Abstract
Gnathophis johnsoni sp. nov. is described on the basis of 15 specimens (138–380 mm TL) from the Emperor–Hawaiian Seamount Chain in the western and central North Pacific. The new species is most similar in morphology to G. bathytopos (Atlantic), G. cinctus (eastern Pacific), and G. smithi (Nazca and Salas-y-Gomez Seamounts in the southeastern Pacific) by the sensory pore configuration and vertebral count, but differs from these species in the following characters in combination: darkly pigmented pectoral fin, dorsal fin with black margin broadened caudally and extended onto the distal half of the caudal fin, relatively long head, jaws, gill slit and caudal fin, and on average a greater preanal distance. Although most of morphometrics overlap between the new species and its closest relatives, multivariate statistical analyses clearly discriminate this species. Molecular analysis shows sister relationships between the new species and G. cinctus, with 1.81% of genetic divergence, which significantly exceeds the differences between the haplotypes belonging to the same species (0.36 and 1.08% of divergence, usually not exceeding 0.9%) and confirms both species as close but distinct. The close relationship of G. johnsoni sp. nov. and G. cinctus represents a rare case of biogeographical relations between western and eastern Pacific demersal fish faunas. Molecular data suggest that some morphologically similar species may represent independently evolved lineages, though the group of Gnathophis possessing elevated lateral-line pores is likely monophyletic.
1. Introduction
The congrid genus Gnathophis Kaup, 1860 is characterized by a moderately elongated body, well-developed pectoral and caudal fins, flanges on the upper and lower lip, snout projecting beyond the lower jaw, and an intermaxillary tooth patch mostly or partly excluded from the mouth; the teeth are moderately small, conical, and in bands [1]. Twenty-seven valid species are currently recognized [2], but Congromuraena musteliceps Alcock, 1894, provisionally placed in Gnathophis by the author of [3], is not included [4]. Only three named species, G. ginanago (Asano, 1958), G. heterognathos (Bleeker, 1858), and G. xenica (Matsubara & Ochiai, 1951), have been previously reported for the western and central North Pacific [5,6], although some unidentified species were also noted [6,7,8].
During a 2019 cruise on the research vessel (RV) Professor Kaganovsky, five specimens of Gnathophis were obtained from the Koko Seamount in the Emperor Chain. As it was not possible to identify them with any known species, we searched for additional material in museum collections to compile a detailed comparison of the Emperor Seamounts eels with other members of the genus. This confirmed that the Emperor Seamounts Gnathophis was a new species, which is described herein.
2. Materials and Methods
Counts, measurements, and terminology follow [9], with the following additions: head width, as the distance between most distant right and left point atop the head; the distance between lower end of pectoral-fin base and lower end of gill slit, as the shortest distance between these points. Measurements were taken point to point from the left side. Vertebral counts were taken from digital radiographs. Morphological data were taken from the formalin-preserved specimens and subsequently transferred to 70–75% ethanol or 50% isopropanol for permanent storage. Fresh coloration was determined from color photographs of the living specimens taken aboard RV Professor Kaganovsky in 2019 and from the freshly defrosted specimens. The holotype and paratypes have been registered in ZooBank (urn:lsid:zoobank.org:act:8950EE8A-CA50-4E25-AD0C-E044B3BAD87D). Catalog numbers and label data are presented under species description. Museum acronyms follow [10]. Other abbreviations: HL, TL, TrL, head length, total length, and trunk length, respectively; RV, FRV, research and fishery research vessel, respectively.
2.1. Comparative Material Examined
Gnathophis bathytopos Smith and Kanazawa, 1977 (4 specimens in total, 265–327 mm TL): USNM 213558, paratypes, 2 (311–327 mm TL), USA, Florida Keys, 25°13′ N, 84°15′ W, 183 m, RV Silver Bay, cruise 17, station 1200, bottom trawl, 9–10 June 1959. USNM 397258, 297 mm TL, USA, Virginia, 37°1.8′ N, 74°39.6′ W, 89–131 m, RV Albatross IV, Cruise AL200502, station 62, 19 February 2005; USNM 466022, 265 mm TL, USA, 38°32.9′ N, 73°17.7′ W, 129–135 m, RV Henry R. Bigelow, Cruise 202204, station 9, 11–12 September 2022.
Gnathophis cinctus (Garman, 1899) (17 specimens in total, 207–412 mm TL): SIO 15-1034, 290 mm TL, USA, likely California, H. Lonzaga, bycatch, 1947; SIO 61-17, 2 (365–412 mm TL), USA, off Santa Rosa Island, 34°3′ N, 120°6′ W, 70.4 m, J. Radovitch, seismic testing, 04 June 1949; SIO 62-90, 2 (207–227 mm TL), Mexico, Islas San Benitos, 28°17.5′ N, 115°34.6′ W, RV Black Douglas, cruise B6111, station 2, trawl 29, bottom trawl, 25 November 1961; SIO 63-184, 4 (208–337 mm TL), Mexico, east side of Isla Guadalupe, 28°58.2′ N, 118°13.3′ W, 0–15 m, JR Stewart and party, chemfish and SCUBA, 25 April 1963; SIO 68–94, 291 mm TL, Mexico, east side of Isla Angel de la Guarda, Mexico, 29°19.9–20.4′ N, 113°10.4–12.0′ W, 256–284 m, RV Thomas Washington, cruise MV 68-I, Station MV 68-I-65, bottom trawl, 20 January 1968; SIO 72-203, 5 (246–305 mm TL), Mexico, 29°40.0–39.5′ N, 113°32–56′ W, 330 m, RV Alexander Humboldt, cruise AH72/03, Station 47/3, bottom trawl, 29 February 1972; SIO 76-333, 296 mm TL, California, Redondo Beach, 33°50.45’ N, 118°23.7′ W, GW Boehlert, powerplant intake pumps, 09 March 1975; SIO 22-111, 239 mm TL, Mexico, Salsipuedes Basin, 29°3.22′ N, 113°15.68′ W, 285–296 m, ROV Doc Ricketts, station 735, 25 March 2015.
Gnathophis heterognathos (Bleeker, 1858) (2 specimens in total, 209–271 mm TL): SIO 09-384, 271 mm TL, Taiwan, Ta Shi Fish Market, HJ Walker and party, 08 September 2009. SIO 19-9, 209 mm TL, Taiwan, Kaohsiung City bycatch processing yard, BW Frable and HC Lin, 30 July 2018.
Gnathophis smithi Karmovskaya, 1990 (9 specimens in total, 257–403 mm TL): IORAS P.00562, paratype, 390 mm TL, 25°45′ S, 85°29′ W, 200–225 m, FRV Ikhtiandr, cruise 5, bottom trawl 26A, 1 November 1979; IORAS P.00564, paratype, 403 mm TL, 25°41′ S, 85°27′ W, 145 m, FRV Ikhtiandr (?), bottom trawl, 12 October 1984; SIO 65-658, 369 mm TL, Chile, south side of Isla Juan Fernandez, 33°45.5′–41.5′ S, 78°54.3′–53.1′ W, 125–200 m, RV Anton Bruun, cruise MV 65-IV, bottom trawl, 15 December 1965; ZMMU P.18045, paratypes, 4 (257–280 mm TL), 25°38′ S, 85°25′ W, 162–190 m, RV Professor Shtokman, cruise 18, station 1923, 26 April 1987; ZMMU P.18046, paratype, 385 mm TL, 25°38′ S, 85°34′ W, 215–240 m, FRV Ikhtiandr, cruise 5, bottom trawl no. 42, 25 November 1979; ZMMU P.18048, paratype, 317 mm TL, 25°45′ S, 85°22′ W, 175–240 m, FRV Ikhtiandr, cruise 6, bottom trawl no. 50, 2 September 1980.
2.2. Statistical Analysis
A principal component analysis (PCA) was conducted on size-standardized morphometrics to identify and visualize the axes of variation between and among the species examined. To remove the effect of body size, an allometric Burnaby correction [11] was applied for TL. This technique log transforms the linear measurements and projects them orthogonally to the first principal component of size. The first two principal components (PC1 and PC2) were visualized with convex hulls constructed around specimens of each species (when possible). A multivariate analysis of variance (MANOVA) was conducted on the first two PC axes to explore whether species differ significant across this variation. Finally, a discriminant function analysis (DFA) was used to explore whether the variation observed separates the putative species and by which variables. All analyses were conducted in R (version 4.4.3) using the FactoMineR packages for PCA, MASS for the DFA, and ggplot2 for visualization.
2.3. Molecular Data and Phylogenetic Analysis
Tissue samples were fixed in a sufficient volume of 96% ethanol. Fixed samples were stored at 20 C; ethanol was changed approximately one month after collection, and again after one year. DNA was extracted using the Wizard SV 96 Genomic DNA Purification System (Promega Corporation, Madison, WI, USA) according to the manufacturer’s manual. DNA extraction, polymerase chain reaction (PCR), PCR product purification, and nucleotide sequencing were performed using standard molecular genetic techniques [12]. Cytochrome oxidase subunit I (COI) fragment was amplified with a primer complex of VF2_t1, FishF2_t1, FishR2_t1, FR1d_t1 [12,13]. Amplification was conducted in a volume of 15 μL with 3–5 μL of total DNA, 1× buffer, 2.5 mM MgCl2, 0.2 mM dNTP, 0.5 mM of each primer, and 0.75 U μL−1 Color Taq polymerase. Cycling consisted of 5 min at 95 °C, followed by 35 cycles of 30 s each at 95 °C, 45 s at 52 °C, 60 s at 72 °C, and a final extension for 12 min at 72 °C. All resulting amplicons were purified by ethanol precipitation [14]. Purified fragments were sequenced from the forward strand by Applied Biosystems BigDye Terminator v3.1. kit (Applied Biosystems, Foster City, CA, USA) with capillary electrophoresis on ABI3500 Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). A molecular analysis was carried out on the basis of Core Shared Research Facilities “Fisheries Genomics” in VNIRO Laboratory of Molecular Genetics.
For a comparative analysis, sequences from the NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 25 November 2024) and BOLD Systems (https://www.boldsystems.org/, accessed on 25 November 2024) databases were used. The resulting sequences were aligned in Geneious 10.0.5 (Biomatters, Auckland, New Zealand) [15]. Sequences were merged into haplotypes using the FaBox 1.41 converter [16]. Haplotype networks were constructed in PopArt v. 1.7 (Allan Wilson Centre Imaging Evolution Initiative, Otago, New Zealand) [17] using the Minimum Spanning Network algorithm.
Plugins for Geneious 10.0.5 were used to build the trees. MrBayes 3.2 was used to perform Bayesian inference analyses (GTR + G model, 1 million generations, three heated chains, sampling frequency—every 1000 generations, the first 25% of trees was discarded as burnin, and the other parameters were default). Maximum-likelihood tree construction was performed using RAxML 8.2.11 [18] (GTR + G model, rapid bootstrapping, 1000 replicates).
The network was built using a 554 bp fragment to include a larger number of samples available for comparison. To construct the trees, a longer fragment of 582 bp was used, but we had to exclude some too short sequences from the analysis. Due to different fragment lengths, the numbering of haplotypes on the network and trees does not match. Albula vulpes (GU224698), Conger cinereus (KF929775), Japonoconger proriger (MF956464), and Nettastoma parviceps (AP010864) sequences from NCBI database were used as an outgroup. The sequences used for molecular analysis are listed in Table 1.

Table 1.
Information about COI sequences and respected samples used for molecular analysis.
3. Results
Gnathophis johnsoni sp. nov.
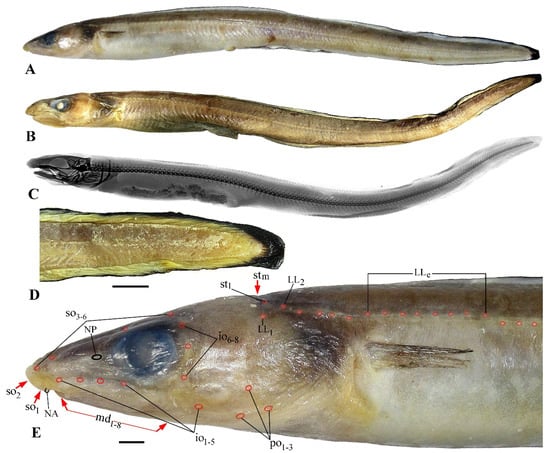
Figure 1.
Gnathophis johnsoni sp. nov., holotype, IORAS P.03671, 321 mm TL. (A–C), lateral view; (A) fresh specimen; (B) preserved specimen; (C) radiograph; (D) caudal fin; (E) head and anteriormost trunk with the configuration of the laterosensory system. Abbreviations: io1–5, infraorbital pores 1–5; io6–8; infraorbital pores 6–8 (postorbital pores); LL1 and LL2, first and second pore of body lateral line; LLe, elevated pores of body lateral line; md1–8, mandibular pores 1–8; NA, anterior nostril; NP, posterior nostril; po1–3, preopercular pores 1–3; so1–6, supraorbital pores 1–6 (so1, ethmoidal pore); stl, lateral supratemporal pore; stm, medial supratemporal pore; arrows indicate pores invisible in this angle. Scale bars: 5 mm.

Table 2.
Measurements of Gnathophis spp. Notes: *, holotype included; M ± m, mean and its error; n, number of specimens; N/A, not applicable; SD, standard deviation.
Holotype: IORAS P.03671, 321 mm TL (Figure 1A–C), Emperor Seamounts, Koko Seamount, 35°10′ N, 171°46′ E, 383–387 m, RV Professor Kaganovsky, bottom trawl no. 101, 9 April 2019.
Paratypes: 11 specimens in total, 138–380 mm TL: IORAS P. 04999, 4 (285–331 mm TL), collected with the holotype; SIO 25-7 (ex USNM 436748), 324 mm TL, Emperor Seamounts, Koko Seamount, 35°22.2′ N, 171°25.2′ E, 20 October 2006; USNM 348130, 2 (138–229 mm TL), USA, Hawaii, Oahu, 21°42′ N, 158°4.2′ W, 100–800 m, RV Townsend Cromwell, cruise 59, station TC 59-2, shrimp trawl, 12 July 1972; USNM 436749, 1 (not measured), Emperor Seamounts, 9 September 2006; USNM 436751, 370 mm TL, Emperor Seamounts, Kammu Seamount, 31°56.85′ N, 173°8.64′ E, 340 m, 13 April 2013; USNM 436750, 380 mm TL, Emperor Seamounts, Yuryaku Seamount, 35°40.8′ N, 172°16.8′ E, 400 m, 5 July 2015; USNM 436752, 351 mm TL, Emperor Seamounts, Kammu Seamount, 32°1.8′ N, 173°9.0′ E, 340 m, 9 July 2015.
Additional non-type specimens: ZIN 47603, 3 (304–363 mm TL), 29°46′ N, 179°03′ E, 280 m, FRV Ekvator, bottom trawl no. 67, 14 December 1975. Not included in statistical analysis.
Diagnosis. A moderately robust species of Gnathophis with 129–135 vertebrae, elevated pores in the anterior portion of the body lateral line, 6 suborbital, 3 preopercular and 31–35 preanal pores, black-margined vertical fins, caudal fin black in distal half (Figure 1D), black stomach, swimbladder extending far beyond anus.
Description. Measurements are shown in Table 2. Elongated body, cylindrical in cross-section, becoming more compressed at the tail; tail tip not attenuated; trunk 2.2–2.5 times the tail length; moderately large head, 1.4 times the trunk length; moderately long snout, depressed, pointed, 3.3–3.5 (3.7 in 1 paratype) times the head length; moderately large eye, oval, 1.2–1.5 times the snout length. Snout projecting well beyond lower jaw, intermaxillary tooth patch exposed by half to one-third when the mouth is closed; tubular anterior nostril, close to snout tip, directed ventrolaterally; oval and slit-like posterior nostril, with a slightly raised rim, close to the eye at mid-eye level; upper lip with a shallow flange between the posterior nostril and the anterior third of the eye; lower lip with a large lateral flange; rictus below the anterior margin to the middle of the pupil. Slender upper- and lower-jaw teeth, pointed, tightly set in a narrow band broadened at symphysis; a nearly rectangular intermaxillary tooth patch; blunt vomerine teeth, in 3 to 5 rows; vomerine tooth patch ending at the middle of the maxillary tooth band.
Cephalic sensory pores (Figure 1E) small; supraorbital canal with six pores: first three pores situated at the tip of the snout, first (ethmoidal) pore opening in front of the anterior nostril, ventral surface of the snout between the ethmoidal pores of the neighboring sides folded; fourth suborbital pore lying on dorsal surface of snout twice closer to anterior than the posterior nostril; fifth and sixth suborbital pores opening close to anterior and posterior ends of orbit, respectively. Infraorbital canal with eight pores, with the first four along the lower lip between the anterior nostril and the anterior third of the eye; slightly enlarged fifth pore, longitudinally extended, opening behind rictus; three postorbital pores. Supratemporal commissure with three conspicuous pores. Eight mandibular and three preopercular pores; the first two preopercular pores are larger, slightly extended longitudinally, forming the ascending line; the third preopercular pore is small, round, shifted anterodorsally, situated above the interspace between first and second pores at the level of the lower margin of the eye. In one paratype and two largest ZIN specimens, this pore is replaced by a free neuromast on both sides (probably under aberrant conditions). Body lateral line complete, its first pore slightly shifted downward, situated slightly in front or below the lateral supratemporal pore; second pore and pores above the adpressed pectoral fin from the 6th–8th to 12th–18th opening dorsally (“elevated”); the lateral line pores before the level of the anterior border of anus are 31–35.
Rather short pectoral fin, 1.2–1.5 times the horizontal eye diameter, narrow, with 12 (1 paratype) or 13 rays. Dorsal fin originating slightly behind to over the pectoral-fin base; 9 or 10 predorsal vertebrae. Total vertebrae 130–134, precaudal vertebrae (38?) 41–43 (29–33% of total vertebrae), preanal vertebrae 35–38. Supraoccipital producing. The posterior third of the swimbladder extends beyond the anus (reaching the level of the 46th to 50th lateral-line pore or first 14–15% of the anal-fin base length; n = 2). The distance between the posterior end of stomach and vent is equal to stomach length.
Coloration. Head and body above lateral line brownish, yellowish below, becoming silvery at the vent; the top and sides of head anterior to orbit and area of gill chamber are darker, contrasting with the paler postorbital area of head; flanks below lateral line with tiny dense melanophore pigmentation invisible to the naked eye, with melanophores becoming very sparse ventrally. Infuscated pectoral fin, often more conspicuously in the distal part; with its rays dark along whole length. Dorsal and anal fin with black margins, more prominent along the dorsal fin; sometimes, the black margin is irregular in the anterior portion of the anal fin, but present in all specimens of this type; most posterior dorsal-fin rays are black for half their length; the distal half of caudal fin is black. Mouth pale; stomach and peritoneum uniformly dusky (smallest specimens 138 and 208 mm TL, scattered darker melanophores emerging in the latter specimen) to dark (jet-black in life, IOM specimens), and the intestine is pale. No external sexual dimorphism (one ripe female and male were each dissected).
Etymology. Named in the memory of G. David Johnson (1945–2024), whose contributions to ichthyology were prolific and need no explanation.
Distribution. The new species probably is distributed throughout the Emperor–Hawaiian Seamount Chain from Koko and Yuryaku Guyots southeastward to Oahu (Figure 2).
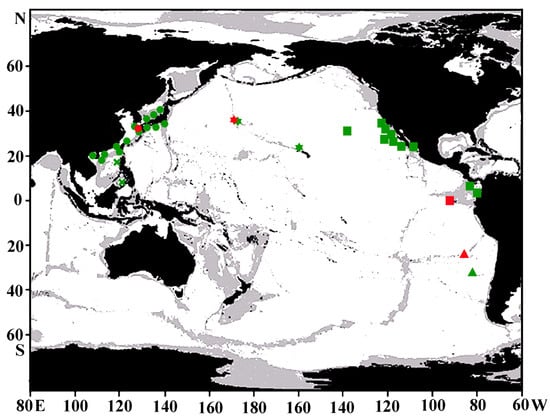
Figure 2.
A distribution map of the four morphologically similar Gnathophis species in the Pacific Ocean based on the specimens examined, the literature data [5,19,20,21,22,23], and web database (www.gbif.org/species/2403303 and www.gbif.org/species/102058300, accessed on 7 April 2025): squares, G. cinctus; stars, G. johnsoni sp. nov.; circles, G. heterognathos (× indicates questionable records in [22]); triangles, G. smithi. Red symbols show holotype localities. A single symbol may indicate more than one capture.
4. Discussion
Gnathophis johnsoni sp. nov. has been previously reported from the Emperor Seamounts as Rhynchocymba nystromi nystromi [24,25], ?Alloconger anagoides [26], and Gnathophis sp. [8]. Hoshino et al. [8] (22) indicated the diagnostic presence of 11 preoperculo-mandibular pores but noted “a typically uniform white anal fin (sometimes with weak dark margin)” for their specimens. All examined specimens have a black-margined anal fin; although this feature may vary individually. Variation in the development of the black margin of the anal fin is also documented in our study for the type series of G. smithi. The nominal taxon R. n. nystromi (originally described by [27] as Leptocephalus nystromi) is a junior synonym of Gnathophis heterognathos, originally described as Myrophis heterognathos by [28] from the same type locality (Nagasaki, Japan) [22,29]. The new species, although superficially similar, can be easily distinguished from G. heterognathos by the presence of three (vs. two) preopercular pores, more elevated lateral-line pores along the section above the adpressed pectoral fin (8–10 vs. 4–6), pigmented (vs. pale) pectoral fin, dark peritoneum (vs. pale with dotted melanophores), and higher vertebral count (129–135 vs. 117–131, not more than 124 in Japanese specimens) [5,20,22]. Karmovskaya [22] (S16) counted 121–131 vertebrae for the four Philippine specimens she referred to as G. heterognathos, clearly higher than in the Japanese specimens (117–124) but partly overlapping with the new species. Although most other characters of the Philippine specimens resemble those in the Japanese fish, the former are characterized by the swimbladder “reaching behind anus” [22] (S17) as opposed to before the anus in the Japanese specimens [20] (54–55, Fig. 27C). In our opinion, the identity of the Philippine specimens requires reconsideration. A genetic divergence of 4.51% (25 nucleotide substitutions) in COI also distinguishes Gnathophis johnsoni sp. nov. from G. heterognathos.
The species of Gnathophis can be roughly separated into two groups: those with and those without elevated lateral-line pores above the adpressed pectoral fin. Thirteen other species possess elevated pores like the new species and G. heterognathos: G. andriashevi Karmovskaya, 1990, G. bathytopos Smith and Kanazawa, 1977, G. castlei Karmovskaya and Paxton, 2000, G. cinctus (Garman, 1899), G. codoniphorus Maul, 1972, G. grahami Karmovskaya and Paxton, 2000, G. heterolinea Kotthaus, 1968, G. macroporis Karmovskaya and Paxton, 2000, G. melanocoelus Karmovskaya and Paxton, 2000, G. microps Karmovskaya and Paxton, 2000, G. mystax (Delaroche, 1809), G. nasutus Karmovskaya and Paxton, 2000, and G. smithi Karmovskaya, 1990. The new species can be easily distinguished by its vertebral count (129–135) from the Atlantic G. codoniphorus and G. mystax (134–144), East African G. heterolinea (120), Australian G. macroporis, G. melanocoelus, G. microps and G. nasutus (120–128), and southeastern Pacific G. andriashevi (139–142) [1,21,30,31,32]. Gnathophis johnsoni sp. nov. differs from the Australian G. castlei by the presence of three (vs. two) preopercular pores and precaudal vertebrae 29–33 vs. 32–35% of total vertebrae [32]. The remaining four species (G. bathytopos, G. cinctus, G. grahami and G. smithi) share the same configuration of the head pores, similar basic meristic traits, and show only slight differences in morphometrics and pigmentation. The new species differs from the Australian G. grahami by the narrower vomerine tooth patch (3–5 vs. 6 or 7 rows in G. grahami), longer trunk region (21.9–26.3 vs. 17.1–23.0% TL) and preanal distance (39.7–43.4 vs. 34.4–40.7% TL), and smaller eyes (16.7–22.0 vs. 20.2–26.9% HL) [32]. A color photograph of G. grahami (https://www.fishbase.se/photos/ThumbnailsSummary.php?ID=60613, accessed on 7 April 2025) also shows a uniformly narrow black margin along the dorsal, caudal and anal s in contrast to broad caudal pigmentation in the new species, and colorless (vs. pigmented) pectoral fins. The most notable differences between the new species, G. bathytopos (western Atlantic), G. cinctus (eastern Pacific: California to Peru) and G. smithi (southeastern Pacific: Nazca and Salas-y-Gomez Ridges) are confined to the pigmentation of fins (Figure 3). The dark pigmentation of the pectoral fin is distinctive for the new species, as the other three possess unpigmented pectorals. The jet-black pigmentation broadens toward the caudal fin, and occupying the distal half-length of the latter is also characteristic for the new species, in contrast to the narrowly margined fins at caudal extremity in G. bathytopos (https://v3.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=89119, accessed on 7 April 2025) and distally pigmented dorsal and anal fins (toward their ending), but unpigmented caudal fin in G. smithi. In addition, the distal margin of the caudal fin is almost truncated in the new species, but narrowed toward the tip in G. bathytopos and G. smithi. The new species is most similar in the caudal-fin shape and pigmentation to G. cinctus. Unfortunately, all available specimens of the latter species are badly faded; however, [33] (196) noted blackish pigmentation was only observed at the tip of the tail, which can also be observed in some examined specimens (Figure 3B). The caudal fin of G. cinctus is likely black in the distal half, as in the new species, but the pigmentation does not extend far forward along the dorsal-fin margin as in the new species (Figure 3).

Figure 3.
Gnathophis cinctus (A–C) and G. smithi (D,E). (A,B,D) habitus, (C,E) caudal fin; (A,C) SIO 72-203, 305 mm TL; (B) SIO 76-233, 296 mm TL; (D,E) ZMMU P.18045, paratype, 280 mm TL. Scale bars: 5 mm.
The new species also can be distinguished from G. cinctus and G. smithi in having a uniformly pigmented peritoneal coloration in adolescent specimens. The peritoneum is pigmented throughout, including the paravertebral area, even in the smallest specimens of G. johnsoni sp. nov. available, dusky in the 138 mm paratype, dusky with scattered darker melanophores in the 208 mm paratype, much darker, uniformly brownish or blackish in the other specimens (jet-black in life). In G. smithi, the peritoneum is silvery with rather sparse melanophore speckling in the smallest 257–280 mm paratypes, becoming solidly black except for a narrow paravertebral stripe retaining pale coloration with melanophore speckling in the 273 mm and 317 mm and larger paratypes. In the largest (403 mm) paratype, a speckled paravertebral area is probably restricted to the anteriormost third of the abdominal cavity only (this specimen was largely eviscerated and its peritoneum is significantly damaged; however, it seems true that it is fully black posteriad). A change in peritoneal pigmentation perhaps occurs at 270–280 mm TL; however, it should be noted that the 273 mm paratype with a black peritoneum, though shorter, has a much larger head and trunk region than the 280 mm paratype with speckled peritoneum; thus, it might be of greater age. Gnathophis cinctus is intermediate in this character: the peritoneum is only pigmented in the anterior half of the cavity, dusky to brownish, becoming pale and speckled with dark melanophores in the posteriad in the specimens less than 250 mm TL and completely dark over 295 mm TL.
Gnathophis johnsoni sp. nov. also has a higher average count of preanal vertebrae in comparison to G. cinctus (35–38, mean 36 vs. 33–36, mean 34), highly overlapping in this respect with G. bathytopos and G. smithi (34–38). Our analysis shows a wider variation in many measurements (Table 2), disproving the diagnostic value of some of them (e.g., head length, eye diameter, gill slit length, preanal distance) taken from the publications [1,21,33,34]. Only a few measurements do not overlap, allowing for the separation of individual specimens. Gnathophis johnsoni sp. nov. has a longer rictus and a longer caudal fin in comparison with G. cinctus (6.2–7.0 and 1.7–2.3% TL vs. 5.0–6.2 and 1.0–1.6% TL) and a narrower interorbital in relation to TL in comparison with G. bathytopos (1.6–3.6 vs. 1.3–1.6% TL). All other morphometrics overlap (Table 2). The principal component analysis (PCA) of the size-standardized morphometrics revealed a clear separation of Gnathophis johnsoni sp. nov. from G. cinctus and G. bathytopos, with G. smithi overlapping all three other species in morphospace (Figure 4A). The first component (PC1) covered 52.7% of the variation, with the largest contributions regarding the interorbital width, caudal-fin length, interbranchial width, body depth at anus, pectoral-fin base length, upper-jaw length, and snout length, while the second component (PC2) covered 13.5% of the variation, with the largest contributions regarding the distance between lower end of pectoral-fin base and lower end of gill slit, gill slit length, body depth at anus, trunk length, and pectoral-fin base length. The MANOVA test revealed a strong species effect (p < 0.001), indicating significant variation in morphometric traits across the four species. Post hoc ANOVA results showed that PC1 differed significantly among species (p < 0.001), while PC2 did not (p = 0.129). Tukey’s pairwise comparisons identified significant differences in PC1 scores between G. johnsoni sp. nov. and G. cinctus (p < 0.001) and G. bathytopos (p = 0.006), but not G. smithi (p = 0.627), although G. smithi differed significantly from G. cinctus (p = 0.001). No significant pairwise differences were detected for PC2.
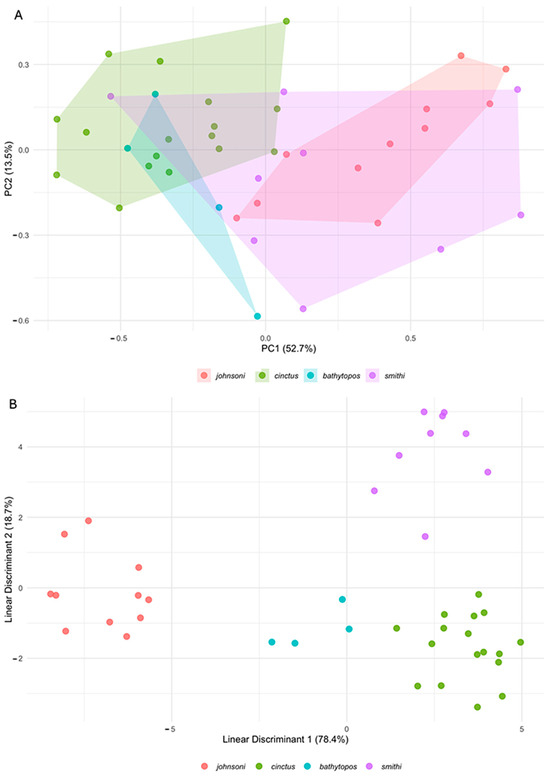
Figure 4.
Plots of multivariate statistical analyses for 17 linear measurements of four Gnathophis eel species: G. johnsoni sp. nov. (n = 11; red), G. cinctus (n = 17; green), G. bathytopos (n = 4; blue), and G. smithi (n = 9; purple). (A) A scatterplot of principal components 1 and 2 (PC1 and PC2) from a principal component analysis of size-corrected measurements. Percentage variation explained in axes. (B) A scatterplot of the linear discriminant axes 1 and 2 from a discriminant function analysis of the four species. Percentage variation is explained in the axes.
The discriminant function analysis (DFA) readily distinguished all four species with 100% classification accuracy (Figure 4B, Table 3). The first two discriminant axes (LD1 and LD2) accounted for 78.4% and 18.7% of the variation, respectively, with preanal distance, trunk length, upper-jaw length, head length, and lower-jaw length contributing most to species differentiation. This aligns with the MANOVA results, which also demonstrated significant morphological separation of the species.

Table 3.
Factor loads from principal component analysis of the morphological characters.
Molecular data inferred from COI support validity of Gnathophis johnsoni sp. nov. and its close relationships with G. cinctus (no molecular samples of G. smithi were available) (Figure 5, Figure 6 and Figure 7). Both species form a monophyletic clade, with a genetic divergence of 1.81% (10 nucleotide substitutions). Although this is the lowest value between the species available for analysis (excluding G. bathytopos/mystax pair, indistinguishable by COI), it is similar to another pair of the morphologically similar species (G. heterognathos/grahami, 2.17%, 12 nucleotide substitutions) and greatly exceeds the differences between the haplotypes of the same species (ranging between 0.36 and 1.08% of genetic divergence, in most cases 2 to 5 (0.9%) nucleotide substitutions, but up to 6 within G. heterognathos) (Figure 5). Surprisingly, despite morphological similarity, the new species is fairly distant from G. bathytopos (3.79% divergence, 21 nucleotide substitutions). The western Atlantic G. bathytopos and eastern Atlantic G. mystax form a single clade with maximum 0.72% of genetic divergence (4 nucleotide substitutions). Although the proximity of these species was pointed out during the original description, G. mystax differs from G. bathytopos by a higher vertebral count (134–141 vs. 128–134) [1,34]. This could support G. bathytopos and G. mystax as being recently diverged.
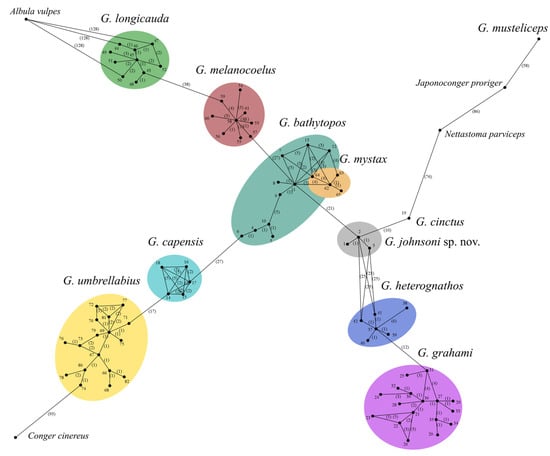
Figure 5.
Phylogenetic relationships of Gnathophis johnsoni sp. nov., its congeners and outgroup by COI mtDNA sequences. Numbers indicate haplotypes (see Table 1 for explanation); numbers of substitutions are given in brackets.
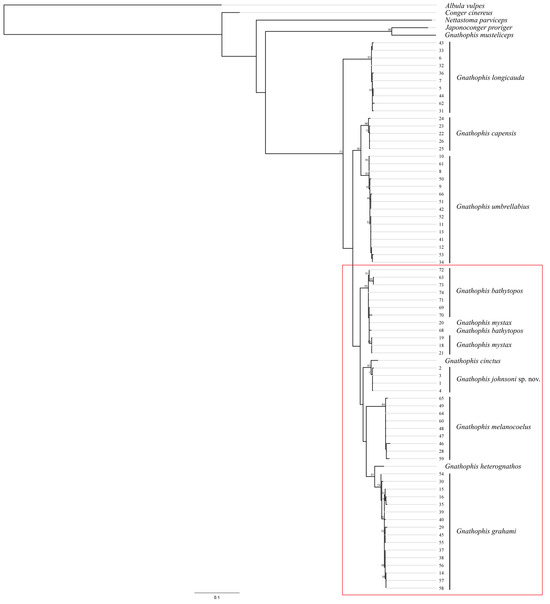
Figure 6.
Tree topology reconstruction of specimens of Gnathophis johnsoni sp. nov., its congeners, and outgroup based on the maximum-likelihood method (GTR + G model, rapid bootstrapping, 1000 replicates) for the mitochondrial cytochrome oxidase subunit I (COI) gene. The numbers beside each branch indicate bootstrap values. The numbers to the right of the branches correspond to the number of haplotypes in Table 1. Red frame combines species with elevated lateral-line pores.
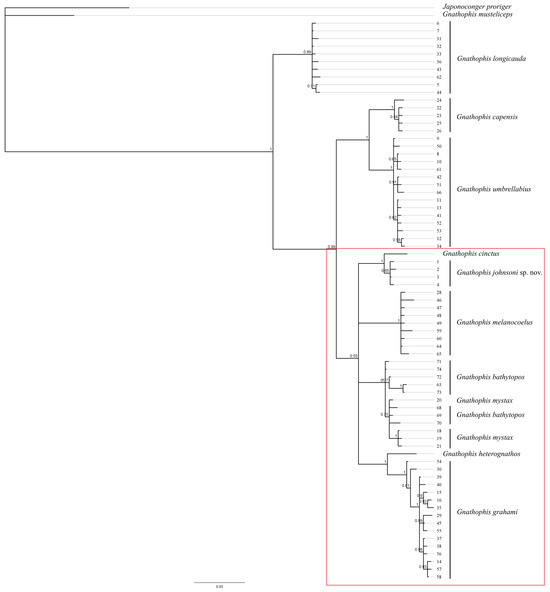
Figure 7.
Tree topology reconstruction of specimens of Gnathophis johnsoni sp. nov., its congeners, and outgroup based on Bayesian inference analyses (GTR + G model, 10 million generations, three heated chains, sampling frequency—every 1000 generations, the first 25% of trees was discarded as burnin, and the other parameters were default) for mitochondrial cytochrome oxidase subunit I (COI) gene. The numbers beside each branch indicate posterior probability. The numbers to the right of the branches correspond to the number of haplotypes in Table 1. Red frame combines species with elevated lateral-line pores.
The COI phylogeny of Gnathophis confirms the isolated position of “G.” musteliceps. According to [4] (39), this species resembles the genus Japonoconger Asano, 1958 but “may not fit in any of the currently recognized genera”. Our data suggest a sister relationship between “G.” musteliceps and J. proriger (Gilbert, 1891); however, the genetic distance between these species (10.47% divergence, 58 nucleotide substitutions) is relatively high for inclusion in the same genus. Interrelationships within the typical members of the genus Gnathophis are highly preliminary, since a restricted number of species were available for the molecular analysis. Both maximum-likelihood and Bayesian analyses support the monophyly of the group of species morphologically characterized by elevated anterior lateral-line pores (possible apomorphy) (Figure 6 and Figure 7).
Other morphological characters such as cephalic sensory pore configuration, vertebral count, and external and internal pigmentation may have independently evolved within the different lineages of Gnathophis, as we found no diagnostic characters supporting the other nodes of the molecular phylogenetic trees.
The close relationships between G. johnsoni sp. nov., G. cinctus, and probably G. smithi indicate an interesting case of biogeographic relations between western and eastern Pacific demersal ichthyofaunas. Springer [35] hypothesized the dispersal of the western Pacific species via the Emperor–Hawaiian island chain and other island groups to the East Pacific Rise on the southeastern portion of the Pacific Plate. Parin [36] demonstrated that the demersal fish fauna of the Nazca and Salas-y-Gomez Seamounts represents the easternmost outpost of the Indo-West Pacific zoogeographical region (including Hawaii), with only a few species in common or showing close resemblance to those inhabiting the western American slope waters. Most of the common species (Tetronarce, Macruronus, Coelorinchus, Notopogon, Helicolenus, Emmelichthys spp.) are believed to disperse from Australian–New Zealand waters eastward across the cold-temperate South Pacific Ocean [37], but this pathway is doubtful for the thermophilic Gnathophis leptocephali. Gnathophis spp. may have dispersed, like Echinorhinus cookei Pietschmann, 1928 (known from Taiwan, Caroline and Hawaiian Islands, New Zealand, Nazca Seamounts, California and Peru) and Ruvettus pretiosus Cocco, 1833 (cosmopolitan in tropical and temperate waters including the Emperor–Hawaiian Chain, islands of Oceania, the Nazcan region, and the western American slope from California to Peru). Although their dispersal pattern through the island groups as the author of [35] suggested seems reasonable, their penetration of the South American slope from the Nazcan region seems doubtful. There are no common tropical demersal fish species known from both the Nazcan region and the Chilean coast at the same latitudes. The cold Humboldt Current likely represents a native barrier for larval dispersion. However, a pathway through the system of the Equatorial Currents and Countercurrent eastward to the Galapagos Islands is possible for the long-living (several months according to [38]) pelagic larvae (like Gnathophis leptocephali) or species that can live for a long time in pelagic waters at the adult stage (like sharks and Ruvettus). The unidentified Gnathophis species are known from Vanuatu (MNHN IC-2009-0760), Marquesas (MNHN IC-2000-4528 and 2001-1162) and Tonga (MNHN IC-2001-1162) Islands. Elucidation of their taxonomic and phylogenetic position may further develop this hypothesis.
The clades of G. bathytopos/mystax, G. heterognathos/grahami/melanocoelus, and G. johnsoni/cinctus may have a Tethyan origin, as suggested for a group of closely related species of unpatterned morays, with a similar distributional pattern: Gymnothorax australicola Lavenberg, 1992 (subtropical South Pacific from Lord Howe to Easter and San Felix Islands), Gy. panamensis Steindachner, 1876 (western American coast from Gulf of California to Panama and the Galapagos Islands) and Gy. bacalladoi Böhlke and Brito, 1987 (Atlantic Ocean off the Canary Islands) [39]. The speciation between G. johnsoni sp. nov., G. cinctus, and perhaps G. smithi was likely more recent due to short genetic distances between the former two species (1.81%). The isolation of the allopatric Gnathophis populations may have occurred during the Pliocene–Pleistocene fluctuations of the sea level, as is commonly proposed for other recently diverged species [40,41].
5. Conclusions
A new species, Gnathophis johnsoni, formerly misidentified as Rhynchocymba nystromi (a junior synonym of G. heterognathos), is described from the Emperor–Hawaiian Seamount Chain in the western and central North Pacific. It is most similar to G. bathytopos (western Atlantic), G. cinctus (eastern Pacific: California to Peru and the Galapagos), and G. smithi (Nazca & Salas-y-Gomez Seamounts in the southeastern Pacific) by the sensory pore configuration and vertebral count, but differs from these species in terms of fin pigmentation and morphometrics. Proportional differences are confirmed by multivariate statistical analysis. Molecular analysis inferred from COI confirms the new species and G. cinctus as a close but distinct species (no public sequences of G. smithi exist). Gnathophis bathytopos is more of a distant species by COI phylogeny, probably diverging from the common ancestor of G. johnsoni sp. nov. and G. cinctus independently from G. heterognathos; the Atlantic–Pacific distribution of this clade may indicate its Tethyan origin. A suggested pathway of dispersion involves the western Pacific eastward through the island chains and further through the Equatorial Current–Countercurrent system to the western American coast.
Molecular phylogeny confirms the separation of “Gnathophis” musteliceps into its own genus, but does not support a separation of the western Atlantic G. bathytopos from the eastern Atlantic and Mediterranean G. mystax, although both these species are distinguishable by the vertebral count. Morphological characteristics separating the species of Gnathophis are likely homoplastic according to COI phylogeny; however, the monophyly of the group of species with elevated anterior lateral-line pores is corroborated by both the maximum-likelihood and Bayesian analyses.
Author Contributions
Conceptualization, A.M.P. and A.M.O.; methodology, A.M.P., B.W.F., O.R.E. and S.Y.S.; software, B.W.F., O.R.E., A.M.O. and S.Y.S.; validation, A.M.P., B.W.F., O.R.E., S.Y.S. and A.M.O.; formal analysis, A.M.P., B.W.F., O.R.E. and S.Y.S.; investigation, A.M.P., B.W.F., O.R.E., S.Y.S. and A.M.O.; resources, A.M.P., B.W.F., O.R.E., S.Y.S. and A.M.O.; data curation, A.M.P., B.W.F., O.R.E., S.Y.S. and A.M.O.; writing—original draft preparation, A.M.P. and A.M.O.; writing—review and editing, A.M.P., B.W.F., O.R.E., S.Y.S. and A.M.O.; supervision, B.W.F. and A.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was prepared within the framework of the Governmental Tasks of A.N. Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences No. 075-01011-23-05, topic “Aquatic communities: biodiversity, invasions, structure and protection” (FFER-2024-0017) (A.M.P.), Vavilov Institute of General Genetics of the Russian Academy of Sciences No. 122022600162-0 “Genetic technologies in biology, medicine, and agriculture” (O.R.E. and S.Y.S.), and the Shirshov Institute of Oceanology of the Russian Academy of Sciences No. FMWE-2024-0022 (A.M.P. and A.M.O.).
Data Availability Statement
The specimens described in this study are available at the National Museum of Natural History, Washington, DC; Shirshov Institute of Oceanology, Russian Academy of Sciences, Moscow; Scripps Institution of Oceanography, La Jolla, CA; Zoological Institute, Russian Academy of Sciences, Saint-Petersburg, and the Zoological Museum of the Lomonosov Moscow State University, Moscow.
Acknowledgments
We are grateful to our colleagues from TINRO (Pacific Branch of VNIRO, Vladivostok, Russia) who took part in the processing of catches during the cruise of the RV Professor Kaganovsky in 2019, storing samples in TINRO and transporting them to Moscow. A.M.O. and S.Y.O. thank Kirill Kolonchin (VNIRO) and Aleksey Baitalyuk (TINRO), who approved their participation in the cruise. We thank Diane Pitassy, Sandra Raredon, and Abby Reft (USNM) for the loan of material and the transfer of a paratype to SIO, and Mikhail Nazarkin (ZIN) and Ekaterina Vasil’eva (ZMMU) for access to the specimens under their care. We also acknowledge the assistance of Gento Shinohara (National Museum of Nature and Science, Tokyo, Japan), Ryo Misawa (Japan Fisheries Research and Education Agency, Yokohama, Japan), and Alexander Zavolokin (North Pacific Fisheries Commission, Tokyo, Japan) for help with some near-inaccessible literary sources. We are sincerely indebted to two anonymous reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smith, D.G. Family Congridae. In Fishes of the Western North Atlantic; Sears Foundation for Marine Research, Yale University: New Haven, CT, USA, 1989; Volume 1, pp. 460–567. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Fong, J.D. Eschmeyer’s Catalog of Fishes: Genera/Species by Family/Subfamily. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp (accessed on 5 March 2025).
- Castle, P.H.J. Alcock’s congrid eels from the “Investigator” collections in Indian seas 1888–1894. Copeia 1995, 3, 706–718. [Google Scholar] [CrossRef]
- Smith, D.G.; Jawad, L.A.; Al-Kharusi, L.H. New records and new information on four eel species from Oman (Teleostei: Anguilliformes: Congridae, Muraenesocidae). J. Ocean Sci. Found. 2017, 28, 34–46. [Google Scholar]
- Hatooka, K. 63. Congridae conger eels. In Fishes of Japan with Pictorial Keys to the Species; Nakabo, T., Ed.; Tokai University Press: Tokyo, Japan, 2002; Volume 1, pp. 227–234. [Google Scholar]
- Mundy, B.C. Checklist of the Fishes of the Hawaiian Archipelago. Bishop Mus. Bull. Zool. 2005, 7, 1–704. [Google Scholar]
- Struhsaker, P.J. A Contribution to the Systematics and Ecology of Hawaiian Bathyal Fishes. Ph.D. Dissertation, University of Hawai‘i at Manoa, Honolulu, HI, USA, 1973; pp. 1–482. [Google Scholar]
- Hoshino, K.; Okamoto, M.; Sawada, K. The Field Guide for Identifications of Fishes of the Emperor Seamount Chain Captured by Bottom Fisheries; NPFC: Tokyo, Japan, 2024; pp. 1–92. [Google Scholar]
- Böhlke, E.B. Methods and Terminology. Anguilliformes and Saccopharyngiformes. In Fishes of the Western North Atlantic; Böhlke, E.B., Ed.; Sears Foundation for Marine Research, Yale University: New Haven, CT, USA, 1989; Volume 1, pp. 1–7. [Google Scholar]
- Sabaj, M.H. Codes for Natural History Collections in Ichthyology and Herpetology (Online Supplement), Version 9.7 (3 Mar 2025); American Society of Ichthyologists and Herpetologists: Washington, WA, USA, 2025; Available online: https://asih.org (accessed on 5 March 2025).
- Burnaby, T.P. Growth-invariant discriminant functions and generalized distances. Biometrics 1966, 22, 96–110. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Phil. Trans. Roy. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Silva Jr, W.A.; Costa, M.C.; Valente, V.; Sousa, J.F.; Paçó-Larson, M.L.; Espreafico, E.M.; Camargo, S.S.; Monteiro, E.; Holanda, A.J.; Zago, M.A.; et al. PCR template preparation for capillary DNA sequencing. Biotechniques 2001, 30, 537–542. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ashton, B.; Buxton, S.; Cheung, M.; Cooper, A.; Duran, C.; Field, M.; Heled, J.; Kearse, M.; Markowitz, S.; et al. Geneious v5.4. 2011. Available online: http://www.geneious.com (accessed on 24 February 2025).
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Garman, S. The Fishes. Reports on an exploration off the west coasts of Mexico, Central and South America, and off the Galapagos Islands by the U. S. Fish Commission steamer “Albatross” during 1891 No. XXVI. Mem. Mus. Comp. Zool. 1899, 24, 1–431. [Google Scholar]
- Asano, H. Studies on the congrid eels of Japan. Bull. Misaki Mar. Biol. Inst. Kyoto Univ. 1962, 1, 1–143. [Google Scholar]
- Karmovskaya, E.S. New species of conger eels (Congridae) from southeastern Pacific Seamounts. J. Ichthyol. 1990, 30, 1–10. [Google Scholar]
- Karmovskaya, E.S. Benthopelagic bathyal conger eels of families Congridae and Nettastomatidae from the western tropical Pacific, with descriptions of ten new species. J. Ichthyol. 2004, 44, S1–S32. [Google Scholar]
- Love, M.S.; Mecklenburg, C.W.; Mecklenburg, T.A.; Thorsteinson, L.K. Resource Inventory of Marine and Estuarine Fishes of the West Coast and Alaska: A Checklist of North Pacific and Arctic Ocean Species from Baja California to the Alaska-Yukon Border; US Geological Survey: Seattle, WA, USA, 2005; pp. ix + 286.
- Iwai, T. Rhynchocymba nystromi nystromi (Jordan et Snyder). In Colored Illustrations of Bottom Fishes Collected by Japanese Trawlers; Hatanaka, H., Ikeda, I., Kawahara, S., Kono, H., Nagai, T., Sasaki, T., Sato, T., Uyeno, T., Eds.; Japan Deep Sea Trawlers Association: Tokyo, Japan, 1976; Volume II, p. 145. [Google Scholar]
- Borets, L.A. Ichthyofauna of the Northwestern and Hawaiian underwater ridges. J. Ichthyol. 1986, 26, 1–13. [Google Scholar]
- Novikov, N.P.; Kodolov, L.S.; Gavrilov, G.M. Preliminary list of fishes of the Emperor Underwater Ridge. In Fishes of the Open Ocean; Institute of Oceanology of the USSR Academy of Sciences: Moscow, USSR, 1981; pp. 32–35. [Google Scholar]
- Jordan, D.S.; Snyder, J.O. A review of the apodal fishes or eels of Japan, with descriptions of nineteen new species. Proc. U.S. Natl. Mus. 1901, 23, 837–890. [Google Scholar] [CrossRef]
- Bleeker, P. Vijfde bijdrage tot de kennis der ichthyologische fauna van Japan. Acta Soc. Reg. Sci. Indo-Neêrland 1858, 5, 1–12. [Google Scholar]
- Castle, P.H.J. The systematics, development and distribution of two eels of the genus Gnathophis (Congridae) in Australasian waters. Zool. Publ. Vic. Univ. Wellingt. 1963, 34, 15–47. [Google Scholar]
- Kotthaus, A. Fische des Indischen Ozeans. A. Systematischer Teil. III. Ostariophysi und Apodes. Meteor Forschungsergebnisse Reihe D Biol. 1968, 3, 14–56. [Google Scholar]
- Maul, G.E. On a new species of eel of the genus Gnathophis (Apodes, Congridae) from the Meteor Seamount. Bocagiana. Mus. Munic. Funchal (História Nat.) 1972, 31, 1–7. [Google Scholar]
- Karmovskaya, E.S.; Paxton, J.R. Revision of the Australian congrid eels of the genus Gnathophis (family Congridae), with descriptions of six new species. J. Ichthyol. 2000, 40, S1–S14. [Google Scholar]
- Wade, C.B. Two new genera and five new species of apodal fishes from the eastern Pacific. In Allan Hancock Pacific Expeditions 1932–40; University of Southern California Press: Los Angeles, CA, USA, 1946; Volume 9, pp. 181–213+24–28 pls. [Google Scholar]
- Smith, D.G.; Kanazawa, R.H. Eight new species and a new genus of congrid eels from the western north Atlantic with redescriptions of Ariosoma analis, Hildebrandia guppyi, and Rhechias vicinalis. Bull. Mari. Sci. 1977, 27, 530–543. [Google Scholar]
- Springer, V.G. Pacific plate biogeography, with special reference to shorefishes. Smith. Contr. Zool. 1982, 367, 1–182. [Google Scholar] [CrossRef][Green Version]
- Parin, N.V. Preliminary review of fish fauna of the Nazca and Sala-y-Gomez submarine ridges (southern East Pacific Ocean). Tr. Inst. Okeanol. USSR Acad. Sci. 1990, 125, 6–36. [Google Scholar]
- McCosker, J.E. A review of the eel genera Leptenchelys and Muraenichthys, with the description of a new genus, Schismorhynchus, and a new species, Muraenichthys chilensis. Pac. Sci. 1970, 24, 506–516. [Google Scholar]
- Smith, D.G. Guide to the Leptocephali (Elopiformes, Anguilliformes, and Notacanthiformes); NOAA Technical Report NMFS CIRC, 424; NOAA: Silver Spring, MD, USA, 1979; pp. 1–39. [Google Scholar]
- Lavenberg, R.J. A New Moray Eel (Muraenidae: Gymnothorax) from Oceanic Islands of the South Pacific. Pac. Sci. 1992, 46, 58–67. [Google Scholar]
- Pillans, B.; Chappell, J.; Naish, T.R. A review of the Milankovitch climatic beat: Template for Plio–Pleistocene sea-level changes and sequence stratigraphy. Sediment. Geol. 1998, 122, 5–21. [Google Scholar] [CrossRef]
- Kukuev, E.I. Formation of extratropical (moderately cold-water and bipolar) ranges of species of mesopelagic fish of high-latitude areas of the World Ocean. J. Ichthyol. 2014, 54, 790–807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).