Abstract
Marine environments provide a unique opportunity to blend offshore wind energy production and marine fishery activities as complementary technologies. This study investigated the morphological characteristics (length and weight) and biomass yield of seaweed (Undaria pinnatifida) in a model marine environment with mariculture within an offshore wind farm in southwestern Korea. The mean lengths in the first cultivation trials of U. pinnatifida sporophytes increased from 1.8 ± 0.1 cm in November 2021 to 120–170 cm in March 2022 (density, 39.8 plants m−1; final wet weight, 98.6–249.1 g (mean 146.8 ± 20.4 g, n = 20 ind.); yield 5842 g m−1). Further, for the second cultivation trial, the length of the sporophytes increased from 1.5 ± 0.1 cm in November 2021 to 120–150 cm in April 2022 (density, 49.3 plants m−1; final wet weight, 83.0–251.6 g (mean 155.7 ± 19.0 g; n = 20 ind.); yield, 7676 g m−1), and, owing to the increase in water temperature and light intensity due to seasonal changes around the offshore wind power farm, the second cultivation trials showed signs of chlorosis. Considering the environment, we judged seaweed growth to be normal. Therefore, when applying this model to grow U. pinnatifida, seasonal temperature changes, the purpose of the product, and the nutritional status of the open-sea area should be considered. These results may improve seaweed farming in offshore wind farms in the future.
1. Introduction
Expanding offshore wind farms worldwide for renewable energy production requires the sustainable coexistence of these energy production farms with other industries that use marine spaces [1,2,3]. Previous studies have identified scientific theories as well as the advantages, concerns, and barriers associated with the efficient sharing of locations and spatial resources. Recommendations for improvement have also been made, with better participation and information sharing being the most discussed recommendations [3]. Recently, large-scale seaweed farming along offshore wind farms has been proposed as a strategy for ensuring food security, providing restorative ecosystem services through nutrients recycling, and realizing carbon capture in response to the negative influence of climate change [4,5,6]. Studies have also been conducted to investigate the cultivation of mussels and seaweed in offshore wind farms along the German North Sea coast [4,7,8]. Further, it was examined whether currently available food and feed safety standards for seaweed can be applied to cultivated in offshore wind farms in the North Sea, because potential hazards may be more apparent for seaweed cultivation from equipment used, the effects of processing on seaweed, the contamination transfer rate to the seaweed, the effect of antifouling or protective coatings on structures near seaweed cultivation [5]. Reynolds [9] also suggested the suitability of offshore cultivation for two native seaweeds, Sugar kelp (Saccharina latissima) and Bladder wrack (Fucus vesiculosus), by conducting a quick scoping review (QSR) to assess the knowledge base surrounding seaweed farms in New Jersey, USA. Taken together, these previous studies suggest that it is necessary to further examine marine food production policies based on a review of the stable production of seaweed in offshore wind farms, because of the possible uptake of physical hazards from equipment used, the effects of processing on seaweed, the contamination transfer rate to the seaweed, and the effect of antifouling or protective coatings on structures near seaweed cultivation [5,6].
The Southwest Sea Offshore Wind Power Demonstration Farm, which is a representative of large-scale seaweed farming along the offshore wind power farm in South Korea, is located approximately 10 km west of the coast of Gochang, Jeongbuk Province, and comprises 20 turbines, each with a capacity of 3 MW. Hence, the total power generation capacity of this pilot offshore farm is 60 MW. Further, there are plans to establish a 400-MW capacity pilot farm and expand its capacity to 2000 MW by 2028, thus generating a total of 2.46 GW [10]. Achieving this goal will require the development of wind farms in the Southwest Sea using a fixed support structure at depths below 50 m and the installation of 800 turbines, each with a capacity of 3 MW. A considerably large area at a suitable location for offshore wind power development will also be required. Thus, there is a high possibility of overlap with the areas where major coastal fishing industry activities are carried out [10,11]. Accordingly, one of the issues that must be addressed during the planning of the expansion of the offshore wind power industry is the coexistence of power generation facilities with the fishing and mariculture industry activities.
Kelp (Undaria pinnatifida, commonly known as wakame), which has been one of the most cultivated seaweeds in northern Asia since the 1980s, is a useful brown algae that is primarily distributed in eastern Asia [12,13,14]. In 2013, the production of U. pinnatifida was 203,099 tonnes wet weight in China, 327,375 tonnes wet weight in Korea, and 43,900 tonnes wet weight in Japan, respectively [15]. It is used as a health food, animal feed, and medical purposes. It is also an important industrial source of alginic acid and fucoidan, which are used as raw materials in food processing [13,16]. Reportedly, kelp exhibits a typical Laminariales life cycle, with alternating gametophytic and sporophytic generations, and in the coastal waters of South Korea, its sporophytes emerge in October and November and grow rapidly from winter to spring when sea temperatures range between 10 and 16 °C [17,18]. At the industrial scale, U. pinnatifida is a major alga that is produced alongside those belonging to the same genera, Porphyra and Saccharina, and since the 2000s, it has served as an important food source in abalone farming, which has been expanding in the southern coastal area. From 2018 to 2022, Korea’s Undaria production is estimated to have ranged from 500,000 to 600,000 tons, with approximately 60% used for food and 40% as feed for abalone. The primary region for Undaria production in Korea is Jeonnam, which accounts for 90% of the total, while Undaria is also produced in other regions such as Busan and Chungnam [19].
U. pinnatifida also plays an important role in the coastal ecosystem as a major component of the underwater forest [20,21], and, in recent years, it has received considerable attention owing to its role in regulating the marine environment and as a functional food source. Thus, policies have been enacted to expand its production.
The possibility of scallop farming on offshore wind farms and the growth characteristics of Zhikong scallop (Chlamys farreri) and Bay scallop (Argopecten irradians) have been highlighted at the same site from March 2021 to July 2021 in a previous study [22]. However, a similar study on U. pinnatifida has not yet been reported. Therefore, in this study, we examined the morphological characteristics (length and weight) and biomass yield of U. pinnatifida within an offshore wind farm to determine the applicability of the long-line farm culture systems on offshore wind farms in southwestern Korea. We expected that the results would provide useful information for identifying suitable cultivation of seaweed species and sites in open sea environmental conditions.
2. Materials and Methods
2.1. Study Site and Environmental Conditions
The cultivation of seaweed (Undaria pinnatifida) was conducted in an offshore wind farm at Gochang, Jeongbuk Province, Korea (35°28′ N, 129°06′ E) between November 2021 and April 2022 (Figure 1). The sea area where the longline farm is installed in the offshore wind farm is 13.5 km from the coast, and the prevailing wind direction is northwest with a wind speed of 10–20 m/s in October and December. In addition, the water depth of the area is in the range of 8–15 m, the significant wave height in the area is in the range of 3–6 m, and the current speed is recorded at 0.98–1.02 m/s [23,24].
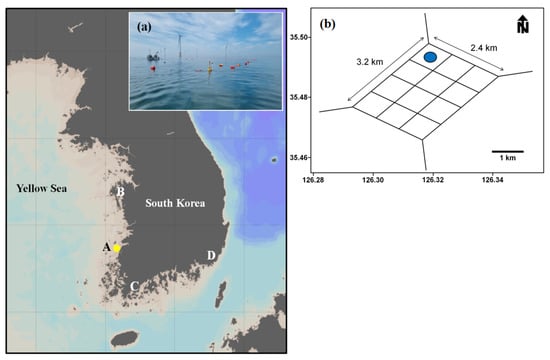
Figure 1.
Map showing the geographic location of the offshore wind farm and major cultivation areas of Undaria pinnatifida, where area (A) is the Gochang area (35°28′ N, 129°06′ E), (B) is Seocheon area (36°40′ N, 126°13′ E), (C) is Wando area (34°18′ N, 126°39′ E), and (D) is Busan area (35°18′ N, 129°20′ E), in South Korean areas slightly modified from Lee et al. [22]. The yellow dot (A) shows the location of the offshore wind farm. (a) Panoramic view of offshore wind farm; (b) location of longline farm (blue dot) installed in offshore wind farm.
To analyze water quality in this area, water temperature, salinity, dissolved oxygen level, pH, total phosphate (PO4-P), and total nitrogen (TN) in seawater were measured at a cultivation water depth of 1 m in November 2021. A conductivity, temperature, and depth instrument (SeaCAT Profiler CTD, SBE 19plus v2, Sea-Bird Electronics, Inc., Bellevue, WA, USA) installed in the upper (top 1 m) water layer was used to measure the above-mentioned parameters. For PO4-P determination in seawater, the Molybdenum blue method was employed [25]. A 50 mL seawater sample was treated with 0.5 mL of ammonium molybdate solution and 0.5 mL of reducing agent, followed by the addition of 1 mL of sulfuric acid. The samples were incubated at 50 °C for 45 min, and the absorbance of the blue color produced was measured at 880 nm. Furthermore, TN in seawater samples was determined using the method described by Suzuki et al. [26]. In this procedure, a 50 mL volume of seawater was digested with concentrated sulfuric acid and a catalyst at elevated temperatures. The resulting digest was then analyzed colorimetrically to quantify the total nitrogen concentration using a standard reagent. Additionally, a HOBO data logger (U24-002-C, ONSET Corp., Boune, MA, USA) was installed at a water depth of 5 m in the longline farm and moored for one year from December 2021 to December 2022 to continuously record water temperature over this period.
2.2. Design of the Culture System, Cultivation and Sampling
The longline farm was constructed by connecting 200 m horizontal and 200 m vertical synthetic polypropylene (PP) ropes, each with a diameter of 48 mm, and to stabilize the structure, 36 mm PP ropes were installed at 50 m intervals. Further, to maintain balance in the cross-anchor line, a 4 kg weight was connected to the longline via the 36 mm PP ropes. The formwork of the longline farm was secured on the ground using 120 kg steel anchors. The frame and mooring control buoys had a volume of 420 L, and the culture system management buoys were installed at the four corners of the aquaculture system as shown in Figure 2.
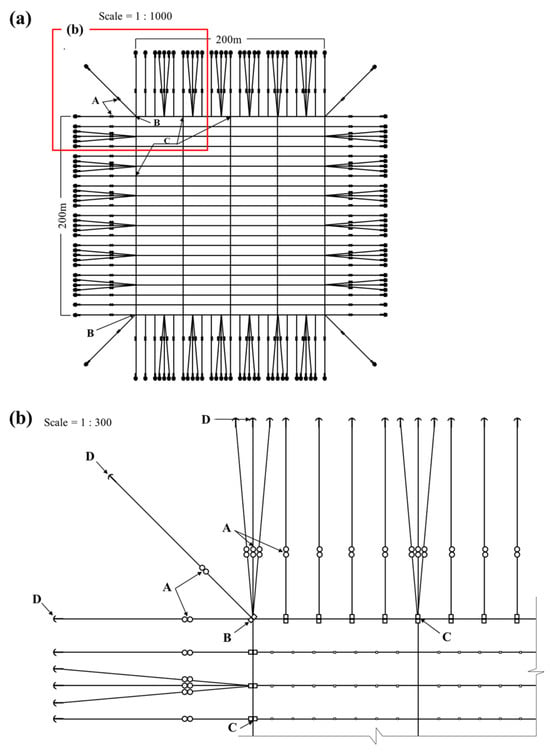
Figure 2.
Schematic representation of the longline farm for Undaria pinnatifida cultivation within the offshore wind farm. (a) Drawing of longline farm (A, balance weight; B, main buoy; C, side support buoy; D, anchor), (b) enlarged image of the upper left of (a).
Juvenile sporophytes of U. pinnatifida, derived from the same strain, were maintained in an indoor nursery under controlled conditions (17–20 °C; 20 μmol m−2s−1 light intensity) and a 14:10 h light-to-dark (L:D) photoperiod at the Seedling Production Center of Wooribada Fishery Corporation located in Wando (site C in Figure 1) from mid-September to late October 2021. The seedlings of U. pinnatifida used for cultivation were cultured in the Wando area (site C in Figure 1). The U. pinnatiufida sporophytes were transplanted and managed for one week at the Wando coastal area farm to allow them to adapt to the coastal water environments before being transplanted to the longline aquaculture farm within the offshore wind farm.
The first seed strings to which the U. pinnatifida juvenile sporophytes adhered were installed at a water depth of 1 m at intervals of 7–10 m on 3 November 2021. The seed strings consisted of eight 200 m long PP ropes, each with a diameter of 9 mm. Then, to construct the seaweed longline, a weft line (length, 4 cm; diameter, 16 mm) was inserted seed string pieces into the PP rope at 50 cm intervals after the weft was separated from the seaweed seedling frame (Figure 2).
The longline farm was established between the wind turbines on the northern side of wind farm; thus, it did not interfere with the operation and management of the wind turbine (Figure 1b). To counter the effects of variations in wave, current, and tide levels on the offshore wind farm, load cases were constructed and the mooring lines and long lines of the marine farm were designed and improved to comply with the standards and recommendations of Det Norske Veritas (DKV; Høvik, Norway) [27].
In the second cultivation trial, U. pinnatifida sporophytes were planted in seven 200 m long longlines on 27 November 2021, following the same procedure that was employed for the planting of the first seed strings. After the planting, the total length (cm/individuals) and final wet weight (g/ind.) of the plants were measured every 2–3 weeks. Then, on 8 March 2022 and 5 April 2022, the first and second cultivation trials of seaweed were harvested, respectively, and their lengths and weights were calculated. The daily growth rate (DGR) [28] and specific growth rate (SGR) [29] were calculated for each mean of 20 individuals using the equation:
in which Wt and W0 are weight of each individual at times of final and initial of the experiment, Lt and L0 are length of each individual at times of final and initial of the experiment.
DGR = [(Wt / W0)1/t − 1] × 100
SGR = (Ln Lt − Ln L0)/t × 100
Next, we performed Student’s t-test to assess differences in length and weigh between the first and second cultivation trials of seaweed, and Pearson’s correlation analysis using SPSS software version 28.0 (IBM Korea Inc., Seoul, Republic of Korea). Plant densities (plants m−1 rope) and yields (g m−1 rope) were determined for 13 replicates.
3. Results and Discussion
3.1. Environmental Conditions
In the aquaculture environment conditions at the study site, surveyed in November 2021, water temperature, salinity, PO4-P, TN, dissolved oxygen, and pH were 13.3 °C, 30.9 psu, 0.025 µM, 0.3010 µM, 8.69 µM, and 8.2, respectively. Further, from December 2021 to April 2022, the lowest water temperature observed during U. pinnatifida cultivation was recorded at 3.3 °C in February 2022, and after, it was increased to 9.2 °C April 8 at the end of the second cultivation trials (Figure 3). The water temperature during the cultivation period (January to mid-March) was 3.7 °C below the minimum optimal level of 7 °C for Undaria growth, as recommended by NIFS [13]. The physical and environmental conditions of our study site are 13.5 km from the coast, and the prevailing wind direction is northwest with a wind speed of 10–20 m/s in October and December. In addition, the water depth of the area is in the range of 8–15 m, the significant wave height in the area is in the range of 3–6 m, and the current speed is recorded at 0.98–1.02 m/s [23,24]. The current speed recorded in the above data is significantly higher than that observed at Undaria farms in Korea to date. According to the NIFS [13], Undaria farms in Korea are primarily located in sheltered bay areas, where they are less exposed to physical disturbances from open sea conditions. These sites are typically characterized by water temperatures ranging from 13 °C to 7 °C in the Undaria culture period, current speeds of approximately 10 cm/s, and water depths between 5 and 40 m.
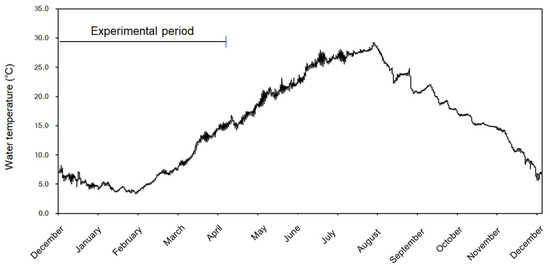
Figure 3.
Monthly changes in surface water temperature at the longline farm within the offshore wind farm from December 2021 to December 2022.
3.2. Morphological Characteristics (Length and Weight) and Biomass Yield
Changes in length, weight, and biomass for the different seaweed cultivation trials over time are shown in Figure 4 and Figure 5 and Table 1. The length of the first cultivation trials of U. pinnatifida sporophytes increased from 1.4~2.9 cm (mean 1.8 ± 0.1 cm; n = 20 individuals) in November 2021 to 120~170 cm (mean 141.4 ± 4.6 cm; n = 20 ind.) in March 2022 (Figure 4 and Figure 5a). Further, the final wet weight at harvest for this first cultivation trial was 98.6~249.1 g (mean 146.8 ± 20.4 g, n = 20 ind.) (Figure 4 and Figure 5b). For the second cultivation trial, the starting plant length increased from 1.2~2.7 cm (mean 1.5 ± 0.1 cm; n = 20 ind.) in November 2021 to 120~150 cm (mean 134 ± 3.5 cm; n = 20 ind.) in April 2022 (harvest) (Figure 5a); and the final wet weight was 83.0~251.6 g (mean 155.7 ± 19.0 g; n = 20 ind.) (Figure 5b). Furthermore, we observed a significant difference in the total length and wet weight between the first and second cultivation trials at each measurement time after 8 January (Figure 5, p < 0.05). This observation could be attributed to the 22-day time difference between when the seed strings of the first and second cultivation trials were inserted into the main culture rope. However, at the time of harvest, after approximately 4 months of cultivation, the two groups showed similar plant sizes, with no significant difference in total length and wet weight (Figure 5, p > 0.05). It has been reported that photoperiod may affect the harvesting time of U. pinnatifida, but it is not related to outplanting [30]. In addition, previous studies reported that growth, morphological features, and maturation of seaweeds, including U. pinnatifida, can be affected by various environmental factors such as water motion, current velocity, and nitrate [8,31,32,33,34]. Increased water motion has been suggested to be definitively beneficial as it has been shown to increase the uptake of nutrients by seaweed such as kelp [35,36,37,38]. However, high-intensity water velocity can be a stressor, because it costs more in terms of survival adaptations than it benefits seaweed growth [35]. Therefore, water movement is a key factor that directly and indirectly affects the yield and quality of kelp and should be considered when determining the optimal location for aquaculture [35]. It has been suggested that the growth of U. pinnatifida had beneficial effects for current velocities not exceeding 0.3 m/s [35]. Gerard and Mann [39] reported that the annual production of Saccharina longicruris (as Laminaria longicruris) was lower in exposed sites (1 m/s) than in sheltered sites (0.5 m/s). In light of this, the conditions of 0.98–1.0 m/s in the current velocity range of the southwestern offshore wind farm in the open sea are close to the 1.0 m/sc range, which is considered to be a stressful condition for the current environment of S. longicruris. They are likely to have been stressful rather than beneficial to U.pinnatifida seaweed growth.

Figure 4.
Cultivation process for first cultivation trials U. pinnatifida at the longline farm within the offshore wind farm. (a) Strings with juvenile sporophytes before adherence at the longline farm, (b) seed strings into a PP rope, view of sporophytes cultivated in (c) January 2022, (d) February 2022, (e) March 2022, (f) the harvesting of sporophytes, (g) the drying of sporophytes.
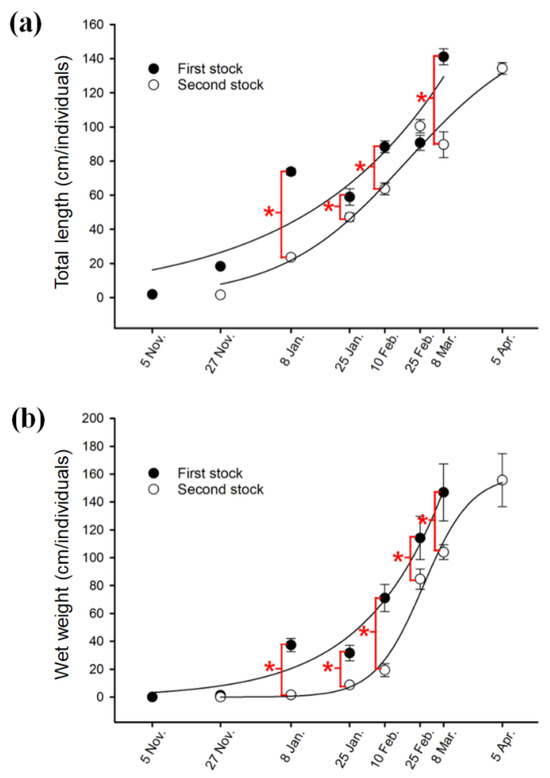
Figure 5.
Changes in total length (a), and wet weight (b) for U. pinnatifida sporophytes from November 2021 to April 2022. * indicates significantly different values between the first and second cultivation trials (p < 0.05, Student’s t-test).

Table 1.
Final Size, weight, density, and yield of Undaria pinnatifida in different seaweed farms along Korean coasts.
At water temperatures in the range of 3.7–9.2 °C, the densities of first and second cultivation trials of U. pinnatifida were 39.8 and 49.3 plants m−1, respectively, and their yields were 5842 and 7676 g m−1, respectively (Table 1). The daily growth rate (DGR) and specific growth rate (SGR) were 8.08 day−1 and 3.49 day −1 in the first cultivation trial, and 8.24 day−1 and 3.42 day −1 in second cultivation trial, respectively. The Pearson’s correlation analysis for water temperature was performed with DGR and SGR of U. pinnatifida. As a result of analyzing the correlation, DSR and water temperature (r = 0.574, p < 0.01), SGR and water temperature (r = 0.549, p < 0.01) appear to be a significant positive correlation during the first cultivation trials. In contrast, DSR and water temperature (r = −0.594, p < 0.01), SGR and water temperature (r = −0.499, p < 0.01) showed a significant negative correlation during the second cultivation trials. These results suggest that changes in water temperature during the second cultivation trials may have hindered seaweed growth.
These values are lower than those obtained for seaweed produced at site C located on the southern coast of South Korea (Wando in Jeonnam Province) at temperatures in the range 8.9–16.5 °C and at site D (southeastern coast of Busan) at temperatures in the range 5.5–13.1 °C, but higher than those of seaweed produced at site B along the west coast in 2013–2014 (Seosan in Chungcheong Province) at temperatures in the range 13.1–15.3 °C for approximately 4 months during the winter season [13] (Figure 1, Table 1). Further, in a previous study conducted in the Sanriku coast in northern Japan, the density and yield of U. pinnatifida, produced at temperatures in the range 5–20 °C from mid-October 2014 to late April 2015 (approximately 4 months), were 14.0 plants m−1 and 4.8 g m−1, respectively [14]. As stated above, these differences in growth and yield characteristics could be attributed to factors such as differences in water temperature in the aquaculture area. High-temperature-tolerant strains of U. pinnatifida showed higher photosynthetic activities and respiration in warmer locations (Naruto, southern Japan) compared to two colder locations (Okirai Bay and Matsushima Bay, northern Japan) [14,40]. Accordingly, if high-temperature tolerant strains can be induced to grow at water temperatures exceeding the 3.7–9.2 °C range observed during the cultivation period in this study, it may be possible to enhance productivity by selecting such strains and optimizing the cultivation schedule. Further investigation in this area is warranted.
Additionally, it has been suggested that the initial transplant density is a major factor affecting aquaculture yield. In previous studies, varying plant densities have been reported. For example, the National Institute of Fisheries Science in Korea employed a plant density of three to nine plants m−1 [13], while Sato et al. [14] employed a plant density of three plants m−1. Further, in this study, the plant density was two plants m−1. In addition, previous study reported that biomass production of U. pinnatifida increased with increasing density, and the maximum possible density exceeded 200 plants m−1 for biomass. However, it has been suggested that between 80 and 120 plants m−1 is an appropriate density range for the optimum production of this particular species in respect to both profitability and quality [41]. In this study, the lowest density level of two plants m−1 was applied, in comparison to previous studies, to obtain baseline data under offshore environmental conditions. However, it is necessary to determine the optimal density in future studies by evaluating growth and productivity at higher density levels.
Color analysis revealed yellowing for the second cultivation trial of harvested seaweed (Figure 6). It has been reported that the tissue concentration and balance of chlorophyll a, chlorophyll c, and fucoxanthin, which comprise the main pigments of Undaria [42,43], are affected by abiotic factors such as nutrient availability, temperature, and irradiance [42,43,44,45]. For example, a decrease in the photosynthetic pigments was associated with co-fading of Undaria in response to low nutrients and high irradiance conditions [44]. In this study, the difference in the occurrence of chlorosis, or the lack thereof, between the first and second trials can be attributed to the abnormality of color due to the increase in water temperature and light intensity according to the seasonal change from winter to spring, considering the results of previous studies [43,46,47]. The water temperature at the harvest time during the first trial increased from 5.3 °C on 8 March 2022, to 9.2 °C on 5 April 2022, the harvest time during the second trial (Figure 3). Unfortunately, we do not have data on the variation in light intensity; it is estimated that the culture water was exposed to higher light intensity than the first culture period due to seasonal changes in the culture water depth 1 m below water depth. Previous studies have reported pigment content with seasonal progression, and differences have been reported between spring and summer when water temperature and light intensity increase [43,46,47]. Light intensity is one of the most important factors affecting the pigment content and color of Undaria, and increased light intensity causes pigments to decrease [43,46,47]. Therefore, this study judged that the color abnormality of seaweed was induced by seasonal changes such as increased water temperature and excessive light exposure during the extended cultivation period until April. Therefore, the cultivation period is suggested as one of the conditions to be considered for successful Undaria cultivation with commercial value in mind.

Figure 6.
Photographs of the drying of Undaria pinnatifida sporophytes. (a,b) First cultivation trial sporophytes of Undaria showing a normal dark brown color. (c,d) Second cultivation trial sporophytes of Undaria showing chlorosis.
To avoid seaweed chlorosis, increasing ocean nutrient levels via artificial means by supplying nutrient-rich water via upwelling and chemical fertilization of surface water around seaweed raft farms have been proposed [13,48,49,50]. In addition, it is necessary to set the cultivation period when the water temperature and light intensity are suitable for the growth of seaweed, taking into account seasonal changes in water temperature and light intensity. However, in large-scale seaweed farming offshore, it is necessary to study the efficiency of artificial nutrient supply and examine whether it is compatible with farm management policies.
Southwestern Korea experiences large tidal differences that are directly or indirectly affected by nearby bays and lakes, which are characterized by strong tidal fronts owing to seasonal environmental changes [51,52]. The surrounding coast, where the offshore pilot wind power farm is located, is geographically adjacent to sites with a developed flat tidal environment comprising Gomso Bay, the largest clam-producing area in the country [53]. Therefore, considering the scale of the offshore wind power demonstration farm, there is an urgent need to present an energy-fisheries and mariculture coexistence model suitable for this region. Indeed, our previous study has found offshore wind farm areas as potential aquaculture locations for scallops [22]. However, since the southwestern offshore wind farm is located in an open sea area with a harsh environment, it is necessary to identify areas for improvement through pilot operation before installing and operating the aquaculture farm.
Depending on the conditions of the sea areas (sheltered or open sites), the culture system was selectively applied using the type of hanging rope culture or horizontal rope culture [38]. In the case of kelp, hanging rope culture has been suggested to be a more efficient system for nutrient uptake by seaweeds than horizontal rope culture in sheltered areas with low current velocities of 0.1 m/s [38]. This is because it provides better water movement, making it easier to maintain an appropriate level of tension for water to flow over the kelp and reducing diffusion across the boundary layer to increase nutrient uptake [38]. However, hanging ropes have a risk of rope tangling and damage to the culture frame in areas with fast currents [38,54]. In contrast, horizontal ropes have strong resistance to water movement and are mainly used in kelp aquaculture performed in Asia. [38,54,55] The water movement of the horizontal rope is suggested to be suitable for the current velocities of 0.2~0.9 m/s, which are found between medium and high environments [38]. In another study, the system used in the offshore wind power plant under the open North Sea condition was designed and tested with S. latissima under very rough conditions of current velocity over 2 m/s, but the horizontal and hanging rope methods were reviewed as not suitable for the offshore wind power plant site where such strong current velocity exists [7,56]. From the above results, it is judged that the horizontal rope culture is a method worth trying in the open-ocean sites of offshore wind farms in southwestern Korea under the water motion conditions of 0.9~1.0 m/s to prevent line tangling; however, discussion on the appropriate aquaculture target species should be further conducted through future studies.
Kelp species generally show morphological differences depending on the degree of exposure to water flow or waves [39,57,58,59,60]. In areas with high-flow, thick, narrow, and flat blades are produced to decrease drag to water flow and prevent breakage. Otherwise, in low-flow areas, thin, wide, and ruffled blades are produced, inducing turbulence and enhancing the mass transport of nutrients [31]. In this study, the length and wet weight of products in site A of the open area were smaller and lighter than Undaria produced in site C with a semi-closed area, and site D near the coast. Also, despite a lack of detailed morphological data (blade width and thickness), it seems that there are thicker and narrower Undaria blade shapes in site A than in sites C and D (Figure 1). In the future, therefore, it may be essential for U. pinnatifida cultivation to control for morphological quality according to water flow conditions.
Sporophytes of U. pinnatifida have commonly been cultivated from November to April [13], and grow fast from December to March in Korea [61]. However, the period during which seawater temperatures increase, especially with high water temperatures persisting until autumn, is shortening the period during which seaweed can be cultivated [40,62]. Thus, variation in the ocean environment may increase variability in the quality of seaweed and increase harvests of Undaria with low marketability for human food consumption. As in this study, Undaria production in offshore areas may have low marketability quality for human food consumption and can instead be considered for the use of Undaria as feed for abalone. The previous studies have suggested that seaweed is useful not only as human food but also for various industrial materials, and in uses such as increasing fishermen’s income by using Undaria as food for sea urchins, or suggesting their use in carbon sequestration [63,64]. On the other hand, in terms of ensuring economic feasibility, Lian et al. [65] found that, for kelp farms with the same number of cultivation lines, construction costs are significantly affected by the cost of mooring lines. Moreover, operational costs typically include expenses for seeding, harvesting, monitoring, and labor for cultivation management, all of which may increase the demand for buoys and mooring lines.
In this study, we report the first attempt at Undaria aquaculture along an offshore wind farm in southwestern Korea. It is concluded that further research is needed to optimize the cultivation period for improved productivity and to design a mooring system that ensures the economic feasibility of aquaculture facilities and management. It is expected that a more advanced model will be developed in the future to establish an optimal aquaculture model for the southwestern offshore wind farm.
4. Conclusions
This study investigated the morphological characteristics (length and weight) and biomass yield of the seaweed Undaria pinnatifida (wakame) in a model marine environment incorporating mariculture within an offshore wind farm in southwestern Korea, from November 2021 to April 2022. Undaria farming was conducted twice, yielding 5842 g m−1 during the first cultivation period and 7676 g m−1 during the second. In the second cultivation trial, signs of chlorosis were presumed to result from seasonal increases in water temperature and light intensity around the offshore wind farm. Future research should focus on optimizing the cultivation timeline to improve productivity and on developing a mooring system that ensures the economic feasibility of aquaculture facility operations and management, in order to establish an optimal offshore aquaculture model.
Author Contributions
Conceptualization, D.C., J.H., J.-Y.H. and Y.-U.C.; Methodology, H.-G.L., Y.-H.J. and D.-W.L.; Validation, H.-G.L., Y.-H.J. and D.-W.L.; Formal Analysis, D.C.; Investigation, H.-G.L., Y.-H.J. and D.-W.L.; Resources, H.-G.L., Y.-H.J. and D.-W.L.; Data Curation, H.-G.L., Y.-H.J. and D.-W.L.; Writing—Original Draft Preparation, D.C. and Y.-U.C.; Writing—Review and Editing, D.C., J.H., J.-Y.H. and Y.-U.C.; Visualization, D.C.; Supervision, J.H. and J.-Y.H.; Project Administration, D.C.; Funding Acquisition, J.H. and J.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korean Institute of Energy Technology Evaluation and Planning (KETEP), the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea, grant number 20203040020130), and the KIOST project, grant number PEA0312.
Institutional Review Board Statement
All experiments were conducted in compliance with the guidelines of the Institutional Animal Care and Experimental Committee of the Korea Institute of Ocean Science and Technology (KIOST) which approved the experimental protocol.
Data Availability Statement
All data generated or analyzed during this study are available from the KIOST data repository. Materials are available upon request by the corresponding author.
Acknowledgments
We also thank Keum-Seok Kang and Hoyeop Lee (KEOCO Research Institute) for their generous assistance in longline farm operations.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
References
- Kafas, A. Offshore Wind and Commercial Fisheries in the East Coast of Scotland. 2017. Available online: https://maritime-spatial-planning.ec.europa.eu/media/12372 (accessed on 20 May 2024).
- Letschert, J.; Stollberg, N.; Rambo, H.; Kempf, A.; Berkehagen, J.; Stelzenmüller, V. The uncertain future of the Norway lobster fisheries in the North Sea calls for new management strategies. ICES J. Mar. Sci. 2021, 78, 3639–3649. [Google Scholar] [CrossRef]
- Bonsu, P.O.; Letschert, J.; Yates, K.L.; Svendsen, J.C.; Berkenhagen, J.; Rozemeijer, M.J.C.; Kerkhove, T.R.H.; Rehren, J.; Stelzenmüller, V. Co-location of fishes and offshores wind farms: Current practies and enabling condition in the North Sea. Mar. Policy 2024, 159, 105941. [Google Scholar] [CrossRef]
- Michler-Cieluch, T.; Kodeih, S. Mussel and seaweed cultivation in offshore wind farms: An opinion survey. Coast Manag. 2008, 36, 392–411. [Google Scholar] [CrossRef]
- Banach, J.L.; van den Burg, S.W.K.; van der Fels-Klerx, H.J. Food safety during seaweed cultivation at offshore wind farms: An exploratory study in the North Sea. Mar. Policy 2020, 120, 104082. [Google Scholar] [CrossRef]
- Maar, M.; Holbach, A.; Boderskov, T.; Thomsen, M.; Buck, B.J.; Kotta, J.; Bruhn, A. Multi-use offshore wind farms with low-tropic aquaculture can help achieve global sustainability goals. Commun. Earth Environ. 2023, 4, 447. [Google Scholar] [CrossRef]
- Buck, B.H.; Buchholz, C.M. The offshore-ring: A new system design for the open ocean aquaculture of macroalgae. J. Appl. Phycol. 2004, 16, 355–368. [Google Scholar] [CrossRef]
- Buck, B.H.; Buchholz, C.M. Response of offshore cultivated Laminaria saccharina to hydrodynamic forcing in the North Sea. Aquaculture 2005, 250, 674–691. [Google Scholar] [CrossRef]
- Reynolds, B.D. Assessing the Feasibility of Seaweed Farm-Offshore Wind Co-Location in New Jersey. Master’s Thesis, Montclair State University, Montclair, NJ, USA, 2024. [Google Scholar]
- NARS-National Assembly Research Service (NARS). Issues and Perspectives. Available online: https://www.nars.go.kr/report/view.do?cmsCode=CM0043&brdSeq=40974 (accessed on 12 May 2024). (In Korean).
- Carbon Trust. Unlocking the Potential: Challenges and Opportunities for South Korean Offshore Wind Supply Chain. 2023. Available online: https://www.plan15.org/report/?bmode=view&idx=17300313 (accessed on 20 March 2024).
- Yamanaka, R.; Akiyama, K. Cultivation and utilization of Undaria pinnatifida (Wakame) as food. J. Appl. Phycol. 1993, 5, 249–253. [Google Scholar] [CrossRef]
- NIFS-National Institute of Fisheries Science (NIFS). The Guideline for Surveying the Suitability of Farm. 2016. Available online: https://nifs.go.kr/board/actionBoard0005View.do?MENU_ID=M0000092&BBS_ID=20211228042022718BBF& (accessed on 20 December 2024). (In Korean).
- Sato, Y.; Hirano, T.; Ichida, H.; Fukunishi, N.; Abe, T.; Kawano, S. Extending the Cultivation Period of Undaria pinnatifida by Using Regional Strains with Phenotypic Differentiation along the Sanriku Coast in Northern Japan. Phycology 2021, 1, 129–142. [Google Scholar] [CrossRef]
- FAO. Undaria pinnatifida Cultured Aquatic Species Information Programme. Text by Shao Jun Pang, Xia Li and Thierry Chopin. In Fisheries and Aquaculture; FAO: Rome, Italy, 2025; Available online: https://www.fao.org/fishery/en/culturedspecies/undaria_pinnatifida/en (accessed on 8 April 2025).
- Watanabe, T.; Nisixawa, K. The utilization of wakame (Undaria pinnatifida) in Japan and manufacture of ‘haiboshi wakame’ and some of its biochemical and physical properties. Hydrobiologia 1984, 116, 106–111. [Google Scholar] [CrossRef]
- Lee, K.Y.; Sohn, C.H. Morphological characteristics and growth of two forms of sea mustard, Undaria pinnatifida f. distans and of U. pinnatifida f. typica. J. Aquac. 1993, 6, 71–87. [Google Scholar]
- Oh, S.H.; Koh, C.H. Growth and photosynthesis of Undaria pinnatifida (Laminariales, Phaeophyta) on a cultivation ground in Korea. Bot. Mar. 1996, 59, 589–599. [Google Scholar] [CrossRef]
- KOSIS-Korean Statistical Information Service (KOSIS). Statistics by Fishery Species. 2023. Available online: https://kosis.kr/index/index.do (accessed on 20 March 2024). (In Korean).
- Brown, M.T.; Lamare, M.D. The distribution of Undaria pinnatifida (Harvey) Suringar within Timaru Habour, New Zealand. Jpn. J. Phycol. 1994, 42, 63–70. [Google Scholar]
- Hwang, E.K.; Baek, J.M.; Park, C.S. The mass cultivation of Ecklonia stolonifera Okamura as a summer feed for the abalone industry in Korea. J. Appl. Phycol. 2009, 21, 585–590. [Google Scholar] [CrossRef]
- Lee, D.W.; Oh, S.Y.; Pa, J.J.C.; Jung, Y.H.; Kim, H.J.; Choi, D.M.; Choi, Y.U.; Han, J. Offshore Wind Farms in South Korea: A Potential Site for Scallop Culture. J. Mar. Sci. Eng. 2023, 11, 1988. [Google Scholar] [CrossRef]
- Seo, J.; Maeng, J.; Lim, E.; Jin, S.; Kim, H.; Kim, T. Marine Environmental Characteristics around the Test Phase of Offshore Wind Farm in the Southwestern Coast of Yellow Sea. J. Environ. Impact Assess. 2019, 5, 457–470. (In Korean) [Google Scholar]
- Oh, S.H.; Jeong, W.M.; Kim, S.I. Analysis of the Observation Data for Winter-Season High Waves Occurred in the West Sea of Korea. J. Korean Soc. Coast. Ocean Eng. 2015, 3, 168–174. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sugimura, Y.; Itoh, T. A catalytic oxidation method for the determination of total nitrogen dissolved in sea-water. Mar. Chem. 1985, 16, 83–97. [Google Scholar] [CrossRef]
- Boo, S.Y.; Shelley, S.A.; Shin, S.H.; Park, J.; Ha, Y.J. Design and Analysis of a Sub-Surface Longline Marine Aquaculture Farm for Co-Existence with Offshore Wind Farm. J. Mar. Sci. Eng. 2023, 11, 1034. [Google Scholar] [CrossRef]
- Yong, Y.S.; Yong, W.T.L.; Anton, A. Analysis of formulae for determination of seaweed growth rate. J. Appl. Phycol. 2013, 25, 1831–1834. [Google Scholar] [CrossRef]
- Luhan, M.R.J.; Sollesta, H. Growing the reproductive cells (carpospores) of the seaweed, Kappaphycus striatum, in the laboratory until outplanting in the field and maturation to tetrasporophyte. J. Appl. Phycol. 2010, 22, 579–585. [Google Scholar] [CrossRef]
- Peteiro, C.; Freire, Ó. Outplanting time and methodologies related to mariculture of the edible kelp Undaria pinnatifida in the Atlantic coast of Spain. J. Appl. Phycol. 2012, 24, 1361–1372. [Google Scholar] [CrossRef]
- Nanba, N.; Fujiwara, T.; Kuwano, K.; Ishikawa, Y.; Ogawa, H.; Kado, R. Effect of water flow velocity on growth and morphology of cultured Undaria pinnatifida sporophytes (Laminariales, Phaeophyceae) in Okirai Bay on the Sanriku coast, Northeast Japan. J. Appl. Phycol. 2011, 23, 1023–1030. [Google Scholar] [CrossRef]
- Peteiro, C.; Freire, Ó. Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. J. Appl. Phycol. 2013, 25, 205–213. [Google Scholar] [CrossRef]
- Gao, X.; Agatsuma, Y.; Taniguchi, K. Effect of nitrate fertilization of gametophytes of the kelp Undaria pinnatifida on growth and maturation of the sporophytes cultivated in Matsushima Bay, northern Honshu, Japan. Aquac. Int. 2013, 21, 53–64. [Google Scholar] [CrossRef]
- Kaga, S.; Kakehi, S.; Naiki, K.; Kodama, T.; Wagawa, T.; Segawa, S.; Watanabe, S.; Musashi, T.; Kuroda, H.; Ito, S.-I. Seasonal variations in nutrient concentrations in Sanriku coastal waters, Japan: Effects on Undaria pinnatifida (Laminariales; Phaeophyta) seaweed farms. Reg. Stud. Mar. Sci. 2022, 54, 102484. [Google Scholar] [CrossRef]
- Wheeler, W.N. Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocrytis pyrifera. Mar. Biol. 1980, 56, 103–110. [Google Scholar] [CrossRef]
- Gerard, V.A. In situ water motion and nutrient uptake by the giant kelp Macrocystis pyrifera. Mar. Biol. 1982, 69, 51–54. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Druehl, L.D. Effect of seawater velocity on inorganic nitrogen uptake by morphologically distinct forms of Macrocrystis integrifolia from wave-sheltered and exposed sites. Mar. Biol. 1996, 126, 205–214. [Google Scholar] [CrossRef]
- Peteiro, C.; Sánchez, N.; Martínez, B. Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlnatic coast of Southern Europe: An overview. Algal Res. 2016, 15, 9–23. [Google Scholar] [CrossRef]
- Gerard, V.; Mann, K.H. Growth and production of Laminaria longicruris populations exposed to different intensities of water movement. J. Phycol. 1979, 15, 33–41. [Google Scholar] [CrossRef]
- Gao, X.; Endo, H.; Taniguchi, K.; Agatsuma, Y. Genetic differentiation of high-temperature tolerance in the kelp Undaria pinnatifida sporophytes from geographically separated populations along the Pacific coast of Japan. J. Appl. Phycol. 2013, 25, 567–574. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, T.; Endo, H. Density regulation of aquaculture production and its effects on commercial profit and quality as food in the cosmopolitan edible seaweed Undaria pinnatifida. Front. Mar. Sci. 2023, 10, 1085054. [Google Scholar] [CrossRef]
- Endo, H.; Okumura, Y.; Sato, Y.; Agatsuma, Y. Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga Undaria pinnatifida. J. Appl. Phycol. 2016, 29, 1683–1693. [Google Scholar] [CrossRef]
- Campbell, J.; Bite, J.; Burridge, T.R. Seasonal patterns in the photosynthetic capacity, tissue pigment and nutrient content of different developmental stages of undaria pinnatifida (Phaeophyta: Laminariales) in port Phillip Bay, south-eastern Australia. Bot. Mar. 1999, 42, 231–241. [Google Scholar] [CrossRef]
- Endo, H.; Suzuki, A.; Sato, Y.; Agatsuma, Y. Effects of nutrient enrichment, irradiance control, and boiling on the color of the brown alga Undaria pinnatifida. Fish. Sci. 2017, 83, 811–817. [Google Scholar] [CrossRef]
- Dean, P.R. The Nutrient and Photosynthetic Eco-Physiology of Undaria pinnatifida, with Applications to Aquaculture. Master’s Thesis, University of Otago, Dubedin, New Zealand, 1998. Available online: http://hdl.handle.net/10523/2920 (accessed on 3 November 2024).
- Gerasimenko, N.I.; Skriptsova, A.V.; Busarova, N.G.; Moiseenko, O.P. Effects of the season and growth stage on the contents of lipids and photosynthetic pigments in brown alga Undaria pinnatifida. Russ. J. Plant Physiol. 2011, 58, 885–891. [Google Scholar] [CrossRef]
- Arijón, M.; Raffo, M.P.; Sánchez, N.; Dellatorre, F.G. Photosynthetic pigments and color of wild Undaria pinnatifida for wakame production (Chubut, Patagonia Argentina). Algal Res. 2023, 69, 102918. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.; Kim, Y.Y.; Choi, S.Y. Analysis of factors underlying Pyropia Chlorosis near Geumgang estuary. J. Korean Soc. Mar. Environ. Energy 2018, 21, 381–386. (In Korean) [Google Scholar] [CrossRef]
- Natoya, M. Production of biofuel by macroalgae with preservation of marine resources environment. In Seaweeds and Their Role in Globally Changing Environments; Seckbach, J., Einav, R., Israel, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 217–228. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Goldberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Seung, Y.H.; Chung, J.H.; Park, Y.C. Oceanographic studies related to the tidal front in the mid Yellow Sea off Korea: Physical aspects. J. Oceanol. Soc. Korea 1990, 25, 84–95. (In Korean) [Google Scholar]
- Choi, H.Y.; Lee, S.H.; Oh, I.S. Quantitative analysis of the thermal front in the mid-eastern coastal area of the yellow sea. J. Korean Soc. Oceanogr. 1998, 3, 1–8. (In Korean) [Google Scholar]
- Lim, H.S. Growth of the manila clam (Ruditapes philippinarum) cultured in Gomso tidal flat. Korea. Korean J. Malacol. 2016, 32, 189–195. (In Korean) [Google Scholar] [CrossRef]
- Neushul, M.; Benson, J.; Harger, B.W.W.; Charters, A.C. Macroalgal farming in the sea: Water motion and nitrate uptake. J. Appl. Phycol. 1992, 4, 255–265. [Google Scholar] [CrossRef]
- Akiyama, K.; Kurogi, M. Cultivation of Undaria pinnatifida (Harvey) Suringar, the decrease in crops from natural plants following crops increase from cultivation. Bull. Tohoku Reg. Fish. Res. Lab. 1988, 44, 91–100. [Google Scholar]
- Tseng, C.K. Laminaria mariculture in China. In Case Studies of Seven Commercial Seaweed Resources; Doty, M.S., Caddy, J.F., Santelices, B., Eds.; FAO Fisheries Technical Paper No. 281, Electronic Edition; FAO: Rome, Italy, 1987; pp. 239–263. [Google Scholar]
- Druehl, L.D.; Kemp, L. Morphological and growth responses of geographically isolated Macrocystis integrifolia populations when grown in a common environment. Can. J. Bot. 1982, 60, 1409–1413. [Google Scholar] [CrossRef]
- Koehl, M.A.R.; Alberte, R.S. Flow, flapping, and photosynthesis of Nereocystis luetkeana: A functional comparison of undulate and flat blade morphologies. Mar. Biol. 1988, 99, 435–444. [Google Scholar] [CrossRef]
- Kawamata, S. Adaptive mechanical tolerance and dislodgement velocity of the kelp Laminaria japonica in wave-induced water motion. Mar. Ecol. Prog. Ser. 2001, 211, 89–104. [Google Scholar] [CrossRef]
- Roberson, L.M.; Coyer, J.A. Variation in blade morphology of the kelp Eisenia arborea: Incipient speciation due to local water motion? Mar. Ecol. Prog. Ser. 2004, 282, 115–128. [Google Scholar] [CrossRef]
- Choi, H.G.; Kim, Y.S.; Lee, S.J.; Nam, K.W. Growth and reproductive patterns of Undaria pinnatifida sporophytes in a cultivation farm in Busan, Korea. J. Appl. Phycol. 2007, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- NIFS-National Institute of Fisheries Science (NIFS). Assessment Report on Fisheries Impacts in a Changing Climate; NIFS: Busan, Republic of Korea, 2019; p. 201. (In Korean)
- Takagi, S.; Murata, Y.; Inomata, E.; Agatsuma, Y. Sporophyll of Undaria pinnatifida: A potential feed for the production of high-quality gonads in the sea urchin Mesocentrotus nudus (A. Agassiz, 1864). J. Appl. Phycol. 2020, 32, 1467–1475. [Google Scholar] [CrossRef]
- Hurd, C.L.; Law, C.S.; Bach, L.T.; Britton, D.; Hovenden, M.; Paine, E.R. Forensic carbon accounting: Assessing the role of seaweeds for carbon sequestration. J. Phycol. 2022, 58, 347–363. [Google Scholar] [CrossRef]
- Lian, Y.; Boamah, S.O.; Pan, Z.; Zheng, J.; Chen, W.; Ma, G.; Yim, S.C. Engineering design and economic analysis of offshore seaweed farm. Front. Mar. Sci. 2024, 11, 1276552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).