Possible Evolutionary Precursors of Mast Cells: The ‘Granular Cell’ Immunocyte of Botrylloides leachii (Tunicata; Ascidiacea)
Abstract
1. Introduction
2. Results
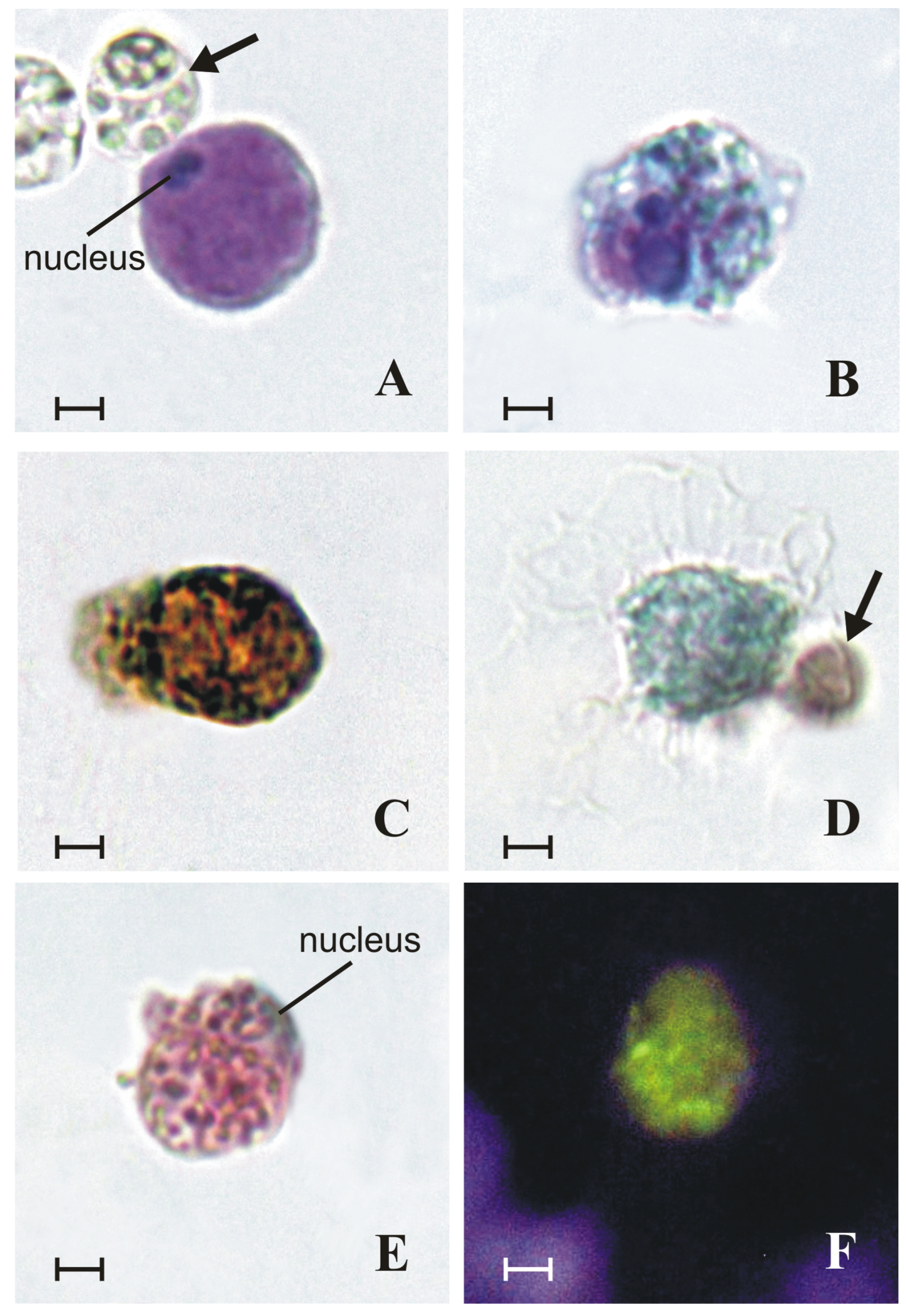
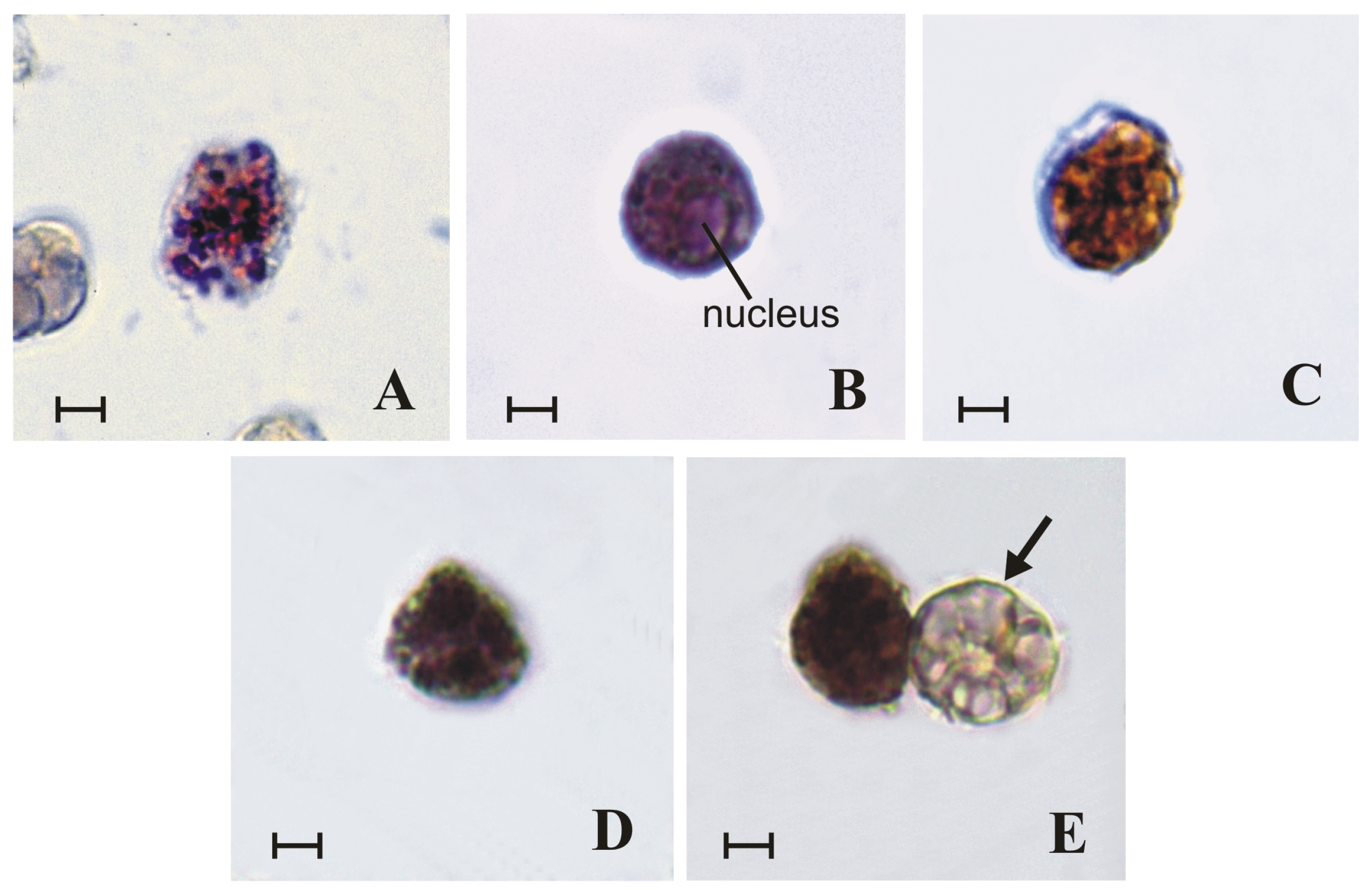
2.1. Granules of Granular Cells Contain a Heparin–Histamine System
2.2. Granules of Granular Cells Contain Mast-Cell Hydrolases
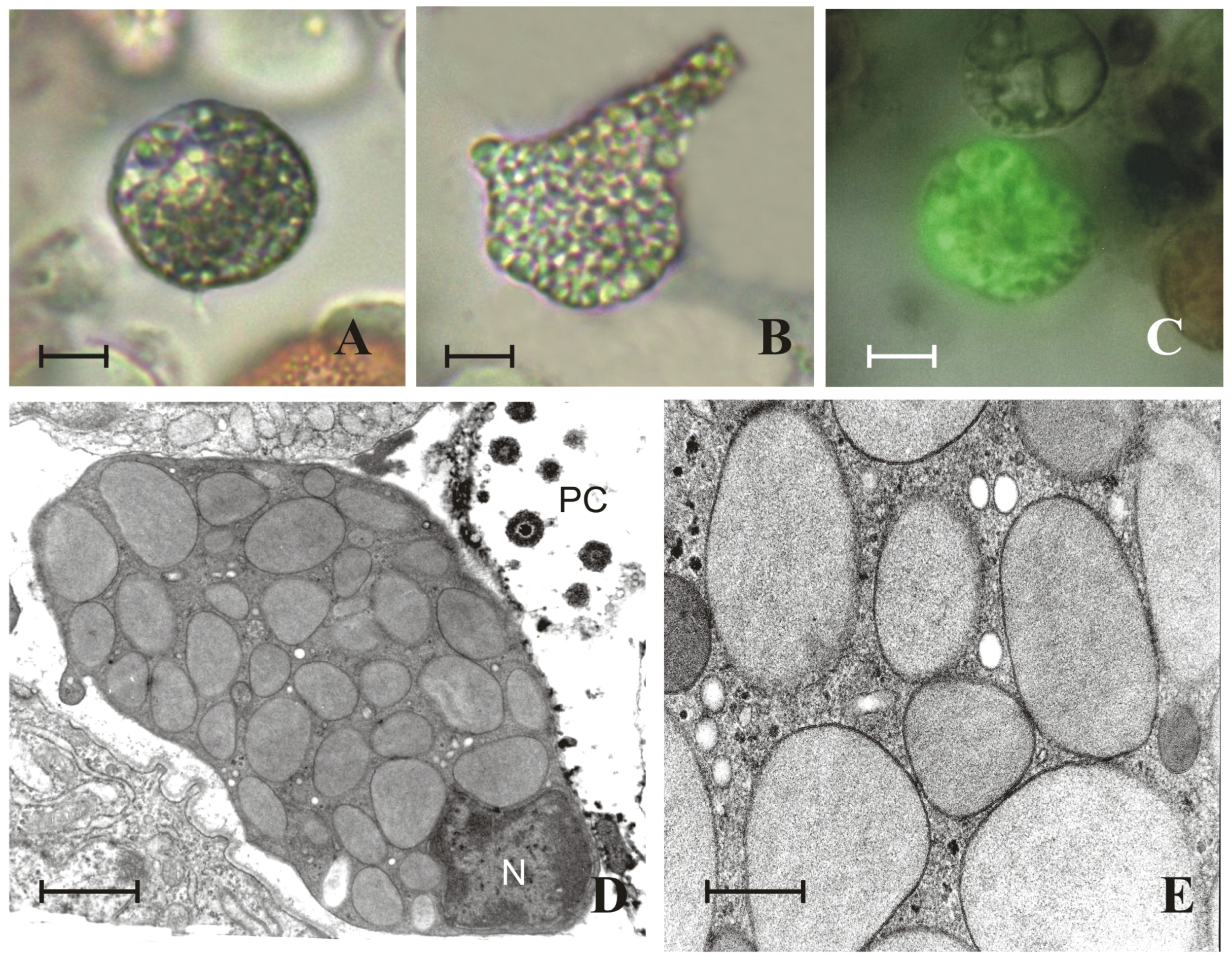
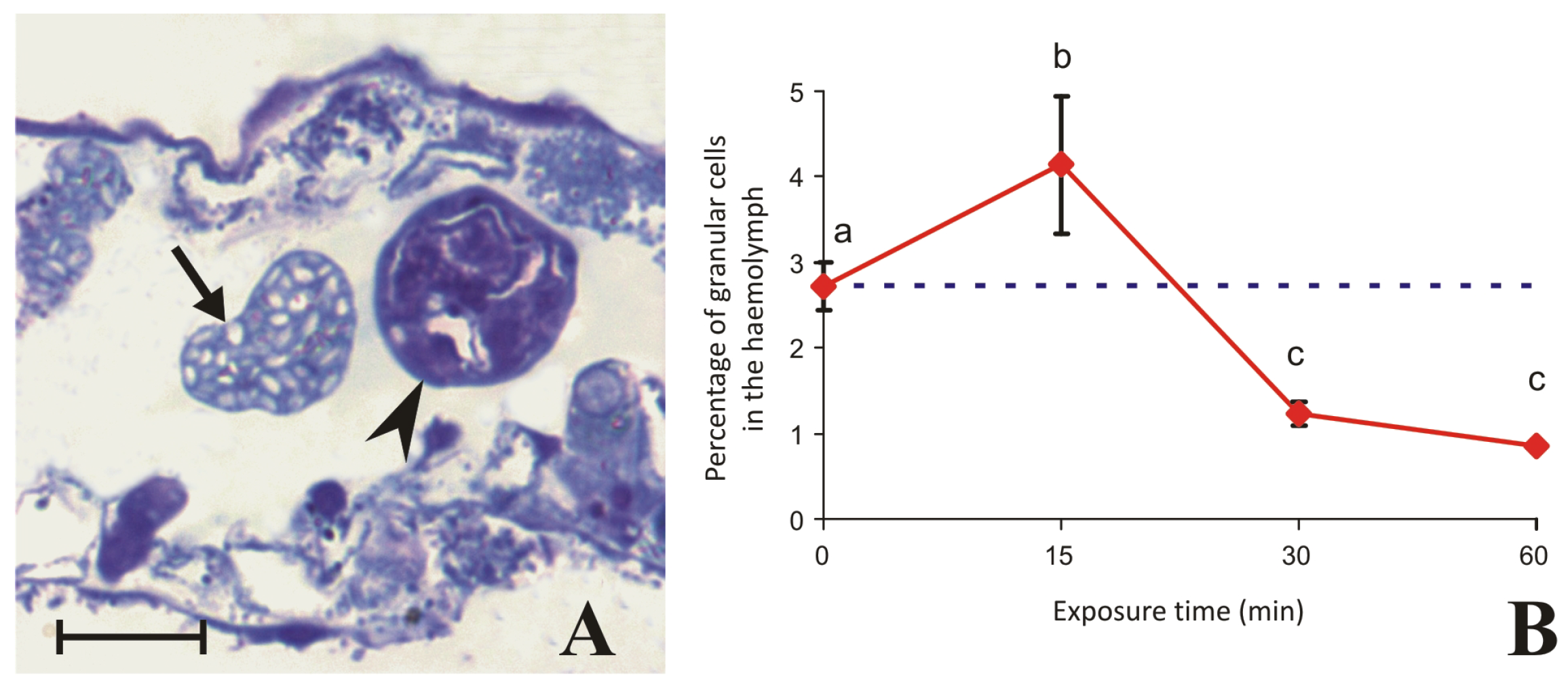
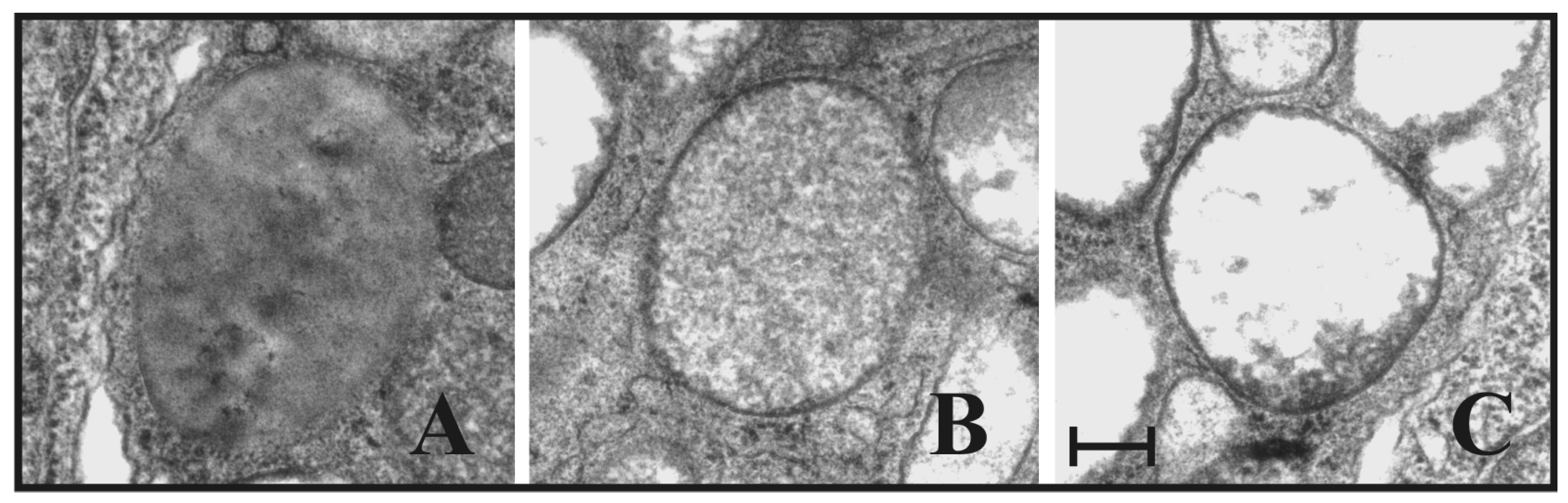
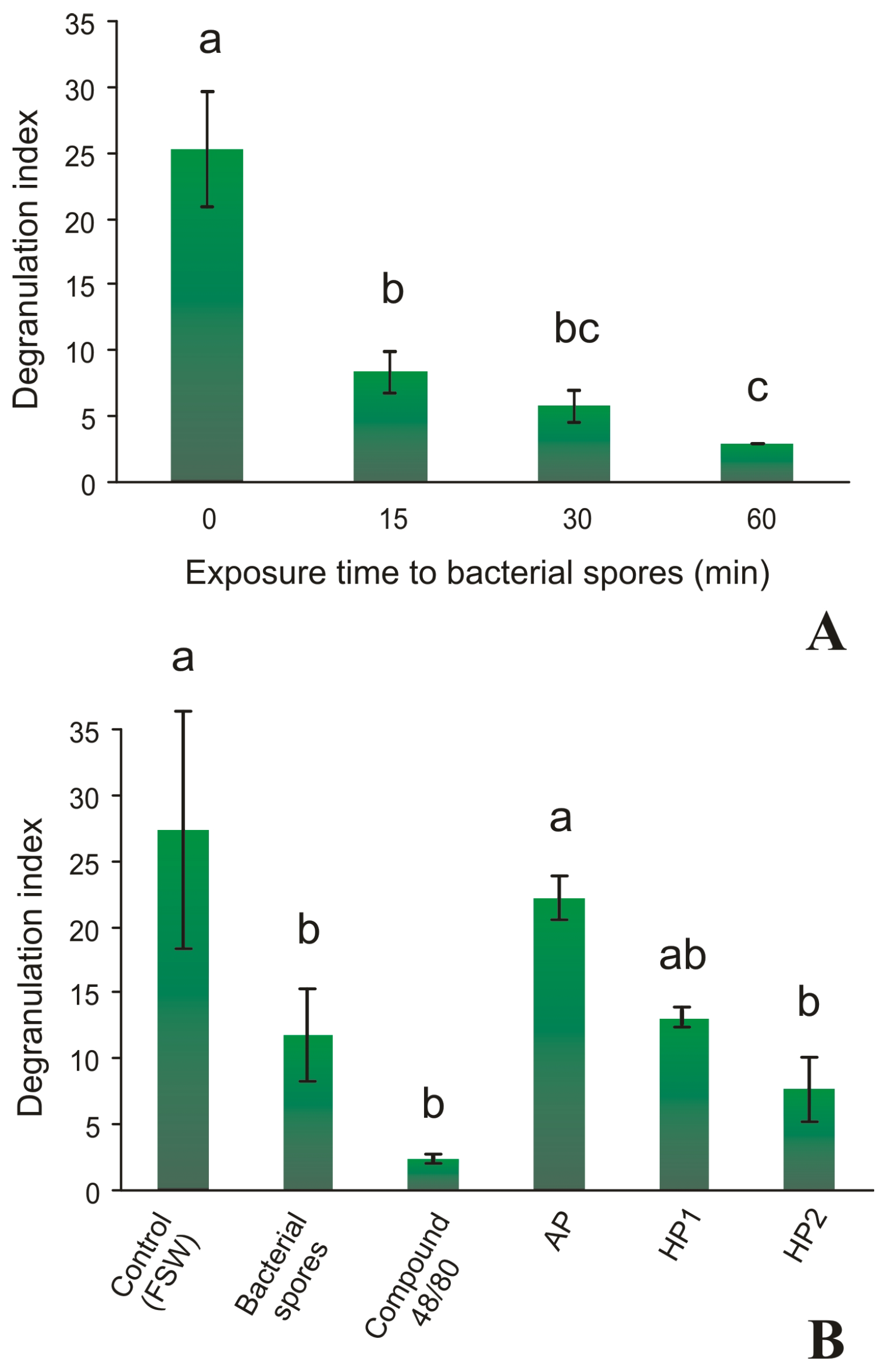
2.3. Granular Cells Have the Ability to Infiltrate Tissues and Degranulate in Response to a Stimulus
3. Discussion
| Basophils (Vertebrates) | Mast Cells (Vertebrates) | Granular Cells (B. leachii) | |
|---|---|---|---|
| 1. GAGs | |||
| Heparin | + | + | + |
| 2. Biogenic amines | |||
| Histamine | + | + | + |
| 3. Hydrolytic enzymes | |||
| β-glucuronidase | − | + | + |
| Chloroacetyl esterase | − | + | + |
| Arylsulphatase | − | + | + |
| Tryptase | + | + | + |
| Chymase | − | + | + |
4. Materials and Methods
4.1. Animals
4.2. Haemocyte Collection
4.3. Fixation of Haemocyte Monolayers
4.4. Histochemical Assays
4.4.1. Staining Methods for Heparin Detection
- (i).
- Metachromatic reaction of toluidine blue. The monolayers were incubated for 20 min in a solution of 0.1% toluidine blue O (Fluka Chemie GmbH, Steinheim, Germany) in 30% ethanol and then washed in 95% ethanol. After quick washing in distilled water, the slides were mounted and observed under an LM. Basophilic substances were stained purple.
- (ii).
- Metachromatic reaction of 1,9-dimethylmethylene blue (DMB). The monolayers were incubated for 30 min in an aqueous solution containing 0.05 M DMB (Sigma-Aldrich, St. Louis, MO, USA), 0.1 M HCl, 0.04 mM glycine, and 0.04 M NaCl and then washed in distilled water. Basophilic substances, such as acidic mucopolysaccharides and heparinoids, were stained violet.
- (iii).
- Ehrlich’s triacid mixture. The monolayers were incubated in Ehrlich’s triacid mixture (12 vol saturated orange G aqueous solution, 8 vol saturated acid fuchsin solution, 10 vol saturated methyl green aqueous solution, 30 distilled water, 18 vol absolute ethanol and 5 vol glycerine) for 15 min and then washed in distilled water. The basophilic granules containing heparinoid substances were stained light green, the acidophilic granules were stained copper red, and the neutrophilic granules were stained violet.
- (iv).
- Csaba’s staining. The monolayers were incubated for 15 min in a solution of 0.36% Alcian blue, 0.18% safranin O, and 0.48% ammonium ferric sulphate in 0.1 M sodium acetate, pH 1.42. Non-sulphated heparin precursor was stained light blue, and highly N-sulphated heparin was stained red.
- (v).
- Geyer’s method for sulphates. The monolayers were incubated for 30 min in an aqueous solution of Fast Blue B (Fluka) (50 mg in 10 mL of 5% acetic acid). The monolayers were then rinsed for 1 min in distilled water at 4 °C and subsequently incubated for 5 min in a cold (4 °C) saturated solution of 1-naphthol (Sigma-Aldrich) in 0.1 M borax buffer, pH 9.4. The monolayers were then rinsed in distilled water for 1 min. The sulphate groups of the glycosaminoglycans were stained brown.
- (vi).
- Berlin and Enerbäck’s method [108] with berberine sulphate. The monolayers were incubated for 20 min in an aqueous solution containing 0.02% berberine sulphate (Sigma-Aldrich) adjusted to pH 4.0 with the addition of 1% citric acid. After being quickly washed in distilled water and mounted, the monolayers were observed under an LM with a UV light excitation source (365 nm). Sulphate-containing polyanions such as heparinoid substances emitted intense blue-green fluorescence.
4.4.2. Assay for Discrimination of Heparin from Heparan Sulphate
4.5. Histoenzymatic Assays
- (i).
- β-glucuronidase. Fixed monolayers were washed in sodium acetate buffer 0.1 M, pH 5.2, for 10 min at 37 °C for 2 h, after which 4 mg of naphthol AS-BI β-glucuronide, which was previously dissolved in 0.25 mL of dimethylformamide (DMF) in 20 mL of buffered hexazonium-p-rosaniline, was added. The monolayers were then washed in distilled water and mounted in Acquovitrex. Positive sites for the enzymatic reaction inside haemocytes were stained magenta.
- (ii).
- Chloroacetyl esterase. Fixed monolayers were washed in PBS, pH 6.5, for 10 min and incubated for 1 h at 20 °C in a reaction mixture consisting of 6 mg of naphthol chloroacetate (Sigma-Aldrich) previously dissolved in 1 mL of DMF and added to 20 mL of PBS containing 20 mg of Fast Blue B. Monolayers were then washed in distilled water and mounted in Acquovitrex. The positive sites inside the haemocytes were stained blue.
- (iii).
- Proteases. Fixed monolayers were incubated for 60 min at 37 °C in a reaction mixture containing 4 mg of the synthetic substrate Z-Ala-Ala-Lys-4-methoxy-2-naphthylamide (Z-AAK-mna, MP Biomedicals), which is specific for tryptase, or Suc-Ala-Ala-Phe-4-methoxy-2-naphthylamide (S-AAF-mna, MP Biomedicals), which is specific for chymase, previously dissolved in 0.5 mL of DMF and then added to 10 mL of 0.1 M Tris–HCl buffer, pH 7.0 for tryptase and 7.8 for chymase, containing 10 mg of Fast Blue BB. This was followed by incubation for 5 min in 1% copper sulphate. Finally, the monolayers were rinsed in distilled water and then mounted with Acquovitrex. The positive sites inside the haemocytes were stained dark blue or black.
- (iv).
- Arylsulphatase. Fixed monolayers were washed in sodium acetate buffer for 10 min and incubated for 2 h at 37 °C in the following reaction mixture: 0.16 g p-nitrocatecholsulphate (Sigma-Aldrich), 4 mL distilled water, 12 mL sodium acetate buffer, and 4 mL of 8% aqueous solution of lead nitrate. After incubation, the monolayers were washed with distilled water and then with an ammonium sulphide solution (diluted 1:100 in distilled water) for 2 min. Finally, the monolayers were washed with distilled water and mounted in Acquovitrex. The positive sites inside the haemocytes were stained brownish black.
4.6. Immunohistochemical Assays for Heparin, Histamine, and Stem Cell Factor Receptor
4.7. Electron Microscopy
4.8. Degranulation Assay
4.9. Exposure of Colonies to Bacteria
4.10. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baccari, G.C.; Pinelli, C.; Santillo, A.; Minucci, S.; Kumar, R.R. Mast cells in nonmammalian vertebrates: An overview. Int. Rev. Cell Mol. Biol. 2011, 290, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast cell-mediated immune regulation in health and disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef] [PubMed]
- Crivellato, E.; Beltrami, C.; Mallardi, F.; Ribatti, D. Paul Ehrlich’s doctoral thesis: A milestone in the study of mast cells. Br. J. Haematol. 2003, 123, 19–21. [Google Scholar] [CrossRef]
- Ghably, J.; Saleh, H.; Vyas, H.; Peiris, E.; Misra, N.; Krishnaswamy, G. Paul Ehrlich’s mastzellen: A historical perspective of relevant developments in mast cell biology. In Mast Cell: Methods and Protocols; Torres, A., Ed.; Methods in Molecular Biology; Humana Press, Springer Science: New York, NY, USA, 2015; Volume 1220, pp. 3–10. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Atiakshin, D.; Samoilova, V.; Buchwalow, I.; Boecker, W.; Tiemann, M. Characterization of mast cell populations using different methods for their identification. Histochem. Cell Biol. 2017, 147, 683–694. [Google Scholar] [CrossRef]
- St. John, A.L.; Rathore, A.P.S.; Ginhoux, F. New perspectives on the origins and heterogeneity of mast cells. Nat. Rev. Immunol. 2023, 23, 55–68. [Google Scholar] [CrossRef]
- da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Metz, M.; Siebenhaar, F.; Maurer, M. Mast cell functions in the innate skin immune system. Immunobiology 2008, 213, 251–269. [Google Scholar] [CrossRef]
- Staby, A.; Sand, M.B.; Hansen, R.G.; Jacobsen, J.H.; Andersen, L.A.; Gerstenberg, M.; Bruus, U.K.; Jensen, I.H. Comparison of chromatographic ion-exchange resins IV. Strong and weak cation-exchange resins and heparin resins. J. Chromatogr. A 2005, 1069, 65–77. [Google Scholar] [CrossRef]
- Bolten, S.N.; Rinas, U.; Scheper, T. Heparin: Role in protein purification and substitution with animal-component free material. Appl. Microbiol. Biotechnol. 2018, 102, 8647–8660. [Google Scholar] [CrossRef]
- Brody, D.; Metcalfe, D.D. Mast cells: A unique and functional diversity. Clin. Exp. Allergy 1998, 28, 1167–1170. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ishizuka, T.; Okayama, Y. Human mast cells and basophils as sources of cytokines. Clin. Exp. Allergy 2000, 30, 1205–1212. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast cell: A multi-functional master cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, Y.C.; Chang, H.A.; Lin, Y.S.; Tsao, C.W.; Shen, M.R.; Chiu, W.T. Differential Ca2+ mobilization and mast cell degranulation by FcεRI- and GPCR-mediated signaling. Cell Calcium 2017, 67, 31–39. [Google Scholar] [CrossRef]
- Ritter, U.; Meissner, A.; Ott, J.; Kömer, H. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-α reveals a essential role for TNF. J. Leukoc. Biol. 2003, 74, 216–222. [Google Scholar] [CrossRef]
- Hershko, A.Y.; Rivera, J. Mast cell and T cell communication; Amplification and control of adaptive immunity. Immunol. Lett. 2010, 128, 98–104. [Google Scholar] [CrossRef]
- Merluzzi, S.; Frossi, B.; Gri, G.; Parusso, S.; Tripodo, C.; Pucillo, C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood 2010, 115, 2810–2817. [Google Scholar] [CrossRef]
- Mekori, Y.A.; Metcalfe, D.D. Mast cells in innate immunity. Immunol. Rev. 2000, 173, 131–140. [Google Scholar] [CrossRef]
- Féger, F.; Varadaradjalou, S.; Gao, Z.; Abraham, S.N.; Arock, M. The role of mast cells in host defense and their subversion by bacterial pathogens. Trends Immunol. 2002, 23, 151–157. [Google Scholar] [CrossRef]
- Von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965–968. [Google Scholar] [CrossRef]
- Delsuc, F.; Philippe, H.; Tsagkogeorga, G.; Simion, P.; Tilak, M.K.; Turon, X.; López-Legentil, S.; Piette, J.; Lemaire, P.; Douzery, E.J.P. A phylogenomic framework and timescale for comparative studies of tunicates. BMC Biol. 2018, 16, 39. [Google Scholar] [CrossRef]
- Fodor, A.; Liu, J.; Turner, L.; Swalla, B.J. Transitional chordates and vertebrate origins: Tunicates. Curr. Top. Dev. Biol. 2021, 141, 149–171. [Google Scholar] [CrossRef]
- Chen, J.Y.; Huang, D.Y.; Peng, Q.Q.; Chi, H.M.; Wang, X.Q.; Feng, M. The first tunicate from the Early Cambrian of South China. Proc. Natl. Acad. Sci. USA 2003, 100, 8314–8318. [Google Scholar] [CrossRef]
- Nanglu, K.; Lerosey-Aubril, R.; Weaver, J.C.; Ortega-Hernández, J. A mid-Cambrian tunicate and the deep origin of the ascidiacean body plan. Nat. Commun. 2023, 14, 3832. [Google Scholar] [CrossRef]
- Donaghy, L.; Hong, H.K.; Park, K.I.; Nobuhisa, K.; Youn, S.H.; Kang, C.K.; Choi, K.S. Flow cytometric characterization of hemocytes of the solitary ascidian, Halocynthia roretzi. Fish Shellfish Immunol. 2017, 66, 289–299. [Google Scholar] [CrossRef]
- Undritz, E. Les Cellules Sanguines de L’homme et Dans La Serie Animale. Schweiz. Med. Wochenschr. 1946, 76, 1477. [Google Scholar]
- Steinemann-Hussein, A. Morphologie des Evertebratenblutes Mit Spezieller Berücksichtigung der Ascidien. Ph.D. Thesis, Inaugural Diss, Basel, Switzerland, 1963. [Google Scholar]
- Undritz, E.; Steinemann, A. Die Blutkörperchen der Ascidien. Schweiz. Med. Wochenschr. 1963, 93, 1477. [Google Scholar]
- Pearse, A.G.E. Histochemistry, Theoretical and Applied, 2nd ed.; Churchill Livingstone: Edinburgh, UK, 1960; p. 998. [Google Scholar]
- Zhang, H.; Sawada, T.; Cooper, E.L.; Tomonaga, S. Electron microscopic analysis of tunicate (Halocynthia roretzi) hemocytes. Zool. Sci. 1992, 9, 551–562. [Google Scholar] [CrossRef]
- Fuke, M.; Fukumoto, M. Correlative fine structural, behavioral, and histochemical analysis of ascidian blood cells. Acta Zool. 1993, 74, 61–71. [Google Scholar] [CrossRef]
- Sawada, T.; Zang, J.; Cooper, E.L. Classification and characterization of hemocytes in Styela clava. Biol. Bull. 1993, 184, 87–96. [Google Scholar] [CrossRef]
- de Barros, C.M.; Andrade, L.R.; Allodi, S.; Viskov, C.; Mourier, P.A.; Cavalcante, M.C.M.; Straus, A.H.; Tahahashi, H.K.; Pomin, V.H.; Carvalho, V.F.; et al. The hemolymph of the ascidian Styela plicata (Chordata-Tunicata) contains heparin inside basophil-like cells and a unique sulfated galactoglucan in the plasma. J. Biol. Chem. 2007, 282, 1615–1626. [Google Scholar] [CrossRef]
- Wong, G.W.; Zhuo, L.; Kimata, K.; Lam, B.K.; Satoh, N.; Stevens, R.L. Ancient origin of mast cells. Biochem. Biophys. Res. Commun. 2014, 451, 314–318. [Google Scholar] [CrossRef]
- Cima, F.; Peronato, A.; Ballarin, L. The haemocytes of the colonial aplousobranch ascidian Diplosoma listerianum: Structural, cytochemical and functional analyses. Micron 2017, 102, 51–64. [Google Scholar] [CrossRef]
- Cima, F.; Caicci, F.; Sordino, P. The haemocytes of the salp Thalia democratica (Tunicata, Thaliacea): An ultrastructural and histochemical study in the oozoid. Acta Zool. 2014, 95, 375–391. [Google Scholar] [CrossRef]
- Brunetti, R.; Mastrototaro, F. Ascidiacea of the European Waters; Fauna d’Italia; Calderini/Edagricole: Milan, Italy, 2017; Volume 51, p. 447. [Google Scholar]
- Cima, F.; Perin, A.; Burighel, P.; Ballarin, L. Morpho-functional characterisation of haemocytes of the compound ascidian Botrylloides leachi (Tunicata, Ascidiacea). Acta Zool. 2001, 82, 261–274. [Google Scholar] [CrossRef]
- Hyams, Y.; Paz, G.; Rabinowitz, C.; Rinkevich, B. Insights into the unique torpor of Botrylloides leachi, a colonial urochordate. Dev. Biol. 2017, 428, 101–117. [Google Scholar] [CrossRef]
- Blanchoud, S.; Zondag, L.; Lamare, M.D.; Wilson, M.J. Hematological analysis of the ascidian Botrylloides leachii (Savigny, 1816) during whole-body regeneration. Biol. Bull. 2017, 232, 143–157. [Google Scholar] [CrossRef]
- Hyams, Y.; Panov, J.; Taranenko, E.; Brodsky, L.; Rinkevich, Y.; Rinkevich, B. “Keep on rolling”: Circulating cells in a botryllid ascidian torpor. Front. Ecol. Evol. 2023, 11, 1196859. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Rosner, A.; Rabinowitz, C.; Lapidot, Z.; Moiseeva, E.; Rinkevich, B. Piwi positive cells that line the vasculature epithelium, underlie whole body regeneration in a basal chordate. Dev. Biol. 2010, 345, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Scelzo, M.; Alié, A.; Pagnotta, S.; Legeune, C.; Henry, P.; Gilletta, L.; Hiebert, L.S.; Mastrototaro, F.; Tiozzo, S. Novel budding mode in Polyandrocarpa zorritensis: A model for comparative studies on asexual development and whole body regeneration. EvoDevo 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Hauswirth, A.W.; Florian, S.; Schernthaner, G.H.; Krauth, M.T.; Sonneck, K.; Sperr, W.R.; Valent, P. Expression of Cell Surface Antigens on Mast Cells. In Mast Cell Phenotyping: Methods and Protocols; Krishnaswamy, G., Chi, D.S., Eds.; Humana Press, Springer Science: New York, NY, USA, 2006; Volume 315, pp. 77–90. [Google Scholar] [CrossRef]
- Palomäki, V.A.; Laitinen, J.T. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br. J. Pharmacol. 2006, 147, 596–606. [Google Scholar] [CrossRef]
- Burighel, P.; Brunetti, R.; Zaniolo, G. Hibernation of the colonial ascidian Botrylloides leachi (Savigny): Histological observations. Boll. Zool. 1976, 43, 293–301. [Google Scholar] [CrossRef]
- Hyams, Y.; Panov, J.; Rosner, A.; Brodsky, L.; Rinkevich, Y.; Rinkevich, B. Transcriptome landscapes that signify Botrylloides leachi (Ascidiacea) torpor states. Dev. Biol. 2022, 490, 22–36. [Google Scholar] [CrossRef]
- Ribatti, D. The staining of mast cells: A historical overview. Int. Arch. Allergy Immunol. 2018, 176, 55–60. [Google Scholar] [CrossRef]
- Ribatti, D. Mast cells are at the interface between the external environment and the inner organism. Front. Med. 2024, 10, 1332047. [Google Scholar] [CrossRef]
- Lowe, J.S.; Anderson, P.G.; Stevens, A.S. Lowe’s Human Histology, 4th ed.; Elsevier-Mosby: Philadelphia, PA, USA, 2015. [Google Scholar]
- Filippi, M.D. Mechanism of diapedesis: Importance of the transcellular route. Adv. Immunol. 2016, 129, 25–53. [Google Scholar] [CrossRef]
- Cambel, P.; Conroy, C.E.; Sgouris, J.T. Gastric mast cell diapedesis in the albino rat. Science 1952, 115, 373–374. [Google Scholar] [CrossRef]
- De Winter, B.Y.; van den Wijngaard, R.M.; de Jonge, W.J. Intestinal mast cells in gut inflammation and motility disturbances. Biochim. Biophys. Acta 2012, 1822, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Gaudenzio, N.; Sibilano, R.; Marichal, T.; Starkl, P.; Reber, L.L.; Cenac, N.; McNeil, B.D.; Dong, X.; Hernandez, J.D.; Sagi-Eisenberg, R.; et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Investig. 2016, 126, 3981–3998. [Google Scholar] [CrossRef]
- Cavalcante, M.C.M.; Allodi, S.; Valente, A.P.; Straus, A.; Takahashi, H.K.; Mourão, P.A.S.; Pavão, M.S.G. Occurence of heparin in the invertebrate Styela plicata is restricted to cell layers facing the outside environment: An ancient role in defense? J. Biol. Chem. 2000, 275, 36189–36196. [Google Scholar] [CrossRef]
- Jooyandeh, F.; Moore, J.S.; Davies, J.V. The mechanism of histamine binding to heparin. Int. J. Radiat. Biol. 1979, 35, 177–182. [Google Scholar] [CrossRef]
- Rabenstein, D.L.; Bratt, P.; Peng, J. Quantitative characterization of the binding of histamine by heparin. Biochemistry 1998, 37, 14121–14127. [Google Scholar] [CrossRef]
- Pejler, G.; Hu Frisk, J.M.; Sjöström, D.; Paivandy, A.; Öhrvik, H. Acidic pH is essential for maintaining mast cell secretory granule homeostasis. Cell Death Dis. 2017, 8, e2785. [Google Scholar] [CrossRef]
- García-García, E.; Gómez-González, N.E.; Meseguer, J.; García-Ayala, A.; Mulero, V. Histamine regulates the inflammatory response of the tunicate Styela plicata. Dev. Comp. Immunol. 2014, 46, 382–391. [Google Scholar] [CrossRef]
- Gandra, M.; Cavalcante, M.; Pavão, M. Anticoagulant sulfated glycosaminoglycans in the tissues of the primitive chordate Styela plicata (Tunicata). Glycobiology 2000, 10, 1333–1340. [Google Scholar] [CrossRef]
- Humphries, D.E.; Wong, G.W.; Friend, D.S.; Gurish, M.F.; Qui, W.T.; Huang, C.; Sharpe, A.H.; Stevens, R.L. Heparin is essential for the storage of specific granule proteases in mast cells. Nature 1999, 400, 769–772. [Google Scholar] [CrossRef]
- Zehnder, J.L.; Galli, S.J. Mast-cell heparin demystified. Nature 1999, 400, 714–715. [Google Scholar] [CrossRef] [PubMed]
- Lever, R.; Page, C.P. Novel drug development opportunities for heparin. Nat. Rev. Drug Discov. 2002, 1, 140–148. [Google Scholar] [CrossRef]
- Stevens, R.L.; Adachi, R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity. Immunol. Rev. 2007, 217, 155–167. [Google Scholar] [CrossRef]
- Young, E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008, 122, 743–752. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Denburg, J.A. Human Mast Cells and Basophil/Eosinophil Progenitors. In Mast Cells: Methods and Protocols; Hughes, M., McNagny, K., Eds.; Methods in Molecular Biology; Humana Press, Springer Science: New York, NY, USA, 2015; Volume 1220, pp. 59–68. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef]
- Sasaki, H.; Kurotaki, D.; Tamura, T. Regulation of basophil and mast cell development by transcription factors. Allergol. Int. 2016, 65, 127–134. [Google Scholar] [CrossRef]
- Méndez-Enríquez, E.; Hallgren, J. Mast cells and their progenitors in allergic asthma. Front. Immunol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Li, J.P.; Vlodavsky, I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb. Haemost. 2009, 102, 823–828. [Google Scholar] [CrossRef]
- Pejler, G.; Rönnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef]
- Trivedi, N.N.; Caughey, G.H. Mast cell peptidases: Chameleons of innate immunity and host defense. Am. J. Respir. Cell Mol. Biol. 2010, 42, 257–267. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell proteases as pharmacological targets. Eur. J. Pharmacol. 2016, 778, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Rönnberg, E.; Melo, F.R.; Pejler, G. Mast cell proteoglycans. J. Histochem. Cytochem. 2012, 60, 950–962. [Google Scholar] [CrossRef]
- Pejler, G.; Abrink, M.; Ringvall, M.; Wernersson, S. Mast cell proteases. Adv. Immunol. 2007, 95, 167–255. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Pawankar, R.; Ackerman, S.J.; Akin, C.; Clayton, F.; Falcone, F.H.; Gleich, G.J.; Irani, A.M.; Johansson, M.W.; Klion, A.D.; et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ. J. 2016, 9, 7. [Google Scholar] [CrossRef]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human mast cells and basophils—How are they similar how are they different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef]
- Cavalcante, M.C.M.; Mourãom, P.A.; Pavão, M.S. Isolation and characterization of a highly sulfated heparin sulfate from ascidian test cells. Biochim. Biophys. Acta 1999, 1428, 77–87. [Google Scholar] [CrossRef]
- Cavalcante, M.C.M.; De Aandrade, L.R.; Du Bocage Santos-Pinto, C.; Straus, A.H.; Takahashi, H.K.; Allodi, S.; Pavão, M.S.G. Colocalization of heparin and histamine in the intracellular granules of test cells from the invertebrate Styela plicata (chordata-tunicata). J. Struct. Biol. 2002, 137, 313–321. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef]
- Migalovich-Sheikhet, H.; Friedman, S.; Mankuta, D.; Levi-Schaffer, F. Novel identified receptors on mast cells. Front. Immunol. 2012, 3, 238. [Google Scholar] [CrossRef]
- Halova, I.; Rönnberg, E.; Draberova, L.; Vliagoftis, H.; Nilsson, G.P.; Draber, P. Changing the threshold-Signals and mechanisms of mast cell priming. Immunol. Rev. 2018, 282, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Cildir, G.; Pant, H.; Lopez, A.F.; Tergaonkar, V. The transcriptional program, functional heterogeneity, and clinical targeting of mast cells. J. Exp. Med. 2017, 214, 2491–2506. [Google Scholar] [CrossRef]
- Migliaccio, G.; Migliaccio, A.R.; Valinsky, J.; Langley, K.; Zsebo, K.; Visser, J.W.; Adamson, J.W. Stem cell factor induces proliferation and differentiation of highly enriched murine hematopoietic cells. Proc. Natl. Acad. Sci. USA 1991, 88, 7420–7424. [Google Scholar] [CrossRef]
- Vliagoftis, H.; Worobec, A.S.; Metcalfe, D.D. The protooncogene c-kit and c-kit ligand in human disease. J. Allergy Clin. Immunol. 1997, 100, 435–440. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Malinovschi, A.; Öhrvik, H.; Sandelin, M.; Janson, C.; Alving, K.; Hallgren, J. Lin- CD34hi CD117int/hi FcεRI+ cells in human blood constitute a rare population of mast cell progenitors. Blood 2016, 127, 383–391. [Google Scholar] [CrossRef]
- Hundley, T.R.; Gilfillan, A.M.; Tkaczyk, C.; Andrade, M.V.; Metcalfe, D.D.; Beaven, M.A. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood 2004, 104, 2410–2417. [Google Scholar] [CrossRef]
- Arthur, G.K.; Ehrhardt-Humbert, L.C.; Snider, D.B.; Jania, C.; Tilley, S.L.; Metcalfe, D.D.; Cruse, G. The FcεRIβ homologue, MS4A4A, promotes FcεRI signal transduction and store-operated Ca2+ entry in human mast cells. Cell Signal. 2020, 71, 109617. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef]
- Mousli, M.; Bronner, C.; Landry, Y.; Bockaert, J.; Rouot, B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990, 259, 260–262. [Google Scholar] [CrossRef]
- Chahdi, A.; Fraundorfer, P.F.; Beaven, M.A. Compound 48/80 activates mast cells phospholipase D via heterotrimeric GTP-binding proteins. J. Pharmacol. Exp. Ther. 2000, 292, 122–130. [Google Scholar] [CrossRef]
- Ferry, X.; Brehin, S.; Kamel, R.; Landry, Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides 2002, 23, 1507–1515. [Google Scholar] [CrossRef]
- Koibuchi, Y.; Ichikawa, A.; Nakagawa, M.; Tomita, K. Histamine release induced from mast cells by active components of compound 4880. Eur. J. Pharmacol. 1985, 115, 163–170. [Google Scholar] [CrossRef]
- Crivellato, E.; Ribatti, D. The mast cell: An evolutionary perspective. Biol. Rev. 2010, 85, 347–360. [Google Scholar] [CrossRef]
- Crivellato, E.; Travan, L.; Ribatti, D. The phylogenetic profile of mast cells. In Mast Cells: Methods and Protocols; Hughes, M., McNagny, K., Eds.; Methods in Molecular Biology; Springer Protocols, Humana Press, Springer Science: New York, NY, USA, 2015; Volume 1220, pp. 11–27. [Google Scholar]
- Cardamone, C.; Parente, R.; Feo, G.D.; Triggiani, M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol. Lett. 2016, 178, 10–14. [Google Scholar] [CrossRef]
- Saccheri, P.; Travan, L.; Ribatti, D.; Crivellato, E. Mast cells, an evolutionary approach. Ital. J. Anat. Embryol. 2019, 124, 271–287. [Google Scholar] [CrossRef]
- Vizzini, A.; Di Falco, F.; Parrinello, D.; Sanfratello, M.A.; Mazzarella, C.; Parrinello, N.; Cammarata, M. Ciona intestinalis interleukin 17-like genes expression is upregulated by LPS challenge. Dev. Comp. Immunol. 2015, 48, 129–137. [Google Scholar] [CrossRef]
- Vizzini, A.; Parisi, M.G.; Cardinale, L.; Testasecca, L.; Cammarata, M. Evolution of Ciona intestinalis tumor necrosis factor alpha (CiTNFα): Polymorphism, tissues expression, and 3D modeling. Dev. Comp. Immunol. 2017, 67, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cima, F. Microscopy Methods for Morpho-Functional Characterisation of Marine Invertebrate haemocytes. In Microscopy: Science, Technology, Applications and Education; Mendez-Vilas, A., Alvarez, J.D., Eds.; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 1100–1107. [Google Scholar]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Berlin, G.; Enerbäck, L. Fluorescent berberine binding as a marker of secretory activity in mast cell. Int. Arch. Allergy Appl. Immunol. 1983, 71, 332–339. [Google Scholar] [CrossRef]
- Cima, F. Enzyme Histochemistry for Functional Histology in Invertebrates. In Single Molecule Histochemistry: Methods and Protocols; Pellicciari, C., Biggiogera, M., Eds.; Methods in Molecular Biology; Springer Protocols, Humana Press, Springer Science: New York, NY, USA, 2017; Volume 1560, pp. 69–90. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunelli, N.; Dalle Palle, S.; Cima, F. Possible Evolutionary Precursors of Mast Cells: The ‘Granular Cell’ Immunocyte of Botrylloides leachii (Tunicata; Ascidiacea). J. Mar. Sci. Eng. 2025, 13, 811. https://doi.org/10.3390/jmse13040811
Brunelli N, Dalle Palle S, Cima F. Possible Evolutionary Precursors of Mast Cells: The ‘Granular Cell’ Immunocyte of Botrylloides leachii (Tunicata; Ascidiacea). Journal of Marine Science and Engineering. 2025; 13(4):811. https://doi.org/10.3390/jmse13040811
Chicago/Turabian StyleBrunelli, Nicolò, Stefano Dalle Palle, and Francesca Cima. 2025. "Possible Evolutionary Precursors of Mast Cells: The ‘Granular Cell’ Immunocyte of Botrylloides leachii (Tunicata; Ascidiacea)" Journal of Marine Science and Engineering 13, no. 4: 811. https://doi.org/10.3390/jmse13040811
APA StyleBrunelli, N., Dalle Palle, S., & Cima, F. (2025). Possible Evolutionary Precursors of Mast Cells: The ‘Granular Cell’ Immunocyte of Botrylloides leachii (Tunicata; Ascidiacea). Journal of Marine Science and Engineering, 13(4), 811. https://doi.org/10.3390/jmse13040811







