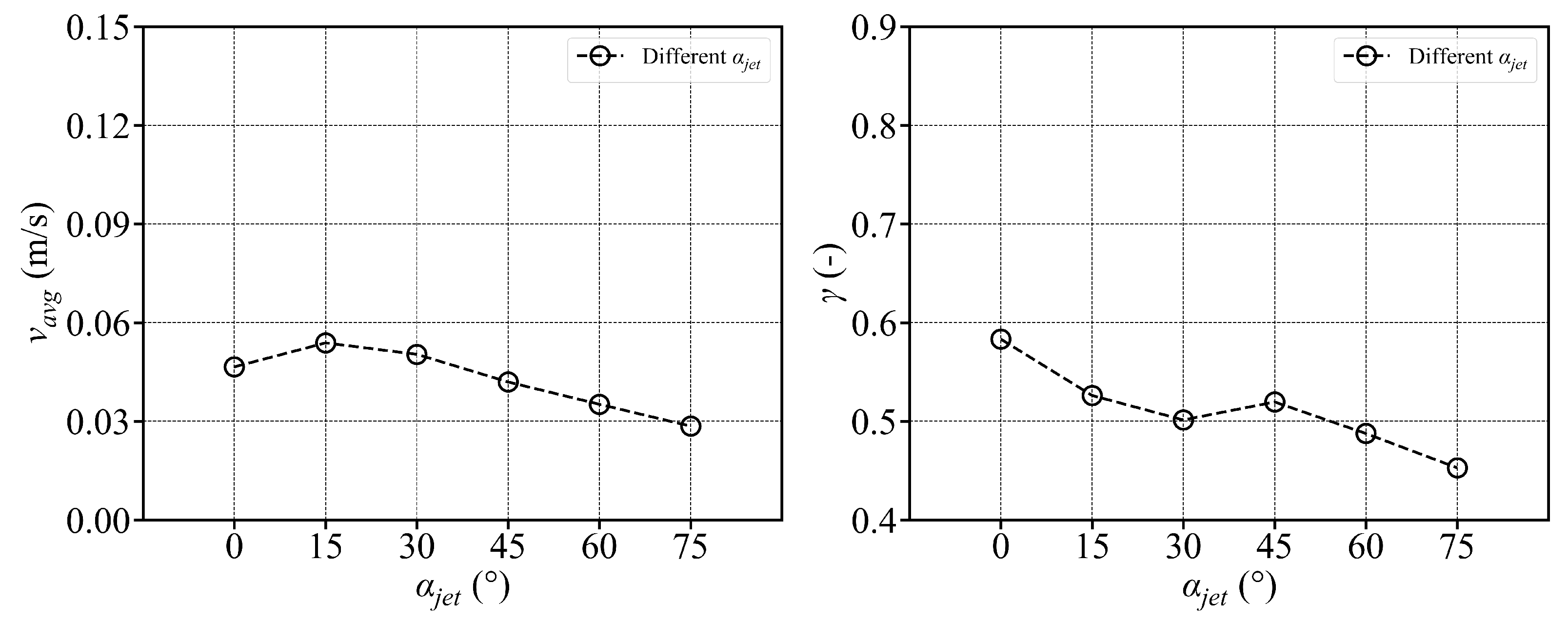
3.3. Circular Fish School Swimming Patterns
4. Conclusions
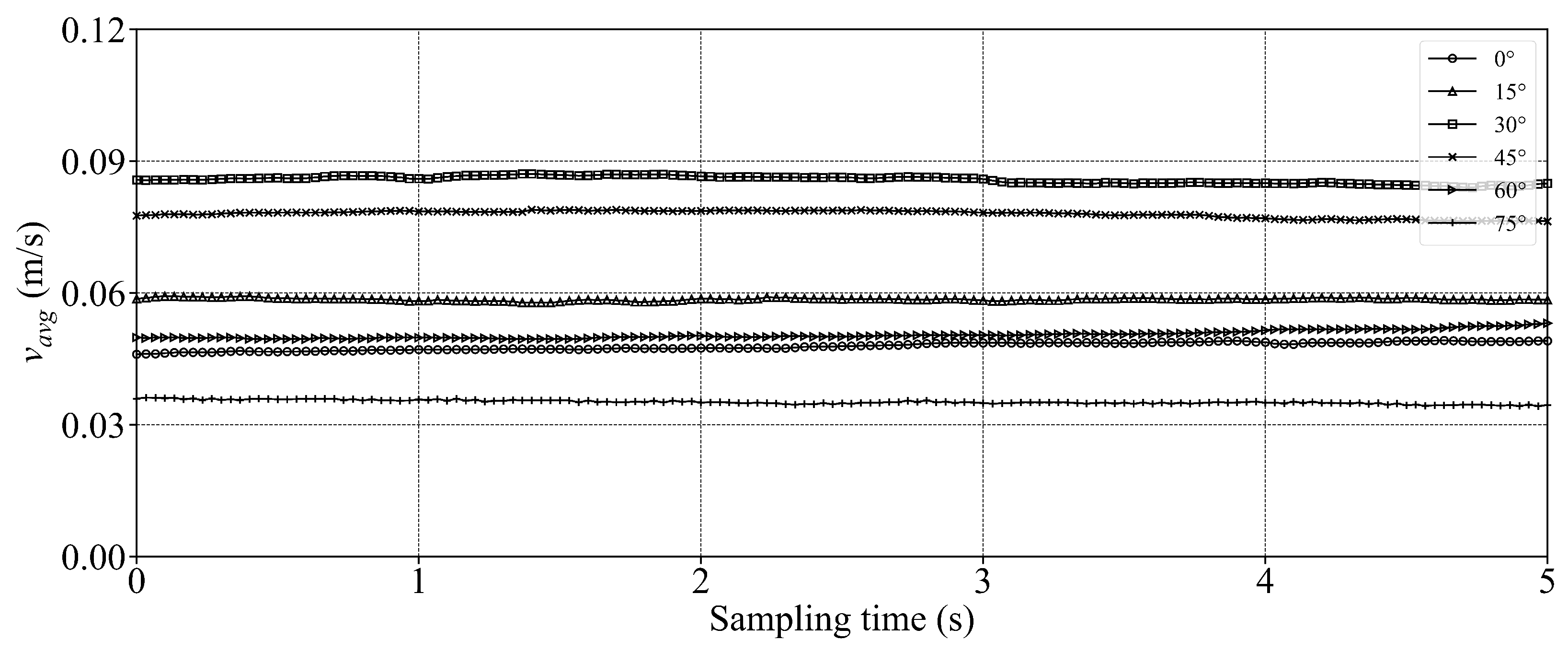
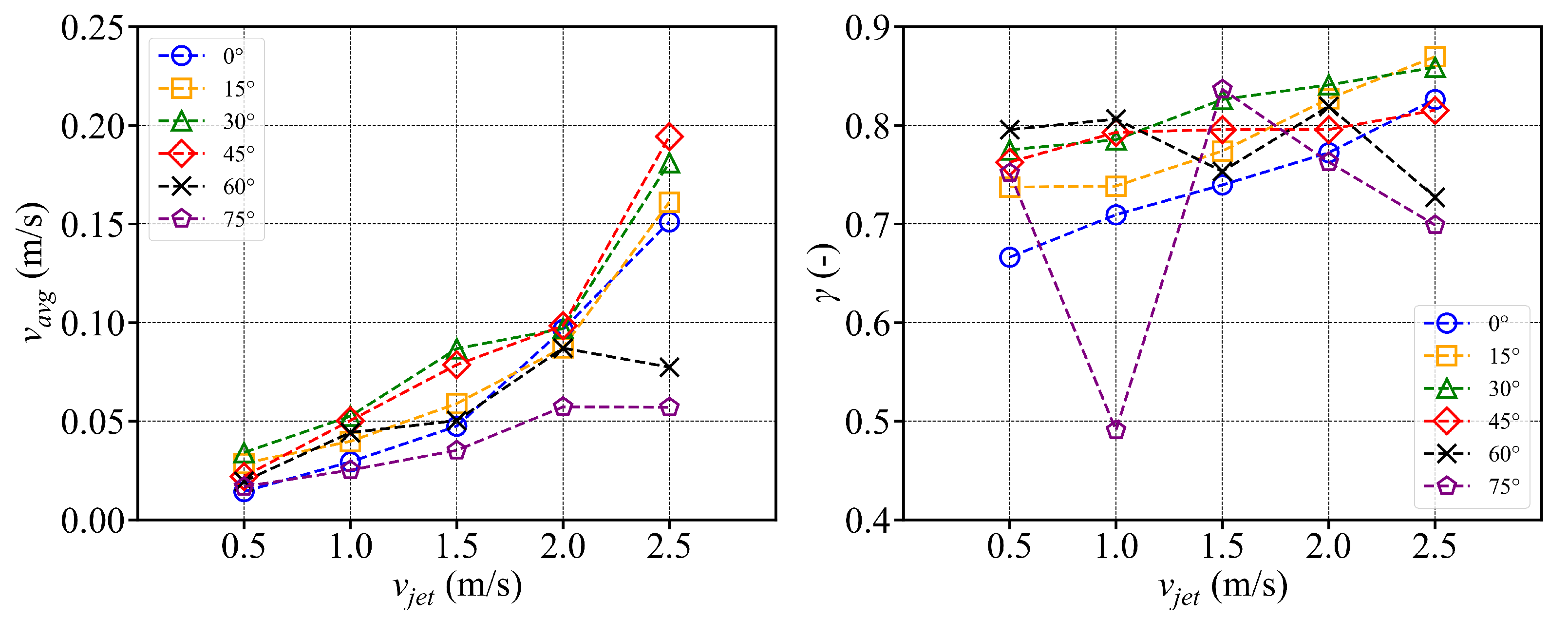
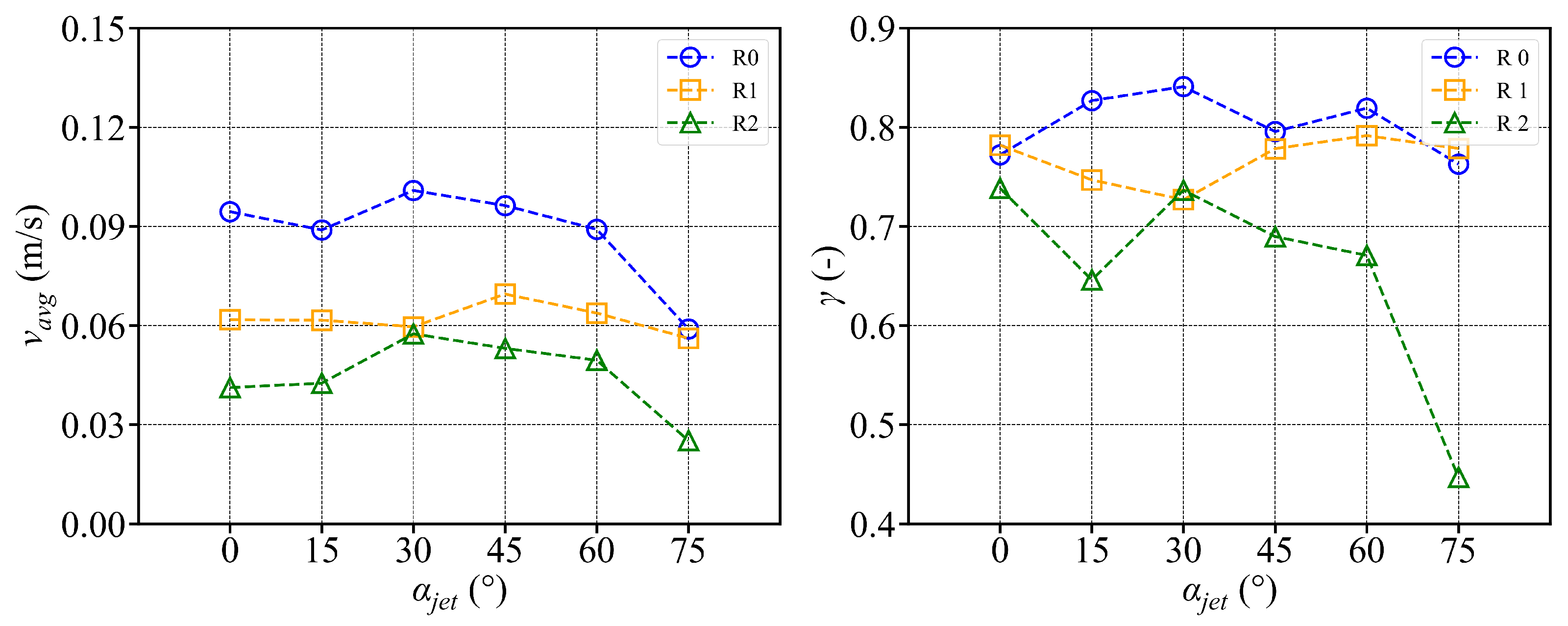
- In the octagonal aquaculture tank, the mean flow velocity increases with jet angle in the range of 0° to 45°, but begins to decline as the angle increases further. The flow velocity peaks at jet angles of 30° and 45°, with particularly significant changes observed at a jet velocity of 2.5 m/s. At smaller jet angles, the flow trend becomes more pronounced as the flow velocity increases. The -value reaches its maximum at 30°, while at 45°, the trend is least sensitive to changes in jet velocity. No consistent trend in -value is observed at higher jet angles (60° and 75°), indicating increased instability. Removing two deflector baffles leads to greater turbulence than removing one. Retaining the deflectors promotes the formation of stable and directional flow, with high mean flow velocity, a clear trend, and uniform distribution—conditions beneficial to fish growth. In contrast, removing both deflectors significantly reduces the flow velocity, disrupts trend clarity, and increases turbulence, which may adversely affect aquaculture.
- Compared with arc-shaped tanks, octagonal aquaculture tanks exhibit higher average flow velocities at jet angles of 30° and 45°, while arc-shaped tanks show peak flow velocities at 0° and 15°. In the arc-shaped tanks, the -value is most stable at 0° and decreases with increasing jet angle. This value is also generally higher under low-jet-velocity conditions. In arc-shaped tanks, retaining deflectors results in higher overall flow velocities, particularly at low jet angles. Removing both deflectors leads to increased turbulence, especially under high-jet-angle conditions. At low jet angles (0°–30°), the mean flow velocity decreases as more deflectors are removed. At high jet angles, removing a single deflector has minimal effect on both the mean flow velocity and the flow field trend. In the case of circular boundaries, Scheme A proves to be more effective. Conversely, removing both deflectors substantially increases flow field turbulence.
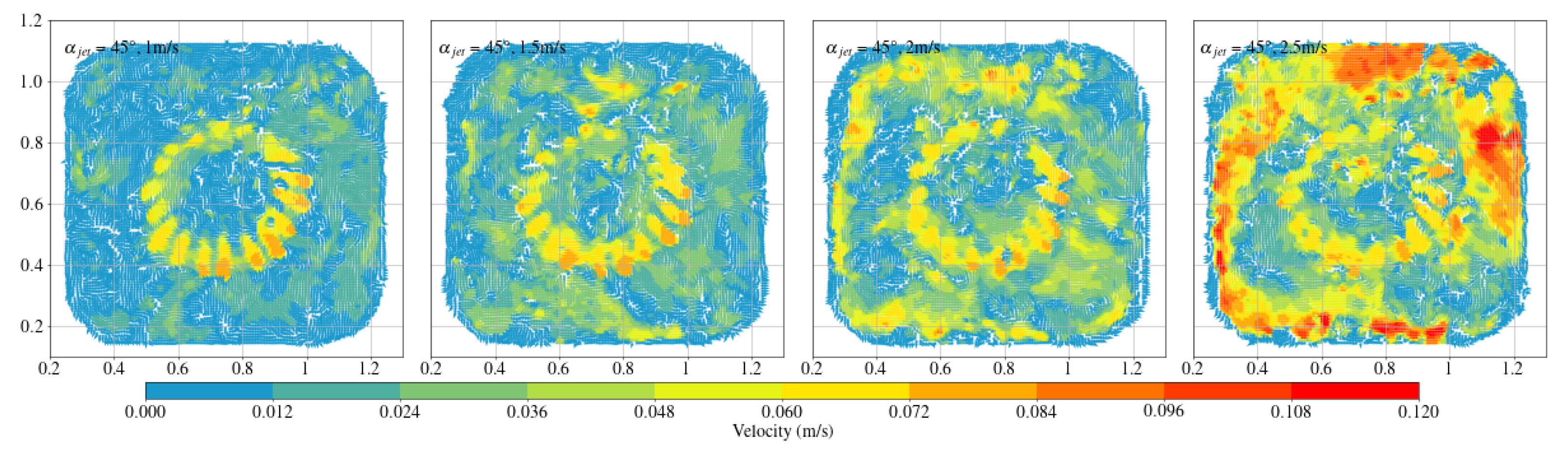
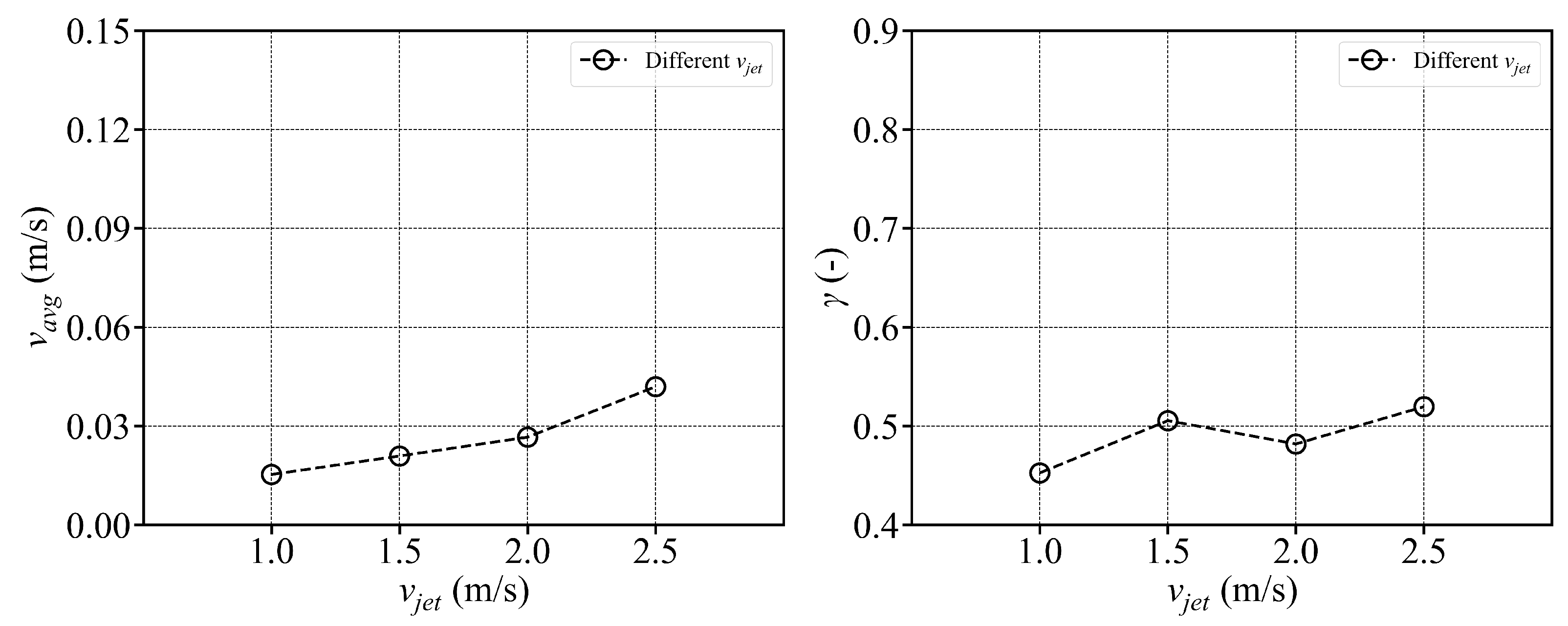
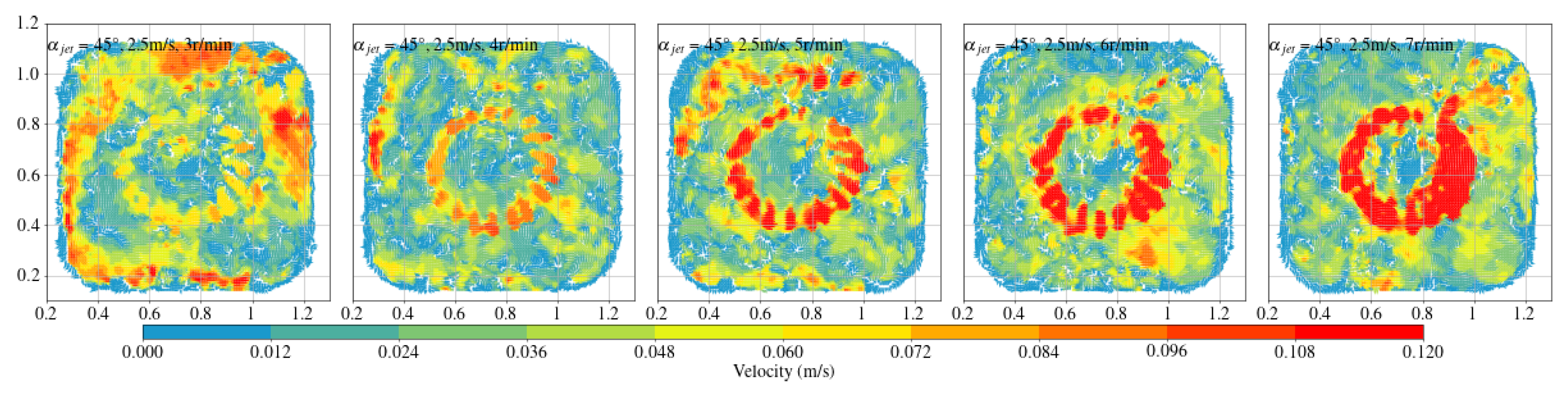
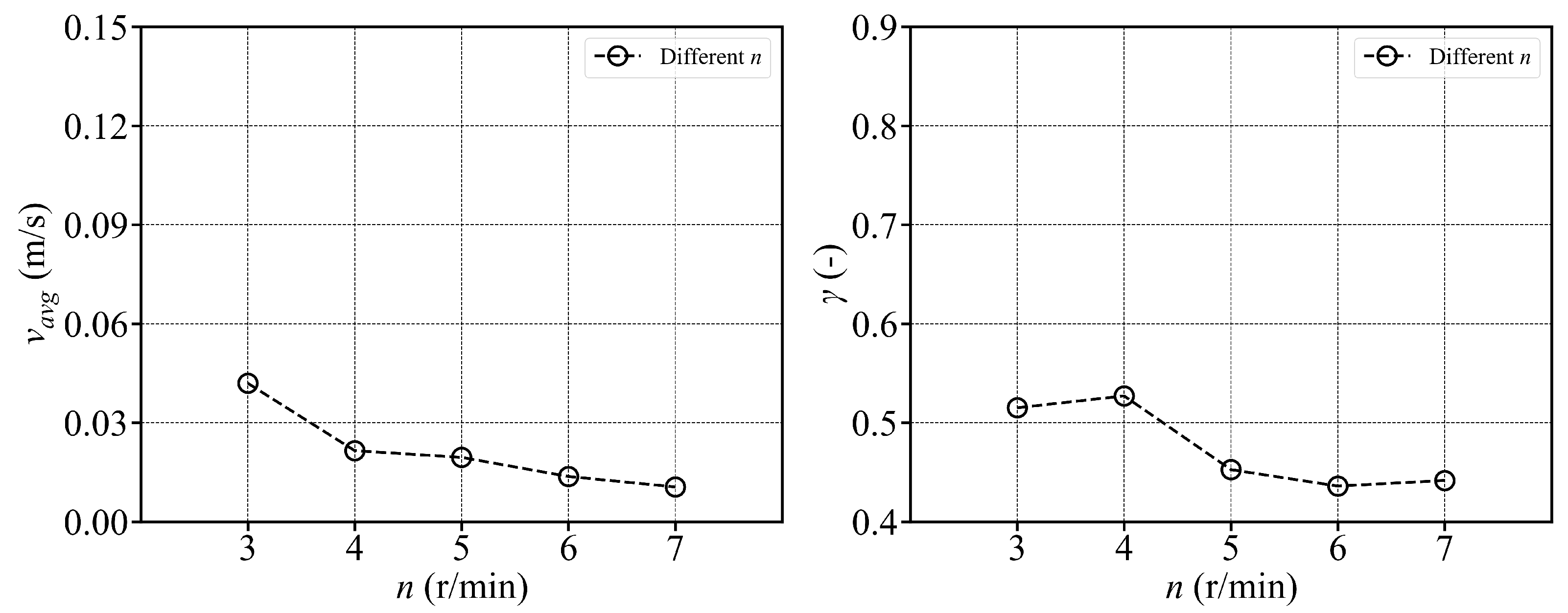
- The fish simulation, experiments were conducted at a jet velocity of 2.5 m/s and a fish rotation speed of 3 rpm under various jet angles, and the trend of mean flow velocity was found to be consistent with that observed in the airspace, peaking at 15°. However, the peak value was 66.875% lower than that in the airspace. Moreover, the overall trend index was 37.5% lower than that in the airspace. These results indicate that fish populations significantly hinder fluid flow and disrupt the stability of the flow field. Under varying jet velocities, the mean flow velocity increased with jet speed, but was reduced by 71.1% at 2.5 m/s compared to that in the airspace. Although the trend index increased overall, it exhibited an average decrease of 34.7% across the four jet velocities. This further confirms that fish disturb the flow field pattern and reduce the mean flow velocity. Disc rotational speeds ranging from 3 rpm to 7 rpm were used to simulate the circular swimming behavior of fish. After removing the high-velocity region near the circular tubes, the overall high-speed region in the flow field was significantly reduced. As the rotational speed increased, flow field disturbance intensified, and the average flow velocity approached zero, while the trend index decreased, indicating that the flow field gradually reached a near-stationary state.
- In aquaculture, when using octagonal tanks and operating under high-jet-velocity conditions, a smaller jet angle (0°–45°) should be selected, and deflector baffles should be retained to maintain a stable flow field, which supports the survival and growth of fish. For arc-shaped tanks, deflectors should be retained at low jet angles (0°–30°) to sustain higher average flow velocities. At higher jet angles, retaining deflectors can help reduce flow field disturbances. This approach helps suppress turbulence in the flow field. In fish farming, jet velocity should be adjusted based on species-specific swimming capabilities to avoid excessive resistance to fish movement.
- This study has certain limitations. The potential influences of fish body size and swimming speed were not fully accounted for in the analysis. Fish of varying body sizes require different spatial allowances within culture tanks; larger fish may demand broader flow field zones to accommodate their swimming behavior, while smaller fish may exhibit different adaptability to flow velocity and field dynamics. Additionally, fish with different inherent swimming speeds may respond differently to variations in jet speed and angle, with fast-swimming species requiring higher flow velocities to mimic natural swimming environments, whereas slow-swimming species may be better suited to lower-velocity conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nyström, M.; Jouffray, J.B.; Norström, A.V.; Crona, B.; Jørgensen, P.S.; Carpenter, S.R.; Bodin, Ö.; Galaz, V.; Folke, C. Anatomy and resilience of the global production ecosystem. Nature 2019, 575, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, J.; Fang, H.; Zhuang, Z.; Liu, H.; Liu, Y.; Xu, Y. Transformation of China’s Marine Fisheries and Strategic Emerging Industry of Deep Blue Fisheries. Fish. Mod. 2020, 47, 1–9. [Google Scholar]
- Masaló, I.; Oca, J. Hydrodynamics in a multivortex aquaculture tank: Effect of baffles and water inlet characteristics. Aquac. Eng. 2014, 58, 69–76. [Google Scholar] [CrossRef]
- Gorle, J.; Terjesen, B.F.; Summerfelt, S.T. Hydrodynamics of octagonal culture tanks with Cornell-type dual-drain system. Comput. Electron. Agric. 2018, 151, 354–364. [Google Scholar] [CrossRef]
- Davidson, J.; Summerfelt, S. Solids flushing, mixing, and water velocity profiles within large (10 and 150 m3) circular ‘Cornell-type’ dual-drain tanks. Aquac. Eng. 2004, 32, 245–271. [Google Scholar] [CrossRef]
- Gorle, J.M.; Terjesen, B.F.; Holan, A.B.; Berge, A.; Summerfelt, S.T. Qualifying the design of a floating closed-containment fish farm using computational fluid dynamics. Biosyst. Eng. 2018, 175, 63–81. [Google Scholar] [CrossRef]
- Nilsen, A.; Hagen, Ø.; Johnsen, C.A.; Prytz, H.; Zhou, B.; Nielsen, K.V.; Bjørnevik, M. The importance of exercise: Increased water velocity improves growth of Atlantic salmon in closed cages. Aquaculture 2019, 501, 537–546. [Google Scholar] [CrossRef]
- Nilsen, A.; Nielsen, K.V.; Bergheim, A. A closer look at closed cages: Growth and mortality rates during production of post-smolt Atlantic salmon in marine closed confinement systems. Aquac. Eng. 2020, 91, 102124. [Google Scholar] [CrossRef]
- Tao, Y.; Zhu, R.; Gu, J.; Li, Z.; Zhang, Z.; Xu, X. Experimental and numerical investigation of the hydrodynamic response of an aquaculture vessel. Ocean Eng. 2023, 279, 114505. [Google Scholar] [CrossRef]
- Xue, B.; Zhao, Y.; Liu, Y.; Cheng, Y. Flow characteristics of an aquaculture vessel with perforated sideboards at various incidence angles. Biosyst. Eng. 2023, 234, 108–124. [Google Scholar] [CrossRef]
- Oca, J.; Masaló, I. Design criteria for rotating flow cells in rectangular aquaculture tanks. Aquac. Eng. 2007, 36, 36–44. [Google Scholar] [CrossRef]
- Gorle, J.; Terjesen, B.; Summerfelt, S.T. Hydrodynamics of Atlantic salmon culture tank: Effect of inlet nozzle angle on the velocity field. Comput. Electron. Agric. 2019, 158, 79–91. [Google Scholar] [CrossRef]
- Xue, B.; Zhao, Y.; Bi, C.; Cheng, Y.; Ren, X.; Liu, Y. Investigation of flow field and pollutant particle distribution in the aquaculture tank for fish farming based on computational fluid dynamics. Comput. Electron. Agric. 2022, 200, 107243. [Google Scholar] [CrossRef]
- Wang, D. Experimental Study on the Effects on Fish of Flow Turbulent; Hohai University: Nanjing, China, 2007; pp. 42–50. (In Chinese) [Google Scholar]
- Song, B.; Lin, X.; Wang, W.; Li, G. Effects of water velocities on rheotaxis behaviour and oxygen consumption rate of tinfoil barbs Barbodes schwanenfeldi. Acta Zool. Sin. 2008, 54, 686–694. [Google Scholar] [CrossRef]
- Tang, M.F.; Xu, T.J.; Dong, G.H.; Zhao, Y.P.; Guo, W.J. Numerical simulation of the effects of fish behavior on flow dynamics around net cage. Appl. Ocean. Res. 2017, 64, 258–280. [Google Scholar] [CrossRef]
- Che, Z.; Ren, X.; Zhang, Q. Research progress on hydrodynamics in a recirculating aquaculture tank system: A review. J. Dalian Ocean Univ. 2021, 36, 886–898. [Google Scholar] [CrossRef]
- Plew, D.R.; Klebert, P.; Rosten, T.W.; Aspaas, S.; Birkevold, J. Changes to flow and turbulence caused by different concentrations of fish in a circular tank. J. Hydraul. Res. 2015, 53, 364–383. [Google Scholar] [CrossRef]
- Masaló, I.; Oca, J. Influence of fish swimming on the flow pattern of circular tanks. Aquac. Eng. 2016, 74, 84–95. [Google Scholar] [CrossRef]
- An, C.H.; Sin, M.G.; Kim, M.J.; Jong, I.B.; Song, G.J.; Choe, C. Effect of bottom drain positions on circular tank hydraulics: CFD simulations. Aquac. Eng. 2018, 83, 138–150. [Google Scholar] [CrossRef]
- Liu, N.; Liu, S.; Yu, G. Numerical simulation of and research on hydrodynamic characteristics of two dual-channel circular aquaculture ponds. Fish. Mod. 2017, 44, 1–6. [Google Scholar]
- Liu, H.; Ren, X.; Zhang, Q.; Bi, C. Numerical modeling of fish movement in a recirculating aquaculture tank: In the case of Schlegel’s black rockfish Sebastes schlegelii. J. Dalian Ocean. Univ. 2021, 36, 995–1002. [Google Scholar] [CrossRef]
- Yu, L.P.; Xue, B.R.; Ren, X.; Liu, Y.; Xu, T.J.; Shi, X.Y.; Hu, Y.X.; Zhang, Q. Influence of single inlet pipe structure on hydrodynamic characteristics in single-drain rectangular aquaculture tank with arc angles. J. Dalian Ocean. Univ. 2020, 35, 134–140. [Google Scholar] [CrossRef]
- Shi, X.Y.; Li, M.; Ren, X.Z.; Feng, D.J.; Liu, H.F.; Zhou, Y.X.; Liu, H.B.; Zhao, C.X. Influence of length width ratio on sewage discharge characteristics in acircular rectangular angle culture tank with double inlet pipe structure. J. Dalian Ocean. Univ. 2023, 38, 707–716. [Google Scholar] [CrossRef]
- Park, S.-G.; Zhou, J.; Dong, S.; Li, Q.; Yoshida, T.; Kitazawa, D. Characteristics of the flow field inside and around a square fish cage considering the circular swimming pattern of a farmed fish school: Laboratory experiments and field observations. Ocean Eng. 2022, 261, 112097. [Google Scholar] [CrossRef]





| Case | Jet Velocity (m/s) | Jet Angle (°) |
|---|---|---|
| 1 | 0.5 | 0, 15, 30, 45, 60, 75 |
| 2 | 1 | 0, 15, 30, 45, 60, 75 |
| 3 | 1.5 | 0, 15, 30, 45, 60, 75 |
| 4 | 2 | 0, 15, 30, 45, 60, 75 |
| 5 | 2.5 | 0, 15, 30, 45, 60, 75 |
| Case | Jet Velocity (m/s) | Jet Angle (°) |
|---|---|---|
| R0 | 2 | 0, 15, 30, 45, 60, 75 |
| R1 | 2 | 0, 15, 30, 45, 60, 75 |
| R2 | 2 | 0, 15, 30, 45, 60, 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Li, G.; Xu, H.; Xie, Y.; Jia, P. An Experimental Study on the Effects of Deflector Baffles and Circular Fish School Swimming Patterns on Flow Field Characteristics in Aquaculture Vessels. J. Mar. Sci. Eng. 2025, 13, 1023. https://doi.org/10.3390/jmse13061023
Zhao C, Li G, Xu H, Xie Y, Jia P. An Experimental Study on the Effects of Deflector Baffles and Circular Fish School Swimming Patterns on Flow Field Characteristics in Aquaculture Vessels. Journal of Marine Science and Engineering. 2025; 13(6):1023. https://doi.org/10.3390/jmse13061023
Chicago/Turabian StyleZhao, Chunhui, Guoqiang Li, Haixiang Xu, Yonghe Xie, and Panpan Jia. 2025. "An Experimental Study on the Effects of Deflector Baffles and Circular Fish School Swimming Patterns on Flow Field Characteristics in Aquaculture Vessels" Journal of Marine Science and Engineering 13, no. 6: 1023. https://doi.org/10.3390/jmse13061023
APA StyleZhao, C., Li, G., Xu, H., Xie, Y., & Jia, P. (2025). An Experimental Study on the Effects of Deflector Baffles and Circular Fish School Swimming Patterns on Flow Field Characteristics in Aquaculture Vessels. Journal of Marine Science and Engineering, 13(6), 1023. https://doi.org/10.3390/jmse13061023






